Molineria recurvata Ameliorates Streptozotocin-Induced Diabetic Nephropathy through Antioxidant and Anti-Inflammatory Pathways
Abstract
:1. Introduction
2. Results
2.1. Phytochemical Properties of the MR Extract
2.2. Acute Toxicity Study of the MR Extract
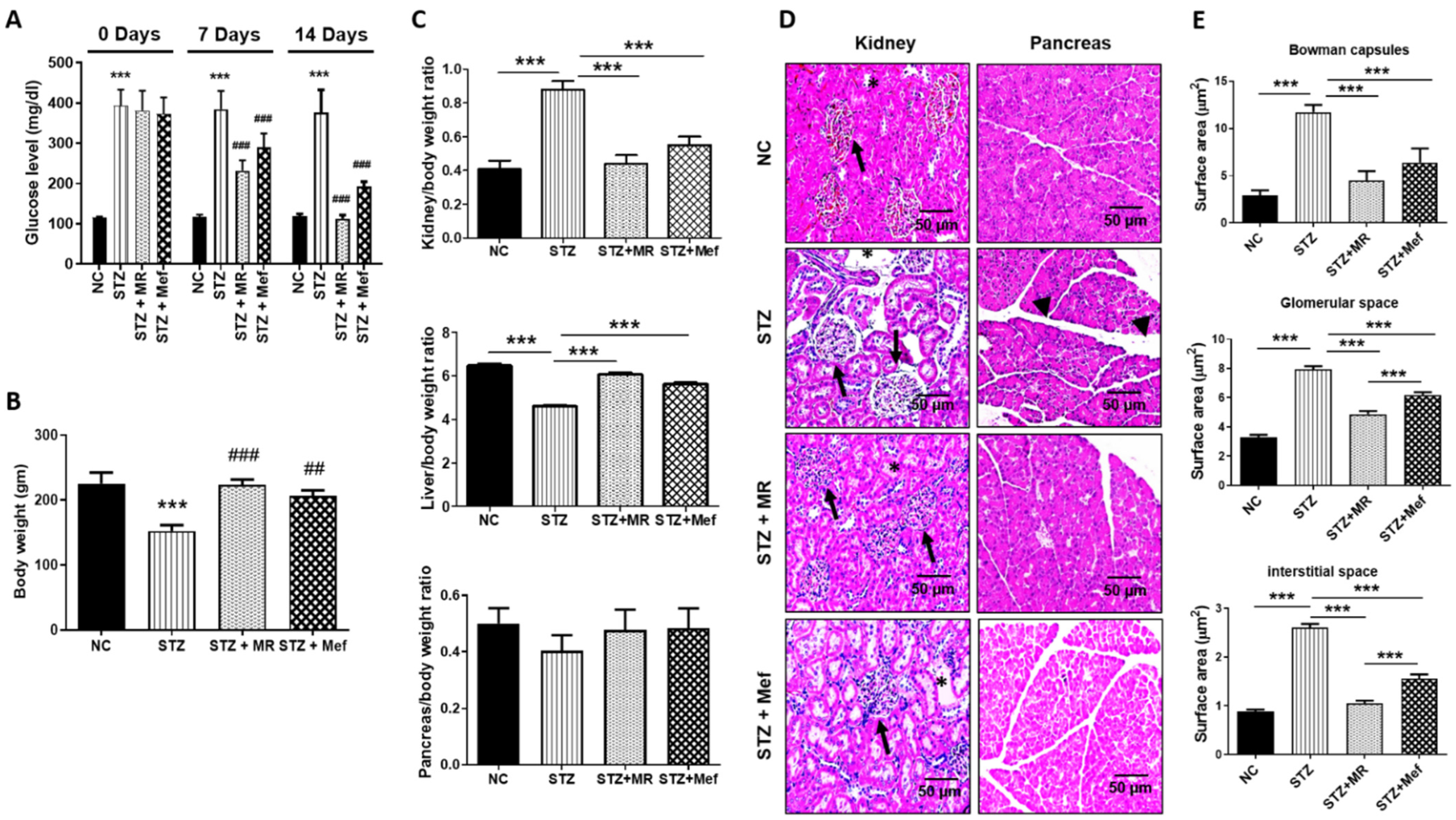
2.3. Protective Impact of the MR Extract on Blood Glucose Concentrations in STZ-Administered Rats
2.4. Protective Effect of the MR Extract on Organ and Body Weight Alterations in STZ-Administered Rats
