Microporous Activated Carbon from Pisum sativum Pods Using Various Activation Methods and Tested for Adsorption of Acid Orange 7 Dye from Water
Abstract
:Highlights
- •
- Pisum sativum pods was used for producing porous activated carbon materials.
- •
- Two chemical methods and two gases were used in the activation processes.
- •
- Higher performances were achieved by applying ZnCl2 and CO2 for activation.
- •
- The products exhibited large surface areas and high AO7 dye adsorption performances.
- •
- RMS study of the AO7 dye removal by produced material was reported.
Abstract
1. Introduction
2. Results and Discussion
2.1. Effect of Impregnation Reagent, Activation Temperature, and Activating Reagent
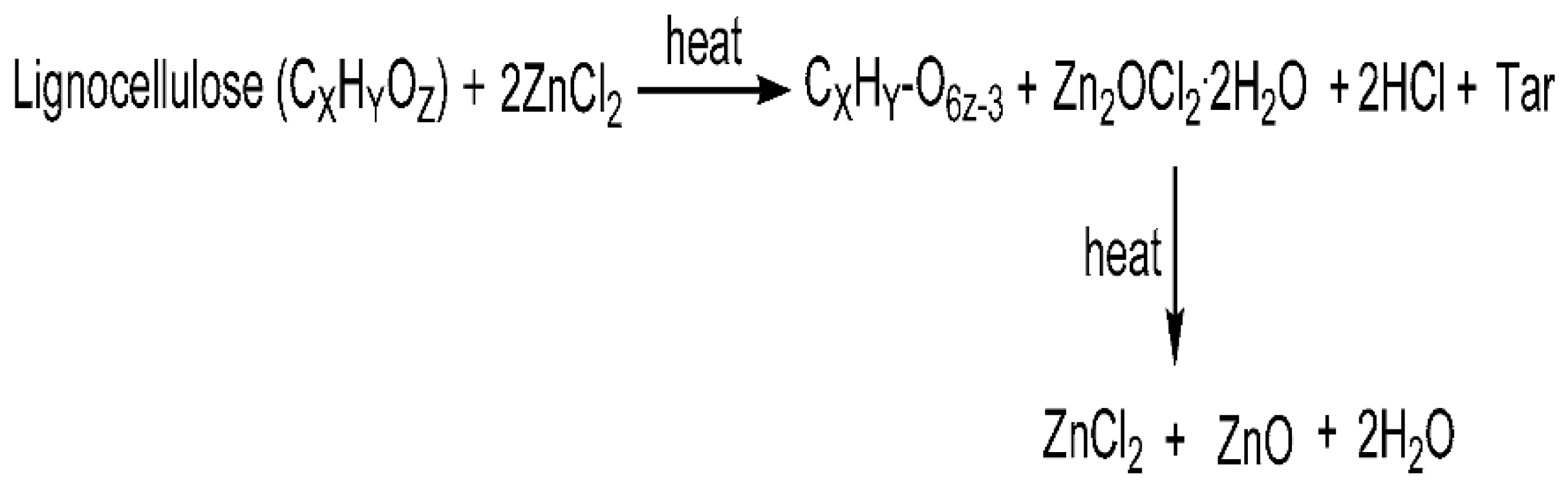
2.2. Characterization of Activated Carbon
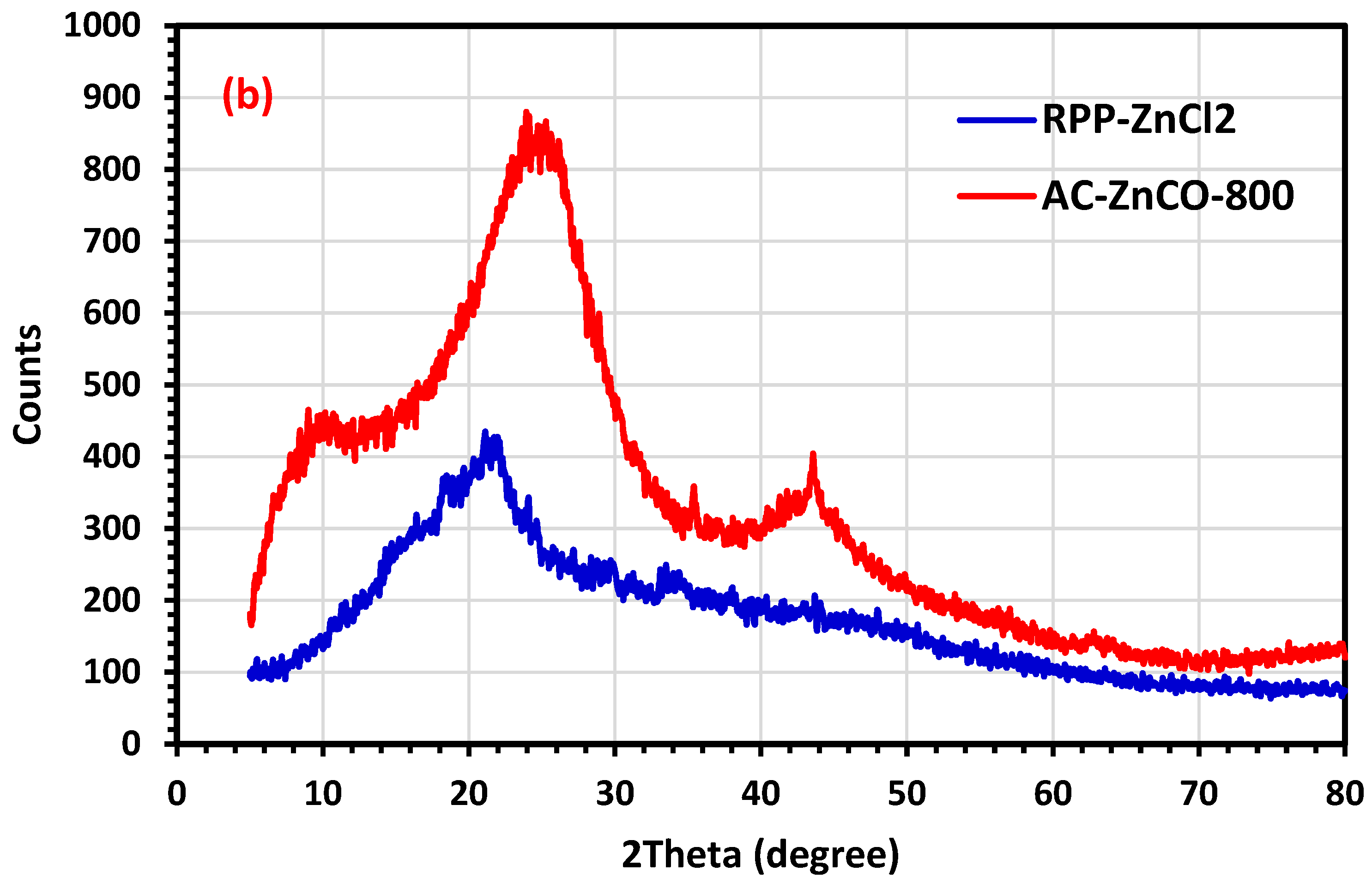
2.2.1. The X-ray Diffraction
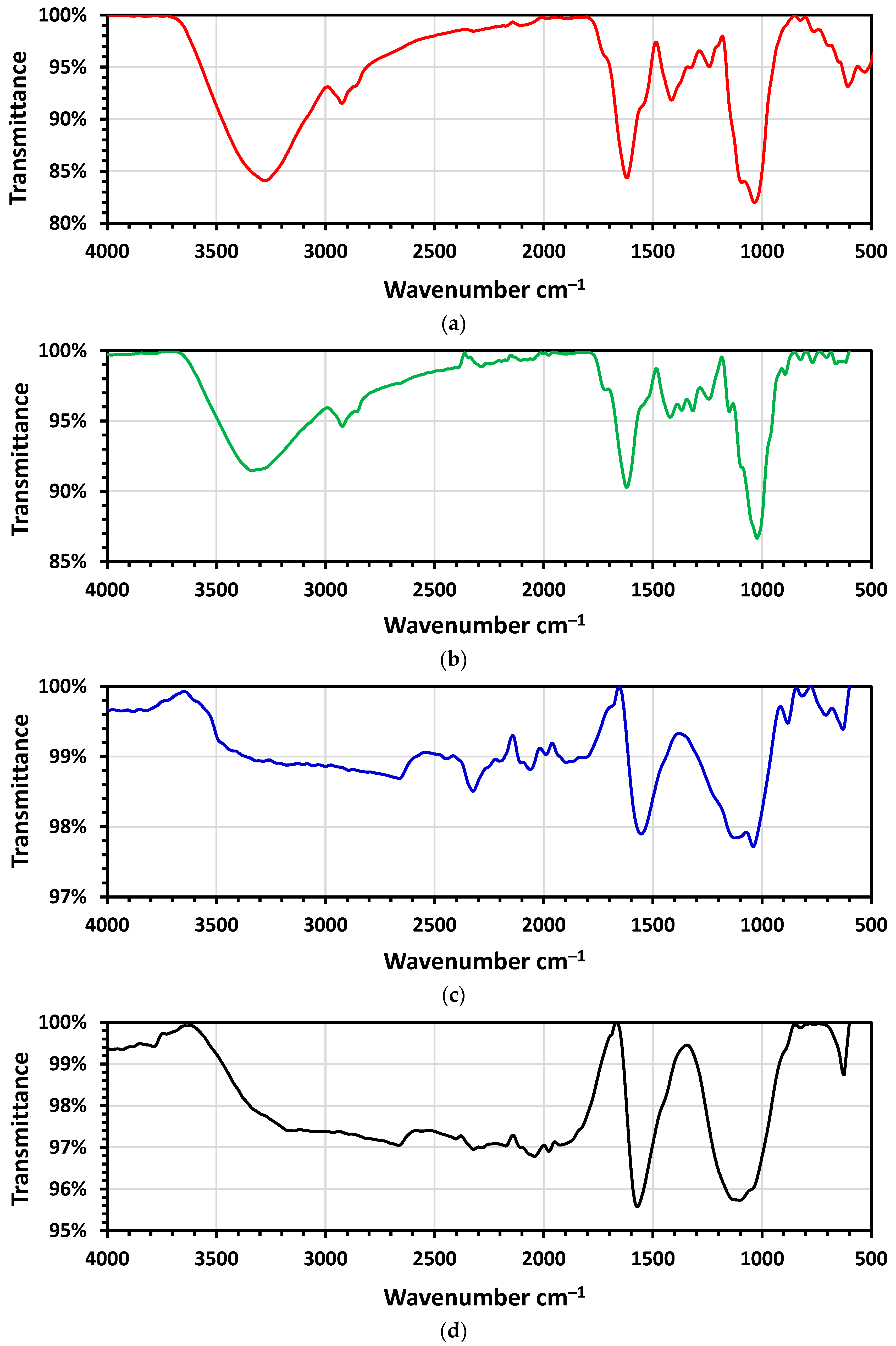
2.2.2. FTIR- Spectra
2.2.3. Scanning Electron Microscopy (SEM)
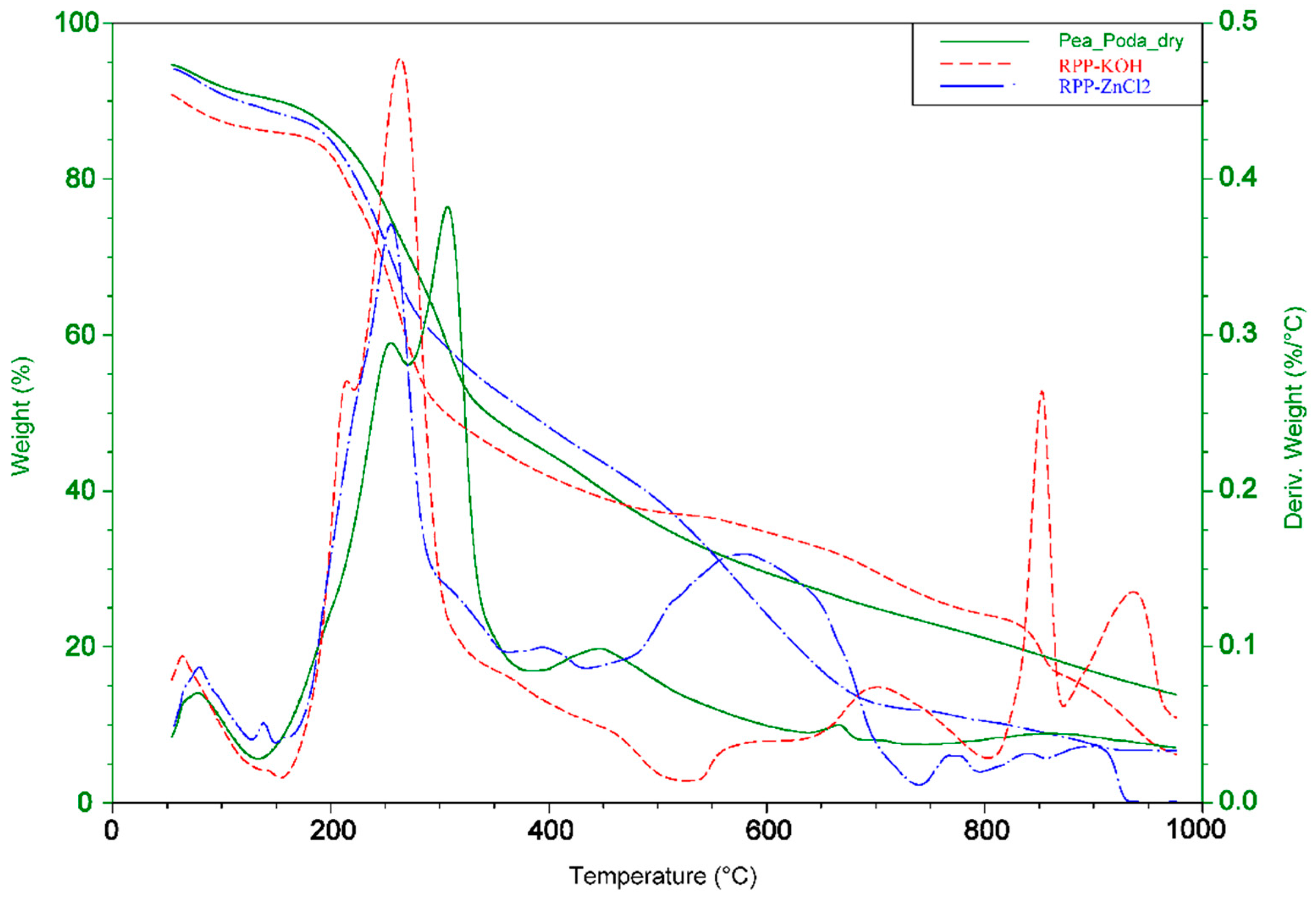
2.2.4. The Thermogravimetric Analysis (TGA and DTA)
2.2.5. Characterizations of Pore Structure
2.3. Acid Orange 7 Dye Adsorption Study
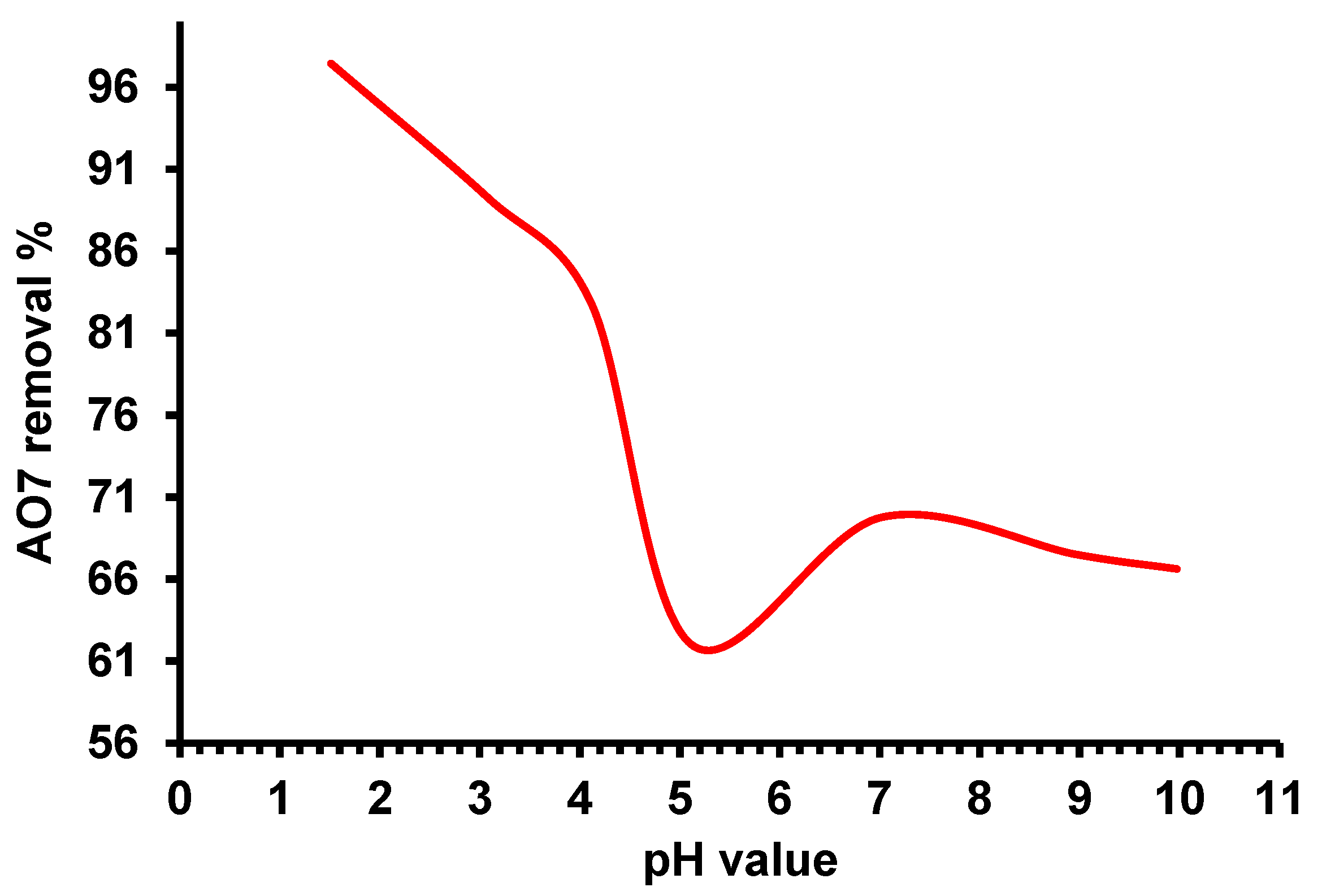
2.3.1. Effect of Solution pH on the Adsorption Process
2.3.2. Impact of Contact Time and Initial Dye Concentration on the Adsorption Process
2.3.3. Adsorption Isotherms
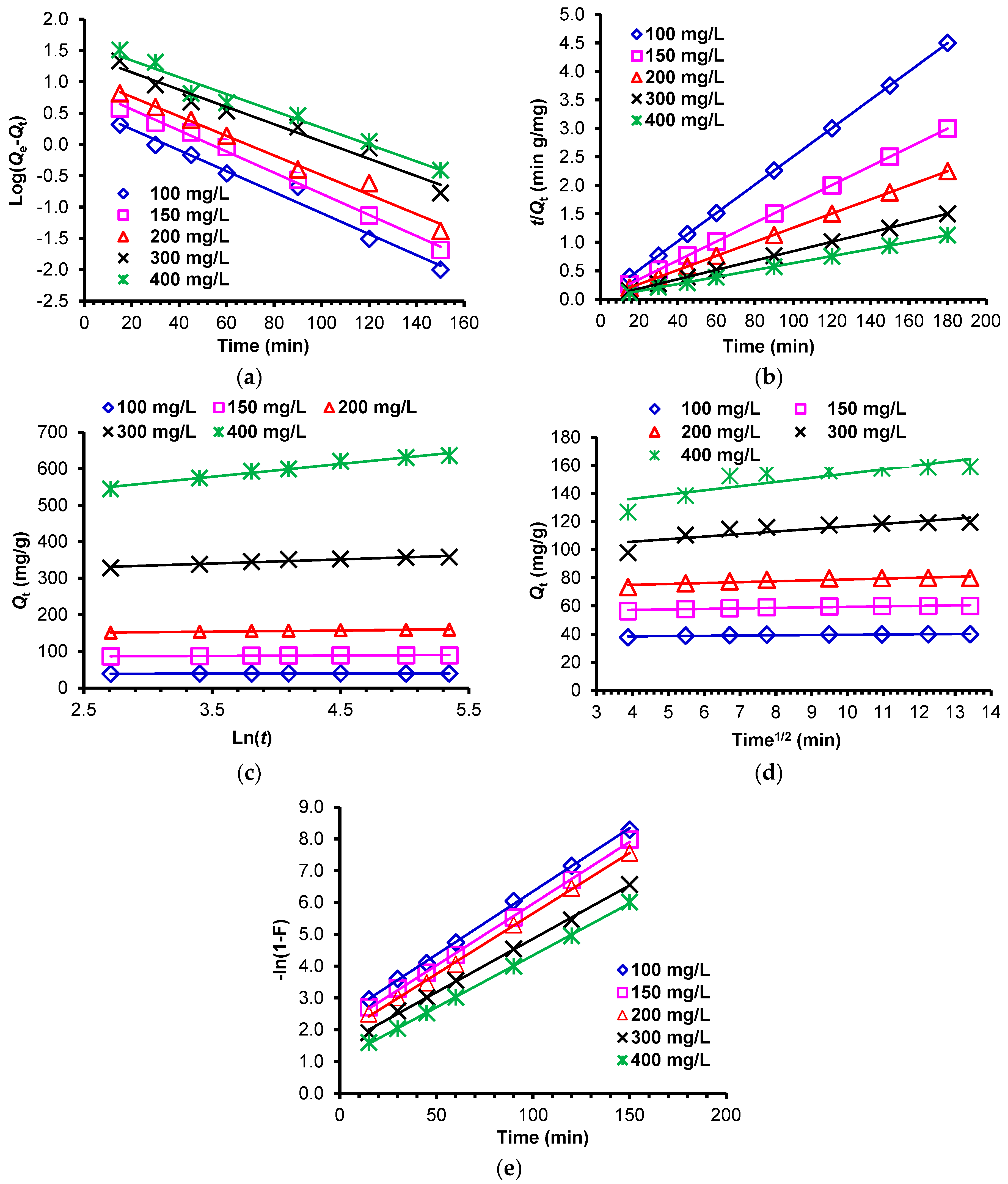
2.3.4. Adsorption Kinetics
The Pseudo-First-Order and Pseudo-Second-Order Kinetic Models
Elovich Kinetic Model
Intraparticle and Film Diffusion Kinetic Models
| Parameter | First-Order Kinetic Model | Second-Order Kinetic Model | |||||||
|---|---|---|---|---|---|---|---|---|---|
| AC (g/L) | AO7 Dye (mg L−1) | qe (exp.) | qe (calc.) | k1 × 103 | R2 | qe (calc.) | k2 × 103 | h | R2 |
| 0.75 | 100 | 133.23 | 101.81 | 46.52 | 0.989 | 138.89 | 0.99 | 19 | 1.000 |
| 150 | 198.77 | 144.18 | 38.69 | 0.992 | 212.77 | 0.48 | 22 | 0.999 | |
| 200 | 264.77 | 185.65 | 28.79 | 0.898 | 285.71 | 2.68 | 219 | 0.999 | |
| 300 | 379.51 | 195.48 | 24.87 | 0.988 | 400.00 | 0.22 | 36 | 1.000 | |
| 400 | 467.16 | 223.87 | 19.81 | 0.963 | 500.00 | 0.15 | 38 | 0.999 | |
| 1.0 | 100 | 99.97 | 36.53 | 39.61 | 0.981 | 103.09 | 2.26 | 24 | 1.000 |
| 150 | 149.71 | 64.46 | 37.77 | 0.968 | 156.25 | 1.05 | 26 | 1.000 | |
| 200 | 199.26 | 81.49 | 22.80 | 0.853 | 208.33 | 0.50 | 22 | 1.000 | |
| 300 | 287.24 | 122.01 | 21.88 | 0.994 | 303.03 | 0.32 | 29 | 1.000 | |
| 400 | 356.47 | 138.90 | 16.81 | 0.967 | 370.37 | 0.24 | 33 | 0.999 | |
| 1.5 | 100 | 66.65 | 22.09 | 47.44 | 0.969 | 68.03 | 4.89 | 23 | 1.000 |
| 150 | 99.89 | 47.40 | 46.29 | 0.995 | 103.09 | 2.09 | 22 | 1.000 | |
| 200 | 133.05 | 47.96 | 34.08 | 0.971 | 136.99 | 1.37 | 26 | 1.000 | |
| 300 | 195.44 | 87.24 | 25.56 | 0.912 | 204.08 | 0.58 | 24 | 0.999 | |
| 400 | 248.58 | 96.32 | 21.88 | 0.974 | 263.16 | 3.80 | 263 | 1.000 | |
| 2.0 | 100 | 49.99 | 10.20 | 43.07 | 0.965 | 50.76 | 9.88 | 25 | 1.000 |
| 150 | 74.96 | 17.96 | 42.38 | 0.974 | 76.34 | 4.82 | 28 | 1.000 | |
| 200 | 99.89 | 27.52 | 37.08 | 0.927 | 102.04 | 2.61 | 27 | 1.000 | |
| 300 | 148.75 | 50.69 | 31.78 | 0.965 | 153.85 | 1.24 | 29 | 1.000 | |
| 400 | 198.13 | 84.94 | 27.18 | 0.979 | 208.33 | 0.61 | 26 | 1.000 | |
| 2.5 | 100 | 39.99 | 3.79 | 38.69 | 0.981 | 40.16 | 26.72 | 43 | 1.000 |
| 150 | 59.99 | 7.92 | 38.92 | 0.995 | 60.61 | 12.43 | 46 | 1.000 | |
| 200 | 79.97 | 11.87 | 35.93 | 0.986 | 80.65 | 7.39 | 48 | 1.000 | |
| 300 | 119.57 | 26.85 | 31.78 | 0.968 | 121.95 | 2.62 | 39 | 1.000 | |
| 400 | 159.15 | 40.96 | 31.09 | 0.970 | 163.93 | 1.50 | 40 | 1.000 | |
| AC (g/L) | AO7 conc. | Elovich | Interaparticle Diffusion | Film Diffusion | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| β | α | R2 | Kdif | C | R2 | KFD | C | R2 | ||
| 0.75 | 100 | 0.0542 | 2.23 × 102 | 0.927 | 0.15 | 1.82 | 0.887 | 0.05 | 0.44 | 1.000 |
| 150 | 0.0322 | 1.55 × 102 | 0.960 | 0.17 | 1.93 | 0.927 | 0.04 | 0.47 | 0.999 | |
| 200 | 0.0245 | 1.75 × 102 | 0.993 | 0.18 | 2.03 | 0.982 | 0.33 | 0.41 | 0.999 | |
| 300 | 0.0179 | 3.44 × 102 | 0.990 | 0.17 | 2.21 | 0.975 | 0.26 | 0.63 | 0.999 | |
| 400 | 0.0143 | 1.42 × 101 | 0.977 | 0.18 | 2.27 | 0.971 | 0.21 | 0.68 | 1.000 | |
| 1.0 | 100 | 0.1067 | 3.60 × 103 | 0.906 | 0.09 | 1.80 | 0.871 | 0.04 | 1.03 | 0.999 |
| 150 | 0.0525 | 4.46 × 102 | 0.884 | 0.13 | 1.90 | 0.846 | 0.04 | 0.67 | 1.000 | |
| 200 | 0.0356 | 2.51 × 102 | 0.974 | 0.16 | 1.96 | 0.951 | 0.03 | 0.67 | 0.997 | |
| 300 | 0.0250 | 3.61 × 102 | 0.982 | 0.16 | 2.11 | 0.966 | 0.02 | 0.84 | 0.997 | |
| 400 | 0.0213 | 5.22 × 102 | 0.968 | 0.16 | 2.20 | 0.969 | 0.02 | 0.82 | 0.999 | |
| 1.5 | 100 | 0.1972 | 2.43 × 104 | 0.869 | 0.07 | 1.67 | 0.831 | 0.05 | 1.07 | 0.996 |
