Isolation and Use of Coprothermobacter spp. to Improve Anaerobic Thermophilic Digestion of Grass
Abstract
:1. Introduction
2. Results and Discussion
Anaerobic Digestion Process
3. Materials and Methods
3.1. Material
3.2. Isolation of Thermophilic Bacteria
3.2.1. Genetic Identification
3.2.2. Microscopic Observations and Staining
3.3. Anaerobic Digestion Experiments
3.4. Chemical Analyses
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Hancsók, J.; Sági, D.; Valyon, J. Diesel fuel blending components from mixture of waste animal fat and light cycle oil from fluid catalytic cracking. J. Environ. Manag. 2018, 223, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.F.; Chiueh, P.-T.; Shih, C.H.; Lo, S.L.; Sun, L.; Zhong, Y.; Qiu, C. Microwave pyrolysis of rice straw to produce biochar as an adsorbent for CO2 capture. Energy 2015, 84, 75–82. [Google Scholar] [CrossRef]
- Ho, D.P.; Jensen, P.D.; Batstone, D.J. Methanosarcinaceae and acetate-oxidizing pathways dominate in high-rate thermophilic anaerobic digestion of waste-activated sludge. Appl. Environ. Microbiol. 2013, 79, 6491–6500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merlin Christy, P.; Gopinath, L.R.; Divya, D. A review on anaerobic decomposition and enhancement of biogas production through enzymes and microorganisms. Renew. Sustain. Energy Rev. 2014, 34, 167–173. [Google Scholar] [CrossRef]
