Simultaneous Detoxification of Aflatoxin B1, Zearalenone and Deoxynivalenol by Modified Montmorillonites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Acid Treatment
2.3. Intercalated Montmorillonite Preparation
2.4. Thermal Treatment
2.5. Mycotoxin Adsorption Test
2.6. Adsorption Test at Different Montmorillonite/Mycotoxin Mass Ratio
2.7. Mycotoxin/Nutrient Selective Adsorption Test
2.8. Characterization
3. Results and Discussion
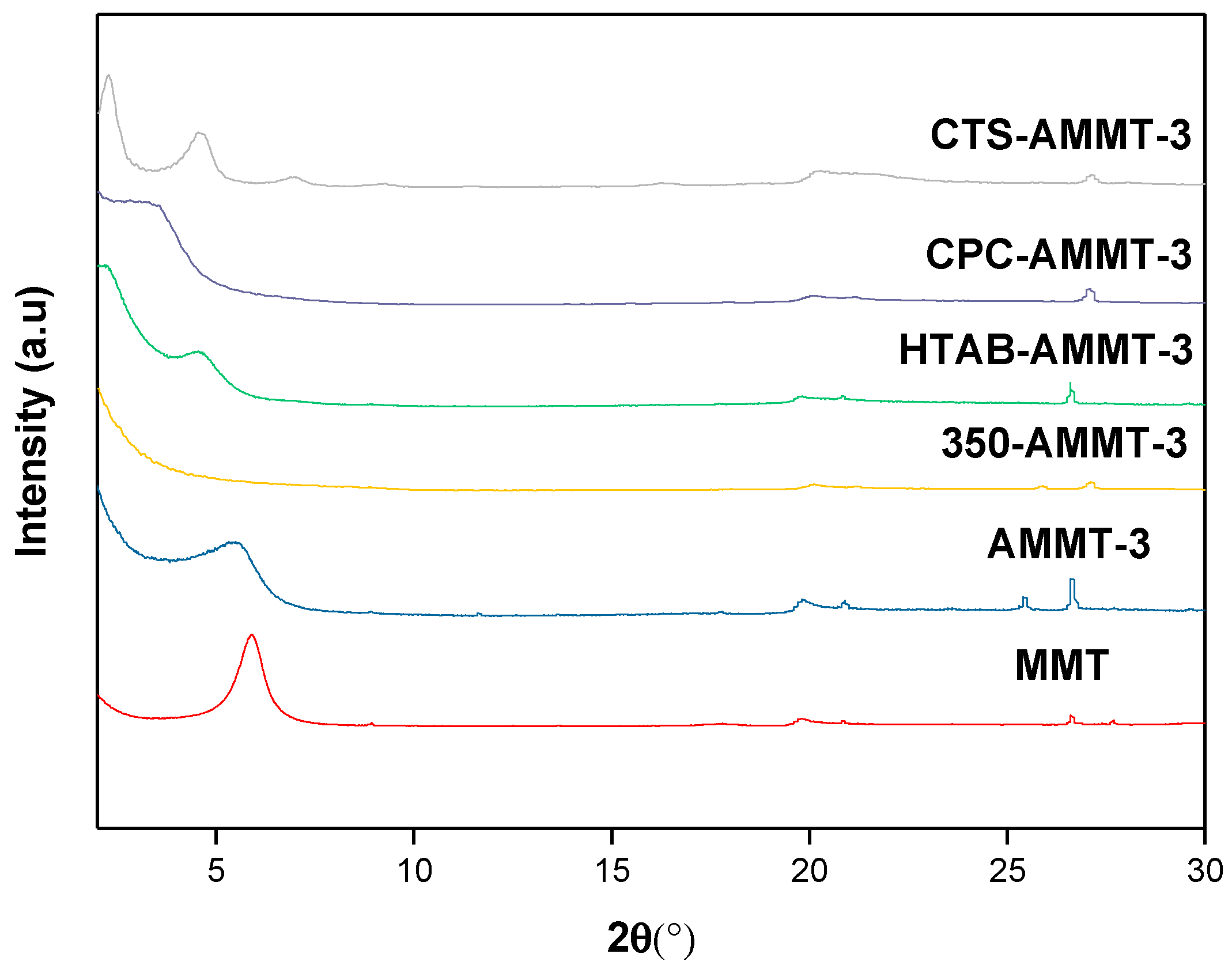
3.1. Structure Analysis
3.2. Elemental Analysis
3.3. Texture Analysis
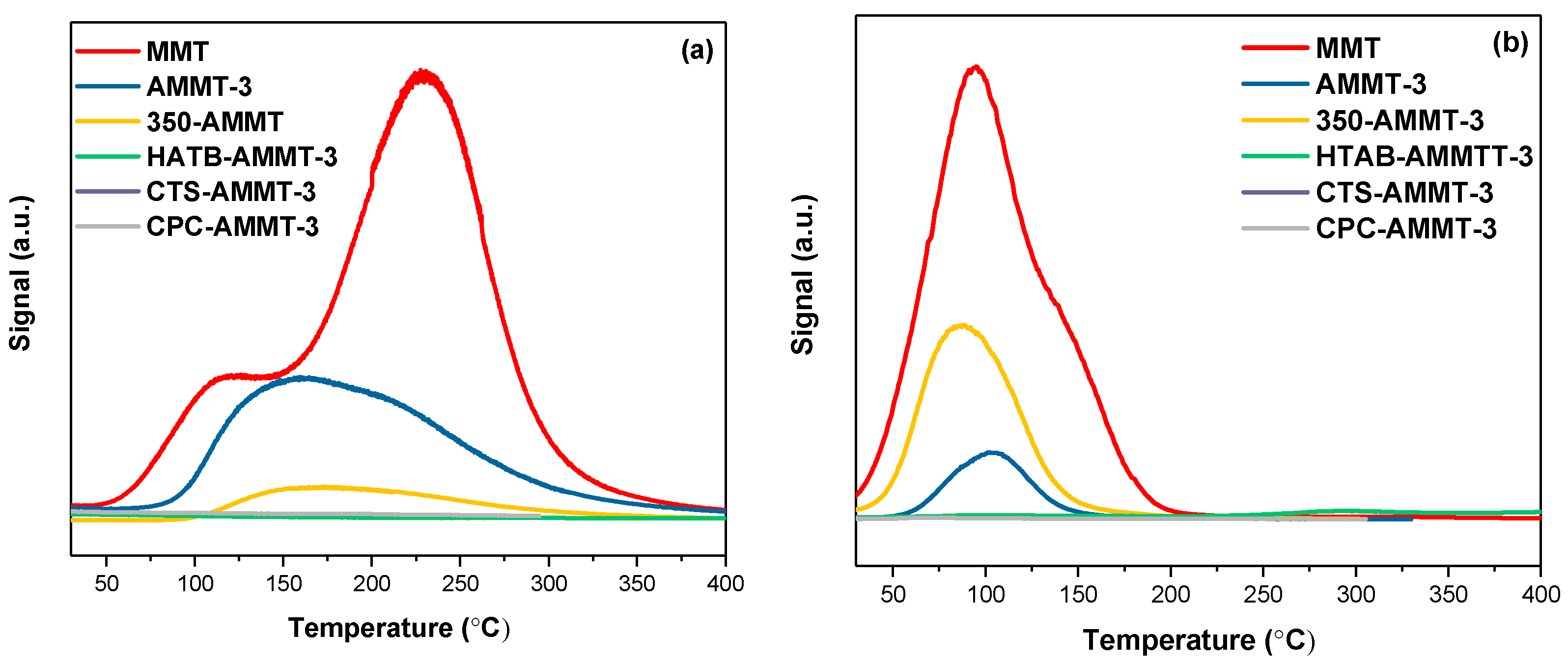
3.4. Acetone/Benzene-TPD
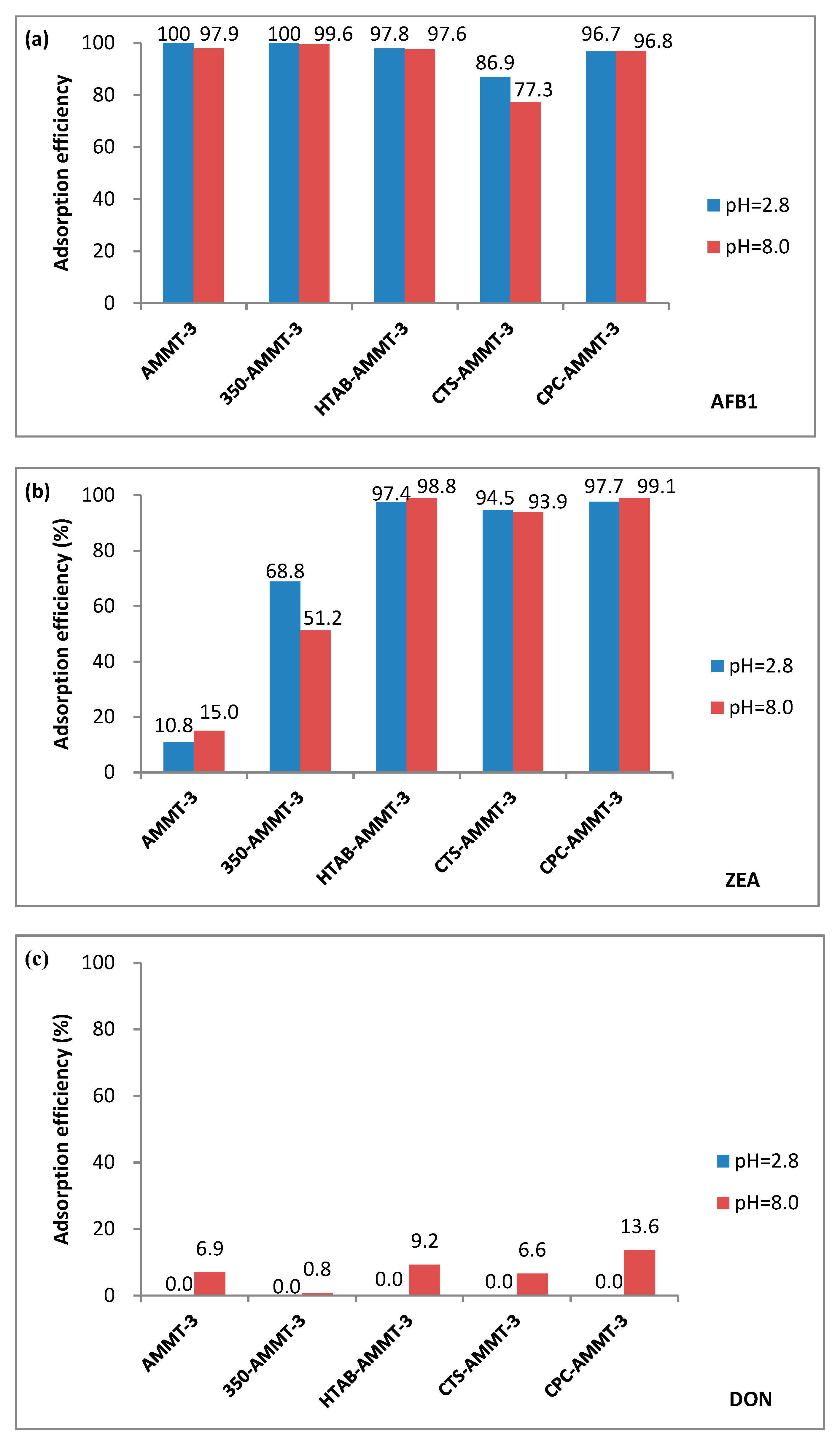
3.5. Adsorption Performance of Mycotoxins
