Influence of Polymer Solvents on the Properties of Halloysite-Modified Polyethersulfone Membranes Prepared by Wet Phase Inversion
Abstract
:1. Introduction
2. Results and Discussion
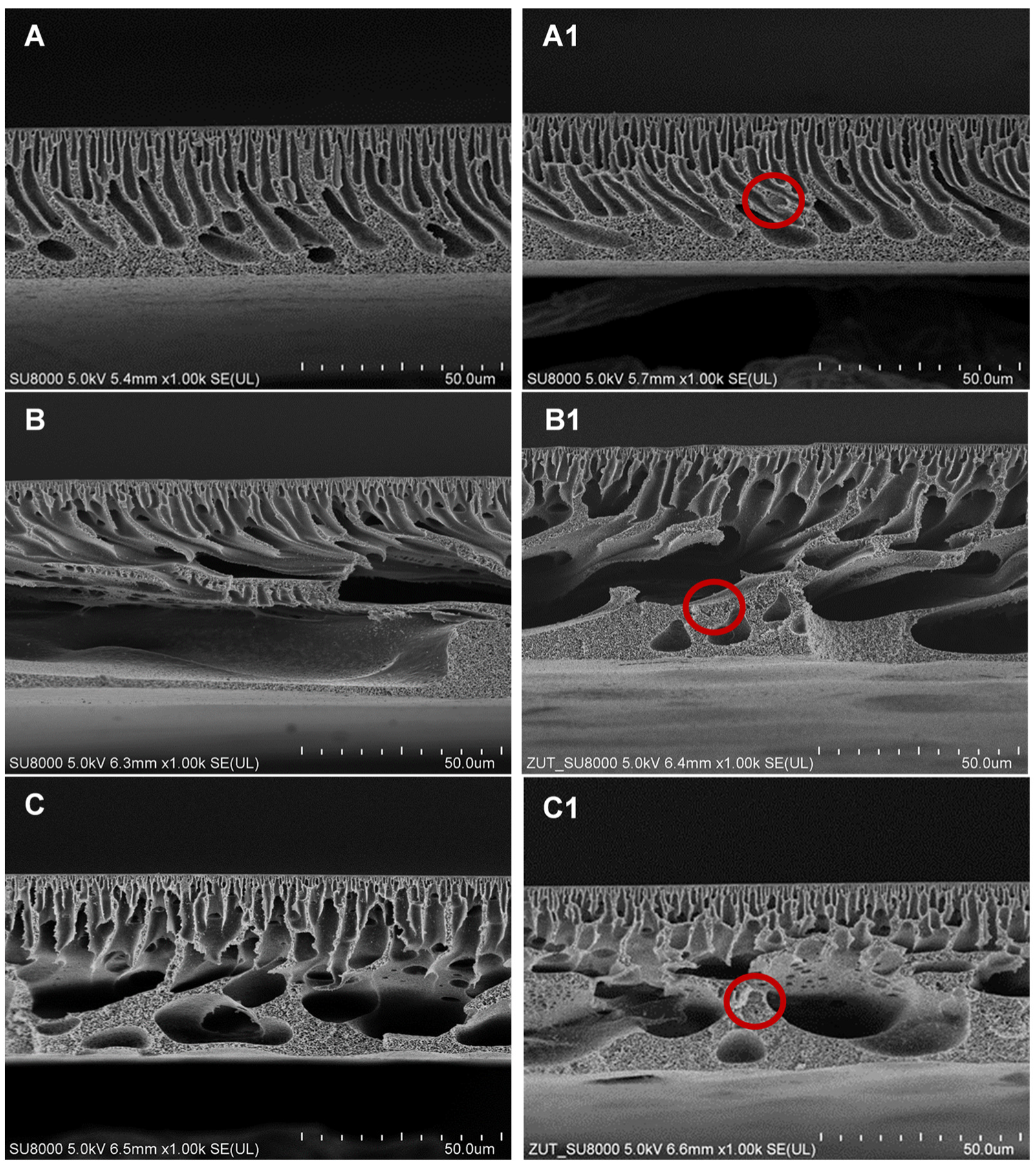
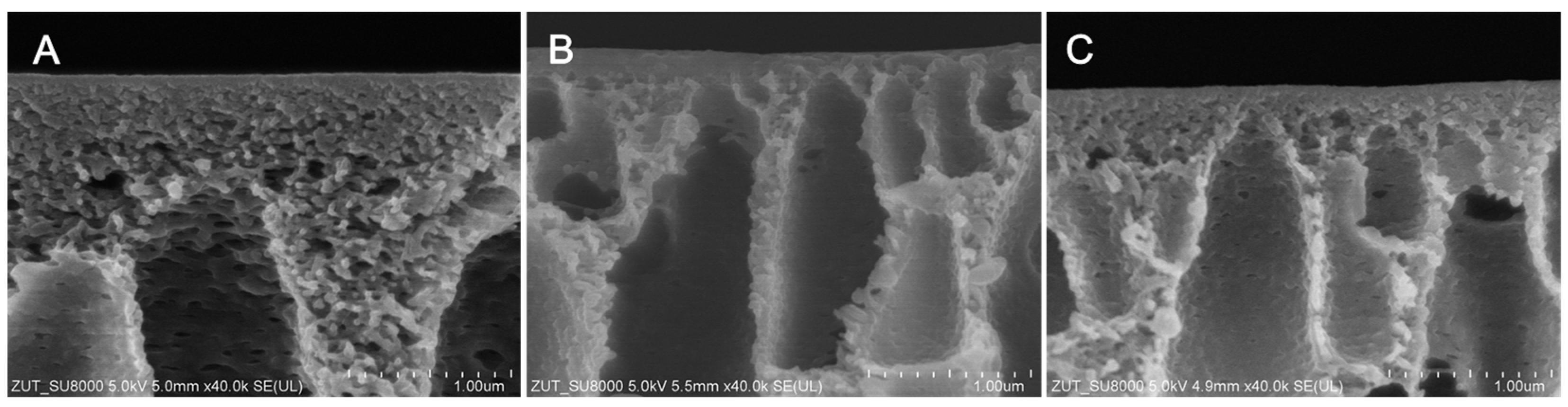
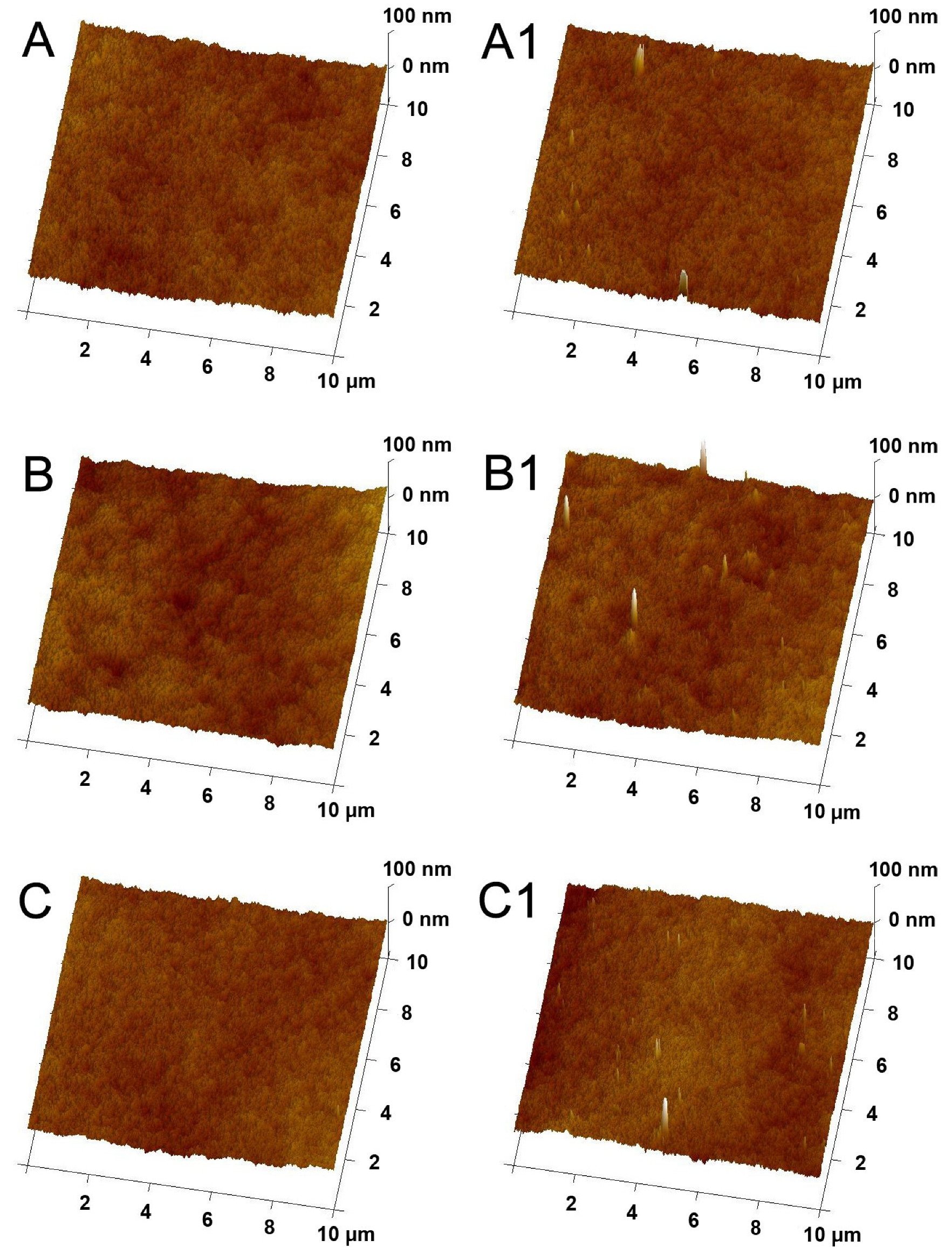
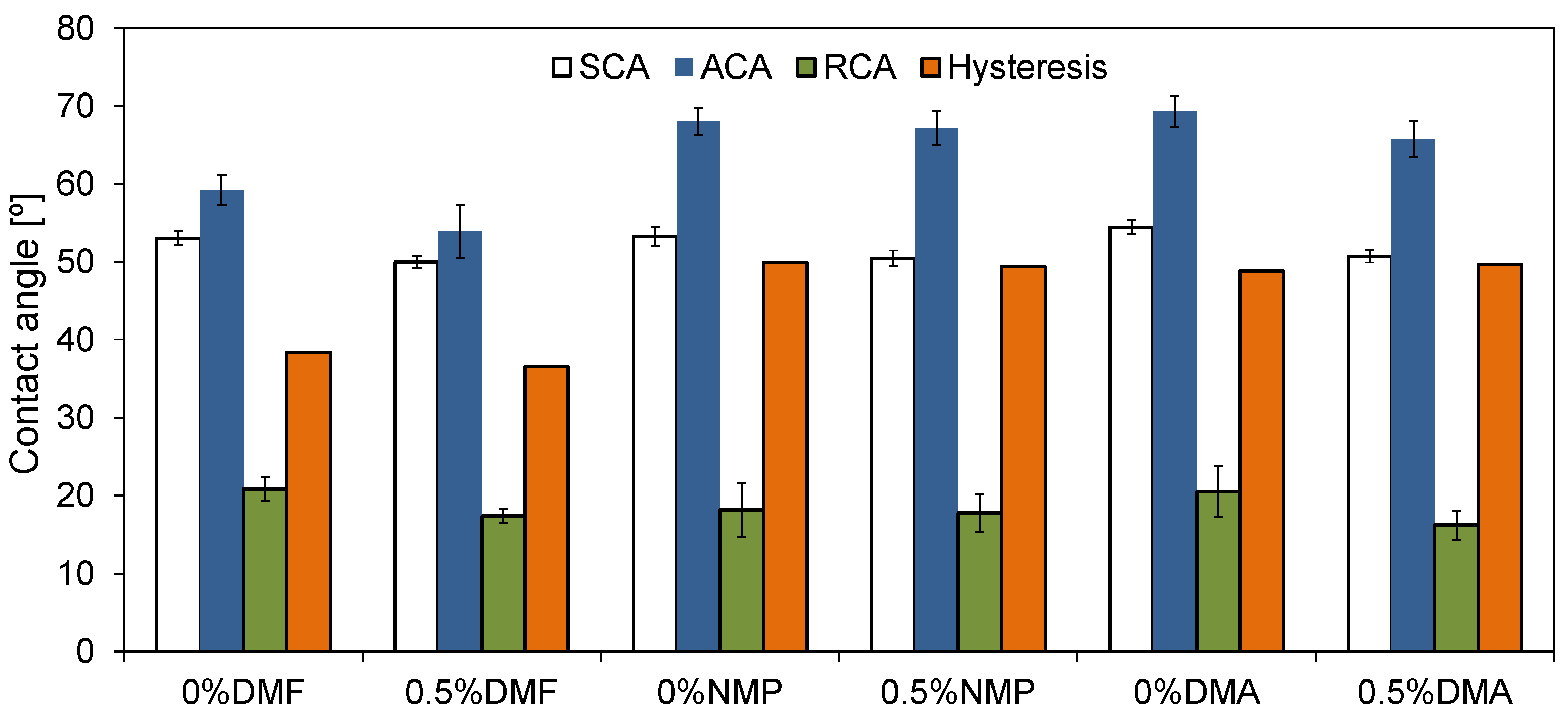
2.1. Physicochemical Properties of Membranes
2.2. Permeability of the Membranes
2.3. Separation Properties
2.4. Antifouling Properties
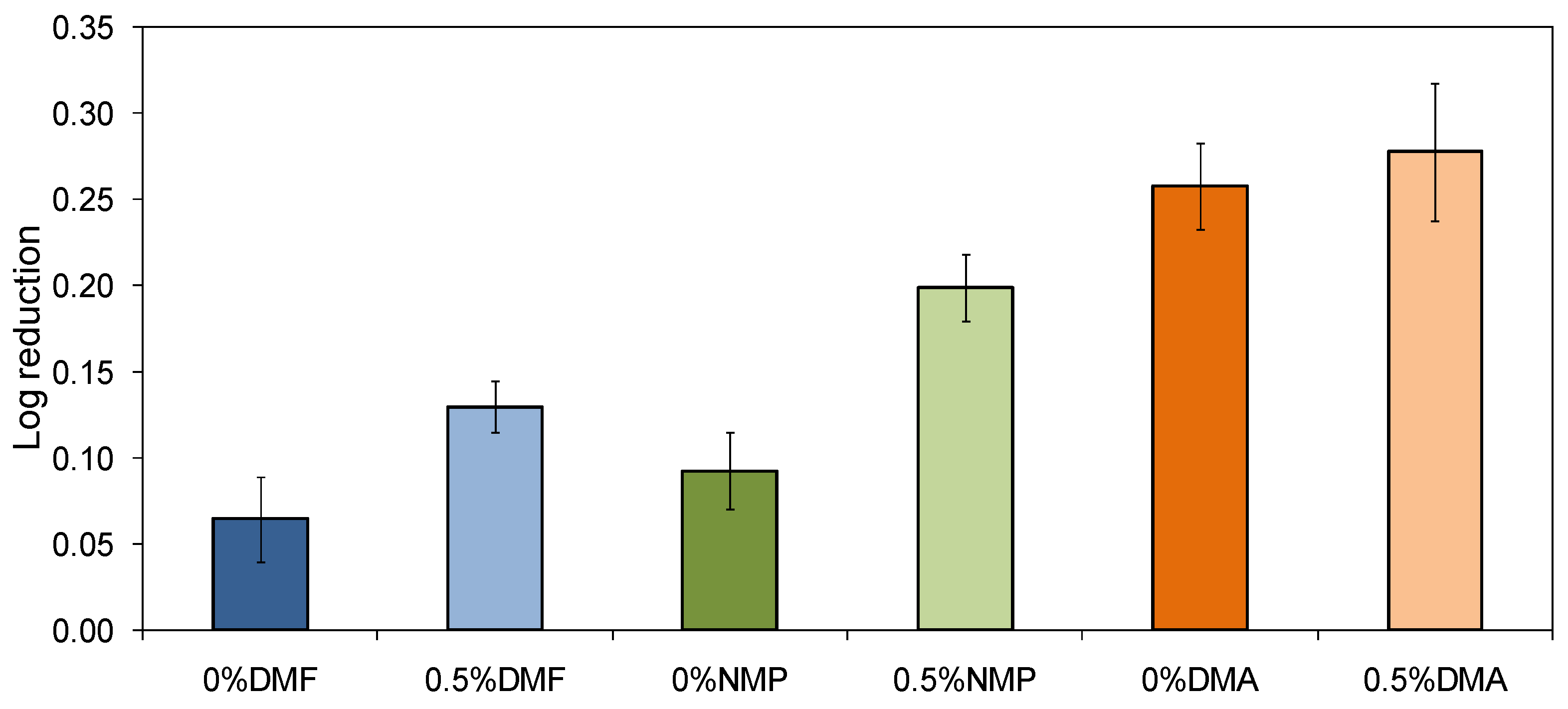
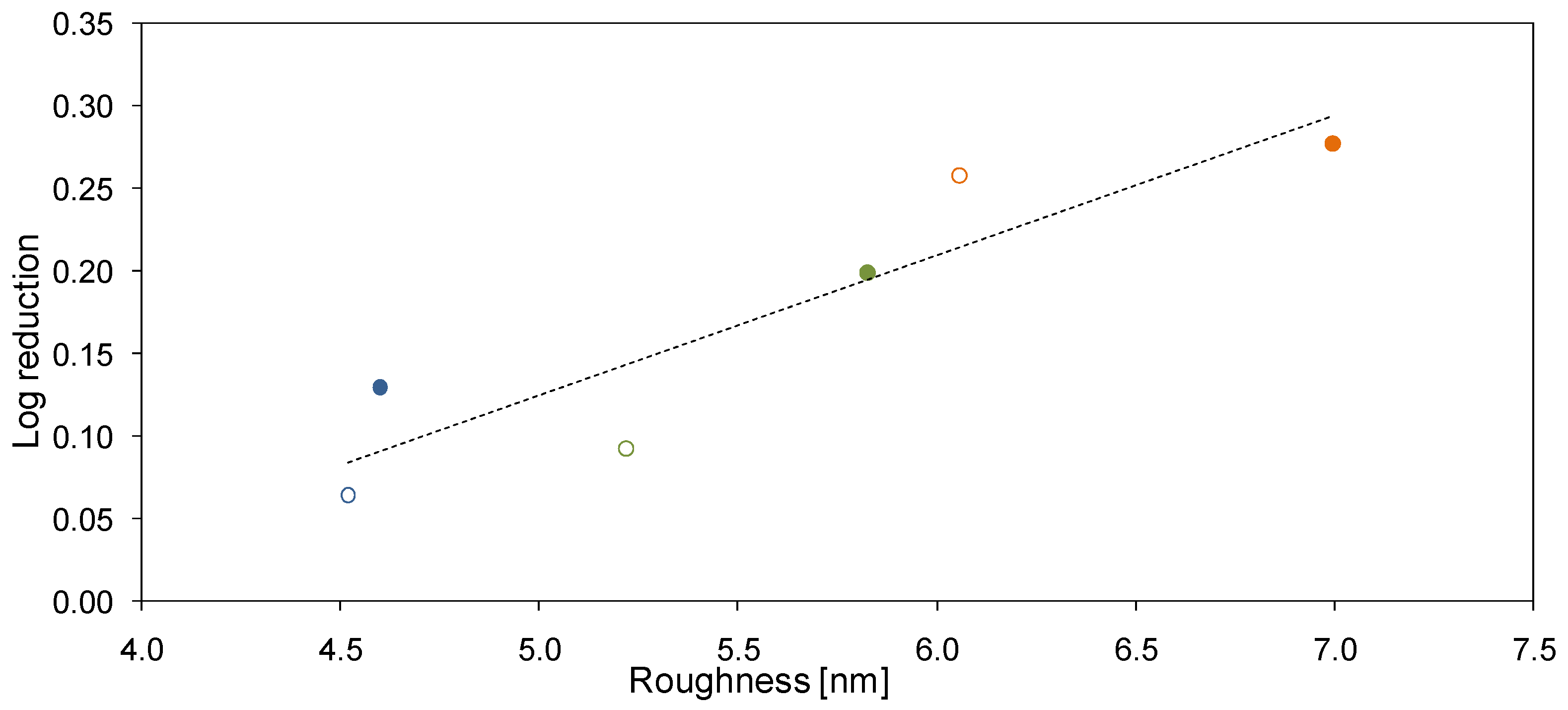
2.5. Antibacterial Properties
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations and Symbols
| A | Number of bacteria in the control sample |
| ACA | Advancing contact angle |
| AFM | Atomic force microscopy |
| B | Number of bacteria in the presence of a membrane |
| BSA | Bovine serum albumin |
| C0 | Concentration of PEG and dextrans in feed |
| CP | Concentration of PEG and dextrans in permeate |
| CA | Contact angle |
| CFU | Colony forming unit |
| CNTs | Carbon nanotubes |
| DMA | N,N-dimethylacetamide |
| DMF | N,N-dimethylformamide |
| DMSO | Dimethyl sulfoxide |
| HNTs | Halloysite nanotubes |
| N | Number of bacteria colonies visible on agar plates |
| NIPS | Non-solvent-induced phase separation |
| NMP | 1-methyl-2-pyrrolidinone |
| NPs | Nanoparticles |
| PEGs | Poly(ethylene glycols) |
| PES | Polyethersulfone |
| PLA | Poly(lactic acid) |
| PWF | Pure water flux |
| R | Rejection |
| Ra | Mean roughness (roughness average) |
| RCA | Receding contact angle |
| SCA | Static contact angle |
| SE | Secondary electrons |
| SEM | Scanning electron microscopy |
