Comparison of Biochemical Composition and Non-Volatile Taste Active Compounds of Back and Abdominal Muscles in Three Marine Perciform Fishes, Chromileptes altivelis, Epinephelus akaara and Acanthopagrus schlegelii
Abstract
:1. Introduction
2. Results and Discussion
2.1. Proximate Compositions
2.2. Fatty Acid Composition
2.3. Comparison of Free Amino Acids (FAAs) and 5′-Nucleotides
2.4. Comparison of Malic and Lactic Acid Contents
2.5. Comparison of Inorganic Ions
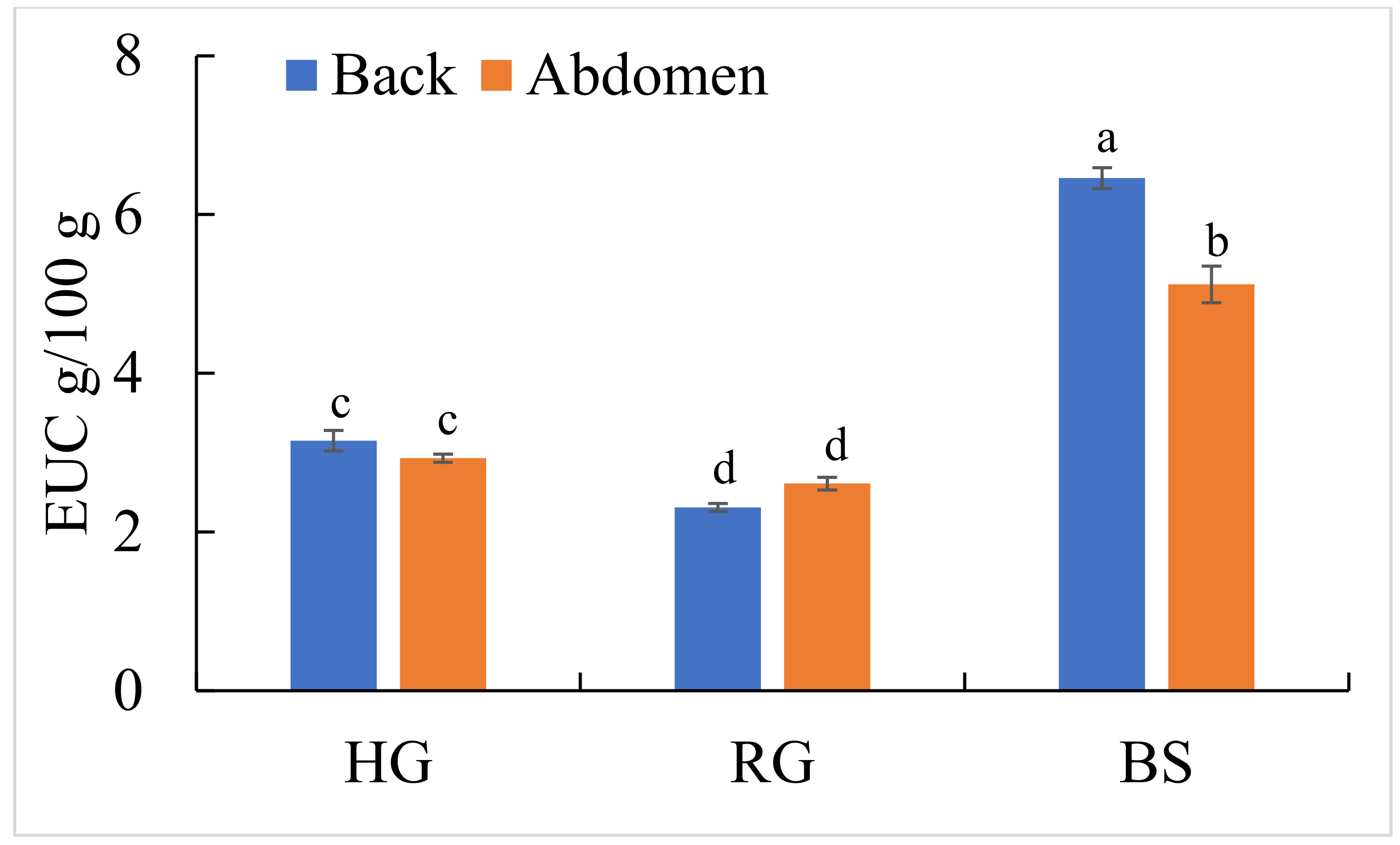
2.6. Comparison of Equivalent Umami Concentration (EUC) Values
3. Materials and Methods
3.1. Collection and Preparation of Three Marine Perciform Fishes
3.2. Proximate Analysis
3.3. Fatty Acid Analysis
3.4. Free Amino Acid (FAA) Analysis
3.5. 5′-Nucleotide Assay
3.6. Equivalent Umami Concentration (EUC)
3.7. Malic, Lactic, Citric and Succinic Acids Assay
3.8. Inorganic Ions Assay
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Rittenschober, D.; Nowak, V.; Charrondiere, U.R. Review of availability of food composition data for fish and shellfish. Food Chem. 2013, 141, 4303–4310. [Google Scholar] [CrossRef] [PubMed]
- Naylor, R.L.; Hardy, R.W.; Buschmann, A.H.; Bush, S.R.; Cao, L.; Klinger, D.H.; Little, D.C.; Lubchenco, J.; Shumway, S.E.; Troel, M. A 20-year retrospective review of global aquaculture. Nature 2021, 591, 551–563. [Google Scholar] [CrossRef] [PubMed]
- Gosline, W.A. The suborders of perciform fishes. Proc. United States Natl. Mus. 1968, 124, 1–78. [Google Scholar] [CrossRef]
- Golden, C.D.; Koehn, J.Z.; Shepon, A.; Passarelli, S.; Free, C.M.; Viana, D.F.; Matthey, H.; Eurich, J.G.; Gephart, J.A.; Fluet-Chouinard, E.; et al. Aquatic foods to nourish nations. Nature 2021, 598, 315–320. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, W.R.; Gladstone, S.; Ng, W.; Zhang, J.Z.; Shao, Q.J. Effects of isoenergetic diets with varying protein and lipid levels on the growth, feed utilization, metabolic enzymes activities, antioxidative status and serum biochemical parameters of black sea bream (Acanthopagrus schlegelii). Aquaculture 2019, 513, 734397. [Google Scholar] [CrossRef]
- CSY. China Statistical Yearbook (2020); China Statistical Publishing House: Beijing, China, 2020. [Google Scholar]
- Sun, Y.; Xiang, Y.J.; He, M.W.; Zhang, X.; Wang, S.F.; Guo, W.L.; Liu, C.S.; Cao, Z.J.; Zhou, Y.C. Evaluation of Lactococcus lactis HNL12 combined with Schizochytrium limacinum algal meal in diets for humpback grouper (Cromileptes altivelis). Fish Shell. Immunol. 2019, 94, 880–888. [Google Scholar] [CrossRef]