| 150 | 0.0966 | 1.47 × 103 | 0.889 | 0.10 | 1.78 | 0.851 | 0.05 | 0.80 | 0.999 | |
| 200 | 0.0668 | 9.62 × 102 | 0.891 | 0.12 | 1.88 | 0.855 | 0.04 | 0.82 | 0.998 | |
| 300 | 0.0456 | 1.04 × 103 | 0.988 | 0.13 | 2.02 | 0.978 | 0.03 | 0.76 | 0.995 | |
| 400 | 0.0295 | 3.76 × 102 | 0.974 | 0.15 | 2.07 | 0.950 | 0.03 | 0.55 | 0.999 | |
| 2.0 | 100 | 0.4188 | 2.66 × 107 | 0.892 | 0.05 | 1.60 | 0.864 | 0.05 | 1.50 | 0.999 |
| 150 | 0.2040 | 1.97 × 105 | 0.873 | 0.06 | 1.74 | 0.843 | 0.04 | 1.40 | 0.998 | |
| 200 | 0.1111 | 5.47 × 103 | 0.874 | 0.09 | 1.81 | 0.837 | 0.04 | 0.85 | 0.998 | |
| 300 | 0.0643 | 1.87 × 103 | 0.901 | 0.11 | 1.94 | 0.872 | 0.03 | 0.90 | 0.997 | |
| 400 | 0.0434 | 8.93 × 102 | 0.975 | 0.13 | 2.02 | 0.960 | 0.03 | 0.83 | 0.998 | |
| 2.5 | 100 | 1.1502 | 7.05 × 1017 | 0.921 | 0.02 | 1.56 | 0.897 | 0.04 | 2.37 | 0.999 |
| 150 | 0.6039 | 7.34 × 1013 | 0.969 | 0.03 | 1.72 | 0.947 | 0.04 | 2.08 | 0.999 | |
| 200 | 0.3456 | 2.43 × 1010 | 0.955 | 0.03 | 1.83 | 0.932 | 0.04 | 1.85 | 0.999 | |
| 300 | 0.1163 | 8.26 × 104 | 0.863 | 0.07 | 1.93 | 0.828 | 0.03 | 1.50 | 0.999 | |
| 400 | 0.0714 | 1.05 × 104 | 0.885 | 0.09 | 2.02 | 0.856 | 0.03 | 1.07 | 1.000 | |
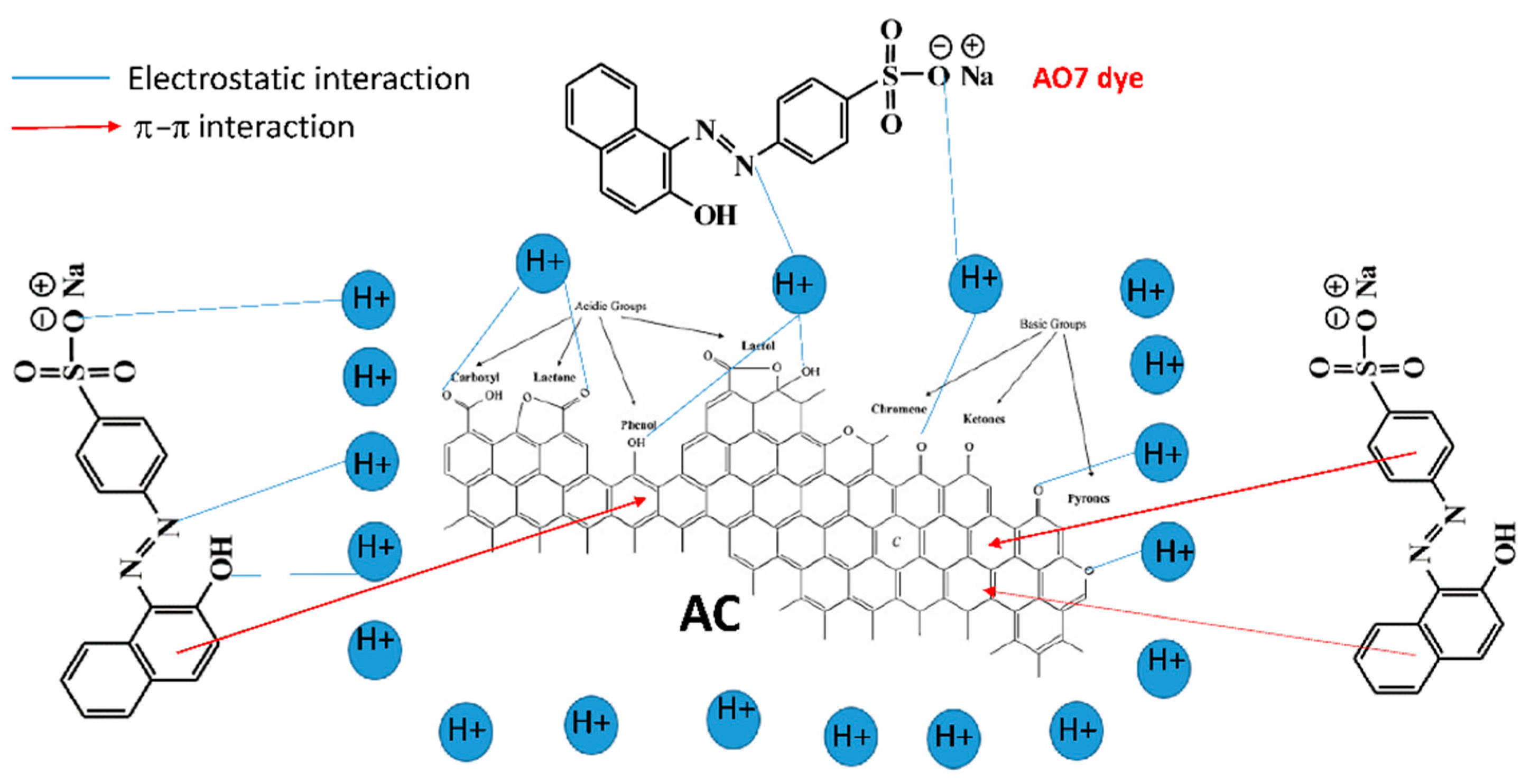
2.3.5. Adsorption Mechanism
2.3.6. Comparison of the Qm of AO7 Dye onto Different ACs
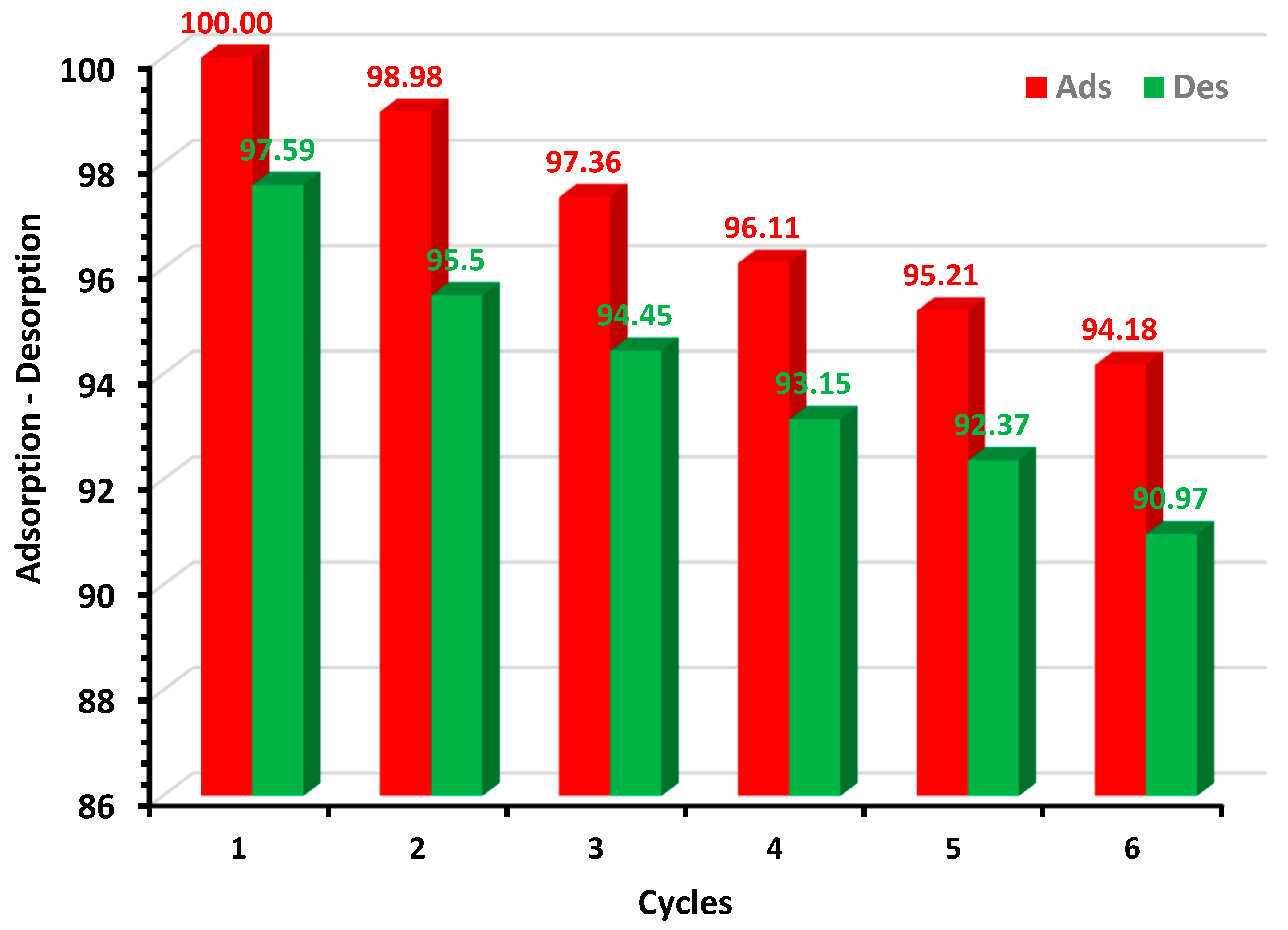
2.3.7. Desorption Studies
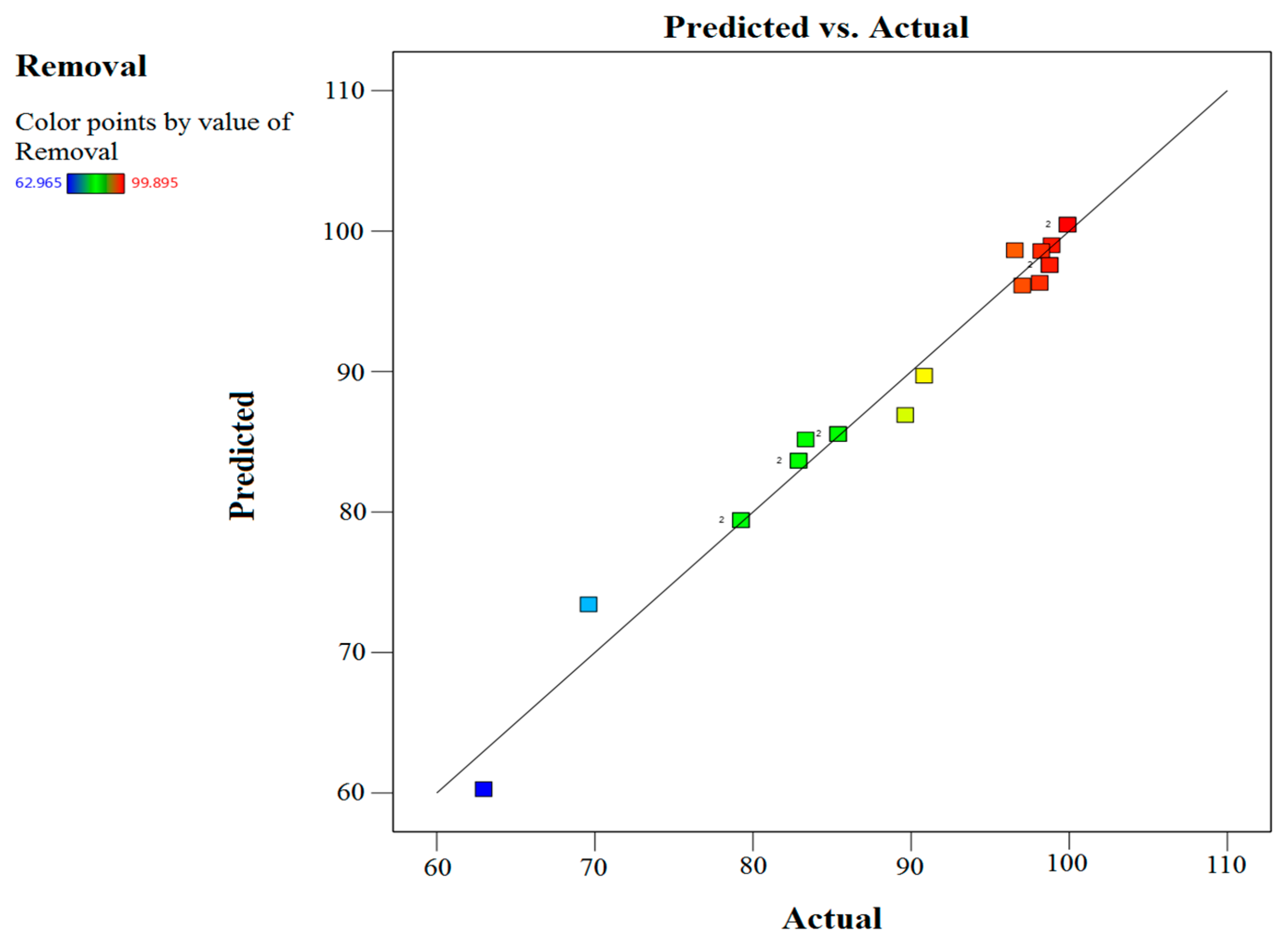
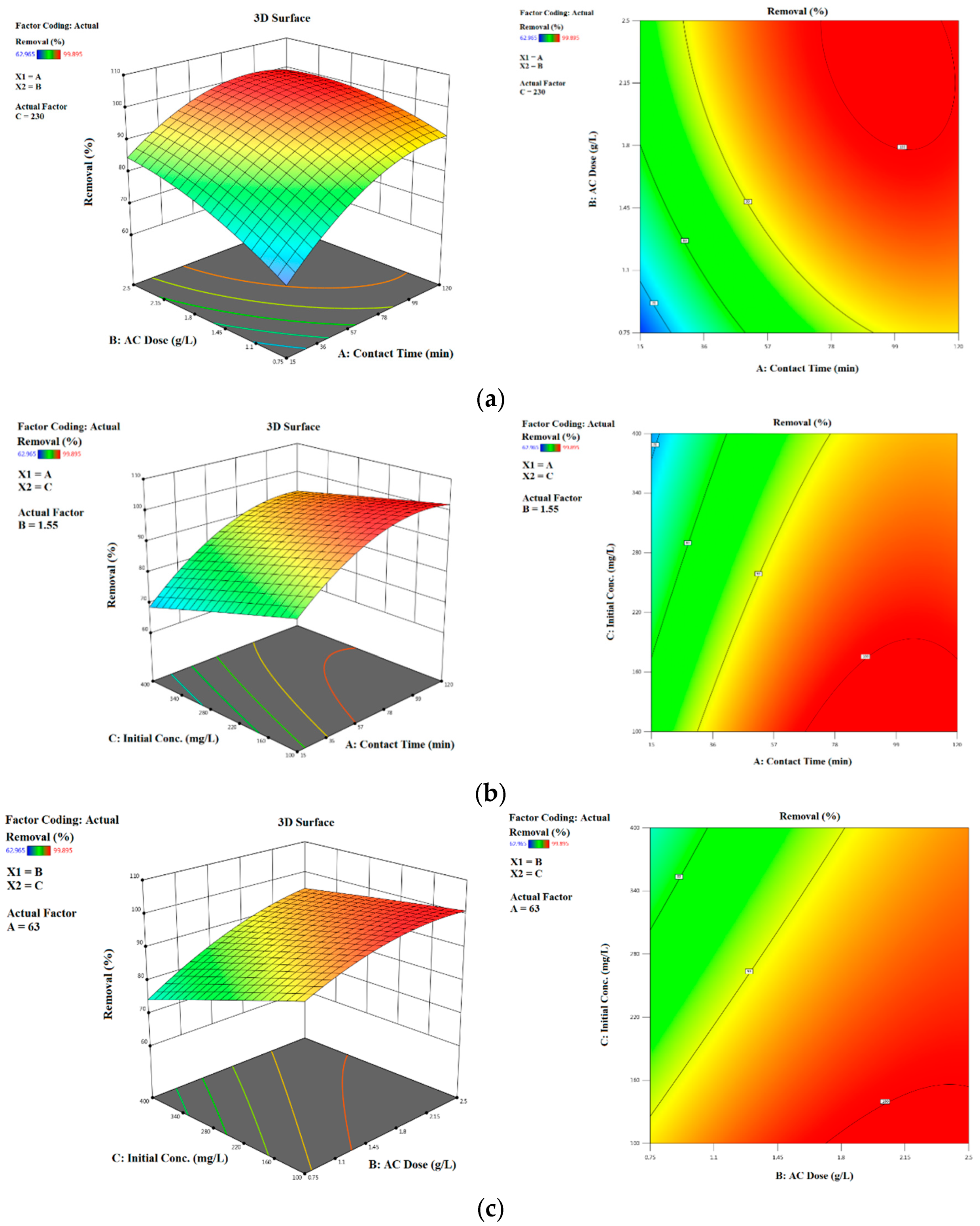
2.4. Optimization Study
Simultaneous Effects of Interactive Adsorption Variables
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Preparation of Activated Carbon
3.2.1. Impregnation with ZnCl2
3.2.2. Impregnation with KOH
3.3. Characterization of the Synthesized Activated Carbons
3.4. Adsorption Experiments
3.5. Isotherm Models Study
3.6. Adsorption Kinetic Models Study
3.7. Optimization Study Response Surface Methodology (RSM)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yuan, X.Z.; Choi, S.W.; Jang, E.; Lee, K.B. Chemically activated microporous carbons derived from petroleum coke: Performance evaluation for CF4 adsorption. Chem. Eng. J. 2018, 336, 297–305. [Google Scholar] [CrossRef]
- Du, Y.; Xu, X.; Liu, Q.; Bai, L.; Hang, K.; Wang, D. Identification of organic pollutants with potential ecological and health risks in aquatic environments: Progress and challenges. Sci. Total. Environ. 2021, 806, 50691. [Google Scholar] [CrossRef] [PubMed]
- Pavithra, K.G.; Jaikumar, V.; Kumar, P.S.; Sundarrajan, P. Removal of emerging pollutants from aquatic system using electrochemical treatment and adsorption: Comparison and analysis. Environ. Technol. Innov. 2021, 23, 101754. [Google Scholar] [CrossRef]
- El Nemr, A. Impact, Monitoring and Management of Environmental Pollution; Nova Science Publishers/Inc. Hauppauge: New York, NY, USA, 2021; p. 638, ISBN-10: 1608764877; ISBN-13: 9781608764877. [Google Scholar]
- Hassaan, M.A.; El Nemr, A.; Madkour, F.F. Testing the advanced oxidation processes on the degradation of Direct Blue 86 dye in wastewater. Egypt. J. Aquat. Res. 2017, 43, 11–19. [Google Scholar] [CrossRef] [Green Version]
- Luo, P.; Zhao, Y.F.; Zhang, B.; Liu, J.D.; Yang, Y.; Liu, J.F. Study on the adsorption of Neutral Red from aqueous solution onto halloysite nanotubes. Water Res. 2010, 44, 1489–1497. [Google Scholar] [CrossRef]
- Acemioğlu, B. Adsorption of congo red from aqueous solution onto calcium-rich fly ash. J. Colloid Interface Sci. 2004, 274, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Sahlabji, T.; El-Nemr, M.A.; El Nemr, A.; Ragab, S.; Alghamdi, M.M.; El-Zahhar, A.A.; Idris, A.M.; Said, T.O. High Surface Area Microporous Activated Carbon from Pisum sativum peels for Hexavalent Chromium Removal from Aquatic Environment. Toxin Rev. 2022, 41, 639–649. [Google Scholar] [CrossRef]
- Ajmal, A.; Majeed, I.; Malik, R.N.; Idriss, H.; Nadeem, M.A. Principles and mechanisms of photocatalytic dye degradation on TiO2 based photocatalysts: A comparative overview. RSC Adv. 2014, 4, 37003–37026. [Google Scholar] [CrossRef]
- Helmy, E.T.; El Nemr, A.; Gomaa, E.; El Dafrawy, S.; Mousa, M. Photocatalytic degradation of textile dyeing wastewater under visible light irradiation using green synthesized mesoporous non-metal doped TiO2. Bull. Mater. Sci. 2021, 44, 30. [Google Scholar] [CrossRef]
- Güzel, F.; Shaygılı, H.; Saygılı, G.A.; Koyuncu, F. New low-cost nanoporous carbonaceous adsorbent developed from carob (Ceratonia siliqua) processing industry waste for the adsorption of anionic textile dye: Characterization, equilibrium and kinetic modeling. J. Mol. Liq. 2015, 206, 244–255. [Google Scholar] [CrossRef]
