Bringing Homogeneous Iron Catalysts on the Heterogeneous Side: Solutions for Immobilization
Abstract
:1. Introduction
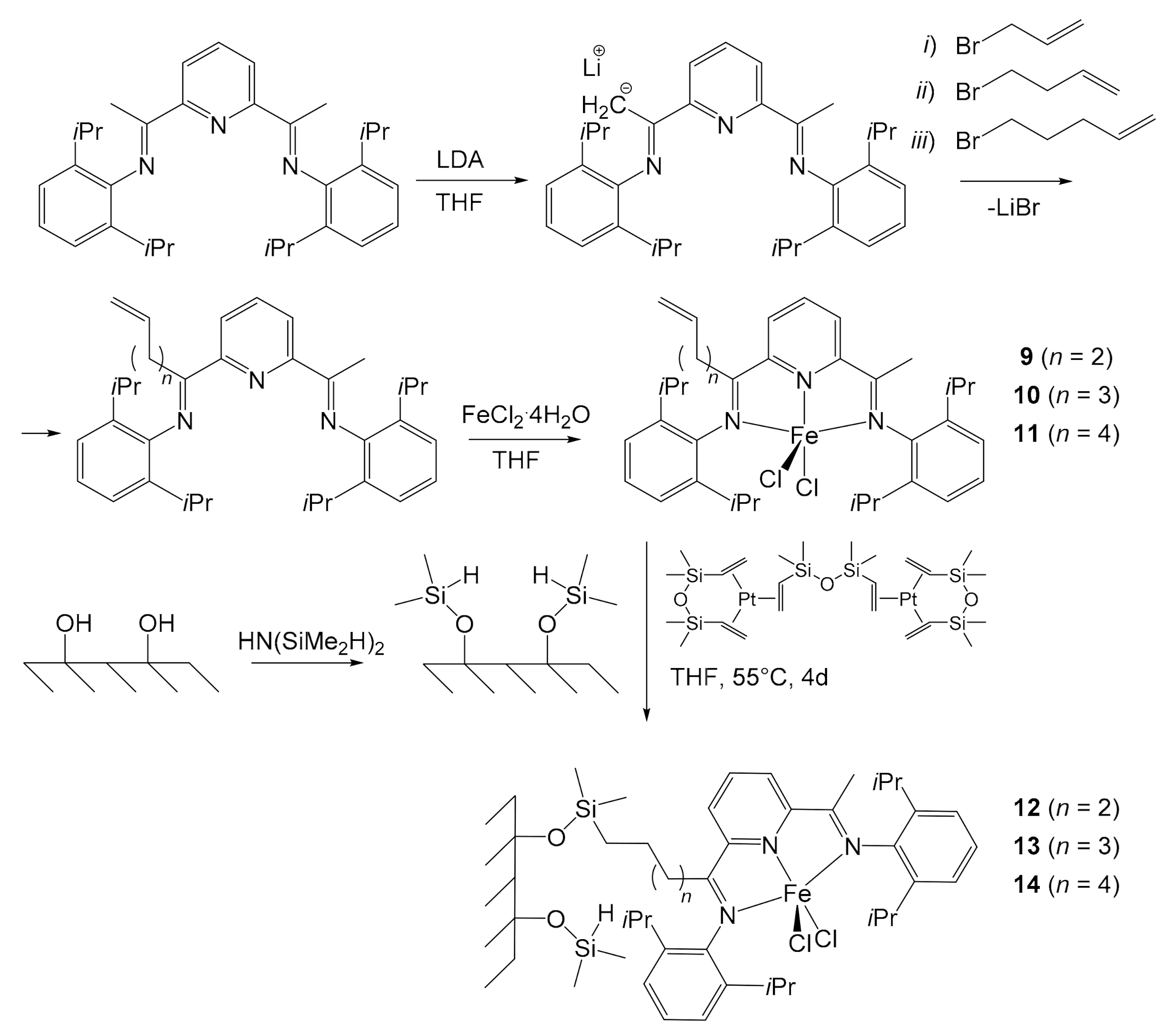
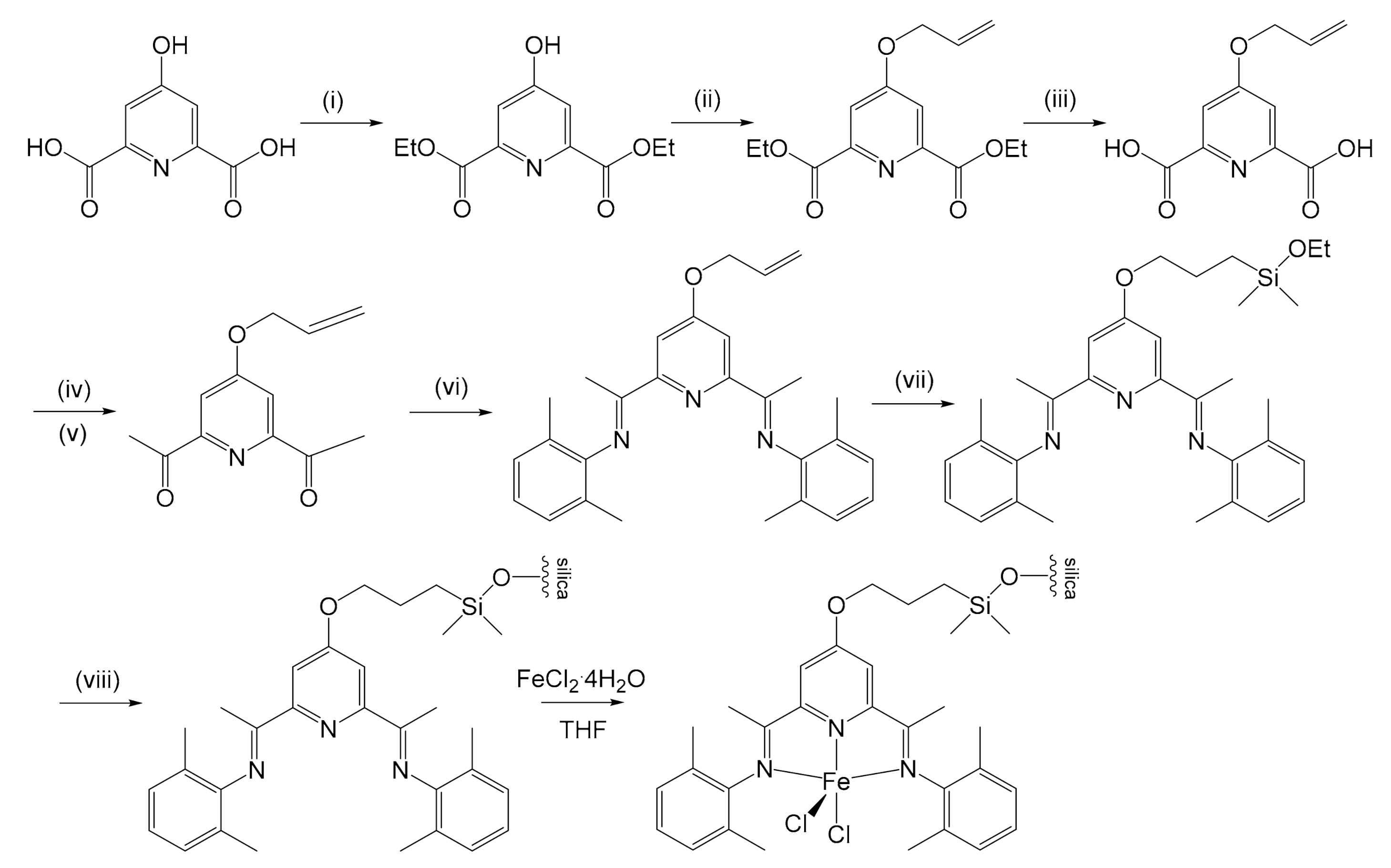
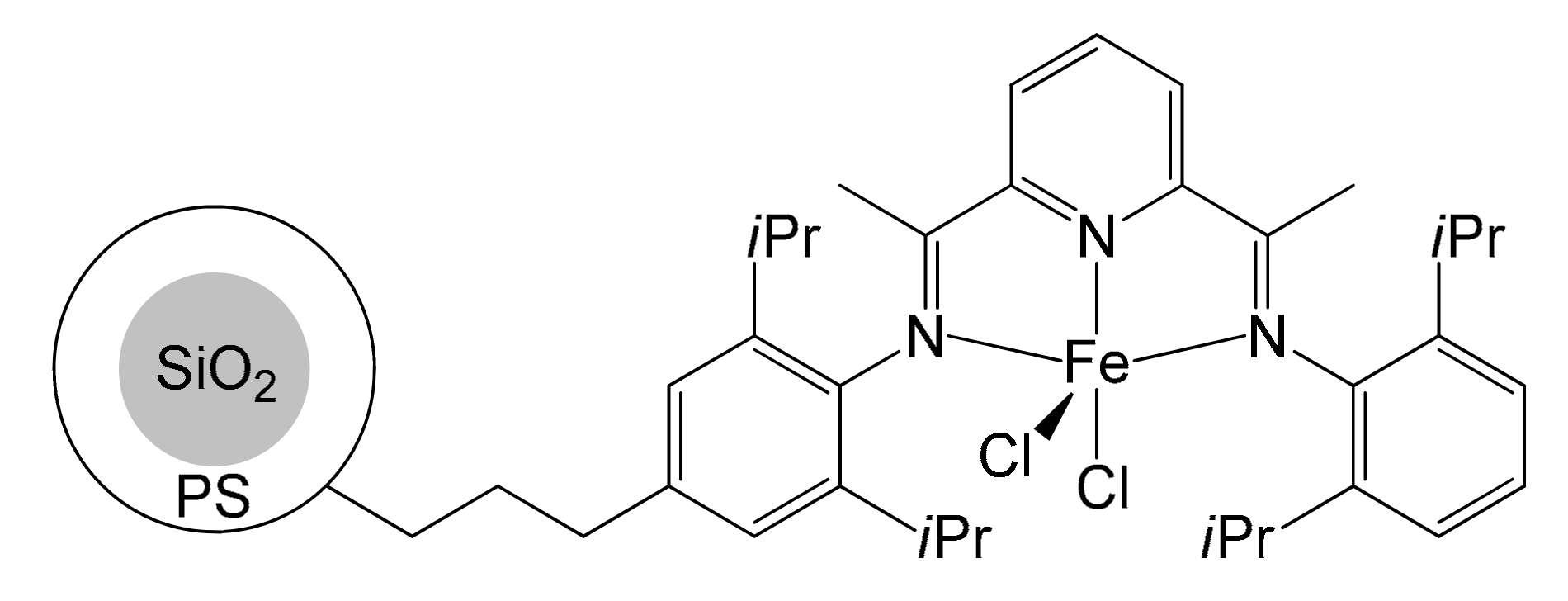
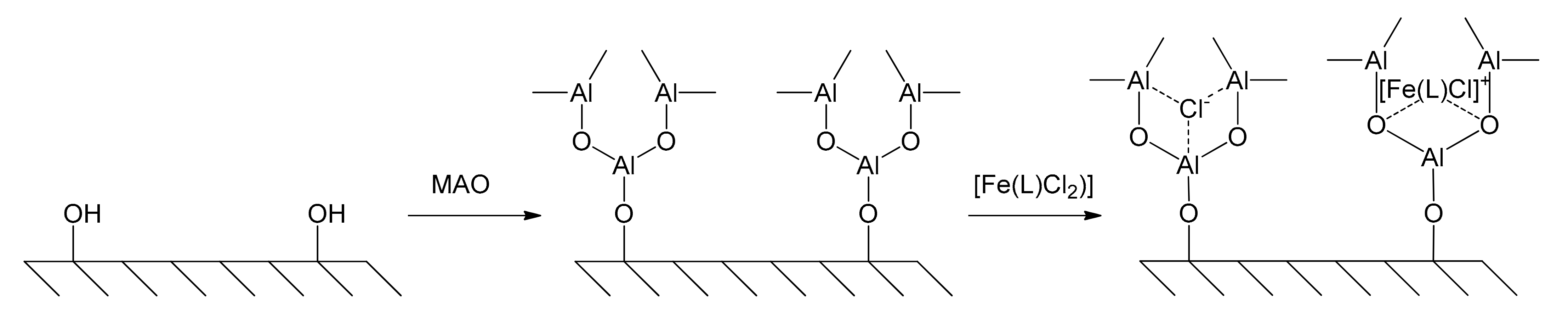
2. Heterogenization of Iron Catalysts for Olefin Polymerization
- (i)
- using MAO as co-catalyst, all the complexes had given good performances in the synthesis of PE, both in terms of yield and of average molecular weight, with excellent results in the case of the most encumbered complexes (catalysts 1 and 3 in Figure 2);
- (ii)
- the synthesis of the bis(arylimino)pyridyl ligands took place with high yields. If we exclude the aldimine derivatives, which had, however, revealed less-interesting performances, all the ligands could be obtained from reagents already available on the market (2,6-diacetylpyridine, anilines and solvents). Finally, the complexation with iron took place in less than 2 h and it was almost quantitative;
- (iii)
- the obtained PE turned out to be linear and of high density, so certainly interesting from an application point of view.