- Morris, A.V.; Roberts, C.M.; Hawkins, J.P. The threatened status of groupers (Epinephelinae). Biodivers. Conserv. 2000, 9, 919–942. [Google Scholar] [CrossRef]
- Sagada, G.; Gray, N.; Wang, L.; Xu, B.Y.; Zheng, L.; Zhong, Z.W.; Ullaha, S.; Tegomo, A.F.; Shao, Q. Effect of dietary inactivated Lactobacillus plantarum on growth performance, antioxidative capacity, and intestinal integrity of black sea bream (Acanthopagrus schlegelii) fingerlings. Aquaculture 2021, 535, 736370. [Google Scholar] [CrossRef]
- Shapawi, R.; Ng, W.; Mustafa, S. Replacement of fish meal with poultry by-product meal in diets formulated for the humpback grouper, Cromileptes altivelis. Aquaculture 2007, 273, 118–126. [Google Scholar] [CrossRef]
- Jeong, D.; Kayano, Y.; Oda, T.; Nakagawa, H. Influence of feeding regime on fatty acid composition in young red-spotted grouper Epinephelus akaara. Fish. Sci. 2003, 69, 569–574. [Google Scholar] [CrossRef]
- Jin, M.; Yuan, Y.; Lu, Y.; Ma, H.; Sun, P.; Li, Y.; Qiu, H.; Ding, L.Y.; Zhou, Q.C. Regulation of growth, tissue fatty acid composition, biochemical parameters and lipid related genes expression by different dietary lipid sources in juvenile black seabream, Acanthopagrus schlegelii. Aquaculture 2017, 479, 25–37. [Google Scholar] [CrossRef]
- Shapawi, R.; Mustafa, S.; Ng, W. Effects of dietary fish oil replacement with vegetable oils on growth and tissue fatty acid composition of humpback grouper, Cromileptes altivelis (Valenciennes). Aquac. Res. 2008, 39, 315–323. [Google Scholar] [CrossRef]
- Wang, J.T.; Han, T.; Li, X.Y.; Yang, Y.X.; Yang, M.; Hu, S.X.; Jiang, Y.D.; Harpaz, S. Effects of dietary protein and lipid levels with different protein-to-energy ratios on growth performance, feed utilization and body composition of juvenile red-spotted grouper, Epinephelus akaara. Aquacult. Nutr. 2017, 23, 994–1002. [Google Scholar] [CrossRef]
- Ou, Y.J.; Li, J.E. Analysis and evaluation of the nutrient contents in the muscle of high finned grouper Cromileptes altivelis. J. Oceanogr. Taiwan Strait 2010, 29, 503–509, (In Chinese with English Abstract). [Google Scholar]
- Selli, S.; Cayhan, G.G. Analysis of volatile compounds of wild gilthead sea bream (Sparus aurata) by simultaneous distillation–extraction (SDE) and GC–MS. Microchem. J. 2009, 93, 232–235. [Google Scholar] [CrossRef]
