Designing Novel Strategy to Produce Active Nanohybrids in Sunlight for Purification of Water Based on Inorganic Nanolayers, Magnetic Nanocomposites and Organic Species
Abstract
:1. Introduction
2. Results
2.1. Characterization of the Prepared Filler
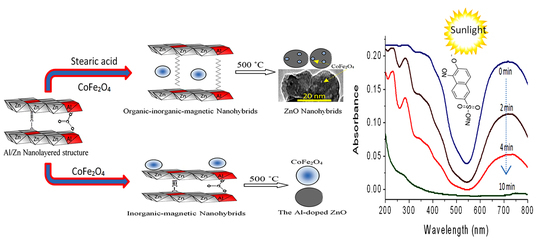
2.2. Design of Organic-Inorganic-Magnetic Nanohybrids
2.3. Design of Nanohybrids Based on Oxides
2.4. Optical Properties
2.5. Optical Activity
3. Discussion
- By sunlight, the surface of Photocatalyst was excited through absorption of enough energy for transferring electrons from valance band to conduction band creating holes in the valance band.Sunlight + ZNH → h+ (valance band) + e− (conduction band)
- At the same time, oxygen molecules physically adsorbed on the surface of the photocatalyst ZNH which captured the electron from the conduction band producing ions.(O2)ads + e− → O2•−
- The holes in valance band neutralized the hydroxyl groups which produced from water molecules producing hydroxyl radicals.(H2O ↔ H+ + OH−) + h+ → OH• + H+
- Neutralization of O2•−O2− + H+ → HO2•
- Production of hydrogen peroxide2HO2• → O2 + H2O2
- Hydrolysis of hydrogen peroxideH2O2 → OH− + OH•The free radicals of hydroxyl groups (•OH) and superoxide radical anion (•O2−) are strong oxidizing agents. The second stage focuses on oxidation reactions of the pollutants by different kinds of the oxidizing agents.
- The superoxide radical anions oxidized and decomposed the green dyesAG1 + •O2− → Degradation compounds + CO2 + H2O
- In addition, the holes started the degradation process of the pollutantsAG1 + h+ → AG1+ → Degradation products
4. Materials and Methods
4.1. Preparation of Magnetic Nanocomposites
4.2. Preparation of Nanohybrids and Nanolayered Structures
4.3. Preparation of Multi-Oxides Nanohybrids
4.4. Physical Characterization
4.5. Photocatalytic Activity
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Li, Y.; Kuang, J.; Lu, Y.; Cao, W. Facile Synthesis, Characterization of Flower-Like Vanadium Pentoxide Powders and Their Photocatalytic Behavior. Acta Metall. Sin. (Engl. Lett.) 2017, 30, 1017–1026. [Google Scholar] [CrossRef]
- Liu, C.; Xu, H.; Wang, L.; Qin, X. Facile One-Pot Green Synthesis and Antibacterial Activities of GO/Ag Nanocomposites. Acta Metall. Sin. (Engl. Lett.) 2017, 30, 36–44. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Wang, S.; Sun, G.; Gao, H.; Yu, X.; Tang, S.; Zhao, X.; Yi, Z.; Wang, Y.; Wei, Y. Facile preparation of MgAl2O4/CeO2/Mn3O4 heterojunction photocatalyst and enhanced photocatalytic activity. Mater. Today Chem. 2021, 19, 100390. [Google Scholar] [CrossRef]
- Liu, H.; Wang, S.; Gao, H.; Yang, H.; Wang, F.; Chen, X.; Fang, L.; Tang, S.; Yi, Z.; Li, D. A simple polyacrylamide gel route for the synthesis of MgAl2O4 nanoparticles with different metal sources as an efficient adsorbent: Neural network algorithm simulation, equilibrium, kinetics and thermodynamic studies. Sep. Purif. Technol. 2022, 281, 119855. [Google Scholar] [CrossRef]
- Gao, H.J.; Wang, S.F.; Fang, L.M.; Sun, G.A.; Chen, X.P.; Tang, S.N.; Yang, H.; Sun, G.Z.; Li, D.F. Nanostructured spinel-type M(M = Mg, Co, Zn)Cr2O4 oxides: Novel adsorbents for aqueous Congo red removal. Mater. Today Chem. 2021, 22, 100593. [Google Scholar] [CrossRef]