- El-Nemr, M.A.; Abdelmonem, N.M.; Ismail, I.M.A.; Ragab, S.; El Nemr, A. Removal of Acid Yellow 11 Dye using novel modified biochar derived from Watermelon Peels. Desal. Desal. Water Treat. 2020, 203, 403–431. [Google Scholar] [CrossRef]
- El-Nemr, M.A.; Abdelmonem, N.M.; Ismail, I.M.A.; Ragab, S.; El Nemr, A. The efficient removal of the hazardous Azo Dye Acid Orange 7 from water using modified biochar from Pea peels. Desal. Desal. Water Treat. 2020, 203, 327–355. [Google Scholar] [CrossRef]
- El Nemr, A. Textiles: Types, Uses and Production Methods; Nova Science Publishers/Inc. Hauppauge: New York, NY, USA, 2012; p. 621, ISBN: 978-1-62100-239-0; ISBN: 978-1-62100-284-0. [Google Scholar]
- Mahmoodi, N.M.; Taghizadeh, M.; Mesoporous, A.T. Mesoporous activated carbons of low-cost agricultural bio-wastes with high adsorption capacity: Preparation and artificial neural network modeling of dye removal from single and multicomponent (binary and ternary) systems. J. Mol. Liq. 2018, 269, 217–228. [Google Scholar] [CrossRef]
- Hassaan, M.A.; El Nemr, A.; El-Zahhar, A.A.; Idris, A.M.; Alghamdi, M.M.; Sahlabji, T.; Said, T.O. Degradation mechanism of Direct Red 23 dye by advanced oxidation processes: A comparative study. Toxin Rev. 2022, 41, 38–47. [Google Scholar] [CrossRef]
- El Nemr, A. Non-Conventional Textile Waste Water Treatment; Nova Science Publishers, Inc. Hauppauge: New York, NY, USA, 2012; p. 267, ISBN: 978-1-62100-079-2; ISBN: 978-1-62100-228-4. [Google Scholar]
- Hosseini, S.A.; Vossoughi, M.; Mahmoodi, N.M.; Sadrzadeh, M. Efficient dye removal from aqueous solution by high-performance electrospun nanofibrous membranes through incorporation of SiO2 nanoparticles. J. Clean. Prod. 2018, 183, 1197–1206. [Google Scholar] [CrossRef]
- El Nemr, A.; Shoaib, A.G.M.; El Sikaily, A.; Ragab, S.; Mohamed, A.E.-D.A.; Hassan, A.F. Utilization of green alga Ulva lactuca for sustainable production of meso-micro porous nano activated carbon for adsorption of Direct Red 23 dye from aquatic environment. Carbon Lett. 2022, 32, 153–168. [Google Scholar] [CrossRef]
- El Nemr, A.; Aboughaly, R.M.; El Sikaily, A.; Ragab, S.; Masoud, M.S.; Ramadan, M.S. Utilization of Citrus aurantium peels for sustainable production of high surface area type I microporous nano activated carbons. Biomass Convers. Biorefin. 2021. [Google Scholar] [CrossRef]
- El Nemr, A.; Aboughaly, R.M.; El Sikaily, A.; Masoud, M.S.; Ramadan, M.S.; Ragab, S. Microporous activated carbons with a high surface area of type I adsorption isotherm derived from sugarcane bagasse impregnated with zinc chloride. Carbon Letter. 2022, 32, 229–249. [Google Scholar] [CrossRef]
- Shoaib, A.G.M.; El-Sikaily, A.; El Nemr, A.; Mohamed, A.E.-D.A.; Hassan, A.A. Testing the carbonization condition for high surface area preparation of activated carbon followed Type IV from green alga Ulva lactuca. Biomass Convers. Biorefin. 2020, 12, 3303–3318 . [Google Scholar] [CrossRef]
- Shoaib, A.G.M.; El-Sikaily, A.; El Nemr, A.; Mohamed, A.E.-D.A.; Hassan, A.A. Preparation and characterization of highly surface area activated carbons followed Type IV from marine red alga (Pterocladia capillacea) by zinc chloride activation. Biomass Convers. Biorefin. 2022, 12, 2253–2265. [Google Scholar] [CrossRef]
- El Nemr, A.; Aboughaly, R.M.; El Sikaily, A.; Ragab, S.; Masoud, M.S.; Ramadan, M.S. Microporous nano activated carbon type I derived from orange peel and its application for Cr(VI) removal from aquatic environment. Biomass Convers. Biorefin. 2020; in press. [Google Scholar] [CrossRef]
- Carrott, P.J.M.; Suhas, R.; Carrott, M.M.L.; Guerrero, C.I.; Delgado, L.A. Reactivity and porosity development during pyrolysis and physical activation in CO2 or steam of kraft and hydrolytic lignins. J. Anal. Appl. Pyrol. 2008, 82, 264–271. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.S.; Zheng, T.; Wang, P.; Guo, L. Preparation and characterization of activated carbon from bamboo by microwave-induced phosphoric acid activation. Ind. Crops Prod. 2010, 31, 233–238. [Google Scholar] [CrossRef]
- Álvarez, P.; Blanco, C.; Granda, M. The adsorption of chromium (VI) from industrial wastewater by acid and base activated lignocellulosic residues. J. Hazard. Mater. 2007, 144, 400–405. [Google Scholar] [CrossRef] [PubMed]
- El Nemr, A. Potential of pomegranate husk carbon for Cr(VI) removal from wastewater: Kinetic and isotherm studies. J. Hazard. Mater. 2009, 161, 132–141. [Google Scholar] [CrossRef]
- Guo, J.; Lua, A.C. Textural and chemical characterisations of activated carbon prepared from oil-palm stone with H2SO4 and KOH impregnation. Microporous Mesoporous Mater. 2007, 32, 111–117. [Google Scholar] [CrossRef]