3.5.1. Adsorption Performance of Modified Montmorillonites for Mycotoxins
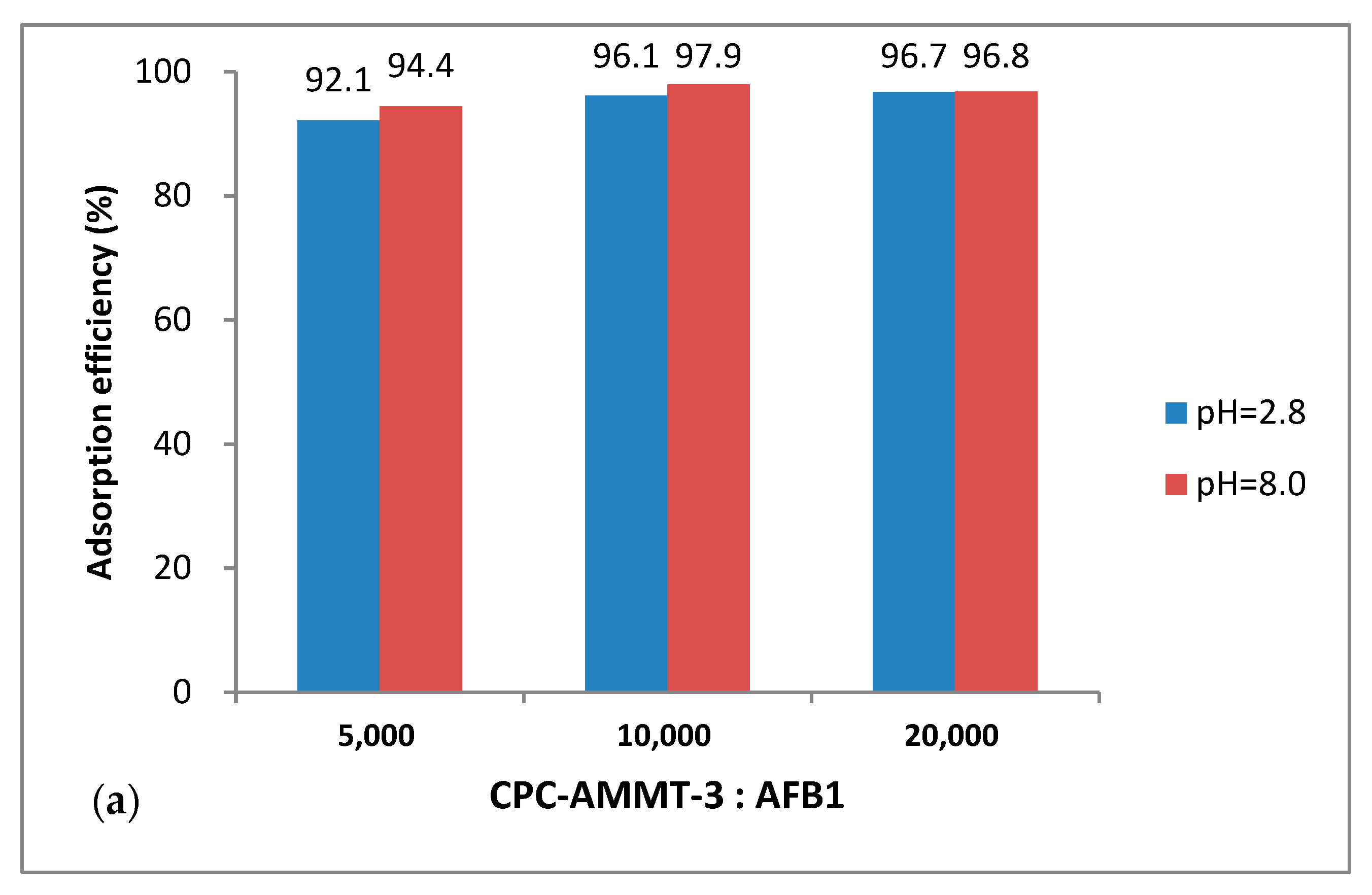
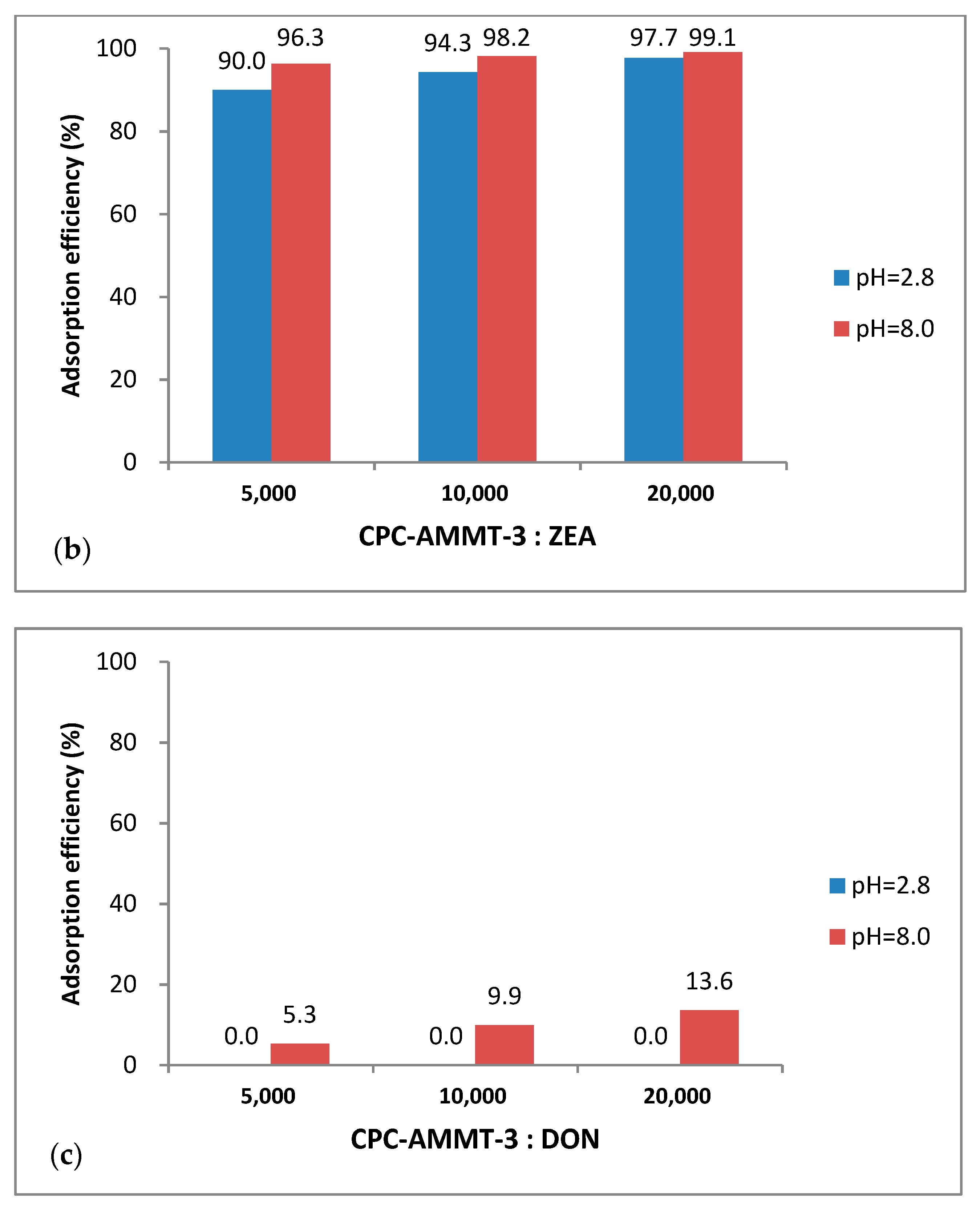
3.5.2. Effect of Different Montmorillonite/Mycotoxin Mass Ratios on Adsorption Performance of CPC-AMMT-3
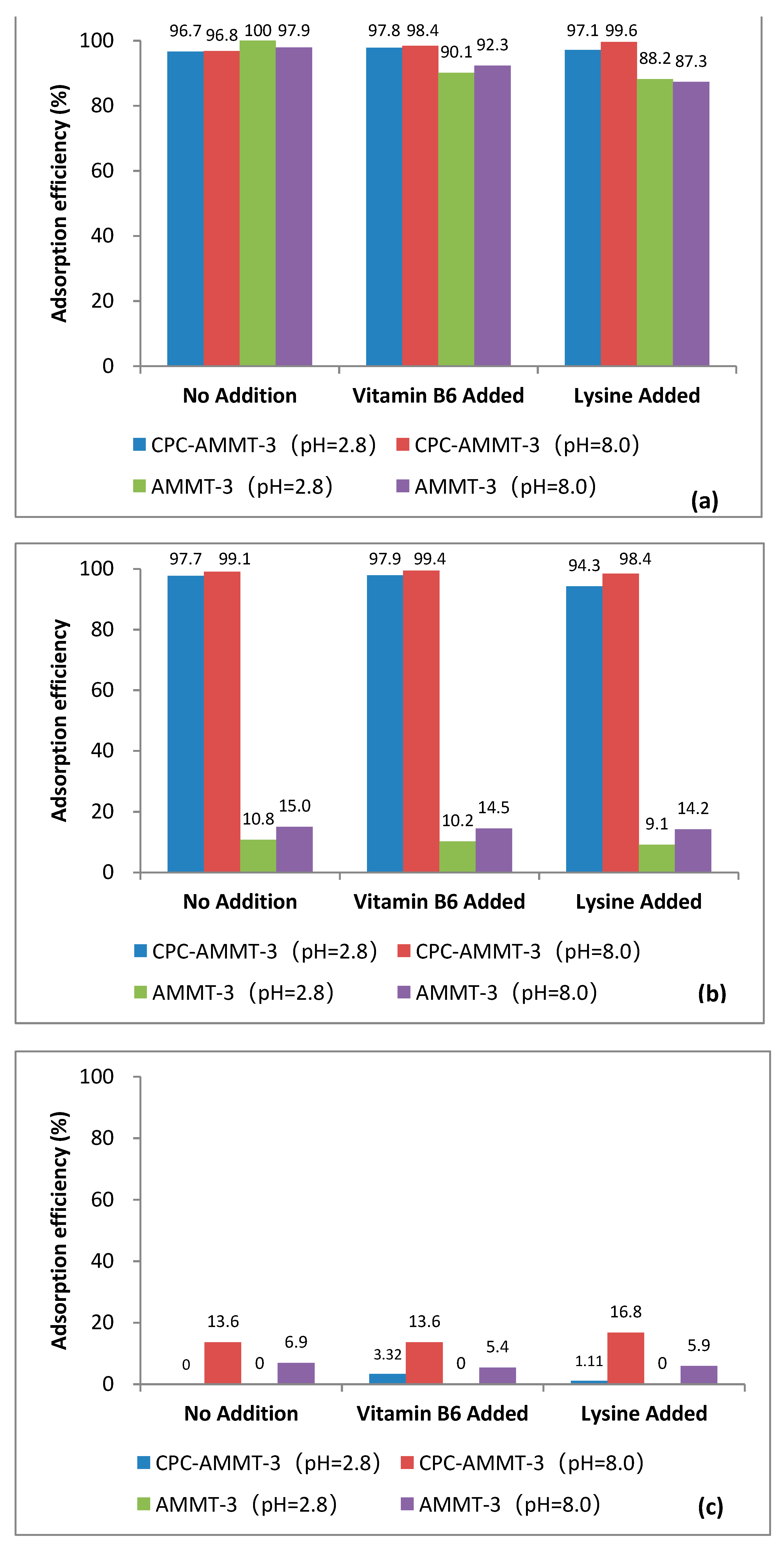
3.5.3. Selective Adsorption Performance of AMMT-3 and CPC-AMMT-3 in Presence of Nutrients
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Aiko, V.; Edamana, P.; Mehta, A. Decomposition and detoxification of aflatoxin B1by lactic acid. J. Sci. Food Agric. 2015, 96, 1959–1966. [Google Scholar] [CrossRef]
