Pt-Chitosan-TiO2 for Efficient Photocatalytic Hydrogen Evolution via Ligand-to-Metal Charge Transfer Mechanism under Visible Light
Abstract
:1. Introduction
2. Results and Discussion
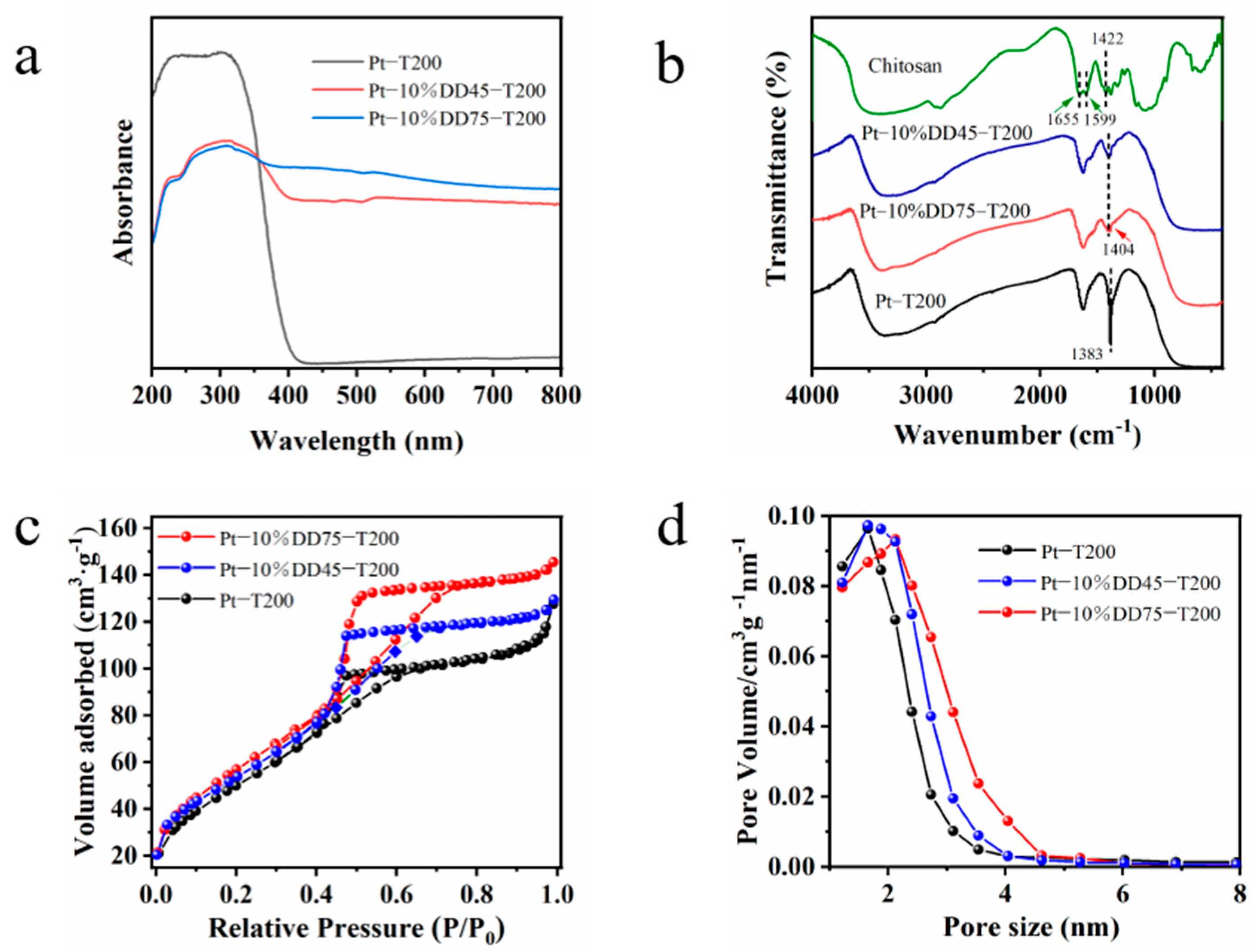
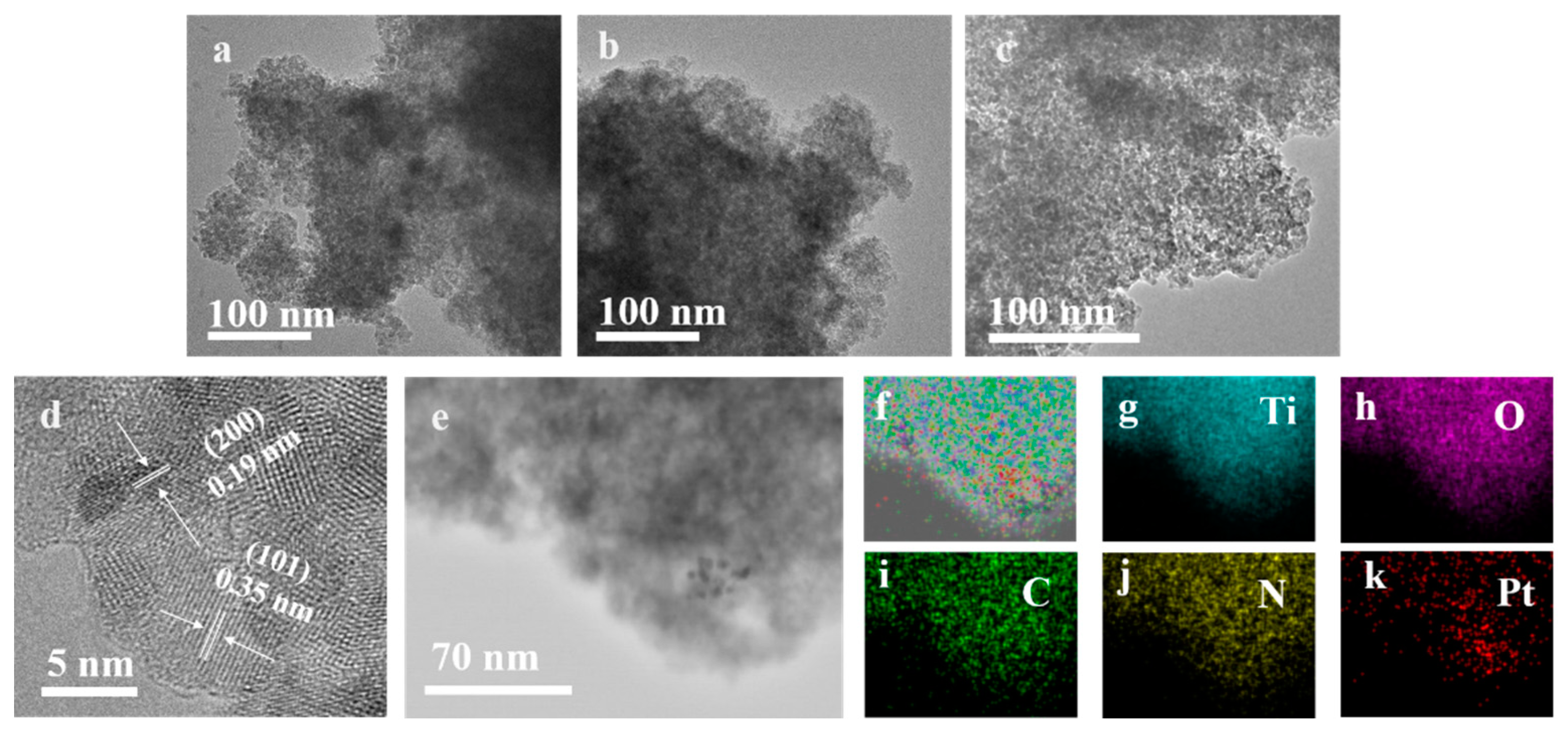
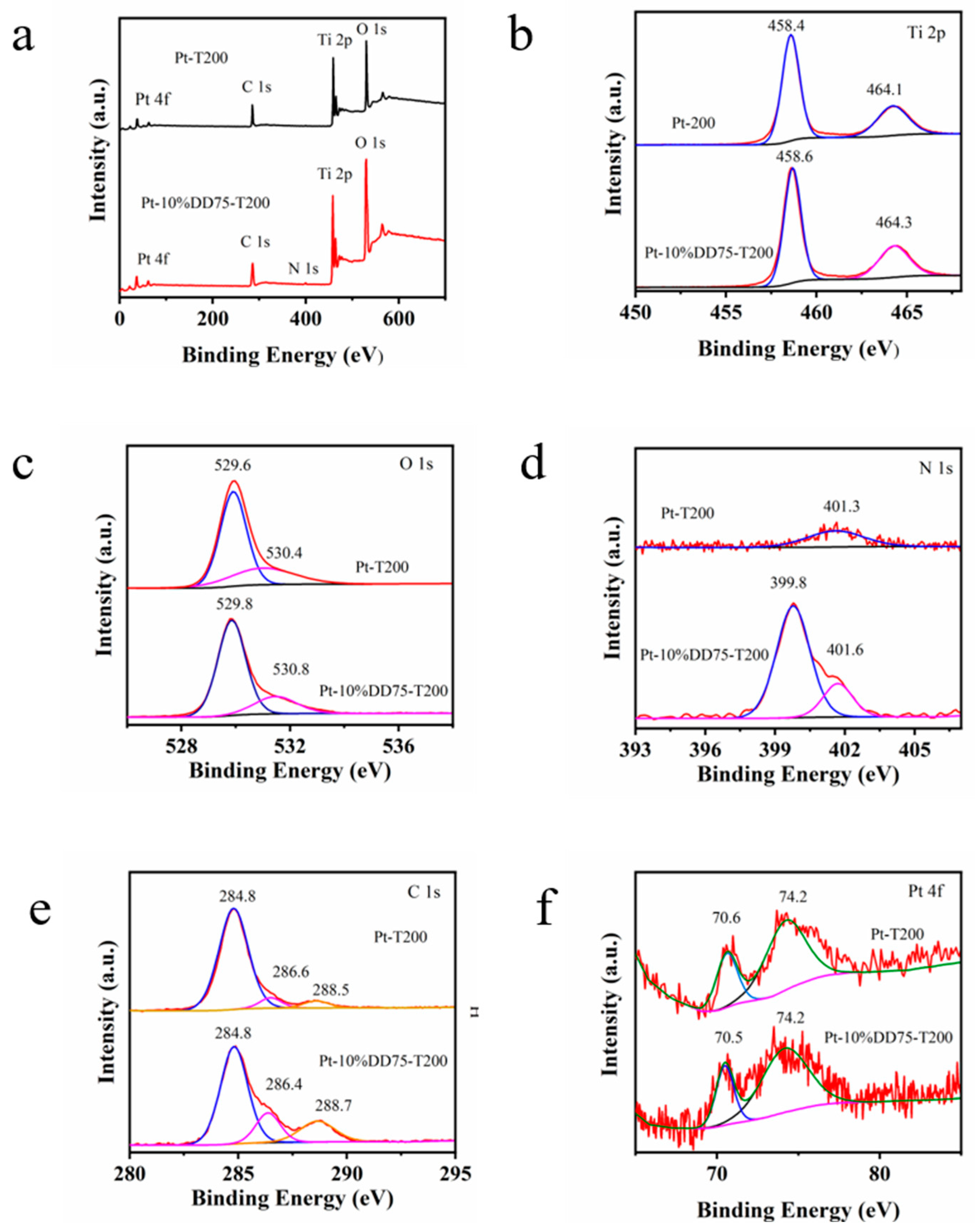
2.1. Physicochemical Properties of the Obtained Samples
2.2. Photocatalytic H2 Evolution Performance
2.3. Mechanism of Photocatalytic Reaction
3. Materials and Methods
3.1. Materials
3.2. Preparation of Photocatalysts
3.3. Determination of Degree of Deacetylation
3.4. Characterization
3.5. Photocatalytic Measurements
3.6. Photoelectrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Liu, J.; Ma, N.; Wu, W.; He, Q. Recent progress on photocatalytic heterostructures with full solar spectral responses. Chem. Eng. J. 2020, 393, 124719. [Google Scholar] [CrossRef]
- Niu, J.N.; Albero, J.; Atienzar, P.; Garcia, H. Porous single-crystal-based inorganic semiconductor photocatalysts for energy production and environmental remediation: Preparation, modification, and applications. Adv. Funct. Mater. 2020, 30, 1908984. [Google Scholar] [CrossRef]
- Mao, J.; Zhong, H.; Liu, X.; Qian, Q.; Luo, Y.; Xiao, L.; Xue, H. Electrospinning preparation of GaN:ZnO solid solution nanorods with visible-light-driven photocatalytic activity toward H2 production. Appl. Sci. 2021, 11, 10854. [Google Scholar] [CrossRef]
- Shukla, B.K.; Rawat, S.; Gautam, M.K.; Bhandari, H.; Garg, S.; Singh, J. Photocatalytic degradation of orange G dye by using bismuth molybdate: Photocatalysis optimization and modeling via definitive screening designs. Molecules 2022, 27, 2309. [Google Scholar] [CrossRef]