| TMP | Transmembrane pressure |
| TNTs | Titanate nanotubes |
| UF | Ultrafiltration |
| Y | Total dilution |
| Z | Volume of bacteria suspension |
References
- Szymański, K.; Darowna, D.; Sienkiewicz, P.; Jose, M.; Szymańska, K.; Zrzebnicki, M.; Mozia, S. Novel polyethersulfone ultrafiltration membranes modified with Cu/titanate nanotubes. J. Water Process. Eng. 2020, 33, 101098. [Google Scholar] [CrossRef]
- Chu, Z.; Chen, K.; Xiao, C.; Ling, H.; Hu, Z. Performance improvement of polyethersulfone ultrafiltration membrane containing variform inorganic nano-additives. Polymer 2020, 188, 122160. [Google Scholar] [CrossRef]
- Amid, M.; Nabian, N.; Delavar, M. Fabrication of polycarbonate ultrafiltration mixed matrix membranes including modified halloysite nanotubes and graphene oxide nanosheets for olive oil/water emulsion separation. Sep. Purif. Technol. 2020, 251, 117332. [Google Scholar] [CrossRef]
- Petrova, D.A.; Filippov, A.N.; Kononenko, N.A.; Shkirskaya, S.A.; Timchenko, M.O.; Ivanov, E.V.; Vinokurov, V.A.; Lvov, Y.M. Perfluorinated hybrid membranes modified by metal decorated clay nanotubes. J. Membr. Sci. 2019, 582, 172–181. [Google Scholar] [CrossRef]
- Aquino, R.R.; Tolentino, M.S.; Millare, J.C.; Balboa, C.D.; Castro, C.J.B.; Basilia, B.A. Fabrication and characterization of electrospun polysulfone (PSf)/halloysite (HAL) nanocomposite membrane. Mater. Sci. Forum 2018, 934, 55–60. [Google Scholar] [CrossRef]
- Bai, Z.; Wang, L.; Liu, C.; Yang, C.; Lin, G.; Liu, S.; Jia, K.; Liu, X. Interfacial coordination mediated surface segregation of halloysite nanotubes to construct a high-flux antifouling membrane for oil-water emulsion separation. J. Membr. Sci. 2021, 620, 118828. [Google Scholar] [CrossRef]