- Chilaka, C.A.; De Boevre, M.; Atanda, O.O.; De Saeger, S. Occurrence of Fusarium Mycotoxins in Cereal Crops and Processed Products (Ogi) from Nigeria. Toxins 2016, 8, 342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, R.; Feussner, K.; Wu, T.; Yan, F.; Karlovsky, P.; Zheng, X. Detoxification of mycotoxin patulin by the yeast Rhodosporidium paludigenum. Food Chem. 2015, 179, 1–5. [Google Scholar] [CrossRef]
- Grenier, B.; Bracarense, A.-P.F.; Schwartz-Zimmermann, H.; Trumel, C.; Cossalter, A.-M.; Schatzmayr, G.; Kolf-Clauw, M.; Moll, W.-D.; Oswald, I. The low intestinal and hepatic toxicity of hydrolyzed fumonisin B1 correlates with its inability to alter the metabolism of sphingolipids. Biochem. Pharmacol. 2012, 83, 1465–1473. [Google Scholar] [CrossRef]
- Bullerman, L.B.; Bianchini, A. Stability of mycotoxins during food processing. Int. J. Food Microbiol. 2007, 119, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Cano-Sancho, G.; Marín, S.; Ramos, A.J.; Sanchis, V. Biomonitoring of Fusarium spp. Mycotoxins: Perspectives for an Individual Exposure Assessment Tool. Food Sci. Technol. Int. 2010, 16, 266–276. [Google Scholar] [CrossRef]
- Wang, X.; Ufer, K.; Kleeberg, R. Routine investigation of structural parameters of dioctahedral smectites by the Rietveld method. Appl. Clay Sci. 2018, 163, 257–264. [Google Scholar] [CrossRef]
- Kosicki, R.; Błajet-Kosicka, A.; Grajewski, J.; Twarużek, M. Multiannual mycotoxin survey in feed materials and feedingstuffs. Anim. Feed Sci. Technol. 2016, 215, 165–180. [Google Scholar] [CrossRef]
