Synthesis of Carvacrol Derivatives as Potential New Anticancer Agent against Lung Cancer
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of Carvacrol Aldehyde (2-Hydroxy-3-methyl-6-(propan-2-yl)benzaldehyde)
2.2. Synthesis of Carvacrol-Derived Schiff Base
2.3. Synthesis of Cu(II)–Schiff Base Complex
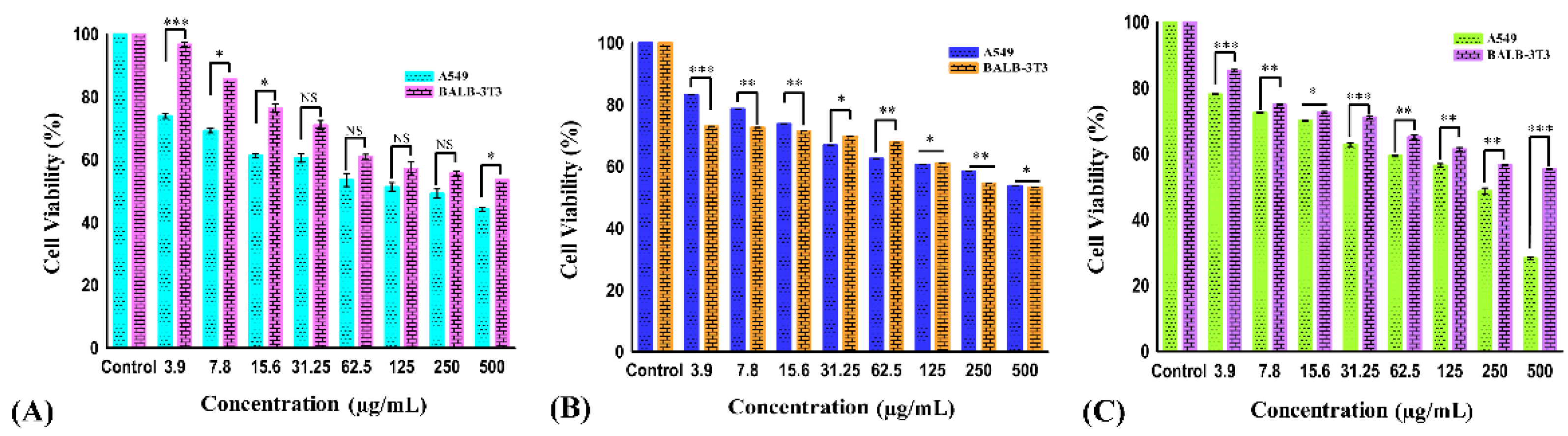
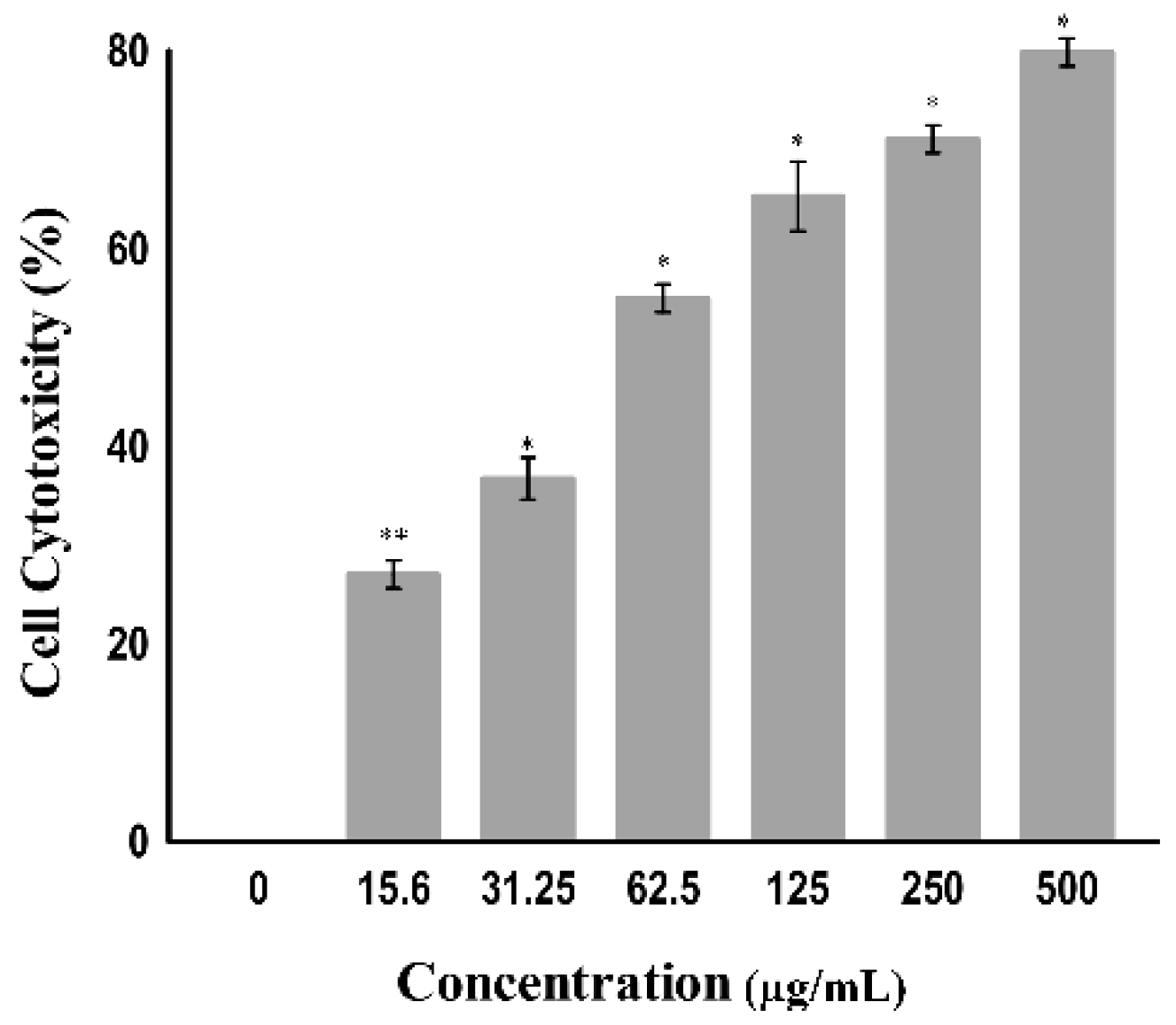
2.4. Effect of Carvacrol Derivatives on Cell Viability
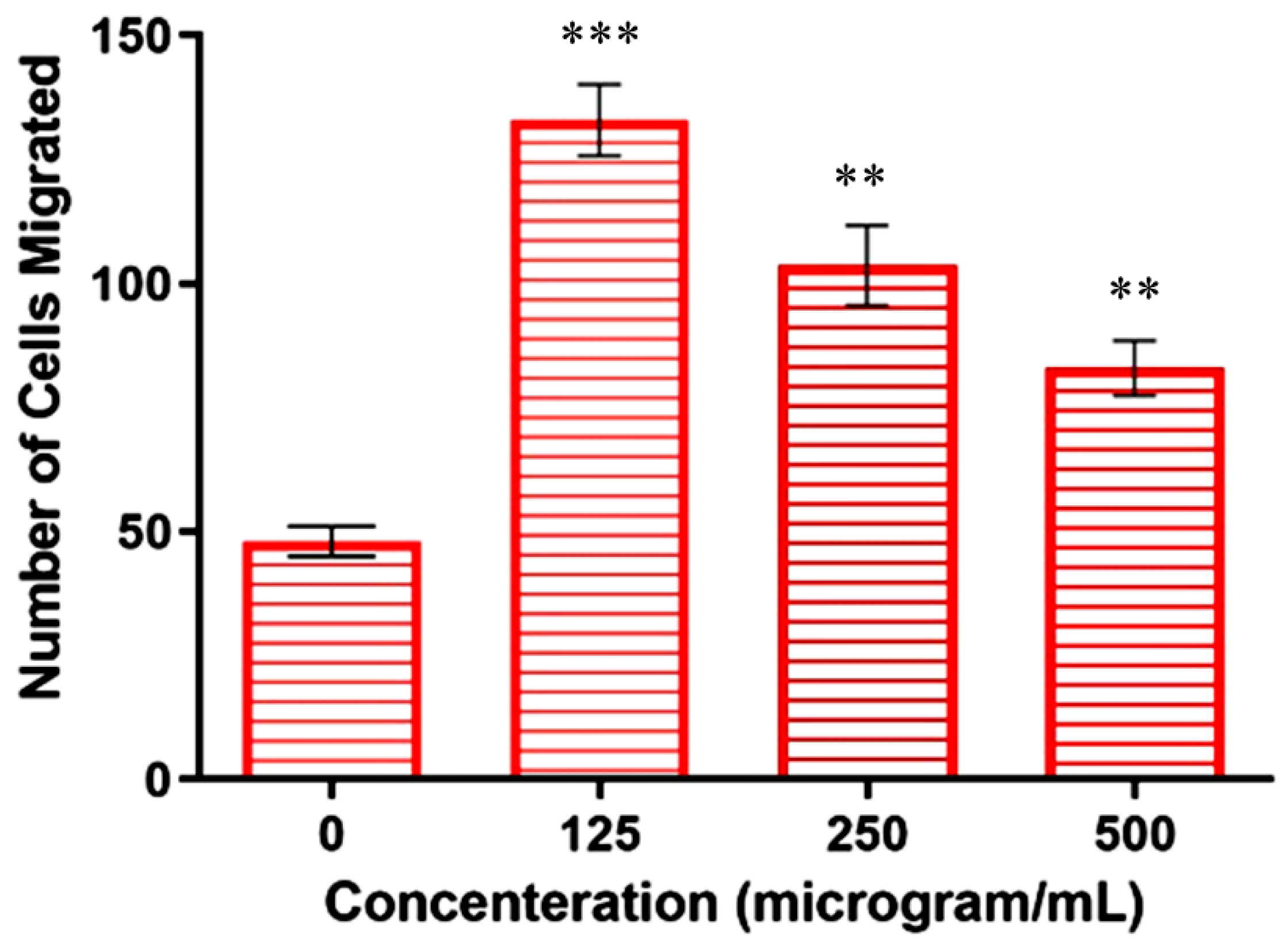
2.5. Inhibition of Cell Invasion by Copper–Schiff Base Complex
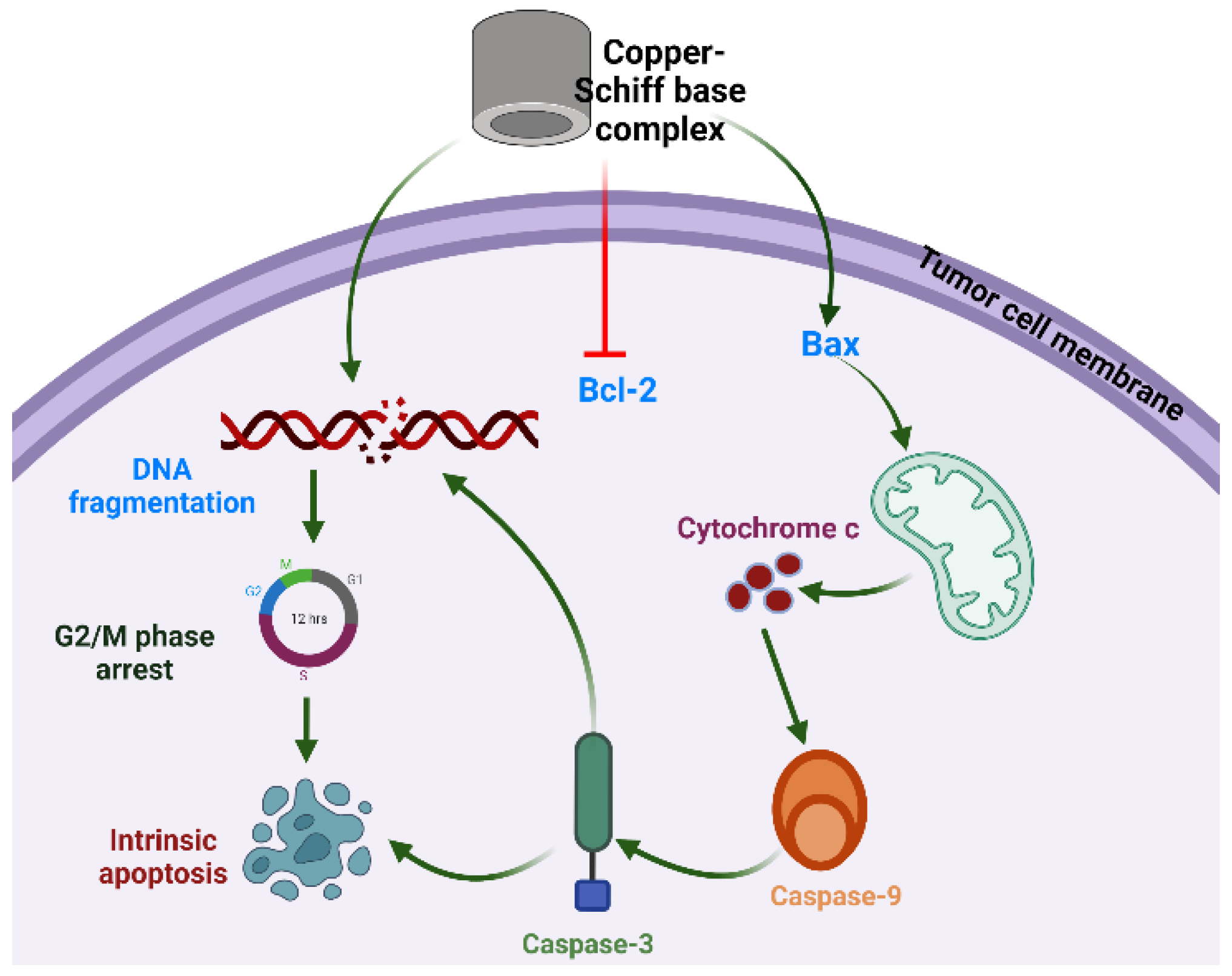
2.6. Cytotoxic Mechanistic Action of Copper–Schiff Base Complex