2.5. Protective Effect of the MR Extract on Histopathological Injury in STZ-Administered Rats
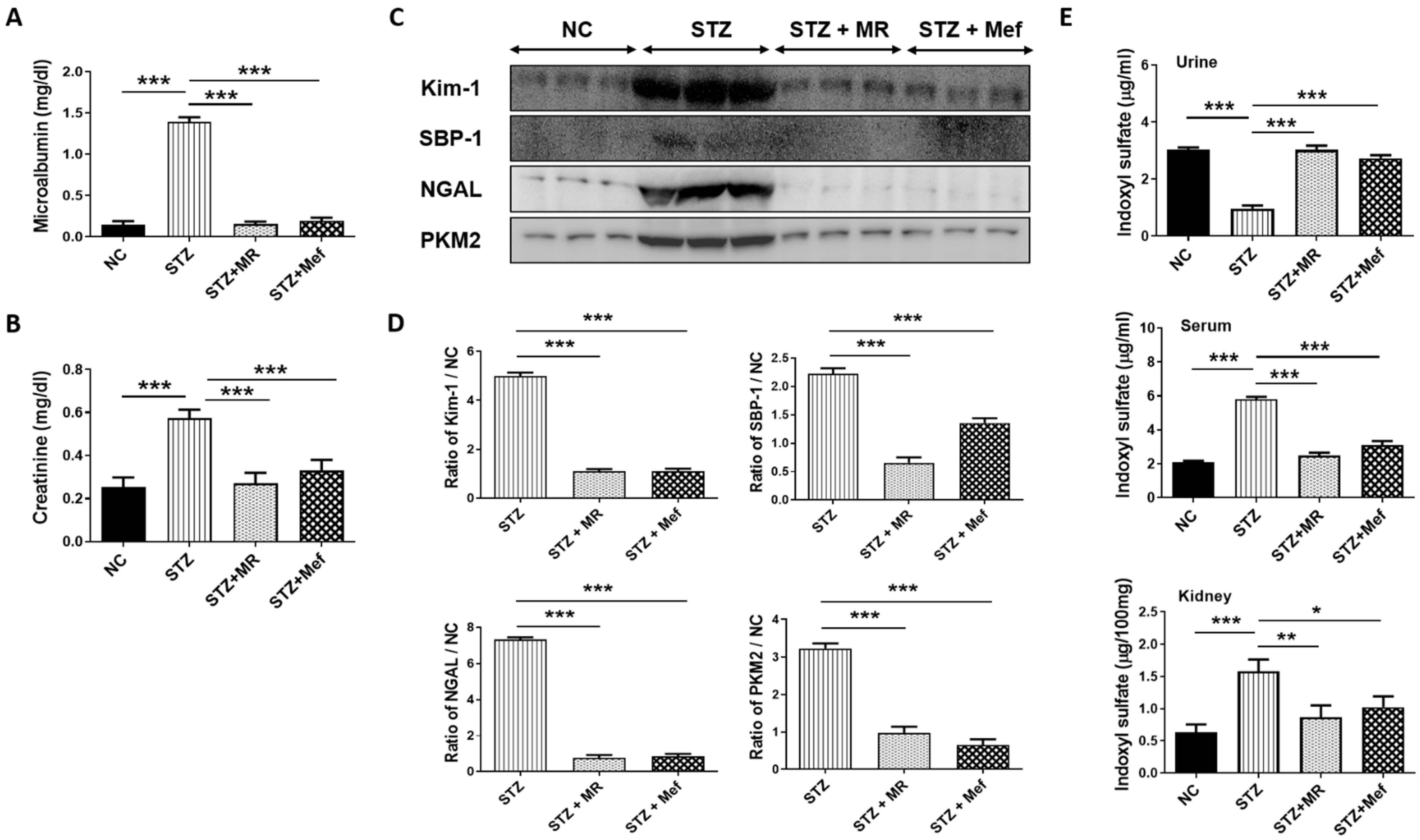
2.6. Protective Effect of the MR Extract on Renal Injury Biomarkers in STZ-Administered Rats
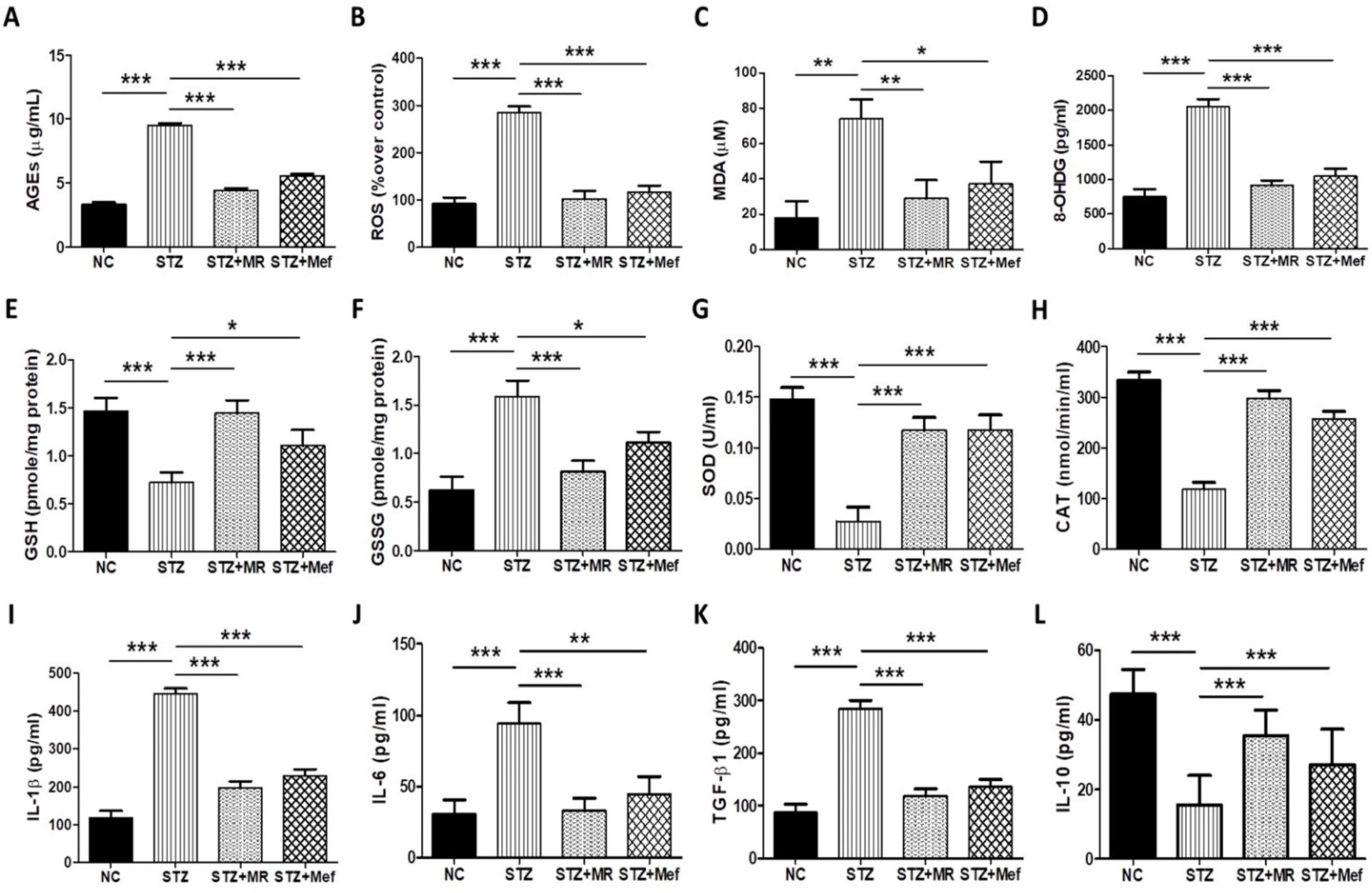
2.7. MR Extract Reduced AGE Levels in STZ-Treated Rats
2.8. Effect of the MR Extract on Oxidative Biomarkers in STZ-Administered Rats
2.9. Protective Effect of the MR Extract on Inflammatory Cytokines in Diabetic Rats