- Saber, O.; Kotb, H.M.; Osama, M.; Khater, H.A. An Effective Photocatalytic Degradation of Industrial Pollutants through Converting Titanium Oxide to Magnetic Nanotubes and Hollow Nanorods by Kirkendall Effect. Nanomaterials 2022, 12, 440. [Google Scholar] [CrossRef]
- Saber, O.; Shaalan, N.M.; Ahmed, F.; Kumar, S.; Alshoaibi, A. One-Step Multi-Doping Process for Producing Effective Zinc Oxide Nanofibers to Remove Industrial Pollutants Using Sunlight. Crystals 2021, 11, 1268. [Google Scholar] [CrossRef]
- Alshoaibi, A.; Saber, O.; Ahmed, F. Enhancement of Optical Activity and Properties of Barium Titanium Oxides to Be Active in Sunlight through Using Hollandite Phase Instead of Perovskite Phase. Crystals 2021, 11, 550. [Google Scholar] [CrossRef]
- Saber, O.; Alshoaibi, A.; Al-Yaari, M.; Osama, M. Conversion of Non-Optical Material to Photo-Active Nanocomposites through Non-Conventional Techniques for Water Purification by Solar Energy. Molecules 2020, 25, 4484. [Google Scholar] [CrossRef]
- Saber, O.; Kotb, H.M. Designing Dual-Function Nanostructures for Water Purification in Sunlight. Appl. Sci. 2020, 10, 1786. [Google Scholar] [CrossRef] [Green Version]
- Saber, O.; Aljaafari, A.; Osama, M.; Alabdulgader, H. Accelerating the Photocatalytic Degradation of Green Dye Pollutants by Using a New Coating Technique for Carbon Nanotubes with Nanolayered Structures and Nanocomposites. ChemistryOpen 2018, 7, 833–841. [Google Scholar] [CrossRef] [PubMed]
- Saber, O.; Alomair, H.; Abu-Abdeen, M.; Aljaafari, A. Fast degradation of green pollutants through nanonets and nanofibers of the Al-doped zinc oxide. Acta Metall. Sin. (Engl. Lett.) 2018, 31, 533–546. [Google Scholar] [CrossRef] [Green Version]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Gui, M.S.; Zhang, W.D. Preparation and modification of hierarchical nanostructured Bi2WO6 with high visible light-induced photocatalytic activity. Nanotechnology 2011, 22, 265601. [Google Scholar] [CrossRef]
- Chen, X.B.; Liu, L.; Yu, P.Y.; Mao, S.S. Increasing solar absorption for photocatalysis with black hydrogenated titanium dioxide nanocrystals. Science 2011, 331, 746–750. [Google Scholar] [CrossRef]
- Dindar, B.; Icli, S. Unusual photoreactivity of ZnO under concentrated sun light. J. Photochem. Photobiol. A Chem. 2001, 140, 263–268. [Google Scholar] [CrossRef]
- Yeber, M.C.; Roderiguez, J.; Freer, J.; Baeza, J.; Duran, N.; Mansilla, H.D. Advanced oxidation of a pulp mill bleaching wastewater. Chemosphere 1999, 39, 1679–1688. [Google Scholar] [CrossRef]
- Behnajady, M.A.; Modirshahla, N.; Hamzavi, R. Kinetic study on photocatalytic degradation of C.I. Acid Yellow 23 by ZnO photocatalyst. J. Hazard. Mater. B 2006, 133, 226–232. [Google Scholar] [CrossRef]
- Alghamdi, Y.G.; Maqsood, B.K.; Malik, A.; Alhayyani, S. Design and Preparation of Biomass-Derived Activated Carbon Loaded TiO2 Photocatalyst for Photocatalytic Degradation of Reactive Red 120 and Ofloxacin. Polymers 2022, 14, 880. [Google Scholar] [CrossRef]
- Ravikumar, S.; Mani, D.; Khan, M.R.; Ahmad, N.; Sylvestre, S.; Surya, C.; Kumar, B.K.; Pandiyan, V.; HoAhn, Y. Ag-TiO2@Pd/C nanocomposites for efficient degradation of Reactive Red 120 dye and ofloxacin antibiotic under UV and solar light and its antimicrobial activity. Environ. Chem. Eng. 2021, 9, 106657. [Google Scholar] [CrossRef]