- Jia, Y.; Liu, P.B.; Wang, Q.Y.; Wu, Y.; Cao, D.D.; Qiao, Q.A. Construction of Bi2S3-BiOBr nanosheets on TiO2 NTA as the effective photocatalysts: Pollutant removal, photoelectric conversion and hydrogen generation. J. Colloid Interface Sci. 2021, 585, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Hunge, Y.; Yadav, A.; Dhodamani, A.; Suzuki, N.; Terashima, C.; Fujishima, A.; Mathe, V. Enhanced photocatalytic performance of ultrasound treated GO/TiO2 composite for photocatalytic degradation of salicylic acid under sunlight illumination. Ultrason. Sonochem. 2019, 61, 104849. [Google Scholar] [CrossRef]
- Liu, Y.L.; Wu, S.S.; Liu, J.; Xie, S.B.; Liu, Y.J. Synthesis of g-C3N4/TiO2 nanostructures for enhanced photocatalytic reduction of U(VI) in water. RSC Adv. 2021, 11, 4810–4817. [Google Scholar] [CrossRef]
- Djellabi, R.; Yang, B.; Wang, Y.; Cui, X.; Zhao, X. Carbonaceous biomass-titania composites with Ti-O-C bonding bridge for efficient photocatalytic reduction of Cr(VI) under narrow visible light. Chem. Eng. J. 2019, 366, 172–180. [Google Scholar] [CrossRef]
- Li, Z.; Wang, S.; Wu, J.; Zhou, W. Recent progress in defective TiO2 photocatalysts for energy and environmental applications. Renew. Sustain. Energy Rev. 2021, 156, 111980. [Google Scholar] [CrossRef]
- Diaz-Angulo, J.; Lara-Ramos, J.; Mueses, M.; Hernández-Ramírez, A.; Puma, G.L.; Machuca-Martínez, F. Enhancement of the oxidative removal of diclofenac and of the TiO2 rate of photon absorption in dye-sensitized solar pilot scale CPC photocatalytic reactors. Chem. Eng. J. 2019, 381, 122520. [Google Scholar] [CrossRef]
- Yang, L.; Bai, X.; Shi, J.; Du, X.; Xu, L.; Jin, P. Quasi-full-visible-light absorption by D35-TiO2/g-C3N4 for synergistic persulfate activation towards efficient photodegradation of micropollutants. Appl. Catal. B-Environ. 2019, 256, 117759. [Google Scholar] [CrossRef]
- Qing, X.; Yuan, L.; Wang, C.; Bi, M.; Wang, Y.; Weng, X. Structural and visible-near infrared optical properties of (Fe, Mo)-co-doped TiO2 for colored cool pigments. J. Alloys Compd. 2020, 826, 153946. [Google Scholar] [CrossRef]