2.6.1. Apoptosis Assessment
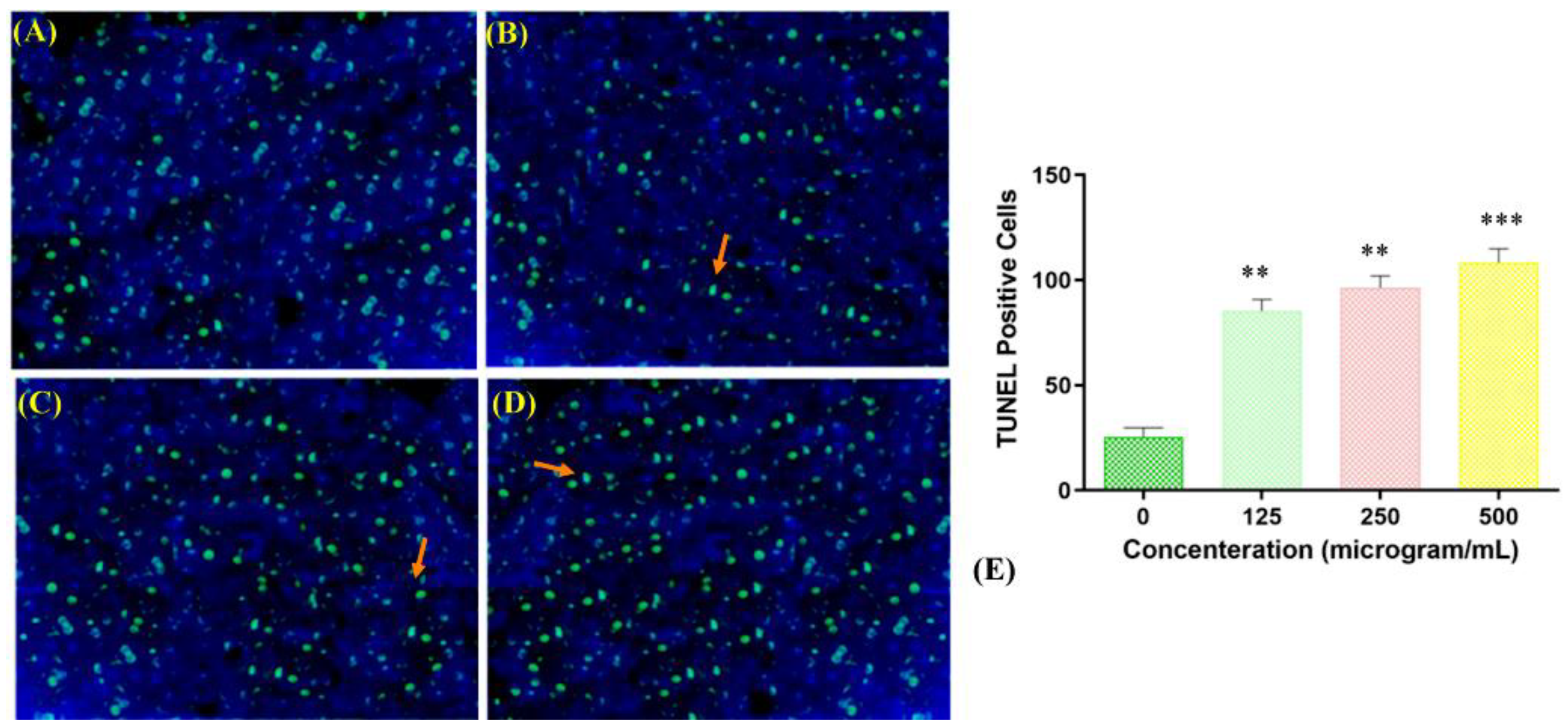
2.6.2. Terminal Deoxynucleotidyl Transferase Nick-End Labeling (TUNEL) Assay
2.7. Effect of Copper–Schiff Base Complex on Various Phases of Cell Cycle
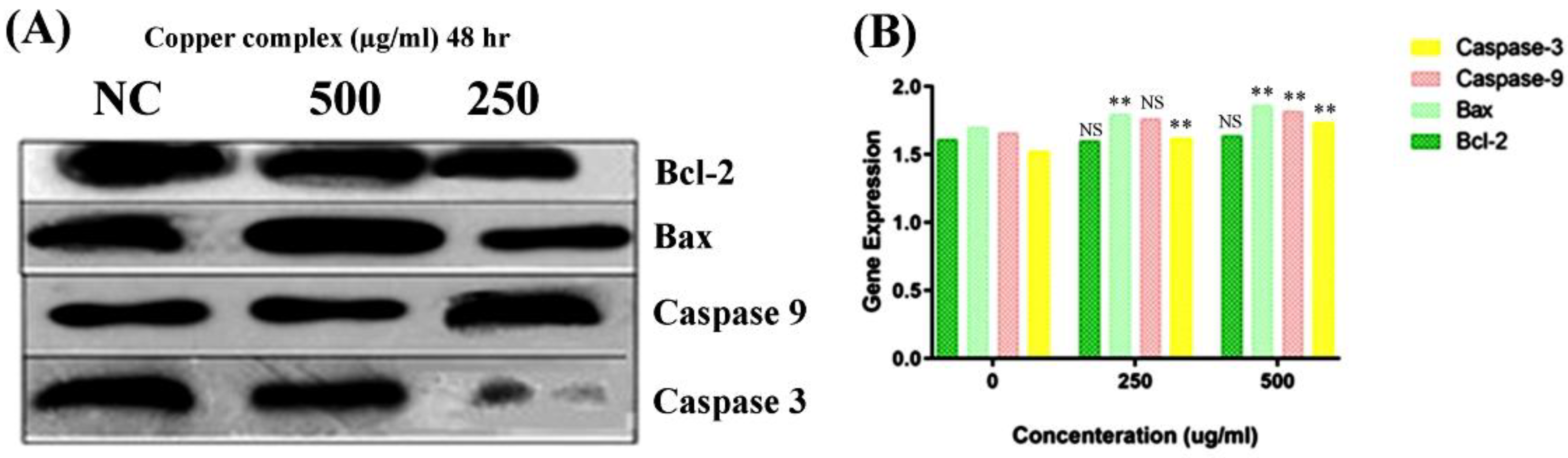
2.8. Copper Complex Elicits Caspase-Dependent Apoptosis in A549 Cell Lines
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Synthesis of Carvacrol Aldehyde (2-Hydroxy-3-methyl-6-(propan-2-yl)benzaldehyde)
3.3. Synthesis of Carvacrol-Derived Schiff Base
3.4. Synthesis of Cu(II)–Schiff Base Complex
3.5. In Vitro Studies
3.6. Detection of Cell Viability Using MTT Assay