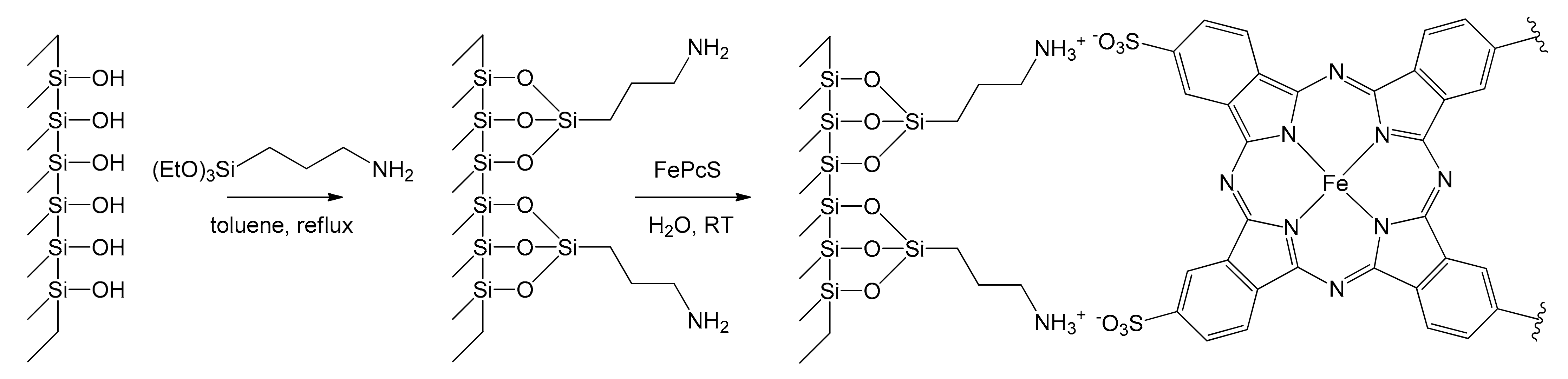
3. Immobilization of Iron Catalysts on Molecular Sieves for Oxidation Reactions
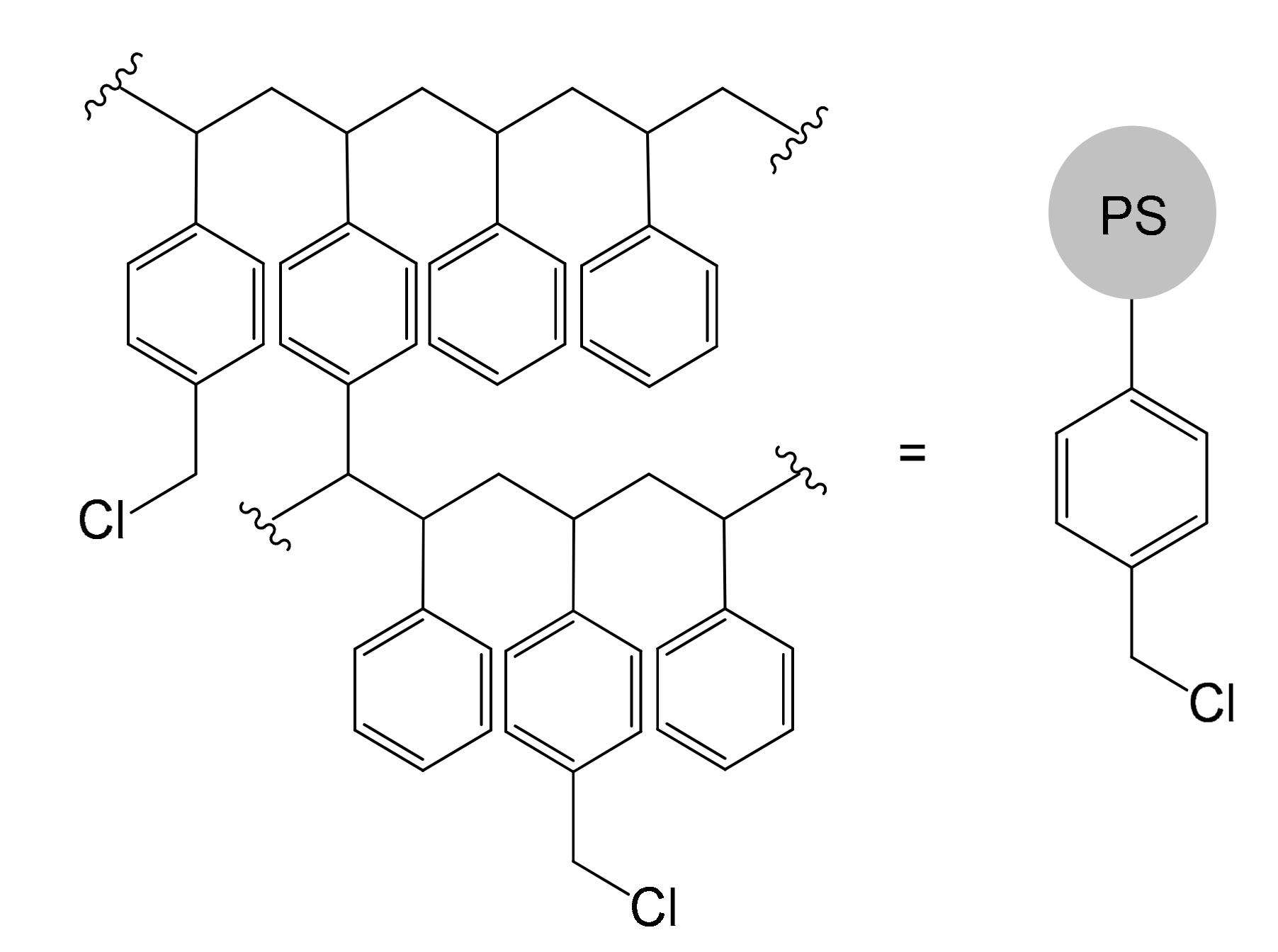
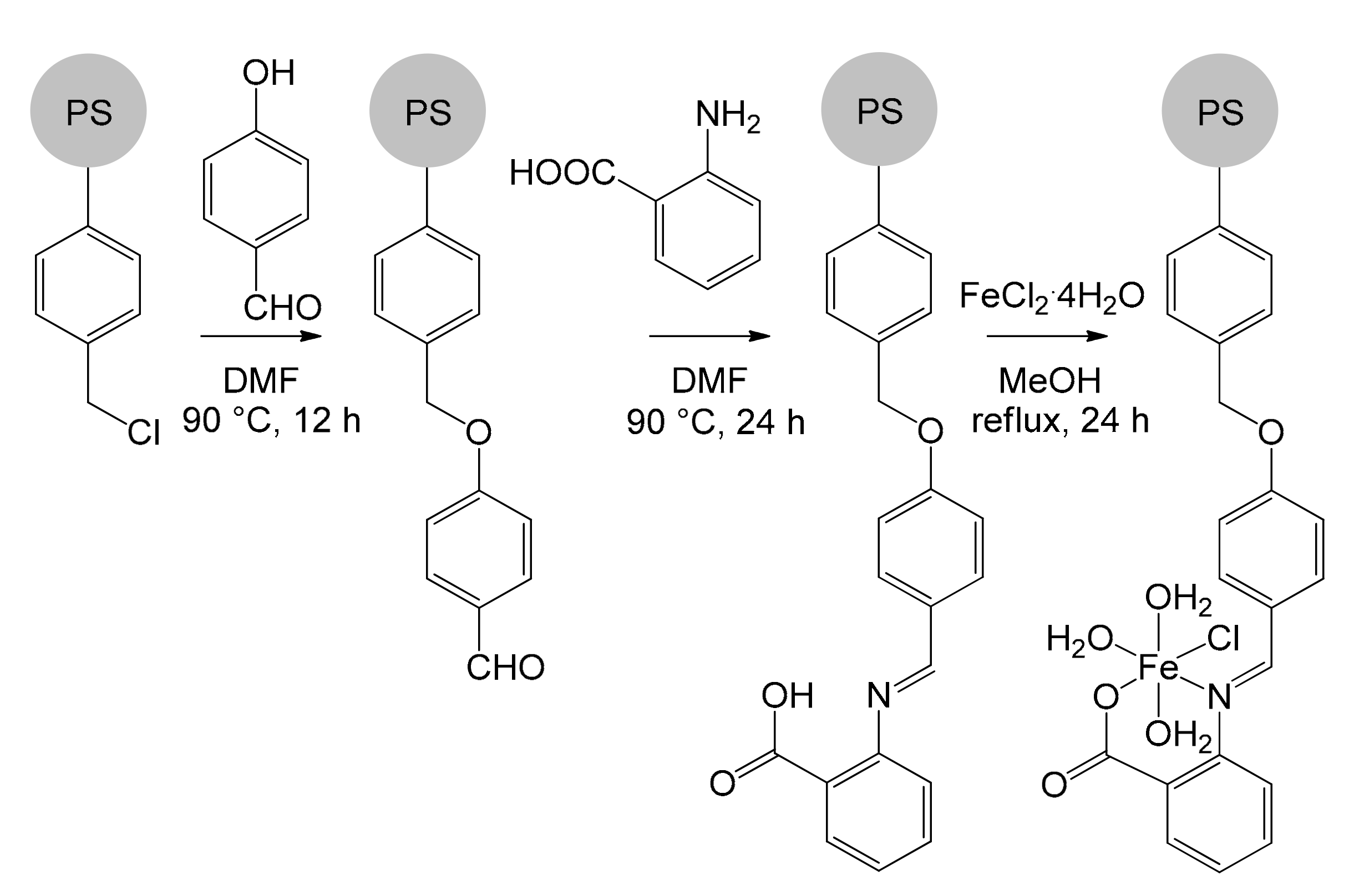
4. Heterogenization of Iron Catalysts on Merrifield Resin
5. Heterogenization through Catalytic SILP Systems
- (i)
- the large quantities of IL compromise the affordability of the process, both for the intrinsic cost of the solvent and for the costs of its disposal and/or recycling;
- (ii)
- separation of the two phases, the polar and the apolar ones, requires the use of organic solvents, increasing costs and lowering the environmental sustainability;
- (iii)
- the typically high viscosity of ILs results in problems related to the transfer of matter, confining the reaction to a thin layer near the interface between the two phases. This leads to substantially inactive catalytic species dispersed in the ILs.