- Li, B.; Kimatu, B.M.; Pei, F.; Chen, S.Y.; Feng, X.; Hu, Q.H.; Zhao, L.Y. Non-volatile flavour components in Lentinus edodes after hot water blanching and microwave blanching. Int. J. Food Prop. 2018, 20, S2532–S2542. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Gu, Z.; Lin, X.; Wang, Y.; Wang, A.; Sun, Y.; Shi, Y. Effects of high hydrostatic pressure (HHP) and storage temperature on bacterial counts, color change, fatty acids and non-volatile taste active compounds of oysters (Crassostrea ariakensis). Food Chem. 2022, 372, 131247. [Google Scholar] [CrossRef]
- Yue, J.; Zhang, Y.F.; Jin, Y.F.; Deng, Y.; Zhao, Y.Y. Impact of high hydrostatic pressure on non-volatile and volatile compounds of squid muscles. Food Chem. 2016, 194, 12–19. [Google Scholar] [CrossRef]
- Guo, Q.Y.; Truonghuynh, T.; Jiang, C.J.; Wang, L.M.; Li, B.G.; Xing, X.L. Quality differences of large yellow croaker (Pseudosciaena crocea) cultured in deep-water sea cages of two China regions. Spine 2018, 9, 1000547. [Google Scholar] [CrossRef]
- Luo, Q.; Wong, M.; Cai, Z. Determination of polybrominated diphenyl ethers in freshwater fishes from a river polluted by e-wastes. Talanta 2007, 72, 1644–1649. [Google Scholar] [CrossRef]
- Xu, H.; Turchini, G.M.; Francis, D.S.; Liang, M.; Mock, T.S.; Rombenso, A.; Ai, Q.H. Are fish what they eat? A fatty acid’s perspective. Prog. Lipid Res. 2020, 80, 101064. [Google Scholar] [CrossRef]
- Shirai, N.; Terayama, M.; Takeda, H. Effect of season on the fatty acid composition and free amino acid content of the sardine Sardinops melanostictus. Comp. Biochem. Phys. B 2002, 131, 387–393. [Google Scholar] [CrossRef]
- Ma, R. Preliminary Study on Relationship between Nutrition and Fish Quality of Farmed Large Yellow Croaker (Pseudosciaena crocea). Ph.D. Thesis, Ocean University of China, Qingdao, China, 2014; pp. 12–60. [Google Scholar]
- Liu, C.S.; Chen, S.Q.; Zhuang, Z.M.; Yan, J.P.; Liu, C.L.; Cui, H.T. Potential of utilizing jellyfish as food in culturing Pampus argenteus juveniles. Hydrobiologia 2015, 754, 189–200. [Google Scholar] [CrossRef]
- Li, G.P.; Sinclair, A.J.; Li, D. Comparison of lipid content and fatty acid composition in the edible meat of wild and cultured freshwater and marine fish and shrimps from China. J. Agr. Food Chem. 2011, 59, 1871–1881. [Google Scholar] [CrossRef]
- Wei, Y.T.; Shen, H.H.; Xu, W.Q.; Pan, Y.; Chen, J.; Zhang, W.B.; Mai, K.S. Replacement of dietary fishmeal by Antarctic krill meal on growth performance, intestinal morphology, body composition and organoleptic quality of large yellow croaker Larimichthys crocea. Aquaculture 2019, 512, 734281. [Google Scholar] [CrossRef]
- Wang, Z.; Qian, X.Q.; Xie, S.Q.; Yun, B. Changes of growth performance and plasma biochemical parameters of hybrid grouper (Epinephelus lanceolatus ♂ × Epinephelus fuscoguttatus ♀) in response to substitution of dietary fishmeal with poultry by-product meal. Aquacult. Rep. 2020, 18, 100516. [Google Scholar] [CrossRef]