- Carvalho, A.P.; Gomes, M.; Mestre, A.S.; Pires, J.; de Carvalho, M.B. Activated carbons from cork waste by chemical activation with K2CO3. Application to adsorption of natural gas components. Carbon 2004, 42, 672–674. [Google Scholar] [CrossRef]
- Hayashi, J.; Kazehaya, A.; Muroyama, K.; Watkinson, A.P. Preparation of activated carbon from lignin by chemical activation. Carbon 2000, 38, 1873–1878. [Google Scholar] [CrossRef]
- Dias, J.M.; Alvim-Ferraz, M.C.; Almeida, M.F.; Rivera-Utrill, J.; Sánchez-Polo, M. Waste materials for activated carbon preparation and its use in aqueous phase treatment: A review. J. Environ. Manag. 2007, 85, 833–846. [Google Scholar] [CrossRef]
- Ogungbenro, A.E.; Quang, D.V.; Al-Ali, K.; Abu-Zahra, M.R. Activated carbon from date seeds for CO2 capture application. Energ. Procedia 2017, 114, 2313–2321. [Google Scholar] [CrossRef]
- Tan, X.; Liu, S.; Liu, Y.; Gu, Y.; Zeng, G.; Hu, X. Biochar as potential sustainable precursors for activated carbon production: Multiple applications in environmental protection and energy storage. Bioresour. Technol. 2017, 227, 359–372. [Google Scholar] [CrossRef] [PubMed]
- Kadirvelu, K.; Kavipriya, M.; Karthika, C.; Radhika, M.; Vennilamani, N.; Pattabhi, S. Utilization of various agricultural wastes for activated carbon preparation and application for the removal of dyes and metal ions from aqueous solutions. Bioresour. Technol. 2003, 87, 129–132. [Google Scholar] [CrossRef]
- Hameed, B.H.; Ahmad, A.L.; Latiff, K.N.A. Adsorption of basic dye (methylene blue) onto activated carbon prepared from rattan sawdust. Dye. Pigment. 2007, 75, 143–149. [Google Scholar] [CrossRef]
- Kumar, B.G.P.; Miranda, L.R.; Velan, M. Adsorption of bismark brown dye on activated carbons prepared from rubberwood sawdust (Hevea brasiliensis) using different activation methods. J. Hazard. Mater. 2005, 126, 63–70. [Google Scholar] [CrossRef]
- Garg, V.K.; Amita, M.; Kumar, R.; Gupta, R. Basic dye (methylene blue) removal from simulated wastewater by adsorption using Indian Rosewood sawdust: A timber industry waste. Dyes Pigment. 2004, 63, 243–250. [Google Scholar] [CrossRef]
- Senthilkumaar, S.; Varadarajan, P.R.; Porkodi, K.; Subbhuraam, C.V. Adsorption of methylene blue onto jute Fiber carbon: Kinetics and equilibrium studies. J. Coll. Interface Sci. 2005, 284, 78–82. [Google Scholar] [CrossRef]
- Attia, A.A.; Girgis, B.S.; Khedr, S. Capacity of activated carbon derived from pistachio shells by H3PO4 in the removal of dyes and phenolics. J. Chem. Technol. Biotechnol. 2003, 78, 611–619. [Google Scholar] [CrossRef]
- Aygün, A.; Yenisoy-Karakaş, S.; Duman, I. Production of granular activated carbon from fruit stones and nutshells and evaluation of their physical, chemical and adsorption properties. Micropor. Mesopor. Mater. 2003, 66, 189–195. [Google Scholar] [CrossRef]
- Kannan, N.; Meenakshisundaram, M. Adsorption of Congo Red on various activated carbons. Water Air Soil Poll. 2002, 138, 289–305. [Google Scholar] [CrossRef]
- Tan, I.A.W.; Hameed, B.H.; Ahmad, W.A.L. Equilibrium and kinetic studies on basic dye adsorption by oil palm Fiber activated carbon. Chem. Eng. J. 2007, 127, 111–119. [Google Scholar] [CrossRef]
- Krishnamoorthy, R.; Govindan, B.; Banat, F.; Sagadevan, V.; Purushothaman, M.; Show, P.L. Date pits activated carbon for divalent lead ions removal. J. Biosci. Bioeng. 2019, 128, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Sankar, M.; Sekaran, G.; Sadulla, S.; Ramasami, T. Removal of diazo and triphenylmethane dyes from aqueous solutions through an adsorption process. J. Chem. Technol. Biotechnol. 1999, 74, 337–344. [Google Scholar] [CrossRef]
- Kavitha, D.; Namasivayam, C. Experimental and kinetic studies on methylene blue adsorption by coir pith carbon. Bioresour. Technol. 2007, 98, 14–21. [Google Scholar] [CrossRef]
- Vasanth, K.; Kumaran, A. Removal of methylene blue by mango seed kernel powder. Biochem. Eng. J. 2005, 27, 83–93. [Google Scholar]
- Kannan, N.; Sundaram, M.M. Kinetics and mechanism of removal of methylene blue by adsorption on various carbons—A comparative study. Dyes Pigment. 2001, 51, 25–40. [Google Scholar] [CrossRef]
- Macedo, J.S.; Júnior, N.B.; Almeida, L.E. Kinetic and calorimetric study of the adsorption of dyes on mesoporous activated carbon prepared from coconut coir dust. J. Coll. Interface Sci. 2006, 298, 515–522. [Google Scholar] [CrossRef]
- Geçgel, Ü.; Sezer, K.; Kolancılar, H. Removal of Cu(II) ions from aqueous solutions by the activated carbon obtained from pine cone. Asian J. Chem. 2010, 22, 3936–3942. [Google Scholar]