- Stantscheff, R.; Kuever, J.; Rabenstein, A.; Seyfarth, K.; Dröge, S.; König, H. Isolation and differentiation of methanogenic Archaea from mesophilic corn-fed on-farm biogas plants with special emphasis on the genus Methanobacterium. Appl. Microbiol. Biotechnol. 2014, 98, 5719–5735. [Google Scholar] [CrossRef]
- Ollivier, B.M.; Mah, R.A.; Ferguson, T.J. Emendation of the genus thermobacteroides: Thermobacteroides proteolyticus sp. nov., a proteolytic acetogen from a methanogenic enrichment. Int. J. Syst. Bacteriol. 1985, 35, 425–428. [Google Scholar] [CrossRef] [Green Version]
- Cai, H.; Gu, J.; Wang, Y. Protease complement of the thermophilic bacterium Coprothermobacter proteolyticus. In Proceedings of the International Conference on Bioinformatics & Computational Biology (BIOCOMP). The Steering Committee of The World Congress in Computer Science, Computer Engineering and Applied Computing (WorldComp), Las Vegas, NV, USA, 18–21 July 2011. [Google Scholar]
- Hatamoto, M.; Imachi, H.; Yashiro, Y.; Ohashi, A.; Harada, H. Detection of active butyrate-degrading microorganisms in methanogenic sludges by RNA-based stable isotope probing. Appl. Environ. Microbiol. 2008, 74, 3610–3614. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.; Hidaka, T.; Tsuno, H. Two-phased hyperthermophilic anaerobic co-digestion of waste activated sludge with kitchen garbage. J. Biosci. Bioeng. 2009, 108, 408–413. [Google Scholar] [CrossRef]