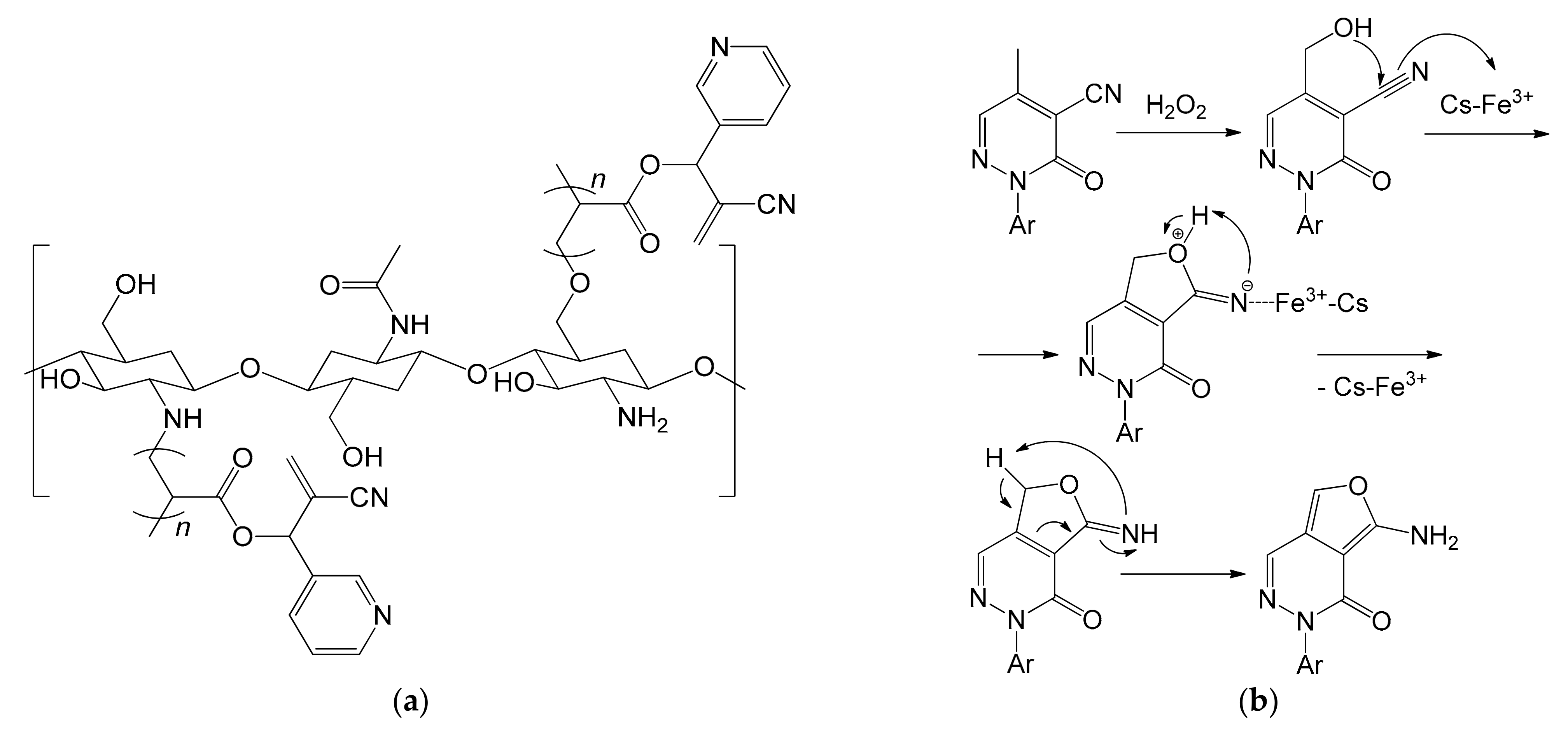
6. Heterogenization of Iron Compounds on Chitosan
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| IL | Ionic Liquid |
| SILP | Supported Ionic Liquid Phase |
| MAO | Methylaluminoxane |
| MMAO | Modified MAO |
| PE | Polyethylene |
| LDA | Diisopropylamide |
| PS | Polystyrene |
| AIBN | Azobisisobutyronitrile |
| TOF | turnover frequency |
| TPP | Tetraphenylporphyrin |
| DBU | 1,5-diazabiciclo(5.4.0)undec-7-ene |
| DAD | Degree of deacetylation |
| CPA | 2-cyano-1-(pyridine-3-yl)allyl acrylate |
| iPrDPI | 2,6-bis(2,6-diisopropylphenylimino)pyridine) |
| THF | Tetrahydrofuran |
| EtOH | Ethanol |
| DMF | Dimethylformammide |
| MeLi | Methyllithium |
| AcOH | Acetic acid |
| Et2O | Diethyl ether |
| MeOH | Methanol |
| PNP | Pincer ligand with phosphorus, nitrogen, phosphorus donor atoms |
| NNN | Pincer ligand with three nitrogen donor atoms |
| DFT | Density Functional Theory |
| bm2im | 1-butyl-2,3-dimethylimidazolium |
| NTf2 | Bis(trifluoromethylsulfonyl)imide |
| MCM-xx | Mobil Composition of Matter n° xx. |
| FePcS | Iron tetrasulfophtalocyanine |
| CPTES | 3-chloropropyltrietoxysilane |
| MAS-NMR | Magic Angle Spinning-Nuclear Magnetic Resonance |
| en | Ethylenediamine |
| bpy | 2,2-bipyridine |
| salen | N,N’-bis(salicylidene)ethylenediamine |
| TMC | 5,7,12,14-tetramethyl-1,4,8,11-tetraazacyclotetradeca-4,6,11,13-tetraene |
References
- Bauer, I.; Knölker, H.-J. Iron Catalysis in Organic Synthesis. Chem. Rev. 2015, 115, 3170–3387. [Google Scholar] [CrossRef] [PubMed]
- Enthaler, S.; Junge, K.; Beller, M. Sustainable Metal Catalysis with Iron: From Rust to a Rising Star? Angew. Chem. Int. Ed. 2008, 47, 3317–3321. [Google Scholar] [CrossRef] [PubMed]
- Bystrzanowska, M.; Petkov, P.; Tobiszewski, M. Ranking of Heterogeneous Catalysts Metals by Their Greenness. ACS Sustain. Chem. Eng. 2019, 7, 18434–18443. [Google Scholar] [CrossRef]
- Chen, J.; Browne, W.R. Photochemistry of Iron Complexes. Coord. Chem. Rev. 2018, 374, 15–35. [Google Scholar] [CrossRef]
- Fürstner, A. Iron Catalysis in Organic Synthesis: A Critical Assessment of What It Takes to Make This Base Metal a Multitasking Champion. ACS Cent. Sci. 2016, 2, 778–789. [Google Scholar] [CrossRef]
- Lindh, L.; Chábera, P.; Rosemann, N.W.; Uhlig, J.; Wärnmark, K.; Yartsev, A.; Sundström, V.; Persson, P. Photophysics and Photochemistry of Iron Carbene Complexes for Solar Energy Conversion and Photocatalysis. Catalysts 2020, 10, 315. [Google Scholar] [CrossRef] [Green Version]
- Wani, W.A.; Baig, U.; Shreaz, S.; Shiekh, R.A.; Iqbal, P.F.; Jameel, E.; Ahmad, A.; Mohd-Setapar, S.H.; Mushtaque, M.; Ting Hun, L. Recent Advances in Iron Complexes as Potential Anticancer Agents. New J. Chem. 2016, 40, 1063–1090. [Google Scholar] [CrossRef]
- Cingolani, A.; Cesari, C.; Zacchini, S.; Zanotti, V.; Cassani, M.C.; Mazzoni, R. Straightforward Synthesis of Iron Cyclopentadienone N-Heterocyclic Carbene Complexes. Dalton Trans. 2015, 44, 19063–19067. [Google Scholar] [CrossRef]
- Bridonneau, N.; Rigamonti, L.; Poneti, G.; Pinkowicz, D.; Forni, A.; Cornia, A. Evidence of Crystal Packing Effects in Stabilizing High or Low Spin States of Iron(II) Complexes with Functionalized 2,6-Bis(Pyrazol-1-Yl)Pyridine Ligands. Dalton Trans. 2017, 46, 4075–4085. [Google Scholar] [CrossRef]
- Rigamonti, L.; Vaccari, M.; Roncaglia, F.; Baschieri, C.; Forni, A. New Silver(I) Coordination Polymer with Fe4 Single-Molecule Magnets as Long Spacer. Magnetochemistry 2018, 4, 43. [Google Scholar] [CrossRef] [Green Version]
- Cingolani, A.; Zanotti, V.; Zacchini, S.; Massi, M.; Simpson, P.V.; Maheshkumar Desai, N.; Casari, I.; Falasca, M.; Rigamonti, L.; Mazzoni, R. Synthesis, Reactivity and Preliminary Biological Activity of Iron(0) Complexes with Cyclopentadienone and Amino-Appended N-Heterocyclic Carbene Ligands: Reactivity and Bio-Activity of Amino-Appended NHC Iron(0) Complexes. Appl. Organomet. Chem. 2019, 33, e4779. [Google Scholar] [CrossRef]
- Mazzoni, R.; Gabiccini, A.; Cesari, C.; Zanotti, V.; Gualandi, I.; Tonelli, D. Diiron Complexes Bearing Bridging Hydrocarbyl Ligands as Electrocatalysts for Proton Reduction. Organometallics 2015, 34, 3228–3235. [Google Scholar] [CrossRef]
- Arrigoni, F.; Bertini, L.; De Gioia, L.; Cingolani, A.; Mazzoni, R.; Zanotti, V.; Zampella, G. Mechanistic Insight into Electrocatalytic H2 Production by [Fe2(CN){μ-CN(Me)2}(μ-CO)(CO)(Cp)2]: Effects of Dithiolate Replacement in [FeFe] Hydrogenase Models. Inorg. Chem. 2017, 56, 13852–13864. [Google Scholar] [CrossRef]
- Arrigoni, F.; Bertini, L.; De Gioia, L.; Zampella, G.; Mazzoni, R.; Cingolani, A.; Gualandi, I.; Tonelli, D.; Zanotti, V. On the Importance of Cyanide in Diiron Bridging Carbyne Complexes, Unconventional [FeFe]-Hydrogenase Mimics without Dithiolate: An Electrochemical and DFT Investigation. Inorg. Chim. Acta 2020, 510, 119745. [Google Scholar] [CrossRef]
- Cingolani, A.; Gualandi, I.; Scavetta, E.; Cesari, C.; Zacchini, S.; Tonelli, D.; Zanotti, V.; Franchi, P.; Lucarini, M.; Sicilia, E.; et al. Cyclopentadienone–NHC Iron(0) Complexes as Low Valent Electrocatalysts for Water Oxidation. Catal. Sci. Technol. 2021, 11, 1407–1418. [Google Scholar] [CrossRef]
