A New Approach to the Determination of Silicon in Zinc, Lead-Bearing Materials and in Waste Using the ICP-OES Method
Abstract
:1. Introduction
2. Results
2.1. Mineralization and ICP-OES Measurement Parameters
- 25 min to reach 190 °C
- 10 min to reach 220 °C
- 15 min for mineralization at 220 °C
- 40 min for cooling
2.2. Radial and Axial ICP-OES Systems
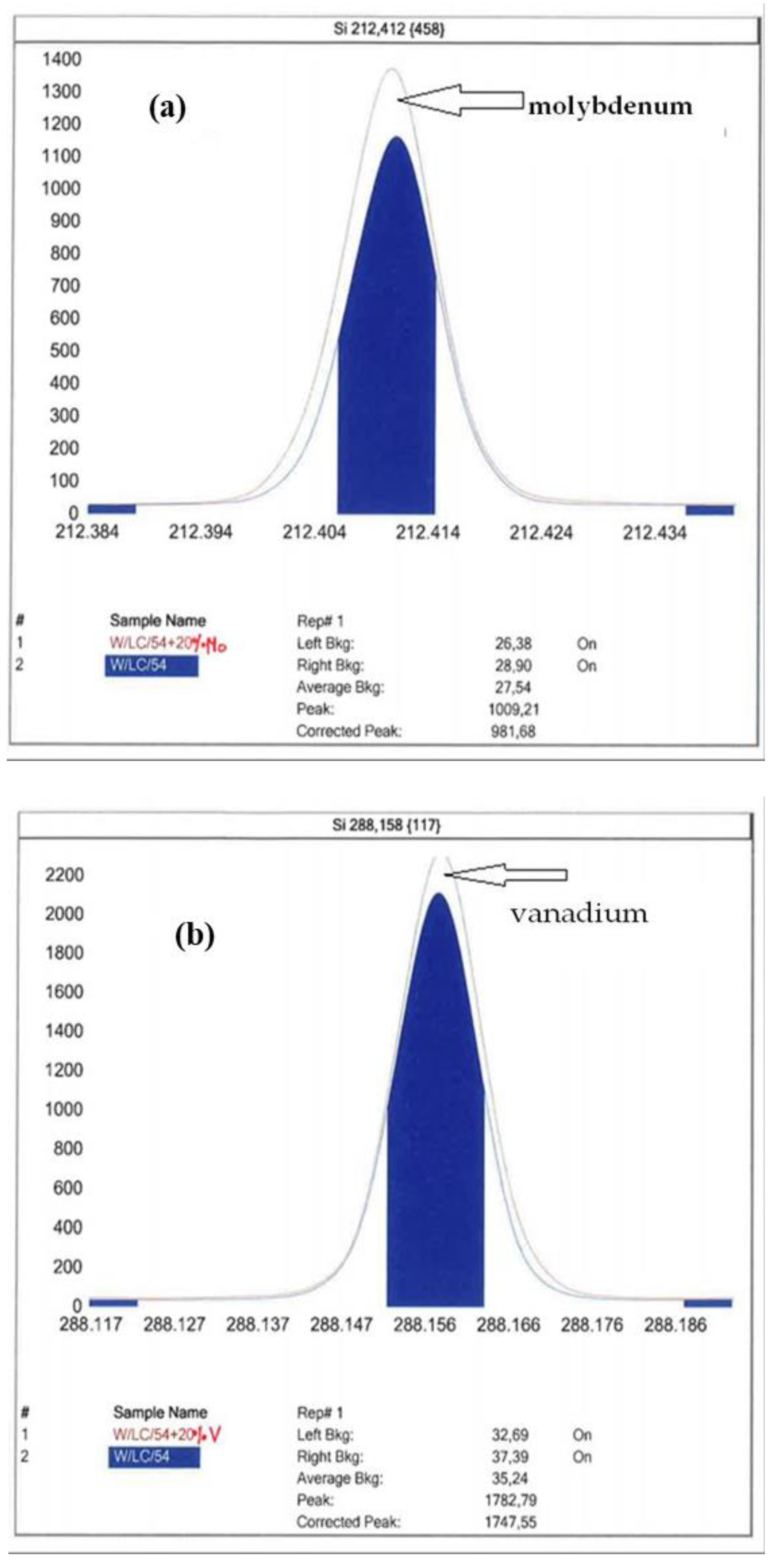
2.3. Spectral Interference
2.4. Validation Parameters
2.5. Silicon Content in Certified Reference Materials and Test Samples
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Research Material
- CRM_1—Zinc concentrate (zinc sulfide) with a silicon content of 0.122%—produced by the Institute of Non-Ferrous Metals, Gliwice, Poland;
- CRM_2—Zinc concentrate (zinc sulfide) with a silicon content of 0.295%—produced by the Canadian Certified Reference Materials Project, Ottawa, Canada;
- CRM_3—Lead concentrate (lead sulfide) with a silicon content of 0.305%—produced by the Canadian Certified Reference Materials Project, Ottawa, Canada;
- CRM_4—Zinc oxide with a silicon content of 2.56%—produced by the Institute of Non-Ferrous Metals, Gliwice, Poland;
- CRM_5—Zinc concentrate (zinc sulfide) with a silicon content of 9.30%—manufactured by the China National Analysis Center for Iron and Steel, Beijing, China;
- CRM_6—Zinc ore with a silicon content 38.77%—manufactured by the China National Analysis Center for Iron and Steel, Beijing, China.