- Park, S.; Yang, E.; Park, H.; Choi, H. Fabrication of functionalized halloysite nanotube blended ultrafiltration membranes for high flux and fouling resistance. Environ. Eng. Res. 2020, 25, 771–778. [Google Scholar] [CrossRef] [Green Version]
- Otitoju, T.A.; Ahmed, A.L.; Ooi, B.S. Recent advances in hydrophilic modification and performance of polyethersulfone (PES) membrane via additive blending. RSC Adv. 2018, 8, 22710–22728. [Google Scholar] [CrossRef] [Green Version]
- Grylewicz, A.; Mozia, S. Polymeric mixed-matrix membranes modified with halloysite nanotubes for water and wastewater treatment: A review. Sep. Purif. Technol. 2021, 256, 117827. [Google Scholar] [CrossRef]
- Buruga, K.; Kalathi, J.T.; Kim, K.; Ok, Y.S.; Danil, B. Polystyrene-halloysite nano tube membranes for water purification. J. Ind. Eng. Chem. 2018, 61, 169–180. [Google Scholar] [CrossRef]
- De Silva, R.T.; Pasbakhsh, P.; Sui Mae, L.; Kit, A.Y. ZnO deposited/encapsulated halloysite–poly (lactic acid) (PLA) nanocomposites for high performance packaging films with improved mechanical and antimicrobial properties. Appl. Clay Sci. 2015, 111, 10–20. [Google Scholar] [CrossRef]
- Zhao, Q.; Hou, J.; Shen, J.; Liu, J.; Zhang, Y. Long-lasting antibacterial behavior of a novel mixed matrix water purification membrane. J. Mater. Chem. A 2015, 36, 18696–18705. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y.; Zhang, H.; Liu, J.; Song, C. Biofouling control of halloysite nanotubes-decorated polyethersulfone ultrafiltration membrane modified with chitosan-silver nanoparticles. Chem. Eng. J. 2013, 228, 12–20. [Google Scholar] [CrossRef]