- Bolm, C. A New Iron Age. Nat. Chem. 2009, 1, 420. [Google Scholar] [CrossRef] [Green Version]
- Iron Catalysis in Organic Chemistry: Reactions and Applications; Plietker, B. (Ed.) Wiley-VCH: Weinheim, Germany, 2008; ISBN 978-3-527-31927-5. [Google Scholar]
- Bart, S.C.; Lobkovsky, E.; Chirik, P.J. Preparation and Molecular and Electronic Structures of Iron(0) Dinitrogen and Silane Complexes and Their Application to Catalytic Hydrogenation and Hydrosilation. J. Am. Chem. Soc. 2004, 126, 13794–13807. [Google Scholar] [CrossRef]
- Knölker, H.-J.; Baum, E.; Goesmann, H.; Klauss, R. Demetalation of Tricarbonyl(Cyclopentadienone)Iron Complexes Initiated by a Ligand Exchange Reaction with NaOH—X-Ray Analysis of a Complex with Nearly Square-Planar Coordinated Sodium. Angew. Chem. Int. Ed. 1999, 38, 2064–2066. [Google Scholar] [CrossRef]
- Mikhailine, A.; Lough, A.J.; Morris, R.H. Efficient Asymmetric Transfer Hydrogenation of Ketones Catalyzed by an Iron Complex Containing a P−N−N−P Tetradentate Ligand Formed by Template Synthesis. J. Am. Chem. Soc. 2009, 131, 1394–1395. [Google Scholar] [CrossRef]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From Visualization to Analysis, Design and Prediction. J. Appl. Crystallogr. 2020, 53, 226–235. [Google Scholar] [CrossRef] [Green Version]
- Coggins, M.K.; Zhang, M.-T.; Vannucci, A.K.; Dares, C.J.; Meyer, T.J. Electrocatalytic Water Oxidation by a Monomeric Amidate-Ligated Fe(III)—Aqua Complex. J. Am. Chem. Soc. 2014, 136, 5531–5534. [Google Scholar] [CrossRef]
- Coggins, M.K.; Zhang, M.-T.; Vannucci, A.K.; Dares, C.J.; Meyer, T.J. Correction to “Electrocatalytic Water Oxidation by a Monomeric Amidate-Ligated Fe(III)—Aqua Complex”. J. Am. Chem. Soc. 2014, 136, 7186. [Google Scholar] [CrossRef]
- Okamura, M.; Kondo, M.; Kuga, R.; Kurashige, Y.; Yanai, T.; Hayami, S.; Praneeth, V.K.K.; Yoshida, M.; Yoneda, K.; Kawata, S.; et al. A Pentanuclear Iron Catalyst Designed for Water Oxidation. Nature 2016, 530, 465–468. [Google Scholar] [CrossRef]
- Kottrup, K.G.; D’Agostini, S.; van Langevelde, P.H.; Siegler, M.A.; Hetterscheid, D.G.H. Catalytic Activity of an Iron-Based Water Oxidation Catalyst: Substrate Effects of Graphitic Electrodes. ACS Catal. 2018, 8, 1052–1061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavarelli, G.; Velasquez Ochoa, J.; Caldarelli, A.; Puzzo, F.; Cavani, F.; Dubois, J.-L. A New Process for Maleic Anhydride Synthesis from a Renewable Building Block: The Gas-Phase Oxidehydration of Bio-1-Butanol. ChemSusChem 2015, 8, 2250–2259. [Google Scholar] [CrossRef] [PubMed]
- Vásquez, P.B.; Tabanelli, T.; Monti, E.; Albonetti, S.; Bonincontro, D.; Dimitratos, N.; Cavani, F. Gas-Phase Catalytic Transfer Hydrogenation of Methyl Levulinate with Ethanol over ZrO2. ACS Sustain. Chem. Eng. 2019, 7, 8317–8330. [Google Scholar] [CrossRef]
- Aitchison, H.; Wingad, R.L.; Wass, D.F. Homogeneous Ethanol to Butanol Catalysis—Guerbet Renewed. ACS Catal. 2016, 6, 7125–7132. [Google Scholar] [CrossRef] [Green Version]
- Pellow, K.J.; Wingad, R.L.; Wass, D.F. Towards the Upgrading of Fermentation Broths to Advanced Biofuels: A Water Tolerant Catalyst for the Conversion of Ethanol to Isobutanol. Catal. Sci. Technol. 2017, 7, 5128–5134. [Google Scholar] [CrossRef] [Green Version]
- Tseng, K.-N.T.; Lin, S.; Kampf, J.W.; Szymczak, N.K. Upgrading Ethanol to 1-Butanol with a Homogeneous Air-Stable Ruthenium Catalyst. Chem. Commun. 2016, 52, 2901–2904. [Google Scholar] [CrossRef]
- Xie, Y.; Ben-David, Y.; Shimon, L.J.W.; Milstein, D. Highly Efficient Process for Production of Biofuel from Ethanol Catalyzed by Ruthenium Pincer Complexes. J. Am. Chem. Soc. 2016, 138, 9077–9080. [Google Scholar] [CrossRef]
- Mazzoni, R.; Cesari, C.; Zanotti, V.; Lucarelli, C.; Tabanelli, T.; Puzzo, F.; Passarini, F.; Neri, E.; Marani, G.; Prati, R.; et al. Catalytic Biorefining of Ethanol from Wine Waste to Butanol and Higher Alcohols: Modeling the Life Cycle Assessment and Process Design. ACS Sustain. Chem. Eng. 2019, 7, 224–237. [Google Scholar] [CrossRef]
- Ostwald, W. Uber Katalyse; Akademische Verlagsgesellschaft: Leipzig, Germany, 1923. [Google Scholar]
- Sauer, J.; Freund, H.-J. Models in Catalysis. Catal. Lett. 2015, 145, 109–125. [Google Scholar] [CrossRef] [Green Version]
- Cole-Hamilton, D.J.; Tooze, R.P. Homogeneous Catalysis—Advantages and Problems. In Catalyst Separation, Recovery and Recycling; Cole-Hamilton, D.J., Tooze, R.P., Eds.; Catalysis by Metal Complexes; Springer: Dordrecht, The Netherlands, 2006; Volume 30, pp. 1–8. ISBN 978-1-4020-4086-3. [Google Scholar]
- Busetto, L.; Cassani, M.C.; van Leeuwen, P.W.N.M.; Mazzoni, R. Synthesis of New Poly(Propylenimine) Dendrimers DAB-Dendr-[NH(O)COCH2CH2OC(O)C5H4Rh(NBD)]n {n = 4, 8, 16, 32, 64} Functionalized with Alkoxycarbonylcyclopentadienyl Complexes of Rhodium(I). Dalton Trans. 2004, 2767–2770. [Google Scholar] [CrossRef]
- Zuccaccia, D.; Busetto, L.; Cassani, M.C.; Macchioni, A.; Mazzoni, R. PGSE NMR Studies on DAB-Organo-Rhodium Dendrimers: Evaluation of the Molecular Size, Self-Aggregation Tendency, and Surface Metal Density. Organometallics 2006, 25, 2201–2208. [Google Scholar] [CrossRef]
- Busetto, L.; Buldini, P.L.; Cassani, M.C.; Mazzoni, R. Clean and Efficient Synthesis of Air Stable Polymer-Supported Alkoxycarbonylcyclopentadienyl Rhodium(I) Complexes. J. Organomet. Chem. 2006, 691, 573–578. [Google Scholar] [CrossRef]