- Luo, G.; Wang, W.; Angelidaki, I. Anaerobic digestion for simultaneous sewage sludge treatment and CO biomethanation: Process performance and microbial ecology. Environ. Sci. Technol. 2013, 47, 10685–10693. [Google Scholar] [CrossRef]
- Pervin, H.M.; Dennis, P.G.; Lim, H.J.; Tyson, G.W.; Batstone, D.J.; Bond, P.L. Drivers of microbial community composition in mesophilic and thermophilic temperature-phased anaerobic digestion pre-treatment reactors. Water Res. 2013, 47, 7098–7108. [Google Scholar] [CrossRef]
- Palatsi, J.; Viñas, M.; Guivernau, M.; Fernandez, B.; Flotats, X. Anaerobic digestion of slaughterhouse waste: Main process limitations and microbial community interactions. Bioresour. Technol. 2011, 102, 2219–2227. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Li, Y.Y.; Harada, H. Analysis of microbial community structure and diversity in the thermophilic anaerobic digestion of waste activated sludge. Water Sci. Technol. 2008, 57, 1199–1205. [Google Scholar] [CrossRef] [PubMed]
- Tandishabo, K.; Nakamura, K.; Umetsu, K.; Takamizawa, K. Distribution and role of Coprothermobacter spp. in anaerobic digesters. J. Biosci. Bioeng. 2012, 114, 518–520. [Google Scholar] [CrossRef] [PubMed]