- Liang, X.; Qin, L.; Wang, J.; Zhu, J.; Zhang, Y.; Liu, J. Facile construction of long-lasting antibacterial membrane by using an orientated halloysite nanotubes interlayer. Eng. Chem. Res. 2018, 57, 3235–3245. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y.; Liu, J.; Zhang, H.; Wang, K. Preparation and antibacterial property of polyethersulfone ultrafiltration hybrid membrane containing halloysite nanotubes loaded with copper ions. Chem. Eng. J. 2012, 210, 298–308. [Google Scholar] [CrossRef]
- Duan, L.; Zhao, Q.; Liua, J.; Zhang, Y. Antibacterial behavior of halloysite nanotubes decorated with copper nanoparticles in a novel mixed matrix membrane for water purification. Environ. Sci. Water Res. Technol. 2015, 1, 874–881. [Google Scholar] [CrossRef]
- Duan, L.; Huang, W.; Zhang, Y. High-flux, antibacterial ultrafiltration membranes by facile blending with N-halamine grafted halloysite nanotubes. RSC Adv. 2014, 5, 6666–6674. [Google Scholar] [CrossRef]
- Liu, Z.; Mi, Z.; Jin, S.; Wang, C.; Wang, D.; Zhao, X.; Zhou, H.; Chen, C. The influence of sulfonated hyperbranched polyethersulfone-modified halloysite nanotubes on the compatibility and water separation performance of polyethersulfone hybrid ultrafiltration membranes. J. Membr. Sci. 2018, 557, 13–23. [Google Scholar] [CrossRef]
- Albo, J.; Wang, J.; Tsuru, T. Application of interfacially polymerized polyamide composite membranes to isopropanol dehydration: Effect of membrane pre-treatment and temperature. J. Membr. Sci. 2014, 453, 384–393. [Google Scholar] [CrossRef]