- Gupta, V.K.; Jain, R.; Varshney, S. Removal of Reactofix golden yellow 3 RFN from aqueous solution using wheat husk—An agricultural waste. J. Hazard. Mater. 2007, 142, 443–448. [Google Scholar] [CrossRef]
- Danish, M.; Ahmad, T.; Majeed, S.; Ahmad, M.; Ziyang, L.; Pin, Z.; Iqubal, S.M.S. Use of banana trunk waste as activated carbon in scavenging methylene blue dye: Kinetic, thermodynamic, and isotherm studies. Bioresour. Technol. Rep. 2018, 3, 127–137. [Google Scholar] [CrossRef]
- Jung, K.W.; Choi, B.H.; Hwang, M.J.; Jeong, T.U.; Ahn, K.H. Fabrication of granular activated carbons derived from spent coffee grounds by entrapment in calcium alginate beads for adsorption of acid orange 7 and methylene blue. Bioresour. Technol. 2016, 219, 185–195. [Google Scholar] [CrossRef]
- Ahmed, S.; Ahmed, A.; Rafat, M. Impact of aqueous and organic electrolytes on the supercapacitive performance of activated carbon derived from pea skin. Surf. Coat. Technol. 2018, 349, 242–250. [Google Scholar] [CrossRef]
- Huang, Y.; Ma, E.; Zhao, G. Thermal and structure analysis on reaction mechanisms during the preparation of activated carbon fibers by KOH activation from liquefied wood-based fibers. Ind. Crops Prod. 2015, 69, 447–455. [Google Scholar] [CrossRef]
- Heschel, W.; Klose, E. On the Suitability of Agricultural Byproducts for the Manufacture of Granular Activated Carbon. Fuel 1995, 74, 1786–1791. [Google Scholar] [CrossRef]
- Hsu, L.-Y.; Teng, H. Influence of different chemical reagents on the preparation of activated carbons from bituminous coal. Fuel Processing Technol. 2000, 64, 55–166. [Google Scholar] [CrossRef]
- Ahmadpour, A.; Do, D.D. The preparation of active carbons from coal by chemical and physical activation. Carbon 1996, 34, 471–479. [Google Scholar] [CrossRef]
- Tseng, R.-L.; Tseng, S.-K.; Wu, F.-C.; Hu, C.-C.; Wang, C.-C. Effects of micropore development on the physicochemical properties of KOH-activated carbons. J. Chin. Inst. Chem. Eng. 2008, 39, 37–47. [Google Scholar] [CrossRef]
- Muniandy, L.; Adam, F.; Mohamed, A.R.; Ng, E.-P. The synthesis and characterization of high purity mixed microporous/mesoporous activated carbon from rice husk using chemical activation with NaOH and KOH. Micropor. Mesopor. Mater. 2014, 197, 316–323. [Google Scholar] [CrossRef]
- Mopoung, S.; Moonsri, P.; Palas, W.; Khumpai, S. Characterization and Properties of Activated Carbon Prepared from Tamarind Seeds by KOH Activation for Fe(III) Adsorption from Aqueous Solution. Sci. World J. 2015, 9, 415961. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Yue, Q.; Gao, B.; Sun, Y.; Wang, W.; Li, Q. Preparation of high surface area-activated carbon from lignin of papermaking black liquor by KOH activation for Ni(II) adsorption. Chem. Eng. J. 2013, 217, 345–353. [Google Scholar] [CrossRef]
- Guo, H.; Chen, Y.; Yang, S.; Li, R.; Zhang, X.; Dong, Q.; Li, X.; Ma, X. Lignin-based adsorbent-catalyst with high capacity and stability for polychlorinated aromatics removal. Bioresour. Technol. 2021, 337, 125453. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, J.; Chen, Y.; Hao, X.; Jin, X. Characterization, preparation, and reaction mechanism of hemp stem based activated carbon. Results Phys. 2017, 7, 1628–1633. [Google Scholar] [CrossRef]
- Ragab, S.; El Nemr, A. Nanofiber Cellulose Di-and Tri-acetate Using Ferric Chloride as a Catalyst Promoting Highly Efficient Synthesis under Microwave irradiation. J. Macromol. Sci. Part A Pure Appl. Chem. 2018, 55, 124–134. [Google Scholar] [CrossRef]
- Lozano-Castelló, D.; Calo, J.M.; Cazorla-Amorós, D.; Linares-Solano, A. Carbon activation with KOH as explored by temperature programmed techniques, and the effects of hydrogen. Carbon 2007, 45, 2529–2536. [Google Scholar] [CrossRef]
- Lv, Y.; Gan, L.; Liu, M.; Xiong, W.; Xu, Z.; Zhu, D.; Wright, D.S. A self-template synthesis of hierarchical porous carbon foams based on banana peel for supercapacitor electrodes. J. Power Sour. 2012, 209, 152–157. [Google Scholar] [CrossRef]
- El Nemr, A.; Ragab, S.; El Sikaily, A. Rapid synthesis of cellulose triacetate from cotton cellulose and its effect on specific surface area and particle size distribution. Iran. Polym. J. 2017, 26, 261–272. [Google Scholar] [CrossRef]
- El Nemr, A.; El-Sikaily, A.; Khaled, A. Modeling of adsorption isotherms of Methylene Blue onto rice husk activated carbon. Egypt. J. Aquat. Res. 2010, 36, 403–425. [Google Scholar]
- Anoopkrishnan, K.; Sreejalekshmi, K.G.; Baiju, R.S. Nickel(II) adsorption onto biomass based activated carbon obtained from sugarcane bagasse pith. Bioresour. Technol. 2011, 102, 10239–10247. [Google Scholar] [CrossRef]