- Twining, C.W.; Brenna, J.T.; Hairston, N.G., Jr.; Flecker, A.S. Highly unsaturated fatty acids in nature: What we know and what we need to learn. Oikos 2016, 125, 749–760. [Google Scholar] [CrossRef]
- Taşbozan, O.; Gökçe, M.A. Fatty acids in fish. In Fatty Acids; IntechOpen: London, UK, 2017. [Google Scholar]
- Toledo, J.; Ganzon-naret, E.S.; Nakagawa, H. Comparison of fatty acid profile between cultured and wild-caught grouper Epinephelus coioides. Aquac. Sci. 2005, 53, 127–134. [Google Scholar]
- Mente, E.; Carter, C.G.; Barnes, R.S.; Vlahos, N.; Nengas, I. Post-Prandial Amino Acid Changes in Gilthead Sea Bream. Animals 2021, 11, 1889. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Unuma, T.; Akiyama, T. The influence of dietary protein and fat levels on tissue free amino acid levels of fingerling rainbow trout (Oncorhynchus mykiss). Aquaculture 2000, 182, 353–372. [Google Scholar] [CrossRef]
- Mente, E.; Deguara, S.; Santos, M.B.; Houlihan, D. White muscle free amino acid concentrations following feeding a maize gluten dietary protein in Atlantic salmon (Salmo salar L.). Aquaculture 2003, 225, 25–36. [Google Scholar] [CrossRef]
- Alasalvar, C.; Taylor, K.D.A.; Shahidi, F. Comparative quality assessment of cultured and wild sea bream (Sparus aurata) stored in ice. J. Agr. Food Chem. 2002, 50, 2039–2045. [Google Scholar] [CrossRef]
- Duan, Z.L.; Zhou, Y.G.; Liu, W.J.; Shi, C.; Li, L.; Dong, Y.W.; Gao, Q.F.; Dong, S.L. Variations in flavor according to fish size in rainbow trout (Oncorhynchus mykiss). Aquaculture 2020, 526, 735398. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Ma, X.T.; Dai, Z.Y. Comparison of nonvolatile and volatile compounds in raw, cooked, and canned yellowfin tuna (Thunnus albacores). J. Food Process. Pres. 2019, 43, e14111. [Google Scholar] [CrossRef]
- Zheng, J.; Tao, N.; Gong, J.; Gu, S.; Xu, C. Comparison of non-volatile taste-active compounds between the cooked meats of pre- and post-spawning Yangtze Coilia ectenes. Fish. Sci. 2015, 81, 559–568. [Google Scholar] [CrossRef]
- Zhang, N.L.; Wang, W.L.; Li, B.; Liu, Y. Non-volatile taste active compounds and umami evaluation in two aquacultured pufferfish (Takifugu obscurus and Takifugu rubripes). Food Biosci. 2019, 32, 100468. [Google Scholar] [CrossRef]
- Shirai, T.; Kikuchi, N.; Matsuo, S.; Uchida, S.; Inada, H.; Suzuki, T.; Hirano, T. Taste components of Boreo Pacific gonate squid Gonatopsis borealis. Fish. Sci. 1997, 63, 772–778. [Google Scholar] [CrossRef] [Green Version]