3.7. Lactate Dehydrogenase (LDH) Release Assay
3.8. Transwell Invasion Assay
3.9. Analysis of Apoptosis Using TUNEL Assay
3.10. Detection of Cell-Cycle Arrest Using Flow Cytometry
3.10.1. Copper–Schiff Base Complex Exposure on A549 Cell Line
3.10.2. Cell-Cycle Analysis
3.11. Reverse Transcriptase Polymerase Chain Reaction (RT-PCR) and Quantification PCR (qRT-PCR)
3.11.1. RNA Separation and RNA Purification
3.11.2. Reverse Transcription for Bcl-2, Bax, Caspase-3, and Caspase-9
3.11.3. Polymerase Chain Reaction (PCR) Analysis
3.11.4. Agarose Gel Electrophoresis (AGE)
3.11.5. Real-Time Quantitative PCR (qRT-PCR)
3.12. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Patent
References
- Ferlay, J.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today; International Agency for Research on Cancer: Lyon, France, 2020; Available online: https://gco.iarc.fr/today (accessed on 21 February 2021).
- Kreeger, P.K.; Lauffenburger, D.A. Cancer systems biology: A network modeling perspective. Carcinogenesis 2010, 31, 2–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reichert, W.; Patel, H.; Mazzei, C.; Park, C.-H.; Juliani, H.R.; Simon, J.E. Two New High Essential Oil and Carvacrol Yielding Oregano (Origanum vulgare) Cultivars Pierre and Eli. HortScience 2021, 156, 1610–1613. [Google Scholar] [CrossRef]
- Mazza, G.; Kiehn, F.A.; Marshall, H.H. Monarda: A source of geraniol, linalool, thymol and carvacrol-rich essential oils. In New Crops; Janick, J., Simon, J.E., Eds.; Wiley: New York, NY, USA, 1993; pp. 628–631. [Google Scholar]
- Ceylan, O.; Ugur, A. Chemical composition and anti-biofilm activity of Thymus sipyleus BOISS. subsp. sipyleus BOISS. var. davisianus RONNIGER essential oil. Arch. Pharmacal Res. 2015, 38, 957–965. [Google Scholar] [CrossRef] [PubMed]
- The United States Food and Drug Administration. CFR-Code of Federal Regulations Title 21; The United States Food and Drug Administration: Silver Spring, MD, USA, 2021.