- Renugadevi, N.; Anitha, G.; Lalitha, P. Hexavalent chromium removal using a low-cost activated carbon adsorbent from Areca catechu. Ind. J. Environ. Prot. 2011, 31, 52–58. [Google Scholar]
- Janos, P.; Buchtova, H.; Ryznarova, M. Sorption of Dyes from Aqueous Solutions onto Fly Ash. Water Res. 2003, 37, 4938–4944. [Google Scholar] [CrossRef]
- Janos, P.; Coskun, S.; Pilarova, V.; Rejnek, J. Removal of Basic (Methylene Blue) and Acid (Egacid Orange) Dyes from Waters by Sorption on Chemically Treated Wood Shavings. Bioresour. Technol. 2009, 100, 1450–1453. [Google Scholar] [CrossRef]
- Janos, P.; Sedivy, P.; Ryznarova, M.; Grotschelova, S. Sorption of Basic and Acid Dyes from Aqueous Solutions onto Oxihumolite. Chemosphere 2005, 59, 881–886. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Du, Y.; Deng, B.; Zhu, K.; Zhang, H. Activated carbon adsorptive removal of azo dye and peroxydisulfate regeneration: From a batch study to continuous column operation. Environ. Sci. Pollut. Res. 2017, 24, 4932–4941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerçel, Ö.; Gerçel, H.F. Removal of Acid Dyes from Aqueous Solutions using Chemically Activated Carbon. Sep. Sci. Technol. 2009, 44, 2078–2095. [Google Scholar] [CrossRef]
- Supriya, S.; Palanisamy, P.N. Adsorptive removal of acid orange 7 from industrial effluents using activated carbon and conducting polymer composite–A comparative study. Ind. J. Chem. Technol. 2016, 23, 506–512. [Google Scholar]
- Sawood, G.M.; Mishra, A.; Gupta, S.K. Optimization of Arsenate Adsorption over Aluminum-Impregnated Tea Waste Biochar Using RSM–Central Composite Design and Adsorption Mechanism. J. Hazard. Toxic Radioact. Waste 2021, 25, 04020075. [Google Scholar] [CrossRef]
- Schwaab, M.; Steffani, E.; Barbosa-Coutinho, E.; Júnior, J.B.S. Critical analysis of adsorption/diffusion modelling as a function of time square root. Chem. Eng. Sci. 2017, 173, 179–186. [Google Scholar] [CrossRef]
- Lutzu, G.A.; Ciurli, A.; Chiellini, C.; Di Caprio, F.; Concas, A.; Dunford, N.T. Latest developments in wastewater treatment and biopolymer production by microalgae. J. Environ. Chem. Eng. 2021, 9, 104926. [Google Scholar] [CrossRef]
- Dil, E.A.; Ghaedi, M.; Asfaram, A. Application of hydrophobic deep eutectic solvent as the carrier for ferrofluid: A novel strategy for pre-concentration and determination of mefenamic acid in human urine samples by high performance liquid chromatography under experimental design optimization. Talanta 2019, 202, 526–530. [Google Scholar] [CrossRef]
- Bagheri, R.; Ghaedi, M.; Asfaram, A.; Dil, E.A.; Javadian, H. RSM-CCD design of malachite green adsorption onto activated carbon with multimodal pore size distribution prepared from Amygdalus scoparia: Kinetic and isotherm studies. Polyhedron 2019, 171, 464–472. [Google Scholar] [CrossRef]
- Isam, M.; Baloo, L.; Kutty, S.R.M.; Yavari, S. Optimisation and modelling of Pb (II) and Cu (II) biosorption onto red algae (gracilaria changii) by using response surface methodology. Water 2019, 11, 2325. [Google Scholar] [CrossRef] [Green Version]
- Mondal, N.K.; Samanta, A.; Roy, P.; Das, B. Optimization study of adsorption parameters for removal of Cr(VI) using Magnolia leaf biomass by response surface methodology. Sustain. Water Resour. Manag. 2019, 5, 1627–1639. [Google Scholar] [CrossRef]
- Roy, P.; Dey, U.; Chattoraj, S.; Mukhopadhyay, D.; Mondal, N.K. Modeling of the adsorptive removal of arsenic (III) using plant biomass: A bioremedial approach. Appl. Water Sci. 2017, 7, 1307–1321. [Google Scholar] [CrossRef] [Green Version]
- Lingamdinne, L.P.; Koduru, J.R.; Chang, Y.Y.; Karri, R.R. Process optimization and adsorption modeling of Pb(II) on nickel ferrite-reduced graphene oxide nano-composite. J. Mol. Liq. 2018, 250, 202–211. [Google Scholar] [CrossRef]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef] [Green Version]
- Freundlich, H.M.F. Über die adsorption in lösungen. Z. Phys. Chem. 1906, 57A, 385–470. [Google Scholar] [CrossRef]
- Tempkin, M.J.; Pyzhev, V. Kinetics of Ammonia Synthesis on Promoted Iron Catalysts. Acta Physiochim. 1940, 12, 217–222. [Google Scholar]
- Harkins, W.; Jura, G. Surfaces of solids XIII A vapor adsorption method for the determination of the area of a solid without the assumption of a molecular area, and the areas occupied by nitrogen and other molecules on the surface of a solid. J. Am. Chem. Soc. 1944, 66, 1366–1371. [Google Scholar] [CrossRef]
- Halsey, G.D. The role of surface heterogeneity. Adv. Catal. 1952, 4, 259–269. [Google Scholar]
- Tao, X.; Hu, X.; Wen, Z.; Ming, Y.; Li, J.; Liu, Y.; Chen, R. Highly efficient Cr(VI) removal from industrial electroplating wastewater over Bi2S3 nanostructures prepared by dual sulfur-precursors: Insights on the promotion effect of sulfate ions. J. Hazard. Mater. 2022, 424, 127423. [Google Scholar] [CrossRef]