- AOAC. Official Methods of Analysis, 14th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1984. [Google Scholar]
| Compositions (% of Wet Weight) | Humpback Grouper | Red-Spotted Grouper | Black Seabream | |||
|---|---|---|---|---|---|---|
| Back | Abdomen | Back | Abdomen | Back | Abdomen | |
| Moisture | 73.17 ± 1.13 b | 72.81 ± 1.29 b | 74.09 ± 0.88 b | 73.36 ± 1.03 b | 76.13 ± 0.85 a | 76.40 ± 1.02 a |
| Crude protein | 21.42 ± 0.26 a | 21.31 ± 0.67 a | 21.06 ± 0.75 a | 20.15 ± 0.33 a | 19.24 ± 0.75 ab | 17.93 ± 0.80 b |
| Crude lipid | 3.29 ± 0.22 ab | 4.10 ± 0.24 a | 2.82 ± 0.12 bc | 3.98 ± 0.13 a | 2.38 ± 0.31 c | 3.35 ± 0.27 ab |
| Ash | 1.92 ± 0.12 | 1.59 ± 0.21 | 1.67 ± 0.32 | 1.68 ± 0.16 | 1.72 ± 0.10 | 1.58 ± 0.17 |
| Humpback Grouper | Red-Spotted Grouper | Black Seabream | ||||
|---|---|---|---|---|---|---|
| Back | Abdomen | Back | Abdomen | Back | Abdomen | |
| Saturated fatty acids (SFA) | ||||||
| C12:0 | 0.06 ± 0.00 | 0.07 ± 0.00 | 0.05 ± 0.00 | 0.05 ± 0.00 | 0.07 ± 0.01 | 0.07 ± 0.02 |
| C13:0 | 0.07 ± 0.01 | 0.06 ± 0.01 | 0.09 ± 0.01 | 0.05 ± 0.03 | 0.08 ± 0.02 | 0.05 ± 0.02 |
| C14:0 | 3.61 ± 0.11 ab | 4.02 ± 0.30 a | 3.36 ± 0.31 bc | 3.97 ± 0.17 a | 2.60 ± 0.11 d | 3.12 ± 0.09 c |
| C15:0 | 0.68 ± 0.04 a | 0.76 ± 0.04 a | 0.50 ± 0.00 b | 0.53 ± 0.03 b | 0.38 ± 0.05 c | 0.40 ± 0.03 c |
| C16:0 | 24.90 ± 0.77 a | 25.26 ± 0.45 a | 20.80 ± 0.47 b | 18.94 ± 0.58 c | 22.41 ± 0.22 b | 21.56 ± 0.85 b |
| C17:0 | 1.05 ± 0.06 a | 1.11 ± 0.01 a | 0.44 ± 0.02 b | 0.41 ± 0.03 b | 0.51 ± 0.03 b | 0.52 ± 0.05 b |
| C18:0 | 10.90 ± 0.35 a | 10.85 ± 0.25 a | 5.67 ± 0.57 c | 4.37 ± 0.14 d | 7.35 ± 0.29 b | 6.99 ± 0.77 b |
| C20:0 | 0.32 ± 0.02 | 0.36 ± 0.03 | 0.27 ± 0.04 | 0.24 ± 0.02 | 0.29 ± 0.04 | 0.34 ± 0.03 |
| ΣSFA | 41.58 ± 1.27 a | 42.49 ± 1.07 a | 31.18 ± 1.39 bc | 28.56 ± 0.97 c | 33.69 ± 0.76 b | 33.05 ± 1.64 b |
| Mono-unsaturated fatty acids (MUFAs) | ||||||
| C23:0 | 1.18 ± 0.10 a | 1.19 ± 0.08 a | 0.54 ± 0.01 c | 0.39 ± 0.03 c | 0.74 ± 0.18 b | 0.72 ± 0.03 b |
| C14:1n-3 | 0.06 ± 0.01 | 0.05 ± 0.01 | 0.09 ± 0.03 | 0.05 ± 0.02 | 0.09 ± 0.01 | 0.06 ± 0.01 |
| C14:1n-5 | 0.09 ± 0.01 b | 0.08 ± 0.03 b | 0.11 ± 0.01 b | 0.08 ± 0.02 b | 0.18 ± 0.01 a | 0.17 ± 0.03 a |
| C15:1n-6 | 0.09 ± 0.02 b | 0.07 ± 0.02 b | 0.13 ± 0.03 ab | 0.07 ± 0.02 b | 0.19 ± 0.02 a | 0.09 ± 0.04 b |
| C16:1n-9 | 0.52 ± 0.04 a | 0.58 ± 0.02 a | 0.31 ± 0.03 b | 0.29 ± 0.03 b | 0.30 ± 0.05 b | 0.29 ± 0.06 b |
| C16:1n-7(E) | 0.20 ± 0.01 | 0.19 ± 0.03 | 0.22 ± 0.03 | 0.27 ± 0.04 | 0.36 ± 0.04 | 0.43 ± 0.04 |