- Rathod, N.B.; Kulawik, P.; Ozogul, F.; Regenstein, J.M.; Ozogul, Y. Biological activity of plant-based carvacrol and thymol and their impact on human health and food quality. Trends Food Sci. Technol. 2021, 116, 733–748. [Google Scholar] [CrossRef]
- Dias, C.J.; Costa, H.A.; Dias-Filho, C.A.A.; Ferreira, A.C.; Rodrigues, B.; Irigoyen, M.C.; Borges, A.C.R.; de Andadre Martins, V.; Vidal, F.C.B.; Ribeiro, R.M.; et al. Carvacrol reduces blood pressure, arterial responsiveness and increases expression of MAS receptors in spontaneously hypertensive rats. Eur. J. Pharmacol. 2022, 917, 174717. [Google Scholar] [CrossRef] [PubMed]
- Bayramoglu, G.; Senturk, H.; Bayramoglu, A.; Uyanoglu, M.; Colak, S.; Ozmen, A.; Kolankaya, D. Carvacrol partially reverses symptoms of diabetes in STZ-induced diabetic rats. Cytotechnology 2014, 66, 251–257. [Google Scholar] [CrossRef] [Green Version]
- Chinigò, G.; Pla, A.F.; Gkika, D. TRP Channels and Small GTPases Interplay in the Main Hallmarks of Metastatic Cancer. Front. Pharmacol. 2020, 11, 581455. [Google Scholar] [CrossRef]
- Fan, K.; Li, X.; Cao, Y.; Qi, H.; Li, L.; Zhang, Q.; Sun, H. Carvacrol inhibits proliferation and induces apoptosis in human colon cancer cells. Anti-Cancer Drugs 2015, 26, 813–823. [Google Scholar] [CrossRef]
- More, U.B.; Narkhede, H.P.; Dalal, D.S.; Mahulikar, P.P. Synthesis of Biologically Active Carvacrol Compounds using Different Solvents and Supports. Synth. Commun. 2007, 37, 1957–1964. [Google Scholar] [CrossRef]
- Mastelić, J.; Jerković, I.; Blažević, I.; Poljak-Blaži, M.; Borović, S.; Ivančić-Baće, I.; Smrěcki, V.; Žarković, N.; Brčić-Kostic, K.; Vikić-Topić, D.; et al. Comparative Study on the Antioxidant and Biological Activities of Carvacrol, Thymol, and Eugenol Derivatives. J. Agric. Food Chem. 2008, 56, 3989–3996. [Google Scholar] [CrossRef]
- McQuitty, R.J. Metal-based drugs. Sci. Prog. 2014, 97, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Hambley, T.W. Developing new metal-based therapeutics: Challenges and opportunities. Dalton Trans. 2007, 21, 4929–4937. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Yu, G.; Huang, F. Supramolecular chemotherapy based on host–guest molecular recognition: A novel strategy in the battle against cancer with a bright future. Chem. Soc. Rev. 2017, 46, 7021–7053. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Rao, L.; Yu, G.; Cook, T.R.; Chen, X.; Huang, F. Supramolecular cancer nanotheranostics. Chem. Soc. Rev. 2021, 50, 2839–2891. [Google Scholar] [CrossRef]
- Ding, Y.; Tong, Z.; Jin, L.; Ye, B.; Zhou, J.; Sun, Z.; Yang, H.; Hong, L.; Huang, F.; Wang, W.; et al. An NIR Discrete Metallacycle Constructed from Perylene Bisimide and Tetraphenylethylene Fluorophores for Imaging-Guided Cancer Ra-dio-Chemotherapy. Adv. Mater. 2022, 34, 2106388. [Google Scholar] [CrossRef]
- Amptoulach, S.; Tsavaris, N. Neurotoxicity Caused by the Treatment with Platinum Analogues. Chemother. Res. Pract. 2011, 2011, 843019. [Google Scholar] [CrossRef]
- Chen, J.; Du, C.; Kang, J.; Wang, J. Cu2+ is required for pyrrolidine dithiocarbamate to inhibit histone acetylation and induce human leukemia cell apoptosis. Chem. Biol. Interact. 2008, 171, 26–36. [Google Scholar] [CrossRef]
- Fan, C.; Su, H.; Zhao, J.; Zhao, B.; Zhang, S.; Miao, J. A novel copper complex of salicylaldehyde pyrazole hydrazone induces apoptosis through up-regulating integrin β4 in H322 lung carcinoma cells. Eur. J. Med. Chem. 2010, 45, 1438–1446. [Google Scholar] [CrossRef]
- Hansen Vidar, T.; Skattebøl, L. Ortho-formylation of phenols; preparation of 3-bromosalicylaldehyde. Org. Synth. 2005, 82, 64. [Google Scholar]
- Hansen, T.V.; Skattebøl, L. Discussion addendum for: Ortho-formylations of phenols; preparation of 3-Bromosalicylaldehyde. Org. Synth. 2012, 89, 220–229. [Google Scholar]
- Roman, G.; Romani’, G.; Andree, M. New Schiff Bases from Ortho-Hydroxyaryl Aldehydes Thiophene Derivatives View Project Design of Isoselective Heme Oxygenase Inhibitors View Project New Schiff Bases From Ortho-Hydroxy Aryl Al-Dehydes. Artic. Maced. J. Chem. Chem. Eng. 2001, 20, 131–136. [Google Scholar]