- Albo, J.; Wang, J.; Tsuru, T. Gas transport properties of interfacially polymerized polyamide composite membranes under different pre-treatments and temperatures. J. Membr. Sci. 2014, 449, 109–118. [Google Scholar] [CrossRef]
- Albo, J.; Hagiwara, H.; Yanagishita, H.; Ito, K.; Tsuru, T. Structural characterization of thin-film polyamide reverse osmosis membranes. Ind. Chem. Res. 2014, 53, 1442–1451. [Google Scholar] [CrossRef]
- Adams, F.V.; Nxumalo, E.N.; Krause, R.W.M.; Hoek, E.M.V.; Mamba, B.B. The influence of solvent properties on the performance of polysulfone/β-cyclodextrin polyurethane mixed-matrix membranes. J. Appl. Polym. Sci. 2013, 130, 2005–2014. [Google Scholar] [CrossRef]
- Szante, L.; Szejtli, J. Cyclodextrins as food ingredients. Trends Food Sci. Technol. 2004, 15, 137–142. [Google Scholar] [CrossRef]
- Tsai, H.-A.; Huan, D.-H.; Ruaan, R.-C.; Lai, J.-Y. Mechanical properties of asymmetric polysulfone membranes containing surfactant as additives. Ind. Eng. Chem. Res. 2001, 40, 5917–5922. [Google Scholar] [CrossRef]
- Bildyukevich, A.V.; Plisko, T.V.; Shustikov, A.A.; Dzyazko, Y.S.; Rozhdestvenska, L.M.; Pratsenko, S.A. Effect of the solvent nature on the structure and performance of poly(amide-imide) ultrafiltration membranes. J. Mater. Sci. 2020, 55, 9638–9654. [Google Scholar] [CrossRef]
- Arthanareeswaran, G.; Starov, V.M. Effect of solvents on performance of polyethersulfone ultrafiltration membranes: Investigation of metal ion separations. Desalination 2011, 267, 57–63. [Google Scholar] [CrossRef] [Green Version]