4.3. Apparatus
4.4. Selecting the Mineralization Parameters
4.5. Calculation Methods
- 15% of the lower result for a silicon content of 0.1–0.5%;
- 10% of the lower result for a silicon content of 0.51–2%;
- 5% of the result lower for a silicon content of 2.01–10%;
- 2.5% of the lower result for a silicon content above 10%.
4.5.1. Working Range
4.5.2. Linearity
4.5.3. Resistance
4.5.4. Limit of Detection (LOD) and Limit of Quantification (LOQ)
4.5.5. Selectivity
4.5.6. Precision
4.5.7. Correctness
4.5.8. Extended Uncertainty
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Wedepohl, K.H. Composition of the continental crust. Geochim. Cosmochim. Acta 1995, 59, 1217–1232. [Google Scholar] [CrossRef]
- Weiss, C. A Background to Silicon and Its Applications. AZO Mater. 2001. Available online: https://www.azom.com/article.aspx?ArticleID=599 (accessed on 28 March 2022).
- Polish Norm PN-H-04913-05:1975 Chemical Analysis of Zinc Concentrates. Determination of Silica Content (in the Original: PN-H-04913-05:1975 “Analiza Chemiczna Koncentratów Cynku. Oznaczanie Zawartości Krzemionki”). Available online: https://sklep.pkn.pl/pn-h-04914-05-1975p.html (accessed on 28 March 2022).
- Adeeyinwo, C.E.; Adesina, K. Performance characteristic of visible spectrophotometer in Analyzing silicon. J. Appl. Sci. 2002, 5, 3139–3147. [Google Scholar]
- Armstrong, F.A.J. The determination of silicate in sea water. J. Mar. Biolog. Assoc. 1951, 30, 149–160. [Google Scholar] [CrossRef] [Green Version]
- Jeffery, G.H.; Basset, J.; Menham, J.; Denney, R.C. Vogel’s Textbook of Quantitative Chemical Analysis, 5th ed.; Bath Press: Avon, UK, 1909; pp. 309–342. [Google Scholar]
- Rajković, D. Spectrophotometric determination of traces of silicon as molybdenum blue in uranium dioxide. Fresenius’ Z. für Anal. Chem. 1971, 255, 190–194. [Google Scholar] [CrossRef]
- Van Dalen, H.P.J.; Galam, L.D. Formulation of Analytical procedures involving flame Atomic—Absorption Spectrometry. Analyst 1981, 10, 695–701. [Google Scholar] [CrossRef]
- Lim, P.G. Total silica Analysis using a double beam Atomic Absorption Spectrophotometer. In Proceedings of the World Geothermal Congress, Anataly, Turkey, 24–29 April 2005; Available online: https://www.geothermal-energy.org/pdf/IGAstandard/WGC/2005/0812.pdf (accessed on 28 March 2022).