- Lagergren, S. Zur theorie der sogenannten adsorption geloster stoffe. Kungliga Svenska Vetenskapsakademiens. Handlingar 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S.; McKay, G.; Wase, D.A.J.; Foster, C.F. Study of the sorption of divalent metal ions on to peat. Adsorpt. Sci. Technol. 2000, 18, 639–650. [Google Scholar] [CrossRef]
- Zeldowitsch, J. Über den mechanismus der katalytischen oxidation von CO and MnO2. Acta Physicochim. 1934, 1, 364–449. [Google Scholar]
- Weber, W.J.; Morris, J.C. Kinetics of adsorption on carbon from solution. J. Sanit. Eng. Div. Am. Soc. Civ. Eng. 1963, 89, 31–60. [Google Scholar] [CrossRef]
- Boyd, G.E.; Adamson, A.M.; Myers, L.S. The exchange adsorption of ions from aqueous solutions by organic zeolites. J. Am. Chem. Soc. 1949, 69, 2836. [Google Scholar] [CrossRef] [PubMed]
- Amirov, N.; Vakhshouri, A.R. Numerical modeling and optimization of product selectivity and catalyst activity in Fischer-Tropsch synthesis via response surface methodology: Cobalt carbide particle size and H2/CO ratio effects. Int. J. Hydrogen Energy 2020, 45, 31913–31925. [Google Scholar] [CrossRef]




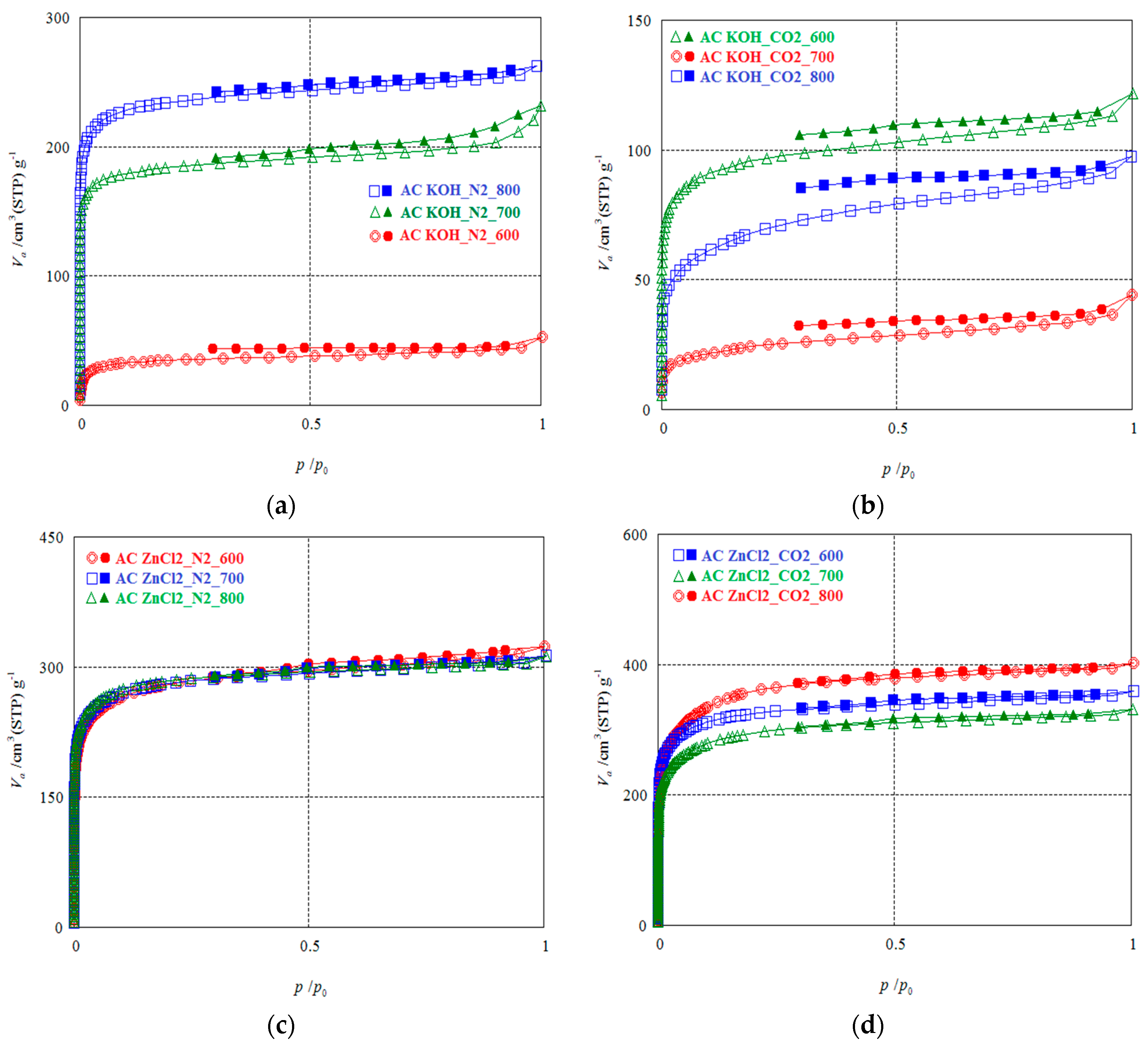







| Analysis Method | BET | t-Plot | MP | BJH ads | BJH des | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample Entry | Carb. Temp. | AC Yield | as, BET (m2/g) | Vm (cm3/g) | Mean Pore Diameter | Total Pore Volume | a1-a2 (m2/g) | V2 | 2t (nm) | a1-a2 (m2/g) | Vp (cm3/g) | Vp | ap | Vp | ap |
| (%) | SBET (m2/g) | Vm (cm3/g) | Dp (nm) | VT (cm3/g) | Smi (m2/g) | Vmi (cm3/g) | 2t (nm) | (m2/g) | (cm3/g) | (cm3/g) | (m2/g) | Vme (cm3/g) | Sme (m2/g) | ||
| KOH/CO2 | 600 | 37.61 | 357.82 | 82.21 | 2.068 | 0.185 | 420.11 | 0.156 | 0.740 | 392.47 | 0.163 | 0.055 | 45.16 | 0.028 | 45.16 |
| 700 | 23.44 | 87.70 | 20.15 | 2.974 | 0.065 | 82.33 | 0.039 | 0.933 | 81.30 | 0.040 | 0.038 | 27.32 | 0.019 | 27.32 | |
| 800 | 18.89 | 241.84 | 55.57 | 2.462 | 0.149 | 254.40 | 0.119 | 0.929 | 244.50 | 0.119 | 0.070 | 67.34 | 0.020 | 67.34 | |
| KOH/N2 | 600 | 36.11 | 197.33 | 45.34 | 2.332 | 0.115 | 205.43 | 0.086 | 0.827 | 212.23 | 0.091 | 0.046 | 35.25 | 0.019 | 5.31 |
| 700 | 24.17 | 716.28 | 164.57 | 1.938 | 0.347 | 879.53 | 0.248 | 0.560 | 804.12 | 0.302 | 0.081 | 48.13 | 0.072 | 31.75 | |
| 800 | 20.94 | 914.58 | 210.13 | 1.772 | 0.405 | 1146.78 | 0.373 | 0.647 | 1029.38 | 0.392 | 0.065 | 56.66 | 0.038 | 21.78 | |
| ZnCl2/CO2 | 600 | 43.61 | 1228.00 | 282.13 | 1.799 | 0.552 | 1480.09 | 0.532 | 0.714 | 1373.09 | 0.544 | 0.089 | 94.87 | 0.053 | 40.38 |
| 700 | 41.35 | 1092.70 | 251.06 | 1.859 | 0.508 | 1281.39 | 0.487 | 0.756 | 1208.81 | 0.498 | 0.097 | 110.18 | 0.052 | 39.60 | |
| 800 | 31.58 | 1299.40 | 298.55 | 1.901 | 0.618 | 1479.36 | 0.595 | 0.799 | 1426.09 | 0.611 | 0.127 | 152.44 | 0.058 | 44.15 | |
| ZnCl2/N2 | 600 | 39.85 | 1039.50 | 238.83 | 1.915 | 0.498 | 1214.95 | 0.455 | 0.745 | 1143.14 | 0.466 | 0.110 | 110.16 | 0.069 | 50.17 |
| 700 | 39.84 | 1064.00 | 244.47 | 1.805 | 0.480 | 1286.20 | 0.457 | 0.706 | 1188.57 | 0.469 | 0.078 | 79.96 | 0.050 | 36.06 | |
| 800 | 39.85 | 1084.20 | 249.11 | 1.767 | 0.479 | 1316.97 | 0.460 | 0.694 | 1217.64 | 0.476 | 0.068 | 69.96 | 0.043 | 31.16 | |
| Isotherm Model | Isotherm Parameter | Pea Pod Activated Carbon (g/L) | ||||
|---|---|---|---|---|---|---|
| 0.75 | 1.00 | 1.50 | 2.00 | 2.50 | ||
| Langmuir | Qm (mg/g) | 473.93 | 250.00 | 358.42 | 153.61 | 124.22 |
| KL × 103 | 0.70 | 1.74 | 1.12 | 12.06 | 23.68 | |
| R2 | 0.996 | 0.996 | 0.995 | 1.000 | 1.000 | |
| Freundlich | 1/n | 0.200 | 0.172 | 0.187 | 0.272 | 0.219 |
| KF (mg1−1/nL1/ng−1) | 214.24 | 185.99 | 135.74 | 142.33 | 135.74 | |
| R2 | 0.995 | 1.000 | 0.998 | 0.992 | 0.998 | |
| Harkins–Jura isotherm | AHJ | 50,000 | 33,333 | 16,667 | 10,000 | 5000 |
| BHJ | 1.50 | 1.33 | 1.33 | 0.80 | 0.20 | |
| R2 | 0.857 | 0.843 | 0.795 | 0.788 | 0.523 | |
| Halsey isotherm | 1/nH | 5.09 | 5.95 | 5.41 | 5.23 | 4.02 |
| KH | 2.87 | 2.42 | 2.49 | 2.52 | 3.34 | |
| R2 | 0.995 | 0.989 | 0.981 | 0.977 | 0.897 | |
| Tempkin | AT | 142.28 | 634.62 | 390.58 | 409.81 | 460.75 |
| BT | 50.60 | 33.45 | 25.90 | 21.67 | 22.81 | |
| bT | 48.96 | 74.07 | 95.67 | 114.36 | 108.64 | |
| R2 | 0.992 | 0.987 | 0.989 | 0.997 | 0.998 | |
| Generalized isotherm | Nb | 0.45 | 0.32 | 0.27 | 0.33 | 0.31 |
| KG | 0.91 | 1.77 | 6.38 | 7.78 | 9.58 | |
| R2 | 0.949 | 0.982 | 0.993 | 0.968 | 0.913 | |
| Adsorbent | Adsorption Capacity (mg/g) | Reference |
|---|---|---|
| Pisum sativum pea pod AC-ZnCl2 | 473.93 | This work |
| Brown coal fly ash | 82.8 | [72] |