- Fahrina, A.; Maimun, T.; Humaira, S.; Rosnelly, C.M.; Lubis, M.R.; Bahrina, I.; Sunarya, R.; Ghufran, A.; Arahman, N. The morphology and filtration performances of poly(ether sulfone) membrane fabricated from different polymer solution. MATEC Web Conf. 2018, 197, 09001. [Google Scholar] [CrossRef] [Green Version]
- Wahab, M.Y.; Muchtar, S.; Arahman, N.; Mulyati, S.; Riza, M. The effects of solvent type on the performance of flat sheet polyethersulfone/Brij58 membranes. IOP Conf. Ser. Mater. Sci. Eng. 2019, 536, 012119. [Google Scholar] [CrossRef]
- Julian, H.; Wenten, I.G. Polysulfone membranes for CO2/CH4 separation: State of the art. IOSR J. Eng. 2012, 2, 484–495. [Google Scholar] [CrossRef]
- Milescu, R.A.; McElroy, R.C.; Farmer, T.J.; Williams, P.M.; Walters, M.J.; Clark, J.H. Fabrication of PES/PVP water filtration membranes using Cyrene®, a safer bio-based polar aprotic solvent. Adv. Polym. Technol. 2019, 2019, 9692859. [Google Scholar] [CrossRef] [Green Version]
- Alvi, M.A.U.R.; Khalid, M.W.; Ahmad, N.M.; Niazi, M.B.K.; Anwar, M.N.; Batool, M.; Cheema, W.; Rafiq, S. Polymer concentration and solvent variation correlation with the morphology and water filtration analysis of polyether sulfone microfiltration membrane. Adv. Polym. Technol. 2019, 2019, 8074626. [Google Scholar] [CrossRef]
- Thuyavan, Y.L.; Anantharaman, N.; Arthanareeswaran, G.; Ismail, A.F. Impact of solvents and process conditions on the formation of polyethersulfone membranes and its fouling behavior in lake water filtration. J. Chem. Technol. Biotechnol. 2015, 91, 2568–2581. [Google Scholar] [CrossRef]
- Xie, D.M.; Liu, D.H.; Zhan, J.A. Temperature optimization for glycerol production by batch fermentation with Candida krusei. J. Chem. Technol. Biotechnol. 2001, 76, 1057–1069. [Google Scholar] [CrossRef]