- Cesari, C.; Conti, R.; Cingolani, A.; Zanotti, V.; Cassani, M.C.; Rigamonti, L.; Mazzoni, R. Synthesis and Reactivity of Poly(Propyleneimine) Dendrimers Functionalized with Cyclopentadienone N-Heterocyclic-Carbene Ruthenium(0) Complexes. Catalysts 2020, 10, 264. [Google Scholar] [CrossRef] [Green Version]
- Cesari, C.; Cingolani, A.; Teti, M.; Messori, A.; Zacchini, S.; Zanotti, V.; Mazzoni, R. Imidazolium Salts of Ruthenium Anionic Cyclopentadienone Complexes: Ion Pair for Bifunctional Catalysis in Ionic Liquids. Eur. J. Inorg. Chem. 2020, 2020, 1114–1122. [Google Scholar] [CrossRef]
- Cingolani, A.; Zanotti, V.; Cesari, C.; Ferri, M.; Mazzocchetti, L.; Benelli, T.; Merighi, S.; Giorgini, L.; Mazzoni, R. Synthesis of Functionalized Iron N-Heterocyclic Carbene Complexes and Their Potential Application as Flame Behavior Modifier in Cross Linked Epoxy Resins. Inorg. Chim. Acta 2021, in press. [Google Scholar] [CrossRef]
- Copéret, C.; Comas-Vives, A.; Conley, M.P.; Estes, D.P.; Fedorov, A.; Mougel, V.; Nagae, H.; Nunez-Zarur, F.; Zhizhko, P.A. Surface Organometallic and Coordination Chemistry toward Single-Site Heterogeneous Catalysts: Strategies, Methods, Structures, and Activities. Chem. Rev. 2016, 116, 323–421. [Google Scholar] [CrossRef]
- Johnson, L.K.; Killian, C.M.; Brookhart, M. New Pd(II)- and Ni(II)-Based Catalysts for Polymerization of Ethylene and Alpha-Olefins. J. Am. Chem. Soc. 1995, 117, 6414–6415. [Google Scholar] [CrossRef]
- Small, B.L.; Brookhart, M.; Bennett, A.M.A. Highly Active Iron and Cobalt Catalysts for the Polymerization of Ethylene. J. Am. Chem. Soc. 1998, 120, 4049–4050. [Google Scholar] [CrossRef]
- Britovsek, G.J.P.; Bruce, M.; Gibson, V.C.; Kimberley, B.S.; Maddox, P.J.; Mastroianni, S.; McTavish, S.J.; Redshaw, C.; Solan, G.A.; Strömberg, S.; et al. Iron and Cobalt Ethylene Polymerization Catalysts Bearing 2,6-Bis(Imino)Pyridyl Ligands: Synthesis, Structures, and Polymerization Studies. J. Am. Chem. Soc. 1999, 121, 8728–8740. [Google Scholar] [CrossRef]
- Kaul, F.A.R.; Puchta, G.T.; Schneider, H.; Bielert, F.; Mihalios, D.; Herrmann, W.A. Immobilization of Bis(Imino)Pyridyliron(II) Complexes on Silica. Organometallics 2002, 21, 74–82. [Google Scholar] [CrossRef]
- Fink, G.; Steinmetz, B.; Zechlin, J.; Przybyla, C.; Tesche, B. Propene Polymerization with Silica-Supported Metallocene/MAO Catalysts. Chem. Rev. 2000, 100, 1377–1390. [Google Scholar] [CrossRef]
- Scavetta, E.; Mazzoni, R.; Mariani, F.; Margutta, R.G.; Bonfiglio, A.; Demelas, M.; Fiorilli, S.; Marzocchi, M.; Fraboni, B. Dopamine Amperometric Detection at a Ferrocene Clicked PEDOT:PSS Coated Electrode. J. Mater. Chem. B 2014, 2, 2861–2867. [Google Scholar] [CrossRef]
- Lewis, L.N.; Stein, J.; Gao, Y.; Colborn, R.E.; Hutchins, G. Platinum Catalysts Used in the Silicones Industry. Their Synthesis and Activity in Hydrosilylation. Platinum Metals Rev. 1997, 41, 66–75. [Google Scholar]
- Kim, I.; Han, B.H.; Ha, C.-S.; Kim, J.-K.; Suh, H. Preparation of Silica-Supported Bis(Imino)Pyridyl Iron(II) and Cobalt(II) Catalysts for Ethylene Polymerization. Macromolecules 2003, 36, 6689–6691. [Google Scholar] [CrossRef]
- Zheng, Z.; Liu, J.; Li, Y. Ethylene Polymerization with Silica-Supported Bis(Imino)Pyridyl Iron(II) Catalysts. J. Catal. 2005, 234, 101–110. [Google Scholar] [CrossRef]
- Liu, C.; Jin, G. Polymer-Incorporated Iron Catalysts for Ethylene Polymerization—A New Approach to Immobilize Iron Olefin Catalysts on Polystyrene Chains. New J. Chem. 2002, 26, 1485–1489. [Google Scholar] [CrossRef]
- Paulino, I.S.; Schuchardt, U. Ethylene Polymerization Using Iron Catalysts Heterogenized in MCM-41. Catal. Commun. 2004, 5, 5–7. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, H.; Guo, C.; Ma, Z.; Dong, J.; Ke, Y.; Hu, Y. Ethylene Polymerization with Iron-Based Diimine Catalyst Supported on MCM-41. Polym. Int. 2005, 54, 274–278. [Google Scholar] [CrossRef]
- Pirouzmand, M.; Amini, M.M.; Safari, N. Immobilization of Iron Tetrasulfophthalocyanine on Functionalized MCM-48 and MCM-41 Mesoporous Silicas: Catalysts for Oxidation of Styrene. J. Colloid Interface Sci. 2008, 319, 199–205. [Google Scholar] [CrossRef]
- Costa, A.A.; Ghesti, G.F.; de Macedo, J.L.; Braga, V.S.; Santos, M.M.; Dias, J.A.; Dias, S.C.L. Immobilization of Fe, Mn and Co Tetraphenylporphyrin Complexes in MCM-41 and Their Catalytic Activity in Cyclohexene Oxidation Reaction by Hydrogen Peroxide. J. Mol. Catal. A Chem. 2008, 282, 149–157. [Google Scholar] [CrossRef]
- Adam, F.; Kueh, C.-W.; Ng, E.-P. The Immobilization of Iron(III) Aminopyridine Complex on MCM-41: Its Preparation and Characterization. J. Porous Mater. 2013, 20, 1337–1343. [Google Scholar] [CrossRef]
- Fan, B.; Li, H.; Fan, W.; Jin, C.; Li, R. Oxidation of Cyclohexane over Iron and Copper Salen Complexes Simultaneously Encapsulated in Zeolite Y. Appl. Catal. A Gen. 2008, 340, 67–75. [Google Scholar] [CrossRef]
- Halma, M.; Dias de Freitas Castro, K.A.; Taviotgueho, C.; Prevot, V.; Forano, C.; Wypych, F.; Nakagaki, S. Synthesis, Characterization, and Catalytic Activity of Anionic Iron(III) Porphyrins Intercalated into Layered Double Hydroxides. J. Catal. 2008, 257, 233–243. [Google Scholar] [CrossRef]