- Guo, Y.; Hu, X.; Zhang, X.; Pu, X.; Wang, Y. The Synthesis of a Cu(II) Schiff Base Complex Using a Bidentate N2O2 Donor Ligand: Crystal Structure, Photophysical Properties, and Antibacterial Activities. RSC Adv. 2019, 9, 41737–41744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dirsch, V.M.; Kirschke, S.O.; Estermeier, M.; Steffan, B.; Vollmar, A.M. Apoptosis signaling triggered by the marine alkaloid ascididemin is routed via caspase-2 and JNK to mitochondria. Oncogene 2004, 23, 1586–1593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koparal, A.T.; Zeytinoğlu, M. Effects of Carvacrol on a Human Non-Small Cell Lung Cancer (NSCLC) Cell Line, A549. Cytotechnology 2003, 43, 149–154. [Google Scholar] [CrossRef]
- Forkasiewicz, A.; Dorociak, M.; Stach, K.; Szelachowski, P.; Tabola, R.; Augoff, K. The usefulness of lactate dehydrogenase measurements in current oncological practice. Cell. Mol. Biol. Lett. 2020, 25, 35. [Google Scholar] [CrossRef]
- Al Bawab, A.Q.; Zihlif, M.; Jarrar, Y.; Sharab, A. Continuous Hypoxia and Glucose Metabolism: The Effects on Gene Expression in Mcf7 Breast Cancer Cell Line. Endocr. Metab. Immune Disord.-Drug Targets 2021, 21, 511–519. [Google Scholar] [CrossRef]
- Liberti, M.V.; Locasale, J.W. The Warburg Effect: How Does It Benefit Cancer Cells? Trends Biochem. Sci. 2016, 41, 211–218. [Google Scholar] [CrossRef] [Green Version]
- Pascu, S.I.; Waghorn, P.A.; Kennedy, B.W.C.; Arrowsmith, R.L.; Bayly, S.R.; Dilworth, J.R.; Christlieb, M.; Tyrrell, R.M.; Zhong, J.; Kowalczyk, R.M.; et al. Fluorescent Copper(II) Bis(thiosemicarbazonates): Synthesis, Structures, Electron Paramagnetic Resonance, Radiolabeling, In Vitro Cytotoxicity and Confocal Fluorescence Microscopy Studies. Chem.-Asian J. 2010, 5, 506–519. [Google Scholar] [CrossRef]
- Tasdogan, A.; Ubellacker, J.M.; Morrison, S.J. Redox Regulation in Cancer Cells during Metastasis. Cancer Discov. 2021, 11, 2682–2692. [Google Scholar] [CrossRef]
- Fidler, I.J. The pathogenesis of cancer metastasis: The “seed and soil” hypothesis revisited. Nat. Rev. Cancer 2003, 3, 453–458. [Google Scholar] [CrossRef]
- Hu, J.; Liao, C.; Guo, Y.; Yang, F.; Sang, W.; Zhao, J. Copper(II) complexes inducing apoptosis in cancer cells, and demonstrating DNA and HSA interactions. Polyhedron 2017, 132, 28–38. [Google Scholar] [CrossRef]
- Hu, J.; Liao, C.; Mao, R.; Zhang, J.; Zhao, J.; Gu, Z. DNA Interactions and in Vitro Anticancer Evaluations of Pyri-dine-Benzimidazole-Based Cu Complexes. MedChemComm 2018, 9, 337. [Google Scholar] [CrossRef] [PubMed]
- Mariño, G.; Kroemer, G. Mechanisms of apoptotic phosphatidylserine exposure. Cell Res. 2013, 23, 1247–1248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koopman, G.; Reutelingsperger, C.P.M.; Kuijten, G.A.M.; Keehnen, R.M.J.; Pals, S.T.; Van Oers, M.H.J. Annexin V for Flow Cytometric Detection of Phosphatidylserine Expression on B Cells Undergoing Apoptosis. Blood 1994, 84, 1415–1420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foo, J.B.; Ng, L.S.; Lim, J.H.; Tan, P.X.; Lor, Y.Z.; Loo, J.S.E.; Low, M.L.; Chan, L.C.; Beh, C.Y.; Leong, S.W.; et al. Induction of cell cycle arrest and apoptosis by copper complex Cu(SBCM)2 towards oestrogen-receptor positive MCF-7 breast cancer cells. RSC Adv. 2019, 9, 18359–18370. [Google Scholar] [CrossRef] [Green Version]
- Arora, S.; Tandon, S. DNA fragmentation and cell cycle arrest: A hallmark of apoptosis induced by Ruta graveolens in human colon cancer cells. Homeopathy 2015, 104, 36–47. [Google Scholar] [CrossRef]
- Tabti, R.; Tounsi, N.; Gaiddon, C.; Bentouhami, E.; Desaubry, L. Progress in Copper Complexes as Anticancer Agents. Med. Chem. 2017, 7, 139–143. [Google Scholar] [CrossRef]
- Danial, N.N.; Korsmeyer, S.J. Cell Death: Critical Control Points. Cell 2004, 116, 205–219. [Google Scholar] [CrossRef] [Green Version]