- Kawagoshi, Y.; Hino, N.; Fujimoto, A.; Nakao, M.; Fujita, Y.; Sugimura, S.; Furukawa, K. Effect of inoculum conditioning on hydrogen fermentation and pH effect on bacterial community relevant to hydrogen production. J. Biosci. Bioeng. 2005, 100, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Morita, M.; Sasaki, D.; Nagaoka, J.; Matsumoto, N.; Ohmura, N.; Shinozaki, H. Syntrophic degradation of proteinaceous materials by the thermophilic strains Coprothermobacter proteolyticus and Methanothermobacter thermautotrophicus. J. Biosci. Bioeng. 2011, 112, 469–472. [Google Scholar] [CrossRef] [PubMed]
- Cheon, J.; Hidaka, T.; Mori, S.; Koshikawa, H.; Tsuno, H. Applicability of random cloning method to analyze microbial community in full-scale anaerobic digesters. J. Biosci. Bioeng. 2008, 106, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wang, C.; Sun, F.; Zhu, W.; Wu, W. A comparison of microbial characteristics between the thermophilic and mesophilic anaerobic digesters exposed to elevated food waste loadings. Bioresour. Technol. 2014, 152, 420–428. [Google Scholar] [CrossRef]
- Wagner, A.O.; Lins, P.; Malin, C.; Reitschuler, C.; Illmer, P. Impact of protein-, lipid- and cellulose-containing complex substrates on biogas production and microbial communities in batch experiments. Sci. Total Environ. 2013, 458–460, 256–266. [Google Scholar] [CrossRef]
- Kersters, I.; Maestrojuan, G.M.; Torck, U.; Vancanneyt, M.; Kersters, K.; Verstraete, W. Isolation of Coprothermobacter proteolyticus from an Anaerobic Digest and Further Characterization of the Species. Syst. Appl. Microbiol. 1994, 17, 289–295. [Google Scholar] [CrossRef]
- Sasaki, K.; Haruta, S.; Ueno, Y.; Ishii, M.; Igarashi, Y. Microbial population in the biomass adhering to supporting material in a packed-bed reactor degrading organic solid waste. Appl. Microbiol. Biotechnol. 2007, 75, 941–952. [Google Scholar] [CrossRef]
- Kato, S.; Kosaka, T.; Watanabe, K. Comparative transcriptome analysis of responses of Methanothermobacter thermautotrophicus to different environmental stimuli. Environ. Microbiol. 2008, 10, 893–905. [Google Scholar] [CrossRef] [PubMed]
- Liczbinski, P.; Borowski, S. Hyperthermophilic treatment of grass and leaves to produce hydrogen, methane and VFA-rich digestate: Preliminary results. Energies 2020, 13, 2814. [Google Scholar] [CrossRef]
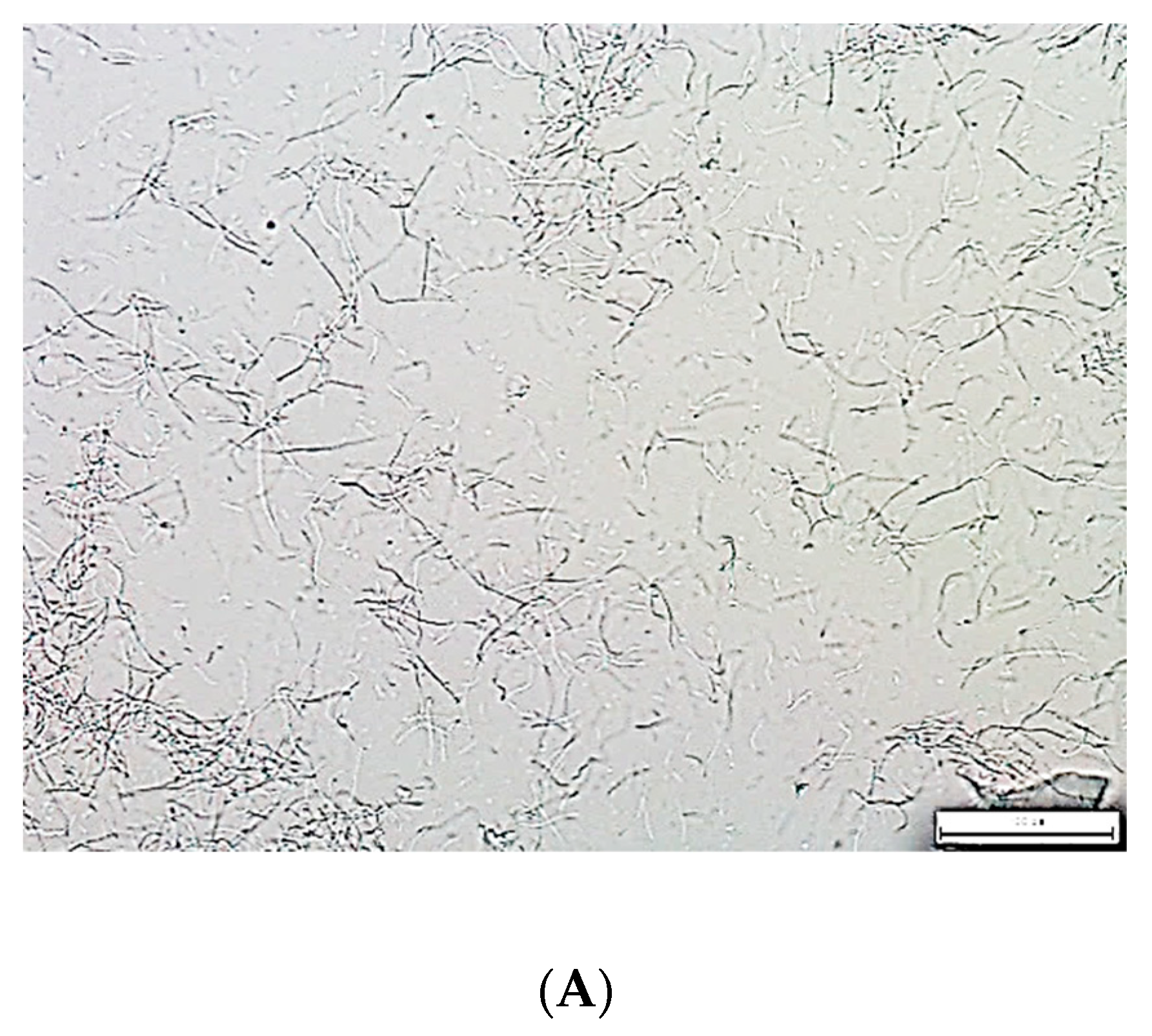
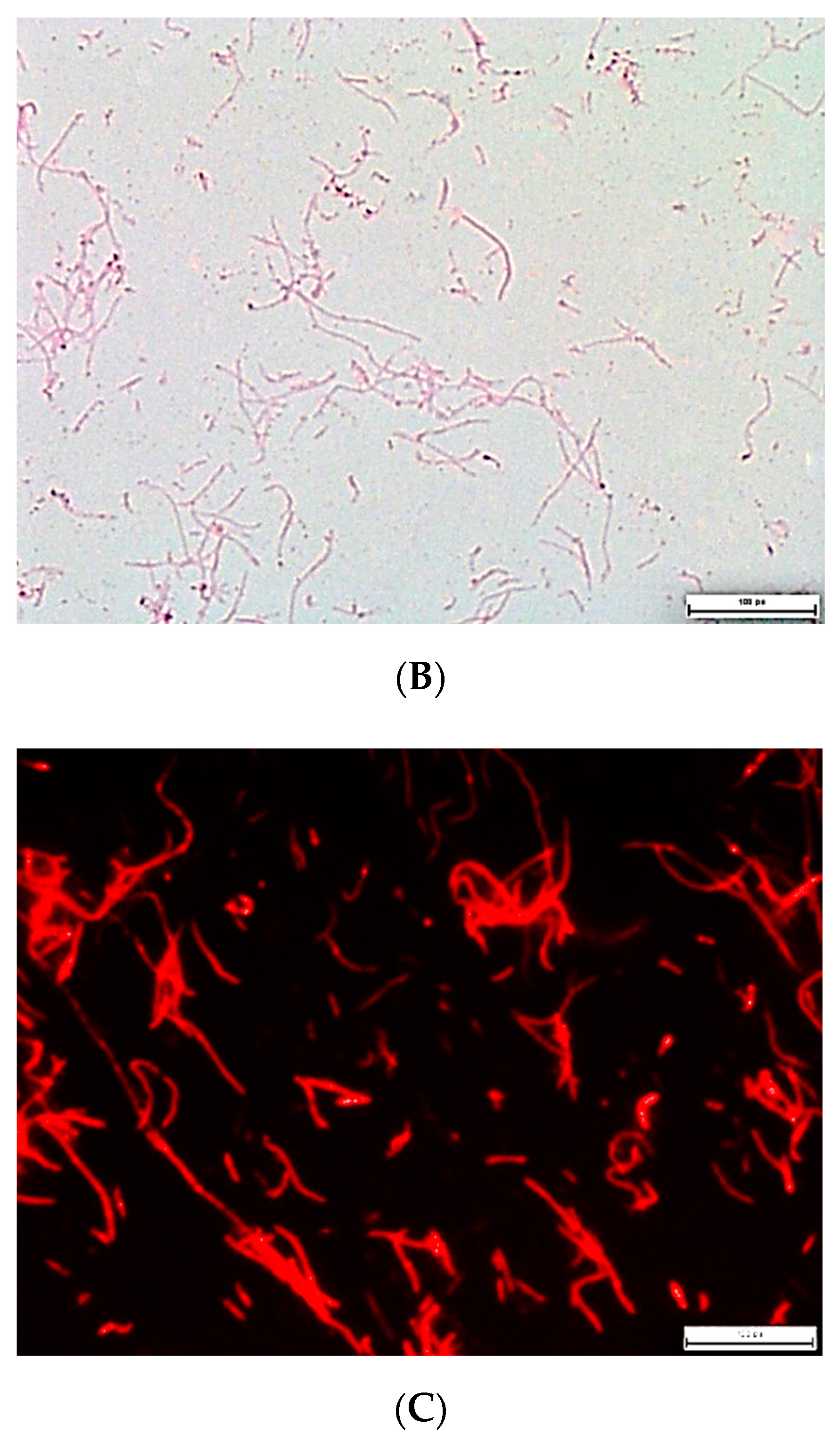
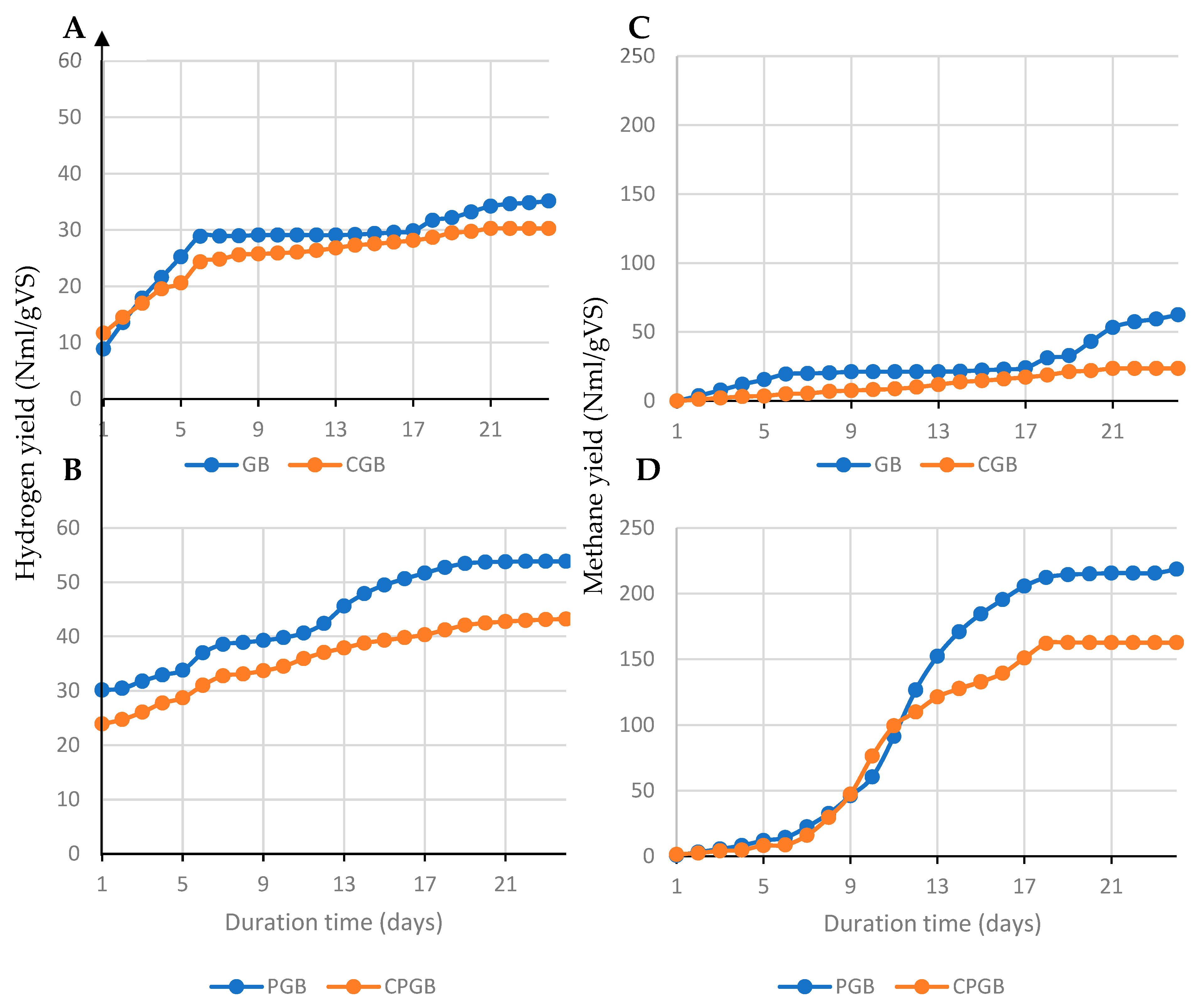
| Name | GB | CGB | PGB | CPGB |
|---|---|---|---|---|