- Zachariasova, M.; Dzuman, Z.; Veprikova, Z.; Hajkova, K.; Jiru, M.; Vaclavikova, M.; Zachariasova, A.; Pospichalova, M.; Florian, M.; Hajslova, J. Occurrence of multiple mycotoxins in European feedingstuffs, assessment of dietary intake by farm animals. Anim. Feed Sci. Technol. 2014, 193, 124–140. [Google Scholar] [CrossRef]
- Mao, J.; Lv, G.; Zhou, R. Effect of acid-treated and hexadecyltrimethylammonium bromide–modified montmorillonites on adsorption performance of mycotoxins. Environ. Sci. Pollut. Res. 2019, 27, 4284–4293. [Google Scholar] [CrossRef]
- Avantaggiato, G.; Greco, D.; Damascelli, A.; Solfrizzo, M.; Visconti, A. Assessment of Multi-mycotoxin Adsorption Efficacy of Grape Pomace. J. Agric. Food Chem. 2014, 62, 497–507. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Xi, Y.; Lian, C.; Sun, Z.; Zheng, S. Simultaneous detoxification of polar aflatoxin B1 and weak polar zearalenone from simulated gastrointestinal tract by zwitterionic montmorillonites. J. Hazard. Mater. 2018, 364, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Shan, M.; Du, H.; Han, X.; Xu, Z. In vitro adsorption of zearalenone by cetyltrimethyl ammonium bromide-modified montmorillonite nanocomposites. Microporous Mesoporous Mater. 2008, 113, 99–105. [Google Scholar] [CrossRef]
- Lertsutthiwong, P.; Noomun, K.; Khunthon, S.; Limpanart, S. Influence of chitosan characteristics on the properties of biopolymeric chitosan–montmorillonite. Prog. Nat. Sci. 2012, 22, 502–508. [Google Scholar] [CrossRef] [Green Version]
- Burton, A.W.; Ong, K.; Rea, T.; Chan, I.Y. On the estimation of average crystallite size of zeolites from the Scherrer equation: A critical evaluation of its application to zeolites with one-dimensional pore systems. Microporous Mesoporous Mater. 2009, 117, 75–90. [Google Scholar] [CrossRef]
- Jiang, Y. Preparation of Al-pillared Bentonite by Microwave Irradiation Method and Its Properties. Non-Met. Mines 2004, 6. Available online: https://en.cnki.com.cn/Article_en/CJFDTotal-FJSK200406007.htm (accessed on 27 November 2021).