- Halma, M.; de Freitas Castro, K.A.D.; Prévot, V.; Forano, C.; Wypych, F.; Nakagaki, S. Immobilization of Anionic Iron(III) Porphyrins into Ordered Macroporous Layered Double Hydroxides and Investigation of Catalytic Activity in Oxidation Reactions. J. Mol. Catal. A Chem. 2009, 310, 42–50. [Google Scholar] [CrossRef]
- de Freitas Castro, K.A.D.; Wypych, F.; Antonangelo, A.; Mantovani, K.M.; Bail, A.; Ucoski, G.M.; Ciuffi, K.J.; Cintra, T.E.; Nakagaki, S. Selective Oxidation Catalysts Obtained by Immobilization of Iron(III) Porphyrins on Thiosalicylic Acid-Modified Mg-Al Layered Double Hydroxides. J. Colloid Interface Sci. 2016, 478, 374–383. [Google Scholar] [CrossRef]
- Rosa, I.L.V.; Manso, C.M.C.P.; Serra, O.A.; Iamamoto, Y. Biomimetical Catalytic Activity of Iron(III) Porphyrins Encapsulated in the Zeolite X. J. Mol. Catal. A Chem. 2000, 160, 199–208. [Google Scholar] [CrossRef]
- Moreira, M.S.M.; Martins, P.R.; Curi, R.B.; Nascimento, O.R.; Iamamoto, Y. Iron Porphyrins Immobilised on Silica Surface and Encapsulated in Silica Matrix: A Comparison of Their Catalytic Activity in Hydrocarbon Oxidation. J. Mol. Catal. A Chem. 2005, 233, 73–81. [Google Scholar] [CrossRef]
- Machado, A.M.; Wypych, F.; Drechsel, S.M.; Nakagaki, S. Study of the Catalytic Behavior of Montmorillonite/Iron(III) and Mn(III) Cationic Porphyrins. J. Colloid Interface Sci. 2002, 254, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Nakagaki, S.; Benedito, F.L.; Wypych, F. Anionic Iron(III) Porphyrin Immobilized on Silanized Kaolinite as Catalyst for Oxidation Reactions. J. Mol. Catal. A Chem. 2004, 217, 121–131. [Google Scholar] [CrossRef]
- Machado, G.S.; Wypych, F.; Nakagaki, S. Immobilization of Anionic Iron(III) Porphyrins onto in Situ Obtained Zinc Oxide. J. Colloid Interface Sci. 2012, 377, 379–386. [Google Scholar] [CrossRef] [PubMed]
- de Faria, E.H.; Ricci, G.P.; Marçal, L.; Nassar, E.J.; Vicente, M.A.; Trujillano, R.; Gil, A.; Korili, S.A.; Ciuffi, K.J.; Calefi, P.S. Green and Selective Oxidation Reactions Catalyzed by Kaolinite Covalently Grafted with Fe(III) Pyridine-Carboxylate Complexes. Catal. Today 2012, 187, 135–149. [Google Scholar] [CrossRef]
- Farzaneh, F.; Poorkhosravani, M.; Ghandi, M. Utilization of Immobilized Biomimetic Iron Complexes within Nanoreactors of Al-MCM-41 as Cyclohexane Oxidation Catalyst. J. Mol. Catal. A Chem. 2009, 308, 108–113. [Google Scholar] [CrossRef]
- Farzaneh, F.; Husseini, F.; Hamidipour, L.; Ghiasi, M. Synthesis, Characterization and Immobilization of Iron(III) Pyridoxinato Complex within Al-MCM-41 as Catalyst for Cycloalkanes Oxidation. J. Porous Mater. 2014, 21, 189–196. [Google Scholar] [CrossRef]
- Merrifield, R.B. Solid Phase Peptide Synthesis. I. The Synthesis of a Tetrapeptide. J. Am. Chem. Soc. 1963, 85, 2149–2154. [Google Scholar] [CrossRef]
- Vaino, A.R.; Janda, K.D. Solid-Phase Organic Synthesis: A Critical Understanding of the Resin. J. Comb. Chem. 2000, 2, 579–596. [Google Scholar] [CrossRef]
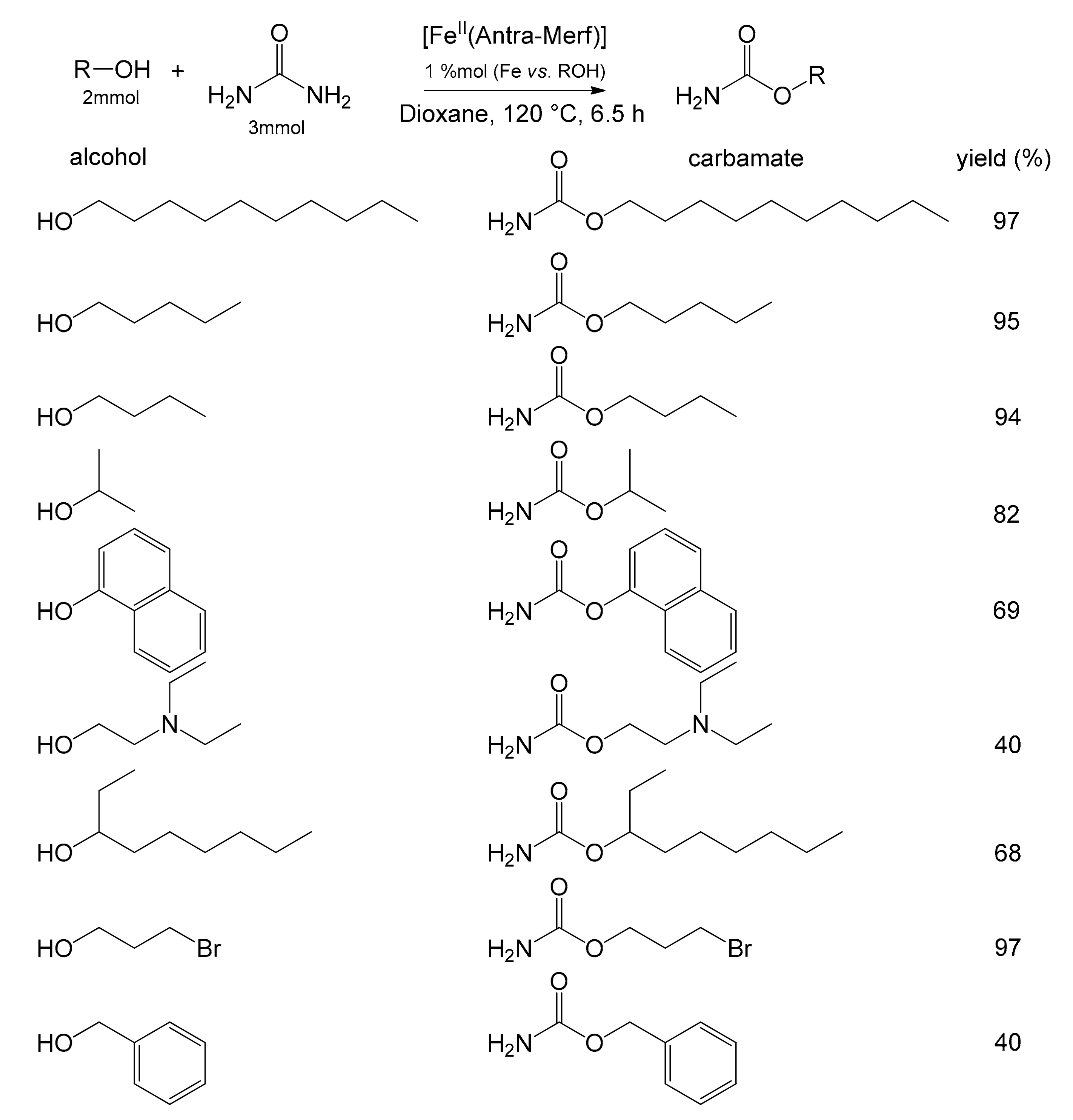
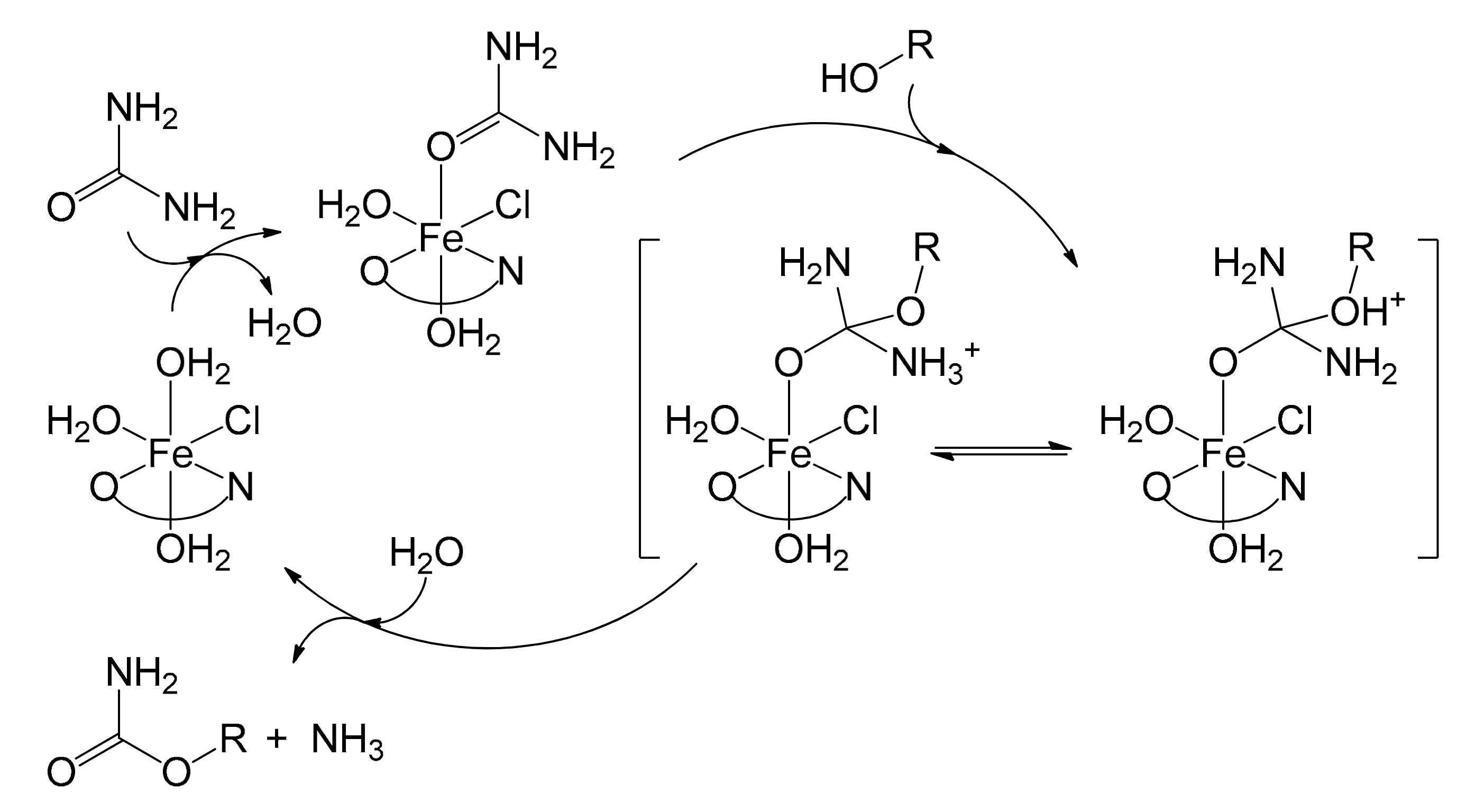
- Basu, P.; Dey, T.K.; Ghosh, A.; Biswas, S.; Khan, A.; Islam, S.M. An Efficient One-Pot Synthesis of Industrially Valuable Primary Organic Carbamates and N-Substituted Ureas by a Reusable Merrifield Anchored Iron(II)-Anthra Catalyst [FeII(Anthra-Merf)] Using Urea as a Sustainable Carbonylation Source. New J. Chem. 2020, 44, 2630–2643. [Google Scholar] [CrossRef]
- Ma, J.; Lu, N.; Qin, W.; Xu, R.; Wang, Y.; Chen, X. Differential Responses of Eight Cyanobacterial and Green Algal Species, to Carbamate Insecticides. Ecotoxicol. Environ. Saf. 2006, 63, 268–274. [Google Scholar] [CrossRef]
- Badreshia, S.; Marks, J.G., Jr. Iodopropynyl Butylcarbamate. Am. J. Contact Dermat. 2002, 13, 77–79. [Google Scholar] [CrossRef]
- Husár, B.; Liska, R. Vinyl Carbonates, Vinyl Carbamates, and Related Monomers: Synthesis, Polymerization, and Application. Chem. Soc. Rev. 2012, 41, 2395–2405. [Google Scholar] [CrossRef]
- Isidro-Llobet, A.; Álvarez, M.; Albericio, F. Amino Acid-Protecting Groups. Chem. Rev. 2009, 109, 2455–2504. [Google Scholar] [CrossRef] [Green Version]
- Watile, R.A.; Bhanage, B.M. Ruthenium Catalyzed Regioselective Coupling of Terminal Alkynes, Amine and Carbon Dioxide Leading to Anti-Markovnikov Adducts. RSC Adv. 2014, 4, 23022–23026. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, X.; Zheng, S. Enantioselective Domino Reaction of CO2, Amines and Allyl Chlorides under Iridium Catalysis: Formation of Allyl Carbamates. Chem. Commun. 2014, 50, 4455. [Google Scholar] [CrossRef]
- Ion, A.; Doorslaer, C.V.; Parvulescu, V.; Jacobs, P.; Vos, D.D. Green Synthesis of Carbamates from CO2, Amines and Alcohols. Green Chem. 2008, 10, 111–116. [Google Scholar] [CrossRef]
- Zhang, Q.; Yuan, H.-Y.; Fukaya, N.; Choi, J.-C. Alkali Metal Salt as Catalyst for Direct Synthesis of Carbamate from Carbon Dioxide. ACS Sustain. Chem. Eng. 2018, 6, 6675–6681. [Google Scholar] [CrossRef]
- Liu, M.; Liang, L.; Li, X.; Gao, X.; Sun, J. Novel Urea Derivative-Based Ionic Liquids with Dual-Functions: CO2 Capture and Conversion under Metal- and Solvent-Free Conditions. Green Chem. 2016, 18, 2851–2863. [Google Scholar] [CrossRef]