- Krishnakumar, B.; Alsalme, A.; Alharthi, F.A.; Mani, D.; Anandan, K.; Amutha, P.; Sobral, A.J. Synthesis, characterization of gelatin assisted ZnO and its effective utilization of toxic azo dye degradation under direct sunlight. Opt. Mater. 2021, 113, 110854. [Google Scholar] [CrossRef]
- Krishnakumar, B.; Ravikumar, S.; Pandiyan, V.; Nithya, V.; Sobral, A.J.F.N. Synthesis, characterization of porphyrin and CdS modified spherical shaped SiO2 for Reactive Red 120 degradation under direct sunlight. Mol. Struct. 2020, 1210, 128021. [Google Scholar] [CrossRef]
- Sun, J.H.; Dong, S.Y.; Wang, Y.K.; Sun, S.P. Preparation and photocatalytic property of a novel dumbbell-shaped ZnO microcrystal photocatalyst. J. Hazard. Mater. 2009, 172, 1520–1526. [Google Scholar] [CrossRef]
- Wang, Q.; Tang, C.; Jiang, C.; Du, D.; Wang, F.; Song, J. Role of Substrate Roughness in ZnO Nanowire Arrays Growth by Hydrothermal Approach. Acta Metall. Sin. Engl. Lett. 2016, 29, 237–242. [Google Scholar] [CrossRef] [Green Version]
- Zhou, W.; Li, X.; Qin, L.; Kang, S. Facile Preparation of Ag2ZnGeO4 Flower-like Hierarchical Nanostructure and Its Photocatalytic Activity. J. Mater. Sci. Technol. 2017, 33, 47–51. [Google Scholar] [CrossRef]
- Bai, X.; Wang, L.; Zong, R.; Lv, Y.; Sun, Y.; Zhu, Y. Performance enhancement of ZnO photocatalyst via synergic effect of surface oxygen defect and graphene hybridization. Langmuir 2013, 29, 3097–3105. [Google Scholar] [CrossRef]
- Leung, Y.; Chen, X.; Ng, A.; Guo, M.; Liu, F.; Djurisic, A.; Chan, W.; Shi, X.; Van Hove, M. Green emission in ZnO nanostructures—Examination of the roles of oxygen and zinc vacancies. Appl. Surf. Sci. 2013, 271, 202–209. [Google Scholar] [CrossRef]
- Wojnarowicz, J.; Chudoba, T.; Koltsov, I.; Gierlotka, S.; Dworakowska, S.; Lojkowski, W. Size control mechanism of ZnO nanoparticles obtained in microwave solvothermal synthesis. Nanotechnology 2019, 29, 06561. [Google Scholar] [CrossRef]
- Pimentel, A.; Ferreira, S.H.; Nunes, D.; Calmeiro, T.; Martins, R.; Fortunato, E. Microwave Synthesized ZnO Nanorod Arrays for UV Sensors: A Seed Layer Annealing Temperature Study. Materials 2016, 9, 299. [Google Scholar] [CrossRef]
- Samadipakchin, P.; Mortaheb, H.R.; Zolfaghari, A. ZnO nanotubes: Preparation and photocatalytic performance evaluation. J. Photochem. Photobiol. A Chem. 2017, 337, 91. [Google Scholar] [CrossRef]
- Zhang, Y.; Ram, M.K.; Stefanakos, E.K.; Goswami, Y.D. Synthesis, Characterization, and Applications of ZnO Nanowires. J. Nanomater. 2012, 12, 624520. [Google Scholar] [CrossRef]
- Diguna, L.J.; Fitriani, A.D.; Liasari, B.R.; Timuda, G.E.; Widayatno, W.B.; Wismogroho, A.S.; Zeng, S.; Birowosuto, M.D.; Amal, M.I. Optical and Photodetection Properties of ZnO Nanoparticles Recovered from Zn Dross. Crystals 2021, 11, 6. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, L.; He, X.; Wang, G.; Wang, X.; Fang, W.; Du, X. In situ Formed Fan-Shaped Nanowires in Biomorphic SiO2: A Multidimensional Composite of Hierarchical Porous Material and Organic Pollutant Adsorption Behavior. Acta Metall. Sin. Engl. Lett. 2017, 30, 104–112. [Google Scholar] [CrossRef] [Green Version]
- Zhong, J.B.; Li, J.Z.; He, X.Y.; Zeng, J.; Lu, Y.; Hu, W.; Lin, K. Improved Photocatalytic Performance of Pd-Doped ZnO. Curr. Appl. Phys. 2012, 12, 998–1001. [Google Scholar] [CrossRef]