- Nones, J.; Nones, J.; Riella, H.; Poli, A.; Trentin, A.; Kuhnen, N.C. Thermal treatment of bentonite reduces aflatoxin b1 adsorption and affects stem cell death. Mater. Sci. Eng. C 2015, 55, 530–537. [Google Scholar] [CrossRef]
- Kong, Q.; Zhang, H.; Zheng, L.; Wang, D.-Y.; Zhang, J. Effect on thermal and combustion behaviors of montmorillonite intercalation nickel compounds in polypropylene/IFR system. Polym. Adv. Technol. 2015, 28, 965–970. [Google Scholar] [CrossRef]
- Kooli, F.; Hian, P.C.; Weirong, Q.; Alshahateet, S.F.; Chen, F. Effect of the acid-activated clays on the properties of porous clay heterostructures. J. Porous Mater. 2006, 13, 319–324. [Google Scholar] [CrossRef]
- Topcu, C.; Caglar, B.; Onder, A.; Coldur, F.; Caglar, S.; Guner, E.K.; Cubuk, O.; Tabak, A. Structural characterization of chitosan-smectite nanocomposite and its application in the development of a novel potentiometric monohydrogen phosphate-selective sensor. Mater. Res. Bull. 2018, 98, 288–299. [Google Scholar] [CrossRef]
- De Mil, T.; Devreese, M.; De Baere, S.; Van Ranst, E.; Eeckhout, M.; De Backer, P.; Croubels, S. Characterization of 27 Mycotoxin Binders and the Relation with in Vitro Zearalenone Adsorption at a Single Concentration. Toxins 2015, 7, 21–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, H.; Frost, R.L.; Bostrom, T.; Yuan, P.; Duong, L.; Yang, D.; Xi, Y.; Kloprogge, T. Changes in the morphology of organoclays with HDTMA+ surfactant loading. Appl. Clay Sci. 2006, 31, 262–271. [Google Scholar] [CrossRef] [Green Version]
- Günister, E.; Pestreli, D.; Ünlü, C.H.; Atıcı, O.; Güngör, N. Synthesis and characterization of chitosan-MMT biocomposite systems. Carbohydr. Polym. 2007, 67, 358–365. [Google Scholar] [CrossRef]
- Wang, G.; Miao, Y.; Sun, Z.; Zheng, S. Simultaneous adsorption of aflatoxin B1 and zearalenone by mono- and di-alkyl cationic surfactants modified montmorillonites. J. Colloid Interface Sci. 2018, 511, 67–76. [Google Scholar] [CrossRef]
- Zeng, L.; Wang, S.P. Adsorption of Zearalenone by Montmorillonite. Adv. Mater. Res. 2013, 683, 343–347. [Google Scholar] [CrossRef]


| Elution Time (min) | HPLC-Grade Water (%) | Acetonitrile (%) |
|---|---|---|
| 0.00 | 90 | 10 |
| 5.00 | 15 | 85 |
| 8.00 | 5 | 95 |
| 10.00 | 5 | 95 |
| 10.50 | 65 | 35 |
| 13.50 | 65 | 35 |
| Samples | 2θ (°) | d001 (Å) | CEC (mmol/g) |
|---|---|---|---|
| MMT | 5.92 | 14.91 | 1.06 |
| AMMT-3 | 5.55 | 15.9 | 0.57 |
| 350-AMMT-3 | 8.73 | 10.1 | 0.38 |
| HTAB-AMMT-3 | 2.23 | 39.0 | 0 |
| CTS-AMMT-3 | 2.29 | 38.4 | 0 |
| CPC-AMMT-3 | 3.42 | 25.8 | 0 |
| Samples | Initial Concentration of Intercalation 1 (CEC) | N% 2 | Load Amount 3 (mmol/g) |
|---|---|---|---|
| HTAB-AMMT-3 | 2.40 | 1.15 | 0.82 |
| CTS-AMMT-3 | 3.16 | 1.73 | 1.24 |
| CPC-AMMT-3 | 2.45 | 1.28 | 0.91 |
| Samples | SBET (m2/g) | V (cm3/g) | Pore Size (Å) |
|---|---|---|---|
| AMMT-3 | 267.0 | 0.30 | 46 |
| 350-AMMT-3 | 251.0 | 0.32 | 51 |
| HTAB-AMMT-3 | 7.4 | 0.04 | 195 |
| CTS-AMMT-3 | 1.4 | 0.01 | 130 |
| CPC-AMMT-3 | 4.8 | 0.03 | 222 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, J.; Zhou, Y.; Lv, G.; Zhou, R. Simultaneous Detoxification of Aflatoxin B1, Zearalenone and Deoxynivalenol by Modified Montmorillonites. Molecules 2022, 27, 315. https://doi.org/10.3390/molecules27010315
Mao J, Zhou Y, Lv G, Zhou R. Simultaneous Detoxification of Aflatoxin B1, Zearalenone and Deoxynivalenol by Modified Montmorillonites. Molecules. 2022; 27(1):315. https://doi.org/10.3390/molecules27010315
Chicago/Turabian StyleMao, Jiaqi, Ying Zhou, Guanglie Lv, and Renxian Zhou. 2022. "Simultaneous Detoxification of Aflatoxin B1, Zearalenone and Deoxynivalenol by Modified Montmorillonites" Molecules 27, no. 1: 315. https://doi.org/10.3390/molecules27010315