- Thavonekham, B. A Practical Synthesis of Ureas from Phenyl Carbamates. Synthesis 1997, 1997, 1189–1194. [Google Scholar] [CrossRef]
- Shi, F.; Deng, Y.; SiMa, T.; Peng, J.; Gu, Y.; Qiao, B. Alternatives to Phosgene and Carbon Monoxide: Synthesis of Symmetric Urea Derivatives with Carbon Dioxide in Ionic Liquids. Angew. Chem. Int. Ed. 2003, 115, 3379–3382. [Google Scholar] [CrossRef]
- Ballini, R.; Fiorini, D.; Maggi, R.; Righi, P.; Sartori, G.; Sartorio, R. TBD-Catalysed Solventless Synthesis of Symmetrically N,N′-Substituted Ureas from Primary Amines and Diethyl Carbonate. Green Chem. 2003, 5, 396–398. [Google Scholar] [CrossRef]
- Moulton, C.J.; Shaw, B.L. Transition Metal–Carbon Bonds. Part XLII. Complexes of Nickel, Palladium, Platinum, Rhodium and Iridium with the Tridentate Ligand 2,6-Bis[(Di-t-Butylphosphino)Methyl]Phenyl. J. Chem. Soc. Dalton Trans. 1976, 1020–1024. [Google Scholar] [CrossRef]
- Dahlhoff, W.V.; Nelson, S.M. Studies on the Magnetic Cross-over in Five-Co-Ordinate Complexes of Iron(II), Cobalt(II), and Nickel(II). Part II. J. Chem. Soc. A 1971, 2184–2190. [Google Scholar] [CrossRef]
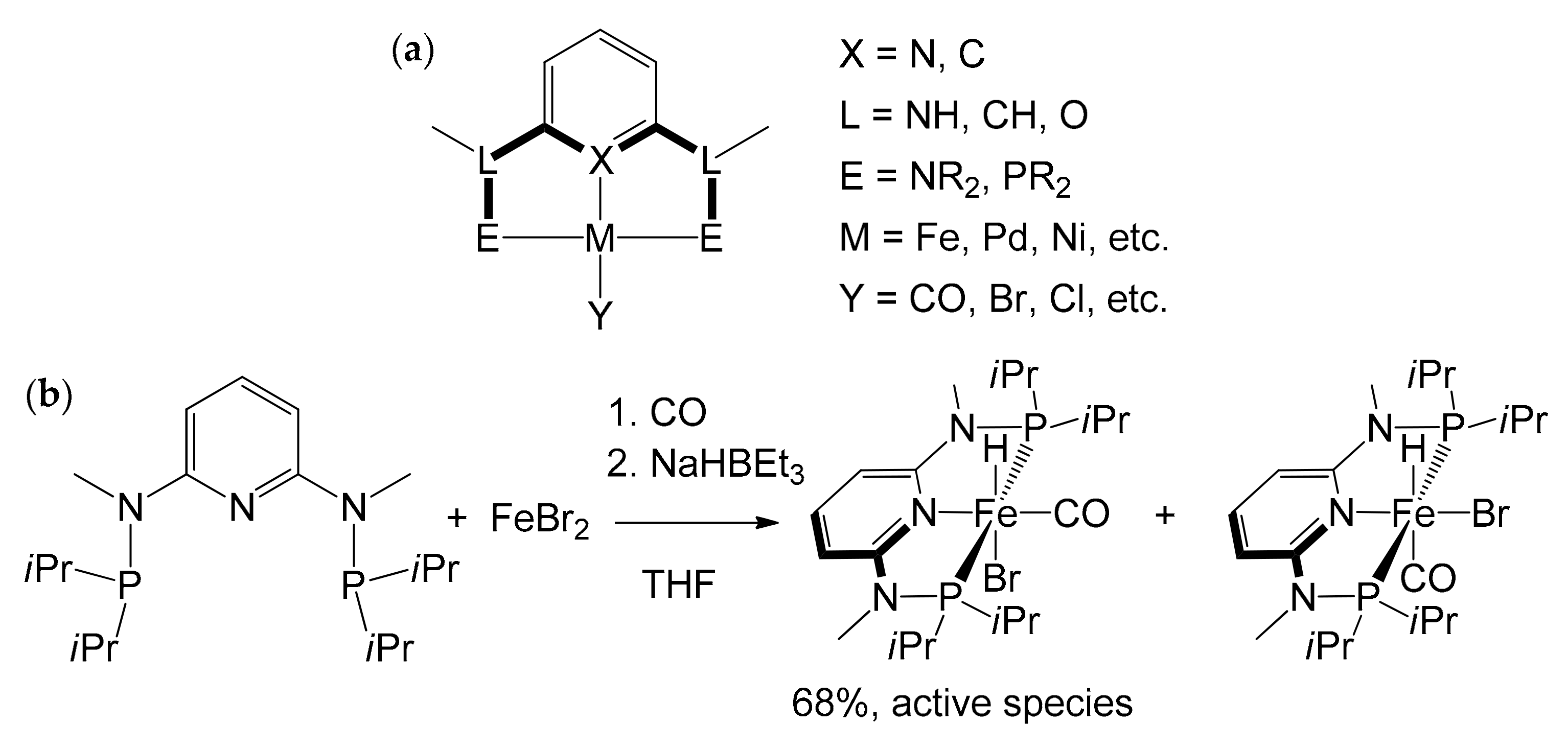
- Bauer, G.; Hu, X. Recent Developments of Iron Pincer Complexes for Catalytic Applications. Inorg. Chem. Front. 2016, 3, 741–765. [Google Scholar] [CrossRef] [Green Version]
- Alig, L.; Fritz, M.; Schneider, S. First-Row Transition Metal (De)Hydrogenation Catalysis Based on Functional Pincer Ligands. Chem. Rev. 2019, 119, 2681–2751. [Google Scholar] [CrossRef]
- González-Sebastián, L.; Morales-Morales, D. Cross-Coupling Reactions Catalysed by Palladium Pincer Complexes. A Review of Recent Advances. J. Organomet. Chem. 2019, 893, 39–51. [Google Scholar] [CrossRef]
- Kostera, S.; Peruzzini, M.; Kirchner, K.; Gonsalvi, L. Mild and Selective Carbon Dioxide Hydroboration to Methoxyboranes Catalyzed by Mn(I) PNP Pincer Complexes. ChemCatChem 2020, 12, 4625–4631. [Google Scholar] [CrossRef]
- Himmelbauer, D.; Stöger, B.; Veiros, L.F.; Pignitter, M.; Kirchner, K. Cr(II) and Cr(I) PCP Pincer Complexes: Synthesis, Structure, and Catalytic Reactivity. Organometallics 2019, 38, 4669–4678. [Google Scholar] [CrossRef]
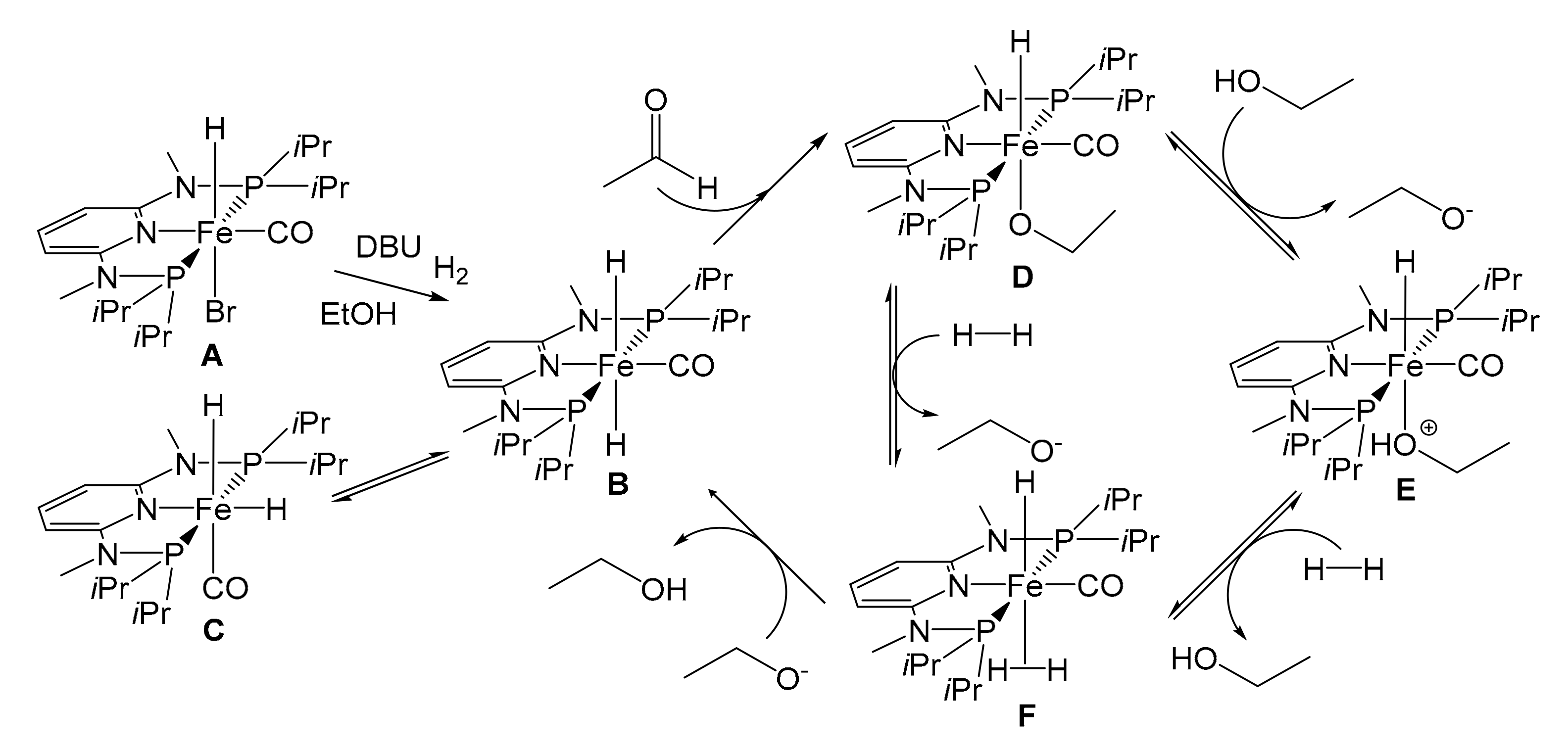
- Gorgas, N.; Stöger, B.; Veiros, L.F.; Pittenauer, E.; Allmaier, G.; Kirchner, K. Efficient Hydrogenation of Ketones and Aldehydes Catalyzed by Well-Defined Iron(II) PNP Pincer Complexes: Evidence for an Insertion Mechanism. Organometallics 2014, 33, 6905–6914. [Google Scholar] [CrossRef]
- Gorgas, N.; Stöger, B.; Veiros, L.F.; Kirchner, K. Highly Efficient and Selective Hydrogenation of Aldehydes: A Well-Defined Fe(II) Catalyst Exhibits Noble-Metal Activity. ACS Catal. 2016, 6, 2664–2672. [Google Scholar] [CrossRef] [Green Version]
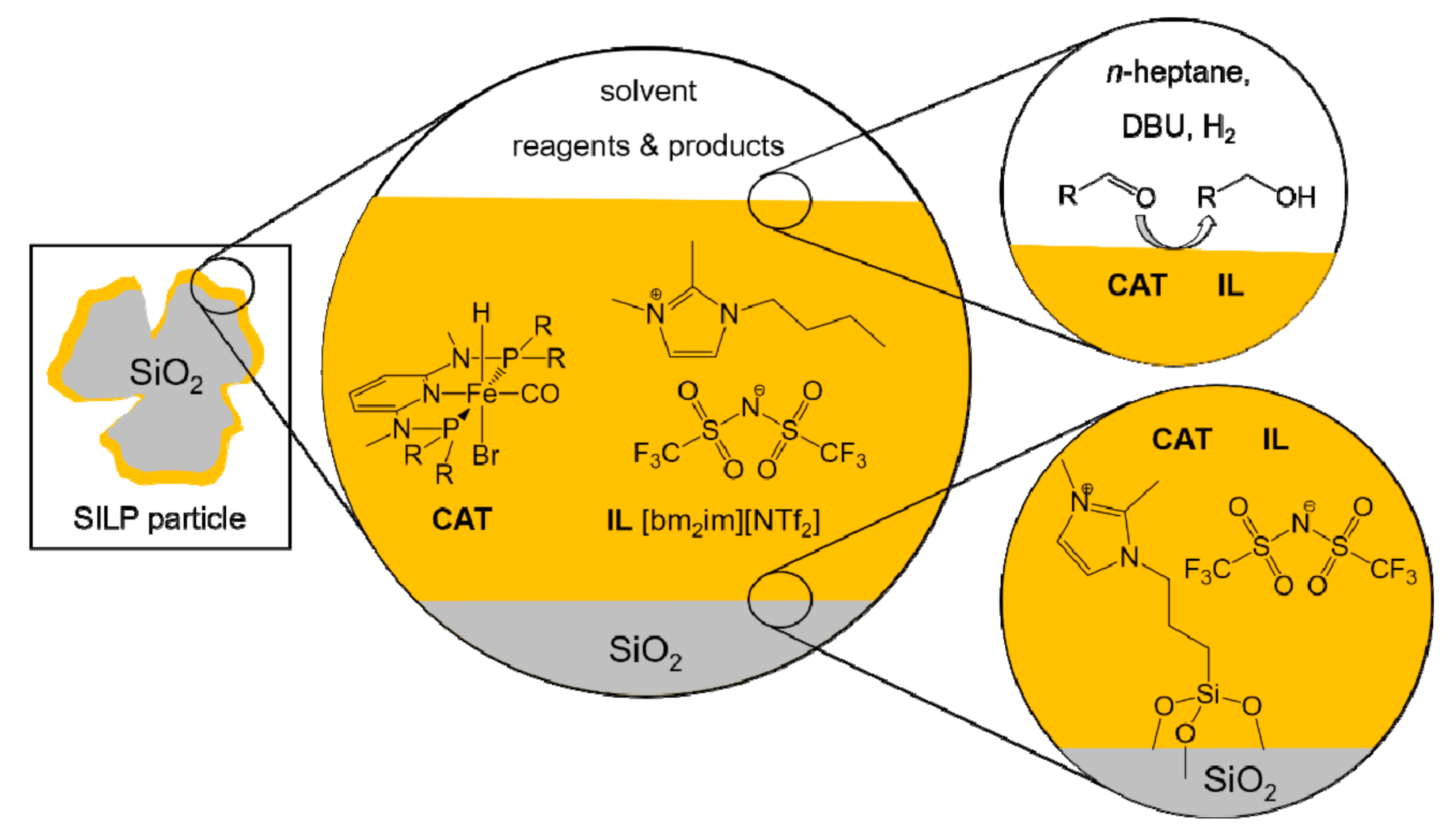
- Brünig, J.; Csendes, Z.; Weber, S.; Gorgas, N.; Bittner, R.W.; Limbeck, A.; Bica, K.; Hoffmann, H.; Kirchner, K. Chemoselective Supported Ionic-Liquid-Phase (SILP) Aldehyde Hydrogenation Catalyzed by an Fe(II) PNP Pincer Complex. ACS Catal. 2018, 8, 1048–1051. [Google Scholar] [CrossRef]
- Van Doorslaer, C.; Wahlen, J.; Mertens, P.; Binnemans, K.; De Vos, D. Immobilization of Molecular Catalysts in Supported Ionic Liquid Phases. Dalton Trans. 2010, 39, 8377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suarez, P.A.Z.; Dullius, J.E.L.; Einloft, S.; De Souza, R.F.; Dupont, J. The Use of New Ionic Liquids in Two-Phase Catalytic Hydrogenation Reaction by Rhodium Complexes. Polyhedron 1996, 15, 1217–1219. [Google Scholar] [CrossRef]
- Wolfson, A.; Vankelecom, I.F.J.; Jacobs, P.A. Co-Immobilization of Transition-Metal Complexes and Ionic Liquids in a Polymeric Support for Liquid-Phase Hydrogenations. Tetrahedron Lett. 2003, 44, 1195–1198. [Google Scholar] [CrossRef]
- Kim, D.W.; Chi, D.Y. Polymer-Supported Ionic Liquids: Imidazolium Salts as Catalysts for Nucleophilic Substitution Reactions Including Fluorinations. Angew. Chem. Int. Ed. 2004, 43, 483–485. [Google Scholar] [CrossRef]
- Bilal, M.; Iqbal, H.M.N. Naturally-Derived Biopolymers: Potential Platforms for Enzyme Immobilization. Int. J. Biol. Macromol. 2019, 130, 462–482. [Google Scholar] [CrossRef]
- Machałowski, T.; Amemiya, C.; Jesionowski, T. Chitin of Araneae Origin: Structural Features and Biomimetic Applications: A Review. Appl. Phys. A 2020, 126. [Google Scholar] [CrossRef]
- Liu, C.; Luan, P.; Li, Q.; Cheng, Z.; Xiang, P.; Liu, D.; Hou, Y.; Yang, Y.; Zhu, H. Biopolymers Derived from Trees as Sustainable Multifunctional Materials: A Review. Adv. Mater. 2020, 2001654. [Google Scholar] [CrossRef]
- Vinod, A.; Sanjay, M.R.; Suchart, S.; Jyotishkumar, P. Renewable and Sustainable Biobased Materials: An Assessment on Biofibers, Biofilms, Biopolymers and Biocomposites. J. Clean. Prod. 2020, 258, 120978. [Google Scholar] [CrossRef]
- Schmitz, C.; Gonzalez Auza, L.; Koberidze, D.; Rasche, S.; Fischer, R.; Bortesi, L. Conversion of Chitin to Defined Chitosan Oligomers: Current Status and Future Prospects. Mar. Drugs 2019, 17, 452. [Google Scholar] [CrossRef] [Green Version]
- Tajik, H.; Moradi, M.; Rohani, S.; Erfani, A.; Jalali, F. Preparation of Chitosan from Brine Shrimp (Artemia Urmiana) Cyst Shells and Effects of Different Chemical Processing Sequences on the Physicochemical and Functional Properties of the Product. Molecules 2008, 13, 1263–1274. [Google Scholar] [CrossRef] [Green Version]