- Sin, J.; Lam, S.; Lee, K.; Mohamed, A. Preparation and photocatalytic properties of visible light-driven samarium-doped ZnO nanorods. Ceram. Int. 2013, 39, 5833–5843. [Google Scholar] [CrossRef]
- Ahmad, M.; Ahmed, E.; Zhang, Y.; Khalid, N.R.; Xu, J.; Ullah, M.; Hong, Z. Preparation of Highly Efficient Al-Doped ZnO Photocatalyst by Combustion Synthesis. Curr. Appl. Phys. 2013, 13, 697–704. [Google Scholar] [CrossRef]
- Huang, L.; Ren, N.; Li, B.; Zhou, M. Effect of Annealing on the Morphology, Structure and Photoelectric Properties of AZO/Pt/FTO Trilayer Films. Acta Metall. Sin. Engl. Lett. 2015, 28, 281–288. [Google Scholar] [CrossRef]
- Haja Sheriff, M.H.; Murugan, S.; Manivasaham, A.; Ashok Kumar, R. Electro spray technique to enhance the physical property of Sulphur doped zinc oxide thin film. Mater. Today Proc. 2021, 47, 1717–1723. [Google Scholar] [CrossRef]
- Riaz, A.; Ashraf, A.; Taimoor, H.; Javed, S.; Akram, M.A.; Islam, M.; Mujahid, M.; Ahmad, I.; Saeed, K. Photocatalytic and Photostability Behavior of Ag- and/or Al- Doped ZnO Films in Methylene Blue and Rhodamine B Under UV-C Irradiation. Coatings 2019, 9, 202. [Google Scholar] [CrossRef] [Green Version]
- Lavand, A.B.; Malghe, Y.S. Synthesis, characterization and visible light photocatalytic activity of nitrogen-doped zinc oxide nanospheres. J. Asian Ceram. Soc. 2018, 3, 305–310. [Google Scholar] [CrossRef] [Green Version]
- Hamrouni, S.; AlKhalifah, M.S.; El-Bana, M.S.; Zobaidi, S.K.; Belgacem, S. Deposition and characterization of spin-coated n-type ZnO thin film for potential window layer of solar cell. Appl. Phys. A 2018, 124, 555. [Google Scholar] [CrossRef]
- Farrag, A.A.-G.; Balboul, M.R. Nano ZnO thin films synthesis by sol–gel spin coating method as a transparent layer for solar cell applications. J. Sol-Gel Sci. Technol. 2016, 82, 269–279. [Google Scholar] [CrossRef]
- Yan, X.; Venkataraj, S.; Aberle, A.G. Wet-Chemical Surface Texturing of Sputter-Deposited ZnO: Al Films as Front Electrode for Thin-Film Silicon Solar Cells. Int. J. Photoenergy 2015, 2015, 548984. [Google Scholar] [CrossRef]
- Islam, M.R.; Rahman, M.; Farhad, S.F.U.; Podder, J. Structural, optical and photocatalysis properties of sol–gel deposited Al-doped ZnO thin films. Surf. Interfaces 2019, 16, 120–126. [Google Scholar] [CrossRef]
- Mahdavi, R.; Talesh, S.S.A. Sol-gel synthesis, structural and enhanced photocatalytic performance of Al doped ZnO nanoparticles. Adv. Powder Technol. 2017, 28, 1418–1425. [Google Scholar] [CrossRef]
- Aydın, C.; AbdEl-sadek, M.S.; Zheng, K.; Yahia, I.S.; Yakuphanoglu, F. Synthesis, diffused reflectance and electrical properties of nanocrystalline Fe-doped ZnO via sol–gel calcination technique. Opt. Laser Technol. 2013, 48, 447–452. [Google Scholar] [CrossRef] [Green Version]
- Vaccari, A. Clays and catalysis: A promising future. Appl. Clay Sci. 1999, 14, 161–198. [Google Scholar] [CrossRef]
- Labajos, F.M.; Rives, V.; Ulibarri, M.A. Effect of hydrothermal and thermal treatments on the physicochemical properties of Mg-Al hydrotalcite-like materials. J. Mater. Sci. 1992, 27, 1546–1552. [Google Scholar] [CrossRef]
- Miyata, S. Anion-exchange properties of hydrotalcite-like compounds. Clays Clay Miner. 1983, 31, 305–311. [Google Scholar] [CrossRef]
- Kruissink, E.C.; Van Reijden, L.L.; Ross, J.R.H. Preparation and properties of Co–Fe mixed oxides obtained by calcinations of layered double hydroxides. J. Chem. Soc. Faraday Trans. 1991, 1, 649–661. [Google Scholar]