- Boussu, K.; Van Der Bruggen, B.; Vandexasteele, C. Evaluation of self-made nanoporous polyethersulfone membranes, relative to commercial nanofiltration membranes. Desalination 2006, 200, 416–418. [Google Scholar] [CrossRef]
- Madaeni, S.S.; Rahimpour, A. Effect of type of solvent and non-solvent on morphology and performance of polysulfone and polyethersulfone ultrafiltration membranes for milk concentration. Polym. Adv. Technol. 2005, 16, 717–724. [Google Scholar] [CrossRef]
- Farahania, M.H.D.A.; Vatanpoura, V. Comparing the effect of incorporation of various nanoparticulate on the performance and antifouling properties of polyethersulfone nanocomposite membranes. J. Water Process. Eng. 2019, 27, 47–57. [Google Scholar] [CrossRef]
- Guan, R.; Dai, H.; Li, C.; Liu, J.; Xu, J. Effect of casting solvent on the morphology and performance of sulfonated polyethersulfone membranes. J. Membr. Sci. 2006, 277, 148–156. [Google Scholar] [CrossRef]
- Ghandashtani, M.B.; Tavangar, T.; Ashtiani, F.Z.; Karimi, M.; Fouladitajar, A. Experimental investigation and mathematical modeling of nano-composite membrane fabrication process: Focus on the role of solvent type. Asia Pac. J. Chem. Eng. 2018, 13, e2260. [Google Scholar] [CrossRef]
- Ashtiani, S.; Khoshnamvand, M.; Shaliutina-Kolesova, A. A fundamental study of morphology prediction for nano and microstructure of PVDF for membrane fabrication and film formation applications. Int. J. Adv. Eng. Nano Technol. 2018, 3. Available online: https://www.ijaent.org/wp-content/uploads/papers/v3i9/I0402043918.pdf (accessed on 3 May 2021).