- Fox, R.L.; Silva, J.A.; Plucknett, D.L.; Teranish, D.Y. Soluble and total silicon in sugarcane. Plant Soil 1969, 30, 81–91. [Google Scholar] [CrossRef]
- Elliot, C.L.; Snyder, G.H. Autoclave-induced digestion for the colorimetric determination of silicon in rice straw. J. Agric. Food Chem. 1991, 39, 1118–1119. [Google Scholar] [CrossRef]
- Nóbrega, J.A.; Donati, G.L. Microwave-Assisted Sample Preparation for Spectrochemistry; Meyers, R.A., Ed.; Encyclopedia of Analytical Chemistry; Wiley: Chichester, UK, 2011. [Google Scholar] [CrossRef]
- Rocha, D.L.; Batista, A.D.; Rocha, F.R.P.; Donati, G.L.; Nóbrega, J.A. Greening sample preparation in inorganic analysis. Trends Anal. Chem. 2013, 45, 79–92. [Google Scholar] [CrossRef]
- Aldabe, J.; Santamaría, C.; Elustondo, D.; Lasheras, E.; Santamaría, J.M. Application of microwave digestion and ICP-MS to simultaneous analysis of major and trace elements in aerosol samples collected on quartz filters. Anal. Methods 2013, 5, 554–559. [Google Scholar] [CrossRef]
- Hauptkorn, S.; Pavel, J.; Seltner, H. Determination of silicon in biological samples by ICP-OES after non-oxidative decomposition under alkaline conditions. Fresenius J. Anal. Chem. 2001, 370, 246–250. [Google Scholar] [CrossRef]
- Liang, F.; Zhang, H.; Jin, Q.; Zhang, D.; Lei, Y. Use of microwave plasma torch atomic emission spectrometry for the determination of silicon. Fresenius J. Anal. Chem. 1997, 357, 384–388. [Google Scholar] [CrossRef]
- Krushevska, A.; Barnes, R.M. Inductively coupled plasma atomic emission spectrometric determination of aluminium, barium, silicon, strontium and titanium in food after sample fusion. Analyst 1994, 119, 131–134. [Google Scholar] [CrossRef]
- Meyer, A.; Friess, M.; Riedel, R.; Harris, M.; Jacob, E.; Tölg, G.; Kaiser, G. Critical comparison of ICP-OES, XRF and fluorine volatilization-FTIR spectrometry for the reliable determination of the silicon main constituent in ceramic materials. Fresenius J. Anal. Chem. 1995, 352, 318–326. [Google Scholar] [CrossRef]
- Wang, C.F.; Tu, F.H.; Jeng, S.L.; Chin, C.J. The determination of silicon in airborne particulate matter by XRF and LA-ICP-MS. J. Radioanal. Nucl. Chem. 1999, 242, 97–103. [Google Scholar] [CrossRef]
- Shoukry, A.F.; Issa, Y.M.; Farghaly, R.A.; Grasserbauer, M.; Puxbaum, H.; Rendl, J. Determination of silicon using electrothermal Zeeman atomic absorption spectrometry in presence of some transition metals as modifiers. Fresenius J. Anal. Chem. 1998, 360, 650–653. Available online: https://scholar.cu.edu.eg/sites/default/files/raafat811964/files/determination_of_silicon_using_electrothermal_zeeman_atomic_absorption_1.pdf (accessed on 28 March 2022). [CrossRef]
- Taddia, M.; Clauser, G. Indirect atomic absorption spectrometric determination of silicon in gallium arsenide using an amplification reaction. Fresenius J. Anal. Chem. 1993, 345, 575–578. [Google Scholar] [CrossRef]
- Minami, H.; Yoshida, T.; Okutsu, K.; Zhang, Q.; Inoue, S.; Atsuya, I. Direct determination of silicon in powdered aluminium oxide by use of slurry sampling with in situ fusion graphite-furnace atomic-absorption spectrometry. Fresenius J. Anal. Chem. 2001, 370, 855–859. [Google Scholar] [CrossRef]
- Boschetti, W.; Dalagnol, L.M.G.; Dullius, M.; Zmozinski, A.V.; Becker, E.M.; Vale, M.G.R.; de Andrade, J.B. Determination of silicon in plant materials using direct solid sample analysis with high-resolution continuum source graphite furnace atomic absorption spectrometry. Microchem. J. 2016, 124, 380–385. [Google Scholar] [CrossRef]
- Nakadia, F.V.; Prodanova, C.; Boschetti, W.; Valea, M.G.R.; Welzb, B.; de Andrade, J.B. Determination of silicon in biomass and products of pyrolysis process via high-resolution continuum source atomic absorption spectrometry. Talanta 2018, 179, 828–835. [Google Scholar] [CrossRef]
- Dravecz, G.; Bencs, L.; Beke, D.; Gali, A. Determination of silicon and aluminum in silicon carbide nanocrystals by high-resolution continuum source graphite furnace atomic absorption spectrometry. Talanta 2016, 147, 271–275. [Google Scholar] [CrossRef]
- Guerra, M.B.B.; Adame, A.; de Almeida, E.; Brasil, M.A.S.; Schaefer, C.E.G.R.; Krug, F.J. In situ Determination of K, Ca, S and Si in Fresh Sugar Cane Leaves by Handheld Energy Dispersive X-Ray Fluorescence Spectrometry. J. Braz. Chem. Soc. 2018, 29, 1086–1093. [Google Scholar] [CrossRef]
- Bradshaw, D.K. Influence of Spectral Interferences on the Reliability of Data When Using Analyte Addition Techniques with ICP-OES. Spectroscopy 2020, 35, 53–56. Available online: https://www.spectroscopyonline.com/view/influence-spectral-interferences-reliability-data-when-using-analyte-addition-techniques-icp-oes (accessed on 28 March 2022).