- Park, B.G. Photocatalytic activity of TiO2-doped Fe, Ag, and Ni with N under visible light irradiation. Gels 2022, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Wu, C.; Jiao, H.; Xu, R.; Wang, Y.; Xie, J.; Guo, Q.; Tang, J. Rational design of high-concentration Ti3+ in porous carbon-doped TiO2 nanosheets for efficient photocatalytic ammonia synthesis. Adv. Mater. 2021, 33, 2008180. [Google Scholar] [CrossRef]
- Ma, Z.L.; Shi, L.Y.; Qu, W.W.; Hu, Q.; Chen, R.F.; Wang, Y.J.; Chen, Z. Microwave-assisted synthesis of an RGO/CdS/TiO2 step-scheme with exposed TiO2 {001} facets and enhanced visible photocatalytic activity. RSC Adv. 2020, 10, 43447–43458. [Google Scholar] [CrossRef]
- Wang, C.J.; Zhao, Y.L.; Xu, H.; Li, Y.F.; Wei, Y.C.; Liu, J.; Zhao, Z. Efficient Z-scheme photocatalysts of ultrathin g-C3N4-wrapped Au/TiO2-nanocrystals for enhanced visible-light-driven conversion of CO2 with H2O. Appl. Catal. B-Environ. 2020, 263, 118314. [Google Scholar] [CrossRef]
- Wang, Y.T.; Zhu, C.Z.; Zuo, G.C.; Guo, Y.; Xiao, W.; Dai, Y.X.; Kong, J.J.; Xu, X.M.; Zhou, Y.X.; Xie, A.M.; et al. 0D/2D Co3O4/TiO2 Z-scheme heterojunction for boosted photocatalytic degradation and mechanism investigation. Appl. Catal. B-Environ. 2020, 278, 119298. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, J.; Zhao, X.Y.; Xiao, X.; Sun, F.Q.; Zuo, X.X.; Nan, J.M. Small-molecule surface-modified bismuth-based semiconductors as a new class of visible-light-driven photocatalytic materials: Structure-dependent photocatalytic properties and photosensitization mechanism. Chem. Eng. J. 2020, 380, 122546. [Google Scholar] [CrossRef]
- Zhang, G.; Kim, G.; Choi, W. Visible light driven photocatalysis mediated via ligand-to-metal charge transfer (LMCT): An alternative approach to solar activation of titania. Energy Environ. Sci. 2014, 7, 954–966. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Wu, B.; Zhang, G.; Gan, Y.; Zhang, S. Enhanced decomplexation of Cu(II)-EDTA: The role of acetylacetone in Cu-mediated photo-fenton reactions. Chem. Eng. J. 2019, 358, 1218–1226. [Google Scholar] [CrossRef]