- Hendrix, K.; Vaneynde, M.; Koeckelberghs, G.; Vankelecom, I.F.J. Synthesis of modified poly(ether ether ketone) polymer for the preparation of ultrafiltration and nanofiltration membranes via phase inversion. J. Membr. Sci. 2013, 447, 96–106. [Google Scholar] [CrossRef]
- Zhang, W.; Wahlgren, M.; Sivik, B. Membrane characterization by the contact angle technique: II. Characterization of UF-membranes and comparison between the captive bubble and sessile drop as methods to obtain water contact angles. Desalination 1989, 72, 263–273. [Google Scholar] [CrossRef]
- Khayet, M.; Suk, D.E.; Narbaitz, R.M.; Santerre, J.P.; Matsuura, T. Study on surface modification by surface-modifying macromolecules and its applications in membrane-separation processes. J. Appl. Polym. Sci. 2003, 89, 2902–2916. [Google Scholar] [CrossRef]
- Wang, J.; Wu, Y.; Cao, Y.; Li, G.; Liao, Y. Influence of surface roughness on contact angle hysteresis and spreading work. Colloid Polym. Sci. 2020, 298, 1107–1112. [Google Scholar] [CrossRef]
- Kaufman, Y.; Chen, S.-Y.; Mishra, H.; Schrader, A.M.; Lee, D.W.; Das, S.; Donaldson, S.H.; Israelachvili, J.N. Simple-to-apply wetting model to predict thermodynamically stable and metastable contact angles on textured/rough/patterned surfaces. J. Phys. Chem. C 2017, 121, 5642–5656. [Google Scholar] [CrossRef]
- Yu, L.; Wang, H.; Zhang, Y.; Zhang, B.; Liu, J. Recent advances in halloysite nanotube derived composites for water treatment. Environ. Sci. Nano 2016, 3, 28–44. [Google Scholar] [CrossRef]
- Vinokurov, V.A.; Stavitskaya, A.V.; Glotov, A.P.; Navikov, A.A.; Zolotukhina, A.V.; Kotelev, M.S.; Gushchin, P.A.; Ivanov, E.V.; Darrat, Y.; Lvov, Y.M. Nanoparticles formed onto/into halloysite clay tubules: Architectural synthesis and applications. Chem. Rec. 2018, 18, 858–867. [Google Scholar] [CrossRef]
- Ghanbari, M.; Emadzadeh, D.; Lau, W.J.; Matsuura, T.; Ismail, A.F. Synthesis and characterization of novel thin film nanocomposite reverse osmosis membranes with improved organic fouling properties for water desalination. RSC Adv. 2015, 5, 21268–21276. [Google Scholar] [CrossRef]
- Ghanbari, M.; Emadzadeh, D.; Lau, W.J.; Matsuura, T.; Davoody, M.; Ismail, A.F. Super hydrophilic TiO2/HNT nanocomposites as a new approach for fabrication of high performance thin film nanocomposite membranes for FO application. Desalination 2015, 371, 104–114. [Google Scholar] [CrossRef] [Green Version]
- Salgin, S.; Takac, S.; Ozdamar, T.H. Adsorption of bovine serum albumin on polyether sulfone ultrafiltration membranes: Determination of interfacial interaction energy and effective diffusion coefficient. J. Membr. Sci. 2006, 278, 251–260. [Google Scholar] [CrossRef]
- Mozia, S.; Sienkiewicz, P.; Szymański, K.; Darowna, D.; Czyżewski, A.; Zgrzebnicki, M. Influence of the procedure of casting solution preparation on the antimicrobial properties of polyethersulfone membranes modified with titanate nanotubes. Desalin. Water Treat. 2021, 214, 273–285. [Google Scholar]
- Gumbi, N.N.; Hu, M.; Mamba, B.B.; Li, J.; Nxumalo, E.N. Macrovoid-free PES/SPSf/O-MWCNT ultrafiltration membranes with improved mechanical strength, antifouling and antibacterial properties. J. Membr. Sci. 2018, 566, 288–300. [Google Scholar] [CrossRef]
- Kang, S.; Pinault, M.; Pfefferle, L.D.; Elimelech, M. Single-walled carbon nanotubes exhibit strong antimicrobial activity. Langmuir 2007, 17, 8670–8673. [Google Scholar] [CrossRef] [PubMed]
- Mozia, S.; Grylewicz, A.; Zgrzebnicki, M.; Darowna, D.; Czyżewski, A. Investigations on the properties and performance of mixed-matrix polyethersulfone membranes modified with halloysite nanotubes. Polymers 2019, 11, 671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khanukaeva, D.Y.; Filippov, A.N.; Bildyukevich, A.V. An AFM study of ultrafiltration membranes: Peculiarities of pore size distribution. Pet. Chem. 2014, 54, 498–506. [Google Scholar] [CrossRef]
- Szymański, K.; Darowna, D.; Czyżewski, A.; Sienkiewicz, P.; Mozia, S. Investiation on ultrafiltration polyethersulfone membranes modified with titanate nanotubes of various characteristics. Desalin. Water Treat. 2021, 214, 302–311. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grylewicz, A.; Szymański, K.; Darowna, D.; Mozia, S. Influence of Polymer Solvents on the Properties of Halloysite-Modified Polyethersulfone Membranes Prepared by Wet Phase Inversion. Molecules 2021, 26, 2768. https://doi.org/10.3390/molecules26092768
Grylewicz A, Szymański K, Darowna D, Mozia S. Influence of Polymer Solvents on the Properties of Halloysite-Modified Polyethersulfone Membranes Prepared by Wet Phase Inversion. Molecules. 2021; 26(9):2768. https://doi.org/10.3390/molecules26092768
Chicago/Turabian StyleGrylewicz, Amanda, Kacper Szymański, Dominika Darowna, and Sylwia Mozia. 2021. "Influence of Polymer Solvents on the Properties of Halloysite-Modified Polyethersulfone Membranes Prepared by Wet Phase Inversion" Molecules 26, no. 9: 2768. https://doi.org/10.3390/molecules26092768