- PN-EN 11885: 2009; Water Quality—Determination of Selected Elements by Optical Emission Spectrometry with Inductively Induced Plasma (ICP-OES) (in Oryginal: PN-EN 11885:2009 “Jakość Wody—Oznaczanie Wybranych Pierwiastków Metodą Optycznej Spektrometrii Emisyjnej z Plazmą Wzbudzoną Indukcyjnie (ICP-OES)”). Available online: https://sklep.pkn.pl/pn-en-iso-11885-2009e.html (accessed on 28 March 2022).
- ISO 12743: 2018; Copper, Lead, Zinc and Nickel Concentrates—Sampling Procedures for Determination of Metal and Moisture Content. Available online: https://www.iso.org/standard/69467.html (accessed on 28 March 2022).
- E-Stat Service. Faculty of Chemistry, University of Warsaw. Available online: http://beta.chem.uw.edu.pl/stat/ (accessed on 28 March 2022).
- Fitzsimmons, J. Analytical method Validation: ICP-OES. Electronic Supplementary Material (ESI) for Journal of Analytical Atomic Spectrometry. The Royal Society of Chemistry 2015. Available online: http://www.rsc.org/suppdata/c5/ja/c5ja00419e/c5ja00419e1.pdf (accessed on 28 March 2022).
- NMKL Report No 8 “Quality Assurance Principles for Chemical Food Laboratories”; Nordic Council of Ministers. Copenhagen, 48E.; Nord: 1990. Available online: https://agris.fao.org/agris-search/search.do?recordID=XF2015012950 (accessed on 28 March 2022).
- Thiem, T.L.; Salter, R.H.; Gardner, J.A. Quantitative simultaneous elemental determinations in alloys using laser-induced breakdown spectroscopy (LIBS) in an ultra-high vacuum. Appl. Spectrosc. 1994, 48, 58–64. [Google Scholar] [CrossRef]
- PN-ISO 5725-1:2002; “Accuracy (Correctness and Precision) of Measurement Methods and Measurement Results—Part 1: General Principles and Definitions” (in Oryginal: PN-ISO 5725-1:2002 “Dokładność (Poprawność i Precyzja) Metod Pomiarowych i Wyników Pomiarów—Część 1: Ogólne Zasady i Definicje”). Available online: https://sklep.pkn.pl/pn-iso-5725-1-2002p.html (accessed on 28 March 2022).