- Choudhury, B.; Bayan, S.; Choudhury, A.; Chakraborty, P. Narrowing of band gap and effective charge carrier separation in oxygen deficient TiO2 nanotubes with improved visible light photocatalytic activity. J. Colloid Interface Sci. 2016, 465, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Karthik, P.; Vinoth, R.; Selvam, P.; Balaraman, E.; Navaneethan, M.; Hayakawa, Y.; Neppolian, B. A visible-light active catechol-metal oxide carbonaceous polymeric material for enhanced photocatalytic activity. J. Mater. Chem. A 2017, 5, 384–396. [Google Scholar] [CrossRef]
- Kim, S.; Moon, G.-H.; Kim, G.; Kang, U.; Park, H.; Choi, W. TiO2 complexed with dopamine-derived polymers and the visible light photocatalytic activities for water pollutants. J. Catal. 2017, 346, 92–100. [Google Scholar] [CrossRef]
- Karthik, P.; Neppolian, B. TiO2-glucose LMCT complex/reduced graphene oxide sheet (rGO): An efficient visible light photocatalyst for Cr(VI) reduction. J. Environ. Chem. Eng. 2018, 6, 3664–3672. [Google Scholar] [CrossRef]
- Kim, G.; Choi, W. Charge-transfer surface complex of EDTA-TiO2 and its effect on photocatalysis under visible light. Appl. Catal. B-Environ. 2010, 100, 77–83. [Google Scholar] [CrossRef]
- Kim, G.; Lee, S.-H.; Choi, W. Glucose-TiO2 charge transfer complex-mediated photocatalysis under visible light. Appl. Catal. B-Environ. 2015, 162, 463–469. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Zhan, C.; Kong, F.; Li, W.; Yang, C.; Hsiao, B.S. Facile synthesis of TiO2/CNC nanocomposites for enhanced Cr(VI) photoreduction: Synergistic roles of cellulose nanocrystals. Carbohydr. Polym. 2020, 233, 115838. [Google Scholar] [CrossRef]
- Arasukumar, B.; Prabakaran, G.; Gunalan, B.; Moovendhan, M. Chemical composition, structural features, surface morphology and bioactivities of chitosan derivatives from lobster (Thenus unimaculatus) shells. Int. J. Biol. Macromol. 2019, 135, 1237–1245. [Google Scholar] [CrossRef]