| C16:1n-7 | 4.93 ± 0.12 c | 4.14 ± 0.35 d | 4.16 ± 0.13 d | 4.73 ± 0.15 cd | 6.53 ± 0.29 b | 7.28 ± 0.37 a |
| C17:1n-7 | 0.53 ± 0.01 bc | 0.59 ± 0.05 ab | 0.50 ± 0.02 bc | 0.48 ± 0.02 c | 0.54 ± 0.05 bc | 0.65 ± 0.04 a |
| C18:1n-7 | 15.79 ± 0.68 d | 15.56 ± 0.75 d | 17.63 ± 0.52 cd | 19.50 ± 0.48 c | 26.30 ± 1.09 b | 29.76 ± 1.42 a |
| C18:1n-9 | 3.20 ± 0.10 ab | 2.90 ± 0.04 b | 2.51 ± 0.11 b | 2.68 ± 0.08 b | 3.45 ± 0.18 a | 3.48 ± 0.08 a |
| ΣMUFA | 26.59 ± 1.01 c | 25.35 ± 1.22 c | 26.20 ± 0.85 c | 28.54 ± 0.88 c | 38.68 ± 1.67 b | 42.93 ± 2.01 a |
| Poly-unsaturated fatty acids (PUFAs) | ||||||
| C16:2n-6 | 0.05 ± 0.01 | 0.05 ± 0.00 | 0.09 ± 0.03 | 0.11 ± 0.02 | 0.04 ± 0.02 | 0.05 ± 0.02 |
| C16:2n-4 | 0.25 ± 0.01 b | 0.26 ± 0.01 b | 0.37 ± 0.01 a | 0.39 ± 0.03 a | 0.25 ± 0.03 b | 0.23 ± 0.03 b |
| C18:2n-6 | 4.06 ± 0.31 b | 3.79 ± 0.34 b | 20.37 ± 0.52 a | 21.23 ± 0.28 a | 4.55 ± 0.50 b | 4.34 ± 0.42 b |
| C18:3n-6 | 0.07 ± 0.00 b | 0.12 ± 0.04 b | 0.06 ± 0.00 b | 0.05 ± 0.01 b | 0.28 ± 0.04 a | 0.25 ± 0.03 a |
| C18:3n-3 | 0.85 ± 0.02 b | 0.89 ± 0.05 b | 1.93 ± 0.13 a | 2.13 ± 0.07 a | 0.70 ± 0.03 b | 0.63 ± 0.03 b |
| C20:1n-9 | 0.70 ± 0.01 c | 0.75 ± 0.09 c | 1.21 ± 0.07 a | 1.40 ± 0.05 a | 0.97 ± 0.10 bc | 1.18 ± 0.08 ab |
| C20:2n-6 | 0.34 ± 0.14 | 0.31 ± 0.07 | 0.71 ± 0.03 | 0.67 ± 0.09 | 0.50 ± 0.05 | 0.38 ± 0.10 |
| C20:3n-6 | 0.30 ± 0.02 | 0.27 ± 0.01 | 0.24 ± 0.03 | 0.23 ± 0.05 | 0.50 ± 0.03 | 0.43 ± 0.07 |
| C20:4n-6 ARA | 1.96 ± 0.21 c | 2.01 ± 0.12 c | 0.52 ± 0.02 d | 0.58 ± 0.05 d | 4.63 ± 0.45 a | 3.53 ± 0.42 b |
| C20:5n-3 EPA | 3.72 ± 0.35 ab | 3.97 ± 0.13 a | 3.78 ± 0.09 ab | 4.05 ± 0.08 a | 3.67 ± 0.17 ab | 3.36 ± 0.16 b |
| C22:6n-3 DHA | 19.51 ± 0.85 a | 19.73 ± 0.81 a | 13.35 ± 1.04 b | 12.06 ± 0.98 b | 11.54 ± 0.89 bc | 9.64 ± 0.73 c |
| ΣPUFA | 31.81 ± 1.81 b | 32.15 ± 1.52 b | 42.63 ± 1.78 a | 42.90 ± 1.53 a | 27.63 ± 2.07 c | 24.02 ± 1.89 c |
| Σn-6 | 6.78 ± 00.56 c | 6.55 ± 0.47 c | 21.99 ± 0.57 a | 22.87 ± 0.44 a | 10.50 ± 0.97 b | 8.98 ± 0.93 b |
| Σn-3 | 24.08 ± 1.13 a | 24.59 ± 0.89 a | 19.06 ± 1.07 b | 18.24 ± 1.06 b | 15.91 ± 1.01 c | 13.63 ± 0.88 c |
| Σn-3/Σn-6 | 3.55 | 3.75 | 0.87 | 0.80 | 1.52 | 1.52 |
| Humpback Grouper | Red-Spotted Grouper | Black Seabream | ||||
|---|---|---|---|---|---|---|
| Back | Abdomen | Back | Abdomen | Back | Abdomen | |
| Aspartic acid 1 | 2.62 ± 0.18 b | 2.53 ± 0.28 b | 3.87 ± 0.14 a | 4.50 ± 0.26 a | 2.32 ± 0.12 b | 2.78 ± 0.12 b |
| Glutamic acid 1 | 8.22 ± 0.31 a | 7.30 ± 0.18 b | 5.61 ± 0.12 c | 6.82 ± 0.23 b | 13.79 ± 0.37 a | 14.00 ± 0.24 a |