| Chemically treated wood shavings | 179.7 | [73] |
| Oxihumolite | 50.0 | [74] |
| Commercial granular activated carbon | 40.0 | [75] |
| Euphorbia macroclada | 455.0 | [76] |
| Casuarina wood activated carbon | 7.80 | [77] |
| Biochar (pea Pisum sativum peel) | 523.12 | [14] |
| Independent Factors | Response % | ||||
|---|---|---|---|---|---|
| Run | Time (A) | Dosage (B) | Concentration (C) | Experiment | Predicted |
| 1 | 120 | 0.75 | 400 | 82.88 | 83.66 |
| 2 | 15 | 2.0 | 100 | 89.63 | 86.91 |
| 3 | 15 | 2.5 | 400 | 79.23 | 79.42 |
| 4 | 15 | 0.75 | 300 | 62.97 | 60.27 |
| 5 | 90 | 1.0 | 300 | 90.83 | 89.71 |
| 6 | 120 | 2.5 | 400 | 98.76 | 97.58 |
| 7 | 60 | 1.5 | 400 | 85.38 | 85.56 |
| 8 | 120 | 2.5 | 400 | 98.76 | 97.58 |
| 9 | 60 | 1.5 | 150 | 97.04 | 96.13 |
| 10 | 120 | 2.5 | 100 | 99.89 | 100.00 |
| 11 | 120 | 0.75 | 400 | 82.88 | 83.66 |
| 12 | 15 | 0.75 | 100 | 69.61 | 73.43 |
| 13 | 60 | 2.5 | 200 | 98.24 | 98.58 |
| 14 | 90 | 2.0 | 300 | 96.55 | 98.64 |
| 15 | 90 | 0.75 | 100 | 98.13 | 96.32 |
| 16 | 30 | 2.0 | 300 | 83.33 | 85.17 |
| 17 | 60 | 1.5 | 400 | 85.38 | 85.56 |
| 18 | 15 | 2.5 | 400 | 79.23 | 79.42 |
| 19 | 120 | 1.5 | 200 | 98.88 | 99.00 |
| 20 | 120 | 2.5 | 100 | 99.89 | 100.00 |
| Source | Value | Sum of Squares | df | Mean Square | F-Value | p-Value | Remarks | Source | SD | R² | Adjusted R² | Predicted R² | PRESS | Remarks |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model | - | 2150.07 | 9 | 238.90 | 50.54 | <0.0001 | significant | - | - | - | - | - | - | - |
| A-Time | - | 1079.40 | 1 | 1079.40 | 228.34 | <0.0001 | - | - | - | - | - | - | - | - |
| B-Dose | - | 513.89 | 1 | 513.89 | 108.71 | <0.0001 | - | - | - | - | - | - | - | - |
| C-Conc | - | 351.46 | 1 | 351.46 | 74.35 | <0.0001 | - | - | - | - | - | - | - | - |
| AB | - | 71.77 | 1 | 71.77 | 15.18 | 0.0030 | - | - | - | - | - | - | - | - |
| AC | - | 19.25 | 1 | 19.25 | 4.07 | 0.0712 | - | - | - | - | - | - | - | - |
| BC | - | 57.94 | 1 | 57.94 | 12.26 | 0.0057 | - | - | - | - | - | - | - | - |
| A² | - | 121.18 | 1 | 121.18 | 25.64 | 0.0005 | - | - | - | - | - | - | - | - |
| B² | - | 26.06 | 1 | 26.06 | 5.51 | 0.0408 | - | - | - | - | - | - | - | - |
| C² | - | 0.0370 | 1 | 0.0370 | 0.0078 | 0.9312 | - | - | - | - | - | - | - | - |
| Residual | - | 47.27 | 10 | 4.73 | - | - | - | - | - | - | - | - | - | |
| Lack of Fit | - | 47.27 | 5 | 9.45 | - | - | - | - | - | - | - | - | - | |
| Pure Error | - | 0.0000 | 5 | 0.0000 | - | - | - | - | - | - | - | - | - | |
| Cor Total | - | 2197.34 | 19 | - | - | - | - | - | - | - | - | - | - | - |
| SD | 2.17 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Mean | 8.88 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| C.V.% | 2.45 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| R² | 0.9785 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Adjusted R² | 0.9591 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Predicted R² | 0.7850 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Adeq Precision | 26.1392 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Linear | 5.62 | 0.7699 | 0.7268 | 0.6104 | 856.03 | |||||||||
| 2FI | 5.24 | 0.8377 | 0.7628 | 0.5614 | 963.71 | |||||||||
| Quadratic | 2.17 | 0.9785 | 0.9591 | 0.7850 | 472.47 | Suggested | ||||||||
| Cubic | 0.0000 | 1.0000 | 1.0000 | - | - | Aliased |
| Independent Variables | Notation | Unit | Range and Levels of Actual and Coded Values | ||||
|---|---|---|---|---|---|---|---|
| −α | −1 | 0 | 1 | α | |||
| Time | A X1 | min | 15 | 30 | 60 | 90 | 120 |
| Dosage | B X2 | g/L | 0.75 | 1 | 1.5 | 2 | 2.5 |
| Concentration | C X3 | mg/L | 100 | 150 | 200 | 300 | 400 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Nemr, M.A.; El Nemr, A.; Hassaan, M.A.; Ragab, S.; Tedone, L.; De Mastro, G.; Pantaleo, A. Microporous Activated Carbon from Pisum sativum Pods Using Various Activation Methods and Tested for Adsorption of Acid Orange 7 Dye from Water. Molecules 2022, 27, 4840. https://doi.org/10.3390/molecules27154840
El-Nemr MA, El Nemr A, Hassaan MA, Ragab S, Tedone L, De Mastro G, Pantaleo A. Microporous Activated Carbon from Pisum sativum Pods Using Various Activation Methods and Tested for Adsorption of Acid Orange 7 Dye from Water. Molecules. 2022; 27(15):4840. https://doi.org/10.3390/molecules27154840
Chicago/Turabian StyleEl-Nemr, Mohamed A., Ahmed El Nemr, Mohamed A. Hassaan, Safaa Ragab, Luigi Tedone, Giuseppe De Mastro, and Antonio Pantaleo. 2022. "Microporous Activated Carbon from Pisum sativum Pods Using Various Activation Methods and Tested for Adsorption of Acid Orange 7 Dye from Water" Molecules 27, no. 15: 4840. https://doi.org/10.3390/molecules27154840