- Sugashini, S.; Gomathi, T.; Devi, R.A.; Sudha, P.N.; Rambabu, K.; Banat, F. Nanochitosan/carboxymethyl cellulose/TiO2 biocomposite for visible-light-induced photocatalytic degradation of crystal violet dye. Environ. Res. 2022, 204, 112047. [Google Scholar] [CrossRef]
- Li, Q.-H.; Dong, M.; Li, R.; Cui, Y.-Q.; Xie, G.-X.; Wang, X.-X.; Long, Y.-Z. Enhancement of Cr(VI) removal efficiency via adsorption/photocatalysis synergy using electrospun chitosan/g-C3N4/TiO2 nanofibers. Carbohydr. Polym. 2021, 253, 117200. [Google Scholar] [CrossRef]
- Balakrishnan, A.; Appunni, S.; Gopalram, K. Immobilized TiO2/chitosan beads for photocatalytic degradation of 2,4-dichlorophenoxyacetic acid. Int. J. Biol. Macromol. 2020, 161, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-H.; Wang, F.-Y.; Mao, C.-F.; Liao, W.-T.; Hsieh, C.-D. Studies of chitosan: II. Preparation and characterization of chitosan/poly(vinyl alcohol)/gelatin ternary blend films. Int. J. Biol. Macromol. 2008, 43, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ge, X.; Lu, Y.; Dong, S.; Zhao, Y.; Zeng, M. Effects of chitosan molecular weight and degree of deacetylation on the properties of gelatine-based films. Food Hydrocolloid. 2012, 26, 311–317. [Google Scholar] [CrossRef]
- Xue, H.; Chen, Y.; Liu, X.; Qian, Q.; Luo, Y.; Cui, M.; Chen, Y.; Yang, D.-P.; Chen, Q. Visible light-assisted efficient degradation of dye pollutants with biomass-supported TiO2 hybrids. Mater. Sci. Eng. C 2018, 82, 197–203. [Google Scholar] [CrossRef]
- Liang, H.; Lv, C.; Chen, H.; Wu, L.; Hou, X. Facile synthesis of chitosan membranes for visible-light-driven photocatalytic degradation of tetracycline hydrochloride. RSC Adv. 2020, 10, 45171–45179. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Liu, H.; Zheng, Y.