| Threonine 2 | 7.42 ± 0.25 c | 7.12 ± 0.26 c | 7.53 ± 0.27 c | 9.12 ± 0.28 ab | 9.64± 0.15 a | 8.69 ± 0.13 b |
| Serine 2 | 9.44 ± 0.31 c | 8.72± 0.23 cd | 12.07± 0.17 b | 16.46 ± 0.22 a | 8.19± 0.35 cd | 7.19 ± 0.53 d |
| Glycine 2 | 75.83 ± 1.68 b | 53.26 ± 1.11 c | 86.30 ± 0.89 a | 71.00± 1.09 b | 44.05 ± 1.32 d | 38.90 ± 0.72 e |
| Alanine 2 | 32.37 ± 0.36 c | 31.40± 0.59 cd | 29.88 ± 0.66 d | 39.26 ± 0.52 a | 35.09 ± 0.82 b | 32.16 ± 0.74 c |
| Arginine 2 | 8.07 ± 0.48 c | 9.32 ± 1.30 c | 8.01 ± 0.94 c | 9.04 ± 0.69 c | 17.55 ± 0.62 a | 14.53 ± 0.47 b |
| Proline 2 | 12.90 ± 0.28 a | 10.14 ± 0.28 b | 13.12 ± 0.70 a | 11.74 ± 0.73 ab | 13.08 ± 0.89 a | 12.81 ± 0.60 a |
| Valine 3 | 5.21 ± 0.12 b | 5.27 ± 0.28 b | 2.18 ± 0.25 c | 2.69 ± 0.16 c | 15.94 ± 0.78 a | 14.16 ± 0.55 a |
| Methionine 3 | 3.88 ± 0.18 b | 3.87 ± 0.15 b | 2.43 ± 0.16 c | 3.45 ± 0.29 b | 8.22 ± 0.40 a | 7.53 ± 0.40 a |
| Isoleucine 3 | 4.56 ± 0.07 b | 4.60 ± 0.24 b | 1.88 ± 0.23 c | 2.15± 0.26 c | 14.15 ± 0.64 a | 12.43 ± 0.79 a |
| Leucine 3 | 8.33 ± 0.37 b | 8.37 ± 0.42 b | 2.99 ± 0.39 c | 3.53 ± 0.14 c | 22.60 ± 0.89 a | 20.02 ± 1.08 a |
| Phenylalanine 3 | 4.33 ± 0.22 b | 4.64 ± 0.13 b | 1.76 ± 0.07 c | 2.24 ± 0.22 c | 14.04 ± 0.52 a | 13.36 ± 0.91 a |
| Histidine 3 | 4.38 ± 0.12 b | 4.36 ± 0.14 b | 3.63 ± 0.26 b | 4.42 ± 0.24 b | 9.44 ± 0.63 a | 9.14 ± 0.59 a |
| Cysteine 4 | 6.05 ± 0.49 | 5.41 ± 0.27 | 5.06 ± 0.32 | 5.78 ± 0.27 | 6.56 ± 0.47 | 5.98 ± 0.16 |
| Lysine 4 | 19.41 ± 0.25 c | 18.08 ± 0.20 c | 16.32 ± 0.45 d | 20.44 ± 1.03 c | 55.37 ± 3.39 a | 46.82 ± 1.05 b |
| Tyrosine 4 | 3.95 ± 0.07 b | 4.08 ± 0.10 b | 2.57 ± 0.22 c | 2.91 ± 0.19 c | 11.44 ± 0.86 a | 10.53 ± 0.84 a |
| Total FAA | 216.97 ± 5.58 c | 188.47 ± 5.29 d | 205.21 ± 5.90 cd | 215.55 ± 6.64 c | 301.59 ± 10.97 a | 271.03 ± 9.09 b |
| MSG-like FAA | 10.84 ± 0.49 bc | 9.83 ± 0.46 cd | 9.48 ± 0.26 d | 11.32 ± 0.49 b | 16.11 ± 0.49 a | 16.78 ± 0.36 a |
| Sweet FAA | 146.03 ± 2.78 b | 119.96 ± 3.01 cd | 156.91 ± 3.13 a | 156.62 ± 3.35 a | 127.72 ± 3.65 c | 114.28 ± 3.02 d |
| Bitter FAA | 30.69 ± 0.98 c | 31.11 ± 1.14 c | 14.87 ± 1.27 d | 18.48 ± 1.13 d | 84.39 ± 3.76 a | 76.64 ± 4.07 b |
| Tasteless FAA | 29.41 ± 0.75 c | 27.57 ± 0.57 c | 23.95 ± 0.78 c | 29.13 ± 1.37 c | 73.37 ± 4.36 a | 63.33 ± 1.96 b |
| Humpback Grouper | Red-Spotted Grouper | Black Seabream | ||||
|---|---|---|---|---|---|---|
| Back | Abdomen | Back | Abdomen | Back | Abdomen | |
| GMP | 1.04 ± 0.09 b | 1.08 ± 0.07 b | 0.65 ± 0.04 b | 0.68 ± 0.04 b | 2.81 ± 0.14 a | 2.40 ± 0.36 a |
| IMP | 302.34 ± 12.77 b | 315.80 ± 18.66 b | 317.90 ± 18.70 b | 296.61 ± 19.68 b | 371.58 ± 18.30 a | 288.50 ± 19.51 b |