| Substrate | Grass + Bacteria | Control | Pretreated Grass + Bacteria | Control |
| Ammonium nitrogen (mg/L) | 540 ± 41.9 | 408 ± 21.5 | 472 ± 14.4 | 423 ± 33.0 |
| TVFA (mg/L) | 9110 ± 548.5 | 8800 ± 565.7 | 6150 ± 393.7 | 5160 ± 224.8 |
| Phosphorus (mg/L) | 482 ± 26.8 | 476 ± 12.1 | 256 ± 41.7 | 240 ± 32.7 |
| pH | 7.4 ± 0.4 | 7.2 ± 0.6 | 7.4 ± 0.5 | 7.38 ± 0.3 |
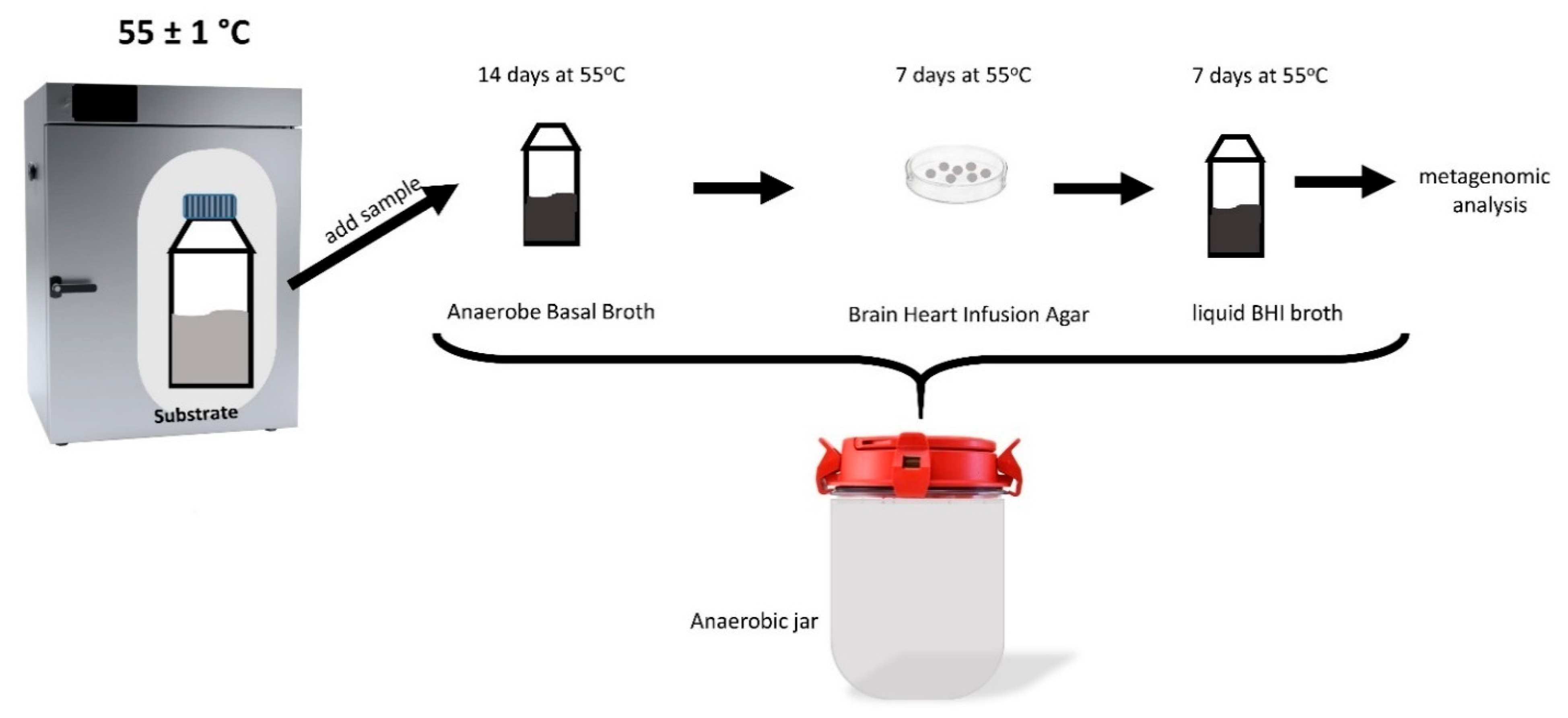
| Cumulative hydrogen yield (NmL/gVS) | 35.1 ± 1.4 | 30.3 ± 0.4 | 54.1 ± 6.3 | 43.2 ± 0.6 |
| Cumulative methane yield (NmL/gVS) | 62.5 ± 2.7 | 23.6 ± 0.8 | 218.6 ± 5.1 | 162.6 ± 2.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liczbiński, P.; Borowski, S.; Nowak, A. Isolation and Use of Coprothermobacter spp. to Improve Anaerobic Thermophilic Digestion of Grass. Molecules 2022, 27, 4338. https://doi.org/10.3390/molecules27144338
Liczbiński P, Borowski S, Nowak A. Isolation and Use of Coprothermobacter spp. to Improve Anaerobic Thermophilic Digestion of Grass. Molecules. 2022; 27(14):4338. https://doi.org/10.3390/molecules27144338
Chicago/Turabian StyleLiczbiński, Przemysław, Sebastian Borowski, and Adriana Nowak. 2022. "Isolation and Use of Coprothermobacter spp. to Improve Anaerobic Thermophilic Digestion of Grass" Molecules 27, no. 14: 4338. https://doi.org/10.3390/molecules27144338