- Galluzzi, L.; Vitale, I.; Abrams, J.M.; Alnemri, E.S.; Baehrecke, E.H.; Blagosklonny, M.V.; Dawson, T.M.; Dawson, V.L.; El-Deiry, W.S.; Fulda, S.; et al. Molecular definitions of cell death subroutines: Recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ. 2012, 19, 107–120. [Google Scholar] [CrossRef]
- Wei, M.C.; Lindsten, T.; Mootha, V.K.; Weiler, S.; Gross, A.; Ashiya, M.; Thompson, C.B.; Korsmeyer, S.J. tBID, a membrane-targeted death ligand, oligomerizes BAK to release cytochrome c. Genes Dev. 2000, 14, 2060–2071. [Google Scholar] [CrossRef]
- Eskes, R.; Desagher, S.; Antonsson, B.; Martinou, J.-C. Bid Induces the Oligomerization and Insertion of Bax into the Outer Mitochondrial Membrane. Mol. Cell. Biol. 2000, 20, 929–935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, M.C.; Zong, W.-X.; Cheng, E.H.-Y.; Lindsten, T.; Panoutsakopoulou, V.; Ross, A.J.; Roth, K.A.; MacGregor, G.R.; Thompson, C.B.; Korsmeyer, S.J. Proapoptotic BAX and BAK: A Requisite Gateway to Mitochondrial Dysfunction and Death. Science 2001, 292, 727–730. [Google Scholar] [CrossRef] [Green Version]
- Llambi, F.; Green, D.R. Apoptosis and oncogenesis: Give and take in the BCL-2 family. Curr. Opin. Genet. Dev. 2011, 21, 12–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimizu, S.; Narita, M.; Tsujimoto, Y. Bcl-2 Family Proteins Regulate the Release of Apoptogenic Cytochrome c by the Mito-chondrial Channel VDAC. Nature 1999, 399, 483–487. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, S.; Kyprianou, N. Targeting anoikis resistance in prostate cancer metastasis. Mol. Asp. Med. 2010, 31, 205–214. [Google Scholar] [CrossRef] [Green Version]
- Cabrera, M.; Gomez, N.; Lenicov, F.R.; Echeverría, E.; Shayo, C.; Moglioni, A.; Fernández, N.; Davio, C.; Chien, M.H. G2/M Cell Cycle Arrest and Tumor Selective Apoptosis of Acute Leukemia Cells by a Promising Benzophenone Thiosemicarbazone Compound. PLoS ONE 2015, 10, e0136878. [Google Scholar] [CrossRef] [Green Version]
- Salvesen, G.S.; Duckett, C.S. IAP proteins: Blocking the road to death’s door. Nat. Rev. Mol. Cell Biol. 2002, 3, 401–410. [Google Scholar] [CrossRef]
- Hassan, A.M.; Nassar, A.M.; Hussien, Y.Z.; Elkmash, A.N. Synthesis, Characterization and Biological Evaluation of Fe (III), Co (II), Ni(II), Cu(II), and Zn(II) Complexes with Tetradentate Schiff Base Ligand Derived from Protocatechualdehyde with 2-Aminophenol. Appl. Biochem. Biotechnol. 2012, 167, 581–594. [Google Scholar] [CrossRef]
- Taş, E.; Kasumov, V.T.; Şahin, Ö.; Özdemir, M. Transition metal complexes with tridentate salicylaldimine derived from 3,5-di-t-butylsalicylaldehyde. Transit. Met. Chem. 2002, 27, 442–446. [Google Scholar] [CrossRef]
- Van Merloo, J.; Kaspers, G.J.L.; Cloos, J. Cell sensitivity assays: The MTT assay. Methods Mol. Biol. 2011, 731, 237–245. [Google Scholar]
- Justus, C.R.; Leffler, N.; Ruiz-Echevarria, M.; Yang, L.V. In vitro Cell Migration and Invasion Assays. J. Vis. Exp. 2014, 88, e51046. [Google Scholar]
- Crowley, L.; Marfell, B.J.; Waterhouse, N.J. Detection of DNA Fragmentation in Apoptotic Cells by TUNEL. Cold Spring Harb. Protoc. 2016, 2016, 900–905. [Google Scholar] [CrossRef] [PubMed]
- Nolan, T.; Hands, R.E.; Bustin, S.A. Quantification of mRNA using real-time RT-PCR. Nat. Protoc. 2006, 1, 1559–1582. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bansal, A.; Saleh-E-In, M.M.; Kar, P.; Roy, A.; Sharma, N.R. Synthesis of Carvacrol Derivatives as Potential New Anticancer Agent against Lung Cancer. Molecules 2022, 27, 4597. https://doi.org/10.3390/molecules27144597
Bansal A, Saleh-E-In MM, Kar P, Roy A, Sharma NR. Synthesis of Carvacrol Derivatives as Potential New Anticancer Agent against Lung Cancer. Molecules. 2022; 27(14):4597. https://doi.org/10.3390/molecules27144597
Chicago/Turabian StyleBansal, Anu, Md. Moshfekus Saleh-E-In, Pallab Kar, Ayan Roy, and Neeta Raj Sharma. 2022. "Synthesis of Carvacrol Derivatives as Potential New Anticancer Agent against Lung Cancer" Molecules 27, no. 14: 4597. https://doi.org/10.3390/molecules27144597