- Schmitt, J.A.; Daniels, F. The Carbon Isotope Effect in the Acid Hydrolysis of Urea. J. Amer. Chem. Soci. 1953, 75, 3564. [Google Scholar] [CrossRef]
- Nakamoto, N. Infrared and Raman Spectra of Inorganic and Coordination Compounds, 4th ed.; John Wiley & Sons: New York, NY, USA, 1986. [Google Scholar]
- Zhang, X.; Zhou, L.; Pi, H.; Guo, S.; Fu, J. Performance of layered double hydroxides intercalated by a UV stabilizer in accelerated weathering and thermal stabilization of PVC. Poly. Degrad. Stab. 2014, 102, 204–211. [Google Scholar] [CrossRef]
- Xu, Z.P.; Zhang, J.; Adebajo, M.O.; Zhang, H.; Zhou, C. Catalytic Applications of Layered Double Hydroxides and Derivatives. Appl. Clay Sci. 2011, 53, 139–150. [Google Scholar] [CrossRef]
- Saber, O. Preparation and characterization of a new nano layered material, Co–Zr LDH. J. Mater. Sci. 2007, 42, 9905–9912. [Google Scholar] [CrossRef]
- Ravichandran, K.; Sindhuja, E.; Uma, R.; Arun, T. Photocatalytic efficacy of ZnO films light intensity and thickness effects. Surf. Eng. 2017, 33, 512–520. [Google Scholar] [CrossRef]
- Parhizkar, J.; Habibi, M.H. Investigation and Comparison of Cobalt ferrite composite nanoparticles with individual Iron oxide and Cobalt oxide nanoparticles in azo dyes removal. J. Water Environ. Nanotechnol. 2019, 4, 17–30. [Google Scholar]
- Singh, A.P.; Kumari, S.; Shrivastav, R.; Dass, S.; Satsangi, V.R. iron doped nanostructured TiO2 for photoelectrochemical generation of hydrogen. Int. J. Hydrogen Energy 2008, 33, 5363–5368. [Google Scholar] [CrossRef]
- Chani, M.T.S.; Khan, S.B.; Rahman, M.M.; Kamal, T.; Asiri, A.M. Sunlight assisted photocatalytic dye degradation using zinc and iron based mixed metal-oxides nanopowders. J. King Saud Univ.—Sci. 2022, 34, 101841. [Google Scholar] [CrossRef]
- Azeez, F.; Al-Hetlani, E.; Arafa, M.; Abdelmonem, Y.; Nazeer, A.A.; Amin, M.O.; Madkour, M. The effect of surface charge on photocatalytic degradation of methylene blue dye using chargeable titania nanoparticles. Sci. Rep. 2018, 8, 7104. [Google Scholar] [CrossRef]
- Chadwick, M.; Goodwin, J.; Lawson, E.; Mills, P.; Vincent, B. Surface charge properties of colloidal titanium dioxide in ethylene glycol and water. Colloids Surf. A 2002, 203, 229–236. [Google Scholar] [CrossRef]















Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saber, O.; Osama, M.; Shaalan, N.M.; Osama, A.; Alshoaibi, A.; Osama, D. Designing Novel Strategy to Produce Active Nanohybrids in Sunlight for Purification of Water Based on Inorganic Nanolayers, Magnetic Nanocomposites and Organic Species. Molecules 2022, 27, 3673. https://doi.org/10.3390/molecules27123673
Saber O, Osama M, Shaalan NM, Osama A, Alshoaibi A, Osama D. Designing Novel Strategy to Produce Active Nanohybrids in Sunlight for Purification of Water Based on Inorganic Nanolayers, Magnetic Nanocomposites and Organic Species. Molecules. 2022; 27(12):3673. https://doi.org/10.3390/molecules27123673
Chicago/Turabian StyleSaber, Osama, Mostafa Osama, Nagih M. Shaalan, Aya Osama, Adil Alshoaibi, and Doaa Osama. 2022. "Designing Novel Strategy to Produce Active Nanohybrids in Sunlight for Purification of Water Based on Inorganic Nanolayers, Magnetic Nanocomposites and Organic Species" Molecules 27, no. 12: 3673. https://doi.org/10.3390/molecules27123673