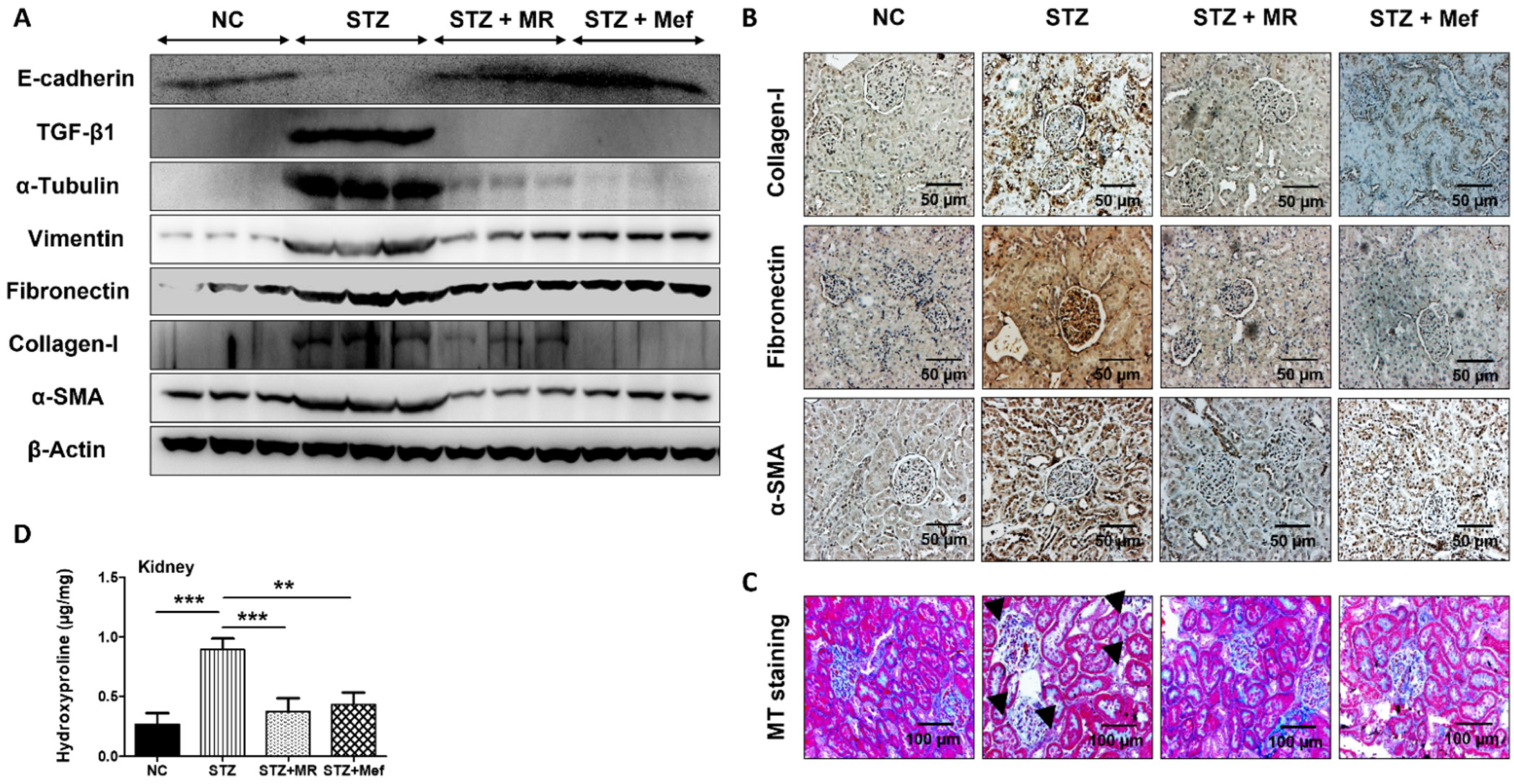
2.10. Protective Effect of the MR Extract on Renal Fibrosis in Diabetic Rats
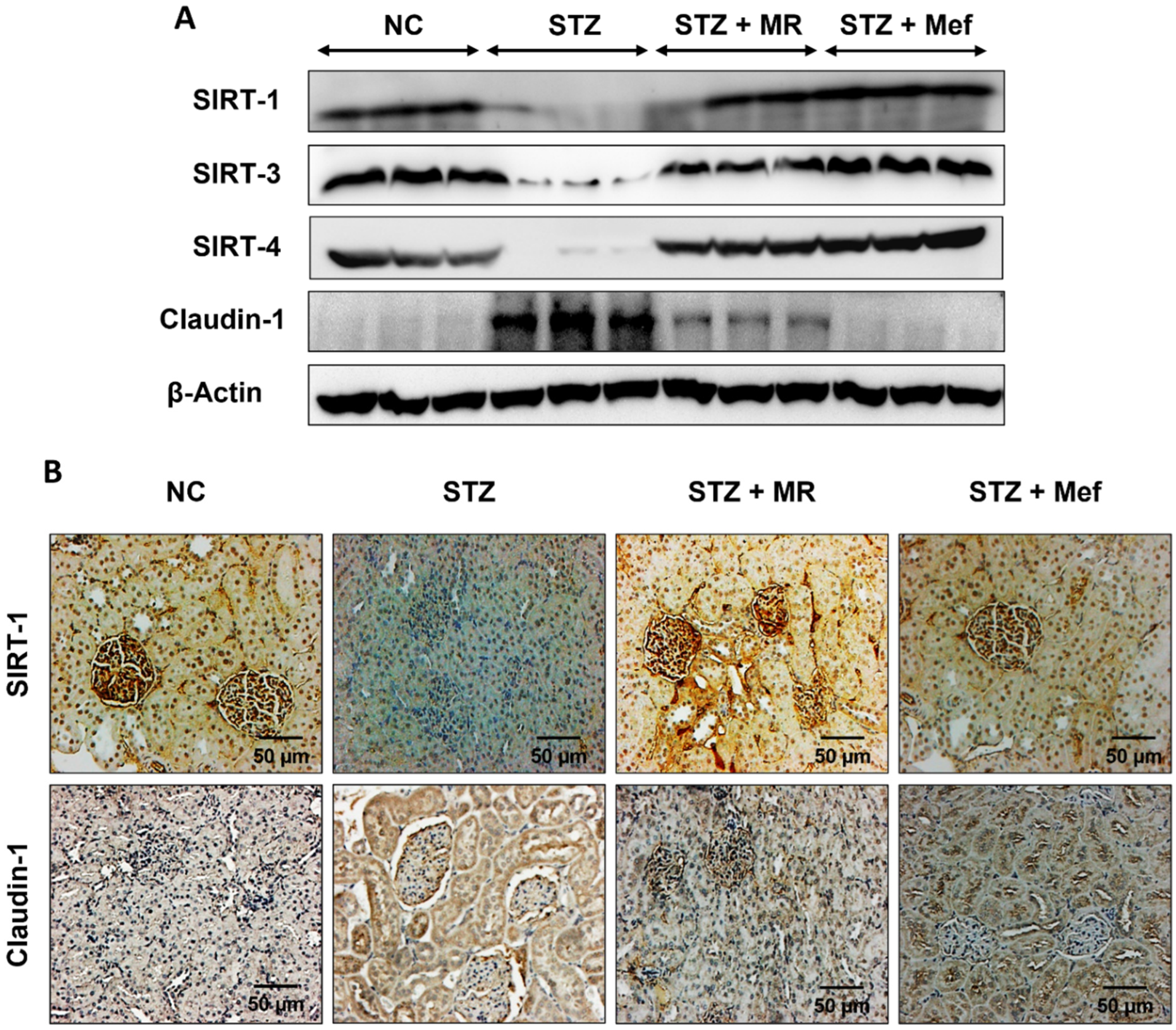
2.11. Effects of the MR Extract on SIRTs and Claudin-1 Expression in the Renal Cortex of STZ-Treated Rats
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Preparation of the MR Extract
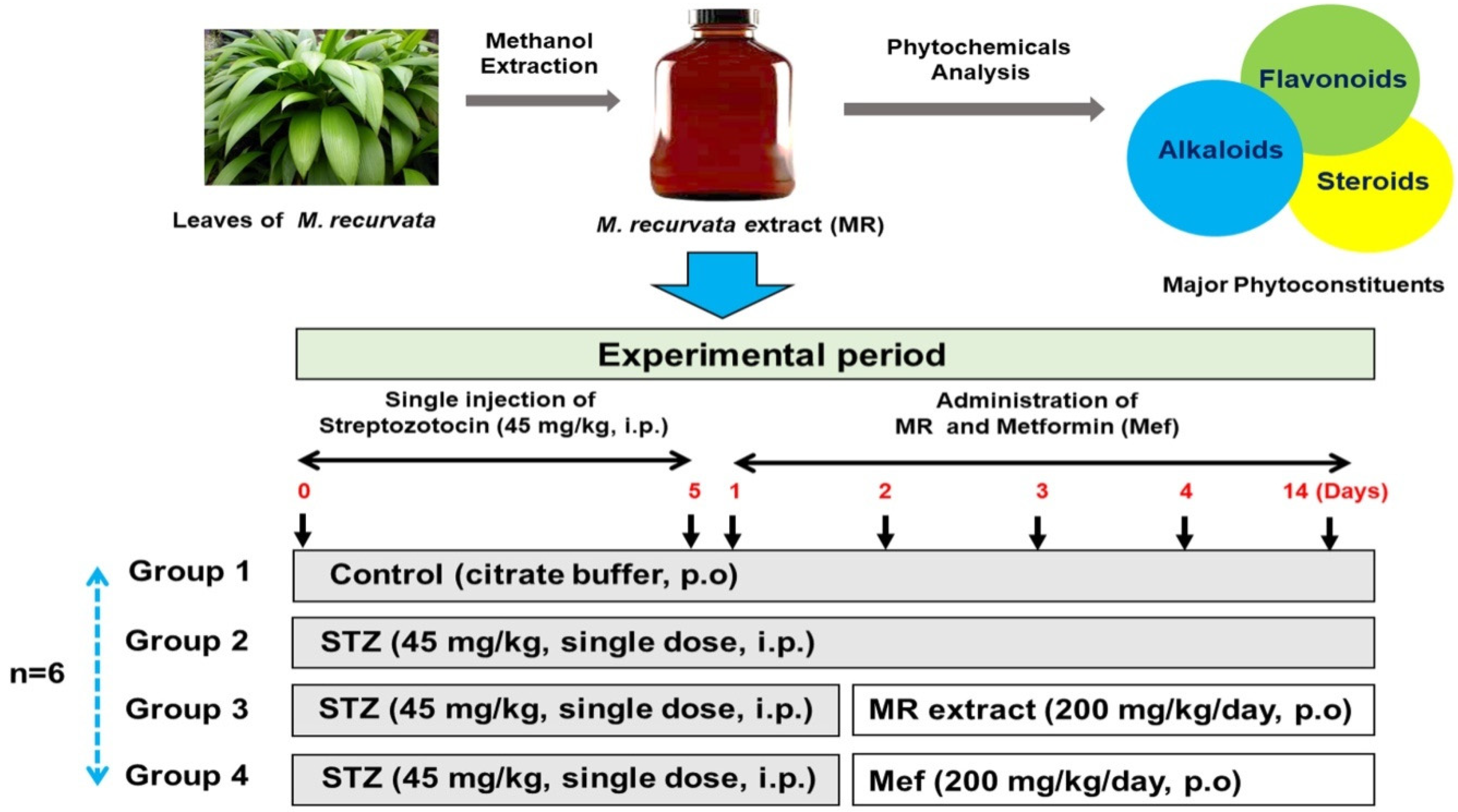
4.3. Design of Animals Experiment and Acute Toxicity Study
4.4. Experimental Design
4.5. Histological Analysis