- Senila, M.; Drolc, A.; Pintar, A.; Senila, L.; Levei, E. Validation and measurement uncertainty evaluation of the ICP-OES method for the multi-elemental determination of essential and nonessential elements from medicinal plants and their aqueous extracts. J. Anal. Sci. Technol. 2014, 5, 37. [Google Scholar] [CrossRef] [Green Version]




| Parameter | Value |
|---|---|
| Radio frequency power (RF) | 1150 W |
| Gas flow in the nebulizer | 0.50 L/min |
| Auxiliary gas flow | 0.5 L/min |
| Plasma gas flow | 12 L/min |
| Pump speed | 50 rpm |
| Purge flow | standard |
| Nebulizer pressure | 210 a |
| Analytical Line, nm | RSD, % | LOQ, mg/L | Selectivity, 1/(mg/L) 1 | |||
|---|---|---|---|---|---|---|
| Axial | Radial | Axial | Radial | Axial | Radial | |
| 212.412 | 0.12 | 0.13 | 0.012 | 0.013 | 1285 | 196 |
| 251.611 | 0.92 | 0.12 | 0.091 | 0.012 | 4915 | 583 |
| 288.158 | 0.29 | 0.16 | 0.029 | 0.016 | 3188 | 374 |
| Parameter | Criteria | Results |
|---|---|---|
| Working range | 0.10–50% | 0.10–50% |
| Linearity | r ≥ 0.999 | r = 1 |
| LOD | LOD ≤ 0.05% | LOD = 0.050% |
| LOQ | LOQ ≤ 0.10% | LOQ = 0.10% |
| Selectivity | Interference may not significantly affect the test results | Molybdenum, chromium and vanadium interference does not statistically significantly affect the test results |
| Precision | 1. RSD < 15% in the range of silicon content of 0.10–0.50 % 2. RSD < 10% in the content of silicon > 0.51% | 1. Silicon content range of 0.10–0.50%: RSDmax = 10.2% 2. Silicon content > 0.51% RSDmax = 6.0% |
| Correctness | 90% ≤ recovery ≤ 110% | 90.0–101.1% |
| Extended uncertainty | 1. U(x) < 50% in the silicon content range of 0.10–0.50% 2. U(x) < 30% in the silicon content > 0.51% | 1. Silicon content range of 0.10–0.50%: U(x)max = 33.2% 2. Silicon content > 0.51%: U(x)max = 19.1% |
| Certified Reference Material | Silicon Content Determined ± U(x), % | Silicon Content Given by the Manufacturer ± U(x), % |
|---|---|---|
| CRM_1 | 0.110 ± 0.035 | 0.122 ± 0.028 |
| CRM_2 | 0.265 ± 0.063 | 0.295 ± 0.019 |
| CRM_3 | 0.303 ± 0.069 | 0.305 ± 0.029 |
| CRM_4 | 2.59 ± 0.29 | 2.56 ± 0.11 |
| CRM_5 | 9.05 ± 0.65 | 9.30 ± 0.06 |
| CRM_6 | 38.02 ± 1.69 | 38.77 ± 0.10 |
| Test Samples | Silicon Content ± U(x), % |
|---|---|
| 1 | 0.190 ± 0.051 |
| 2 | 0.310 ± 0.070 |
| 3 | 0.550 ± 0.103 |
| 4 | 1.65 ± 0.21 |
| 5 | 2.98 ± 0.31 |
| 6 | 5.35 ± 0.46 |
| 7 | 7.56 ± 0.58 |
| 8 | 12.11 ± 0.79 |
| 9 | 25.34 ± 1.29 |
| Sample No. | Compound | Origin | Average Content of Zn and Pb, % | Appearance |
|---|---|---|---|---|
| 1 | ZnO | obtained from zinc bearing waste (steel dust) | Zn ≈ 62; Pb ≈ 3 |  |
| 2 | PbS | galena | Zn ≈ 3; Pb ≈ 63 |  |
| 3 | ZnO | obtained from zinc bearing waste (sludge) | Zn ≈ 57; Pb ≈ 2 |  |
| 4 | ZnS (blende) | imported from the Grot mine in Serbia | Zn ≈ 49; Pb ≈ 2.5 |  |
| 5 | ZnS (blende) | imported from the Lece mine in Serbia | Zn ≈ 51; Pb ≈ 0.6 |  |
| 6 | Zinc-bearing waste | sludge from flotation process (code 190205) | Zn ≈ 13; Pb ≈ 6 |  |
| 7 | Zinc-bearing waste | Singen steel dust (code 100207) | Zn ≈ 35; Pb ≈ 0.1 |  |
| 8 | Zinc-bearing waste | Tiroler Rohre sludge (code 100213) | Zn ≈ 31; Pb ≈ 0.6 |  |
| 9 | Zinc-lead ore | imported from Swedish mines | Zn ≈ 8; Pb ≈ 4 |  |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Przybyła, A.; Kuc, J.; Wzorek, Z. A New Approach to the Determination of Silicon in Zinc, Lead-Bearing Materials and in Waste Using the ICP-OES Method. Molecules 2022, 27, 3059. https://doi.org/10.3390/molecules27103059
Przybyła A, Kuc J, Wzorek Z. A New Approach to the Determination of Silicon in Zinc, Lead-Bearing Materials and in Waste Using the ICP-OES Method. Molecules. 2022; 27(10):3059. https://doi.org/10.3390/molecules27103059
Chicago/Turabian StylePrzybyła, Artur, Joanna Kuc, and Zbigniew Wzorek. 2022. "A New Approach to the Determination of Silicon in Zinc, Lead-Bearing Materials and in Waste Using the ICP-OES Method" Molecules 27, no. 10: 3059. https://doi.org/10.3390/molecules27103059