- Hahn, T.; Tafi, E.; Paul, A.; Salvia, R.; Falabella, P.; Zibek, S. Current State of Chitin Purification and Chitosan Production from Insects. J. Chem. Technol. Biotech. 2020, 95, 2775–2795. [Google Scholar] [CrossRef]
- Molnár, Á. The Use of Chitosan-Based Metal Catalysts in Organic Transformations. Coord. Chem. Rev. 2019, 388, 126–171. [Google Scholar] [CrossRef]
- Khalil, K.; Al-Matar, H.; Elnagdi, M. Chitosan as an Eco-Friendly Heterogeneous Catalyst for Michael Type Addition Reactions. A Simple and Efficient Route to Pyridones and Phthalazines. Eur. J. Chem. 2010, 1, 252–258. [Google Scholar] [CrossRef] [Green Version]
- Khalil, K.; Al-Matar, H. Chitosan Based Heterogeneous Catalyses: Chitosan-Grafted-Poly(4-Vinylpyridne) as an Efficient Catalyst for Michael Additions and Alkylpyridazinyl Carbonitrile Oxidation. Molecules 2013, 18, 5288–5305. [Google Scholar] [CrossRef] [Green Version]
- Ngah, W.S.W.; Ab Ghani, S.; Kamari, A. Adsorption Behaviour of Fe(II) and Fe(III) Ions in Aqueous Solution on Chitosan and Cross-Linked Chitosan Beads. Biores. Technol. 2005, 96, 443–450. [Google Scholar] [CrossRef]
- Burke, A.; Yilmaz, E.; Hasirci, N. Evaluation of Chitosan As a Potential Medical Iron(III) Ion Adsorbent. Turk. J. Med. Sci. 2000, 30, 341–348. [Google Scholar]
- Khalil, K.D.; Ibrahim, E.I.; Al-Sagheer, F.A. A Novel, Efficient, and Recyclable Biocatalyst for Michael Addition Reactions and Its Iron(III) Complex as Promoter for Alkyl Oxidation Reactions. Catal. Sci. Technol. 2016, 6, 1410–1416. [Google Scholar] [CrossRef]
- Bakshi, P.S.; Selvakumar, D.; Kadirvelu, K.; Kumar, N.S. Chitosan as an Environment Friendly Biomaterial—A Review on Recent Modifications and Applications. Int. J. Biol. Macromol. 2020, 150, 1072–1083. [Google Scholar] [CrossRef]
- Domski, G.J.; Rose, J.M.; Coates, G.W.; Bolig, A.D.; Brookhart, M. Living Alkene Polymerization: New Methods for the Precision Synthesis of Polyolefins. Prog. Polym. Sci. 2007, 32, 30–92. [Google Scholar] [CrossRef]
- Dominique, S.; Mostafa, T.; Christophe, B. Polyolefins, a Success Story. Polymers 2017, 9, 185. [Google Scholar] [CrossRef] [Green Version]






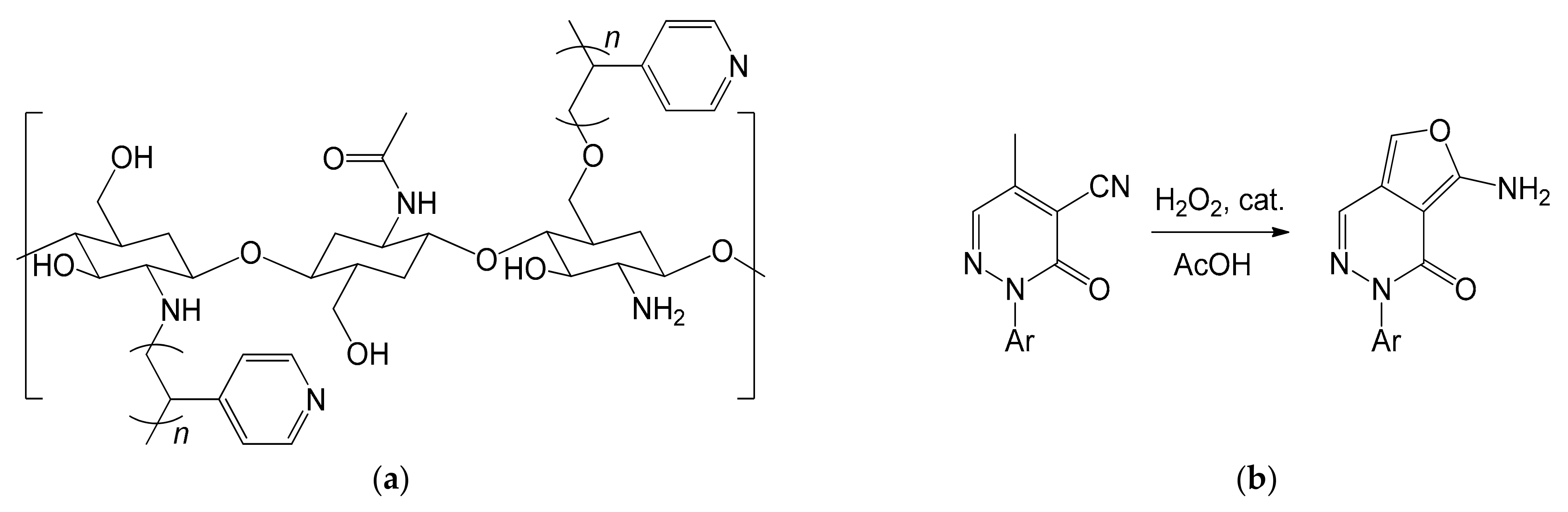
| Complex | Pre-Cat (μmol) | MAO (mmol/eq) | Activity (g/mmol h bar) | Yield (g) | MW | Mn | MW/Mn |
|---|---|---|---|---|---|---|---|
| 1 | 0.5 | 0.5/1000 | 5340 | 26.9 | 611,000 | 64,000 | 9.5 |
| 2 | 0.6 | 0.6/1000 | 9340 | 56.5 | 242,000 | 9600 | 25.3 |
| 3 | 0.6 | 0.6/1000 | 20600 | 123.5 | 148,000 | 14,000 | 10.7 |
| 4 | 0.6 | 0.6/1000 | 3750 | 22.8 | 313,000 | 3000 | 105.1 |
| 5 | 6 | 1.2/200 | 305 | 18.2 | 132,000 | 3400 | 38.9 |
| 6 | 6 | 1.2/200 | 560 | 33.7 | 108,000 | 1900 | 57.3 |
| 7 | 6 | 1.2/200 | 340 | 20.3 | 230,000 | 3900 | 58.4 |
| 8 | 6 | 1.2/200 | 550 | 32.8 | 152,000 | 1800 | 83.5 |
| Catalyst | Reaction Time (h) | Conversion (%) | Selectivity (%) | Yield (%) Benzaldehyde | TOF 1 | |
|---|---|---|---|---|---|---|
| Benzaldehyde | Benzoic Acid | |||||
| FePcS | 6 | 39.3 | 18.7 | 47.3 | 7.3 | 19.7 |
| FePcS | 24 | 57.8 | 36.2 | 36.3 | 20.9 | 28.9 |
| FePcS/NH2-MCM-41 | 6 | 16.7 | 19.1 | 0 | 3.2 | 8.3 |
| FePcS/NH2-MCM-41 | 24 | 46.9 | 20.2 | 0 | 9.5 | 23.5 |
| FePcS/NH2-MCM-48 | 6 | 21.9 | 23.9 | 0 | 5.2 | 10.9 |
| FePcS/NH2-MCM-48 | 24 | 65.5 | 21.4 | 0 | 14.0 | 32.7 |
| Substrate | S/C 2 | Conversion (%) | Yield (%) | 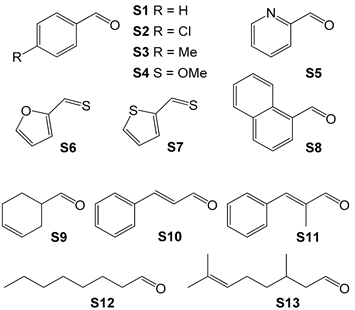 |
| S1 | 20,000 | >99 | 96 | |
| S2 | 20,000 | >99 | >99 | |
| S3 | 15,000 | >99 | >99 | |
| S4 | 15,000 | 98 | 98 | |
| S5 | 20,000 | >99 | 97 | |
| S6 | 20,000 | >99 | >99 | |
| S7 | 20,000 | >99 | >99 | |
| S8 | 10,000 | 97 | 96 | |
| S9 | 10,000 | >99 | 98 | |
| S10 | 10,000 | >99 | >99 | |
| S11 | 20,000 | >99 | >99 | |
| S12 | 10,000 | >99 | >99 | |
| S13 | 10,000 | 99 | 97 |
| SILP System | H2 (atm) | S/C 2 | t (min) | Yield (%) | TON | TOF (h−1) |
|---|---|---|---|---|---|---|
| SILP10 | 10 | 200 | 75 | 16 | 32 | 26 |
| SILP20 | 10 | 200 | 17 | >99 | 200 | 706 |
| SILP30 | 10 | 200 | 75 | 7 | 14 | 11 |
| SILP40 | 10 | 200 | 75 | 3 | 6 | 5 |
| SILP20 | 20 | 200 | 13 | >99 | 200 | 923 |
| SILP20 | 50 | 200 | 8 | >99 | 200 | 1500 |
| SILP20 | 10 | 1000 | 90 | 85 | 850 | 567 |
| SILP20 | 50 | 1000 | 15 | >99 | 1000 | 4000 |
| homogeneous | 10 | 200 | 6 | >99 | 200 | 2000 |
| biphasic | 10 | 200 | 12 | >99 | 200 | 1000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moccia, F.; Rigamonti, L.; Messori, A.; Zanotti, V.; Mazzoni, R. Bringing Homogeneous Iron Catalysts on the Heterogeneous Side: Solutions for Immobilization. Molecules 2021, 26, 2728. https://doi.org/10.3390/molecules26092728
Moccia F, Rigamonti L, Messori A, Zanotti V, Mazzoni R. Bringing Homogeneous Iron Catalysts on the Heterogeneous Side: Solutions for Immobilization. Molecules. 2021; 26(9):2728. https://doi.org/10.3390/molecules26092728
Chicago/Turabian StyleMoccia, Fabio, Luca Rigamonti, Alessandro Messori, Valerio Zanotti, and Rita Mazzoni. 2021. "Bringing Homogeneous Iron Catalysts on the Heterogeneous Side: Solutions for Immobilization" Molecules 26, no. 9: 2728. https://doi.org/10.3390/molecules26092728