-M.; Qu, J.; Chen, J.P. Systematic study of synergistic and antagonistic effects on adsorption of tetracycline and copper onto a chitosan. J. Colloid Interface Sci. 2010, 344, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, X.; Lu, D.; Fang, P.; Xiong, R.; Wei, J.; Pan, C. Carbon deposited TiO2-based nanosheets with enhanced adsorption ability and visible light photocatalytic activity. J. Mol. Catal. A-Chem. 2014, 392, 208–215. [Google Scholar] [CrossRef]
- Vovk, E.I.; Kalinkin, A.V.; Smirnov, M.Y.; Klembovskii, I.O.; Bukhtiyarov, V.I. XPS study of stability and reactivity of oxidized Pt nanoparticles supported on TiOJ. Phys. Chem. C 2017, 121, 17297–17304. [Google Scholar] [CrossRef]
- Nwe, N.; Furuike, T.; Tamura, H. Isolation and characterization of chitin and chitosan from marine origin. Adv. Food Nutr. Res. 2014, 72, 1–15. [Google Scholar]
- Wang, J.; Qian, Q.; Chen, Q.; Liu, X.-P.; Luo, Y.; Xue, H.; Li, Z. Significant role of carbonate radicals in tetracycline hydrochloride degradation based on solar light-driven TiO2-seashell composites: Removal and transformation pathways. Chin. J. Catal. 2020, 41, 1511–1521. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Mao, J.; Huang, Y.; Qian, Q.; Luo, Y.; Xue, H.; Yang, S. Pt-Chitosan-TiO2 for Efficient Photocatalytic Hydrogen Evolution via Ligand-to-Metal Charge Transfer Mechanism under Visible Light. Molecules 2022, 27, 4673. https://doi.org/10.3390/molecules27154673
Liu Y, Mao J, Huang Y, Qian Q, Luo Y, Xue H, Yang S. Pt-Chitosan-TiO2 for Efficient Photocatalytic Hydrogen Evolution via Ligand-to-Metal Charge Transfer Mechanism under Visible Light. Molecules. 2022; 27(15):4673. https://doi.org/10.3390/molecules27154673
Chicago/Turabian StyleLiu, Yanru, Jingyun Mao, Yiwei Huang, Qingrong Qian, Yongjin Luo, Hun Xue, and Songwei Yang. 2022. "Pt-Chitosan-TiO2 for Efficient Photocatalytic Hydrogen Evolution via Ligand-to-Metal Charge Transfer Mechanism under Visible Light" Molecules 27, no. 15: 4673. https://doi.org/10.3390/molecules27154673