4.6. Urine Analysis and Quantification of Biochemical Parameters
4.7. Detection of 3-Indoxyl Sulfate
4.8. Examination of Advanced Glycation End Products
4.9. Evaluation of Oxidative Stress
4.10. Assessment of Inflammatory Cytokines
4.11. Investigation of Hydroxyproline
4.12. Western Blot Analyses
4.13. Immunohistochemical Evaluation
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
| AGEs | Advanced glycation end products |
| CAT | Catalase |
| DAB | Diaminobenzidine tetrahydrochloride |
| DN | Diabetic nephropathy |
| ECM | Extracellular matrix |
| EMT | Epithelial-mesenchymal transition |
| ESRD | End-stage renal disease |
| GSH | Glutathione |
| GPx | Glutathione peroxidase |
| H&E | Hematoxylin and eosin |
| HPLC | High-performance liquid chromatography |
| HRP | Horseradish peroxidase |
| 3-IS | 3-indoxyl sulfate |
| KIM-1 | Kidney injury molecule-1 |
| IL-1β | Interleukin-1β |
| MDA | Malondialdehyde |
| MR | Molineria recurvata |
| ROS | Reactive oxygen species |
| 8-OHdG | 8-Hydroxy-deoxyguanosine |
| PBS | Phospahte-buffered saline |
| PKM2 | Pyruvate kinase M2 |
| PVDF | Polyvinylidene difluoride |
| SBP-1 | Selenium binding protein 1 |
| SDS | Sodium dodecyl sulfate |
| SOD | Superoxide dismutase |
| STZ | Streptozotocin |
| α-SMA | α-Smooth muscle actin |
| TBA | Thiobarbituric acid |
| TGF-β | Transforming growth factor-β |
| TNF-α | Tumor necrosis factor-α |
References
- Kitada, M.; Zhang, Z.; Mima, A.; King, G.L. Molecular mechanisms of diabetic vascular complications. J. Diabetes Investig. 2010, 1, 77–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keane, W.F.; Brenner, B.M.; De Zeeuw, D.; Grunfeld, J.-P.; McGill, J.; Mitch, W.E.; Ribeiro, A.B.; Shahinfar, S.; Simpson, R.L.; Snapinn, S.M. The risk of developing end-stage renal disease in patients with type 2 diabetes and nephropathy: The RENAAL study. Kidney Int. 2003, 63, 1499–1507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qian, X.; Li, X.; Ma, F.; Luo, S.; Ge, R.; Zhu, Y. Novel hydrogen sulfide-releasing