| AMP | 10.20 ± 1.03 a | 12.22 ± 2.34 a | 0.91 ± 0.24 c | 0.95 ± 0.44c | 6.07 ± 1.57 b | 6.42 ± 0.92 b |
| Total | 313.58 ± 13.73 b | 329.10 ± 20.26 b | 319.46 ± 18.15 b | 298.24 ± 19.24 b | 379.83± 19.37 a | 297.32 ± 20.51 b |
| Humpback Grouper | Red-Spotted Grouper | Black Seabream | ||||
|---|---|---|---|---|---|---|
| Back | Abdomen | Back | Abdomen | Back | Abdomen | |
| Organic acids | ||||||
| 1 Malic acid | 0.45 ± 0.06 b | 0.59 ± 0.02 a | 0.19 ± 0.01 d | 0.33 ± 0.02 c | 0.17 ± 0.03 d | 0.18 ± 0.03 d |
| 1 Lactic acid | 3.18 ± 0.09 d | 3.44 ± 0.05 d | 4.90 ± 0.04 c | 5.26 ± 0.14 c | 7.95 ± 0.37 a | 6.35± 0.19 b |
| Mineral ions | ||||||
| 1 Na+ | 0.63 ± 0.04 bc | 0.57 ± 0.06 cd | 0.47 ± 0.05 d | 0.67 ± 0.05 bc | 1.09 ± 0.04 a | 0.72 ± 0.04 b |
| 1 K+ | 4.40 ± 0.19 ab | 4.79 ± 0.15 a | 4.35 ± 0.15 ab | 3.93 ± 0.11 b | 4.35 ± 0.12 ab | 4.64 ± 0.18 a |
| 1 Ca2+ | 0.54 ± 0.11 | 0.39 ± 0.06 | 0.37 ± 0.03 | 0.49 ± 0.14 | 0.40 ± 0.06 | 0.33 ± 0.05 |
| 1 Mg2+ | 0.34 ± 0.03 | 0.34 ± 0.03 | 0.30 ± 0.02 | 0.30 ± 0.02 | 0.33 ± 0.03 | 0.32 ± 0.02 |
| 2 Mn2+ | 0.02 ± 0.01 | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.04 ± 0.01 | 0.02 ± 0.01 | 0.02 ± 0.01 |
| 2 Zn2+ | 0.52 ± 0.13 | 0.39 ± 0.06 | 0.40 ± 0.02 | 0.50 ± 0.08 | 0.44 ± 0.02 | 0.37 ± 0.05 |
| 1 Cl− | 0.56 ± 0.08 c | 0.54 ± 0.03 c | 0.53 ± 0.08 c | 0.70 ± 0. 03 bc | 0.78 ± 0.08 b | 1.34 ± 0.08 a |
| 1 PO43− | 6.12 ± 0.35 bc | 4.86 ± 0.26 d | 6.76 ± 0.48 ab | 5.48 ± 0.18 cd | 7.01 ± 0.13 a | 6.45 ± 0.27 ab |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Chen, G.; Cao, Z.; Liu, C. Comparison of Biochemical Composition and Non-Volatile Taste Active Compounds of Back and Abdominal Muscles in Three Marine Perciform Fishes, Chromileptes altivelis, Epinephelus akaara and Acanthopagrus schlegelii. Molecules 2022, 27, 4480. https://doi.org/10.3390/molecules27144480
Sun Y, Chen G, Cao Z, Liu C. Comparison of Biochemical Composition and Non-Volatile Taste Active Compounds of Back and Abdominal Muscles in Three Marine Perciform Fishes, Chromileptes altivelis, Epinephelus akaara and Acanthopagrus schlegelii. Molecules. 2022; 27(14):4480. https://doi.org/10.3390/molecules27144480
Chicago/Turabian StyleSun, Yun, Guisen Chen, Zhenjie Cao, and Chunsheng Liu. 2022. "Comparison of Biochemical Composition and Non-Volatile Taste Active Compounds of Back and Abdominal Muscles in Three Marine Perciform Fishes, Chromileptes altivelis, Epinephelus akaara and Acanthopagrus schlegelii" Molecules 27, no. 14: 4480. https://doi.org/10.3390/molecules27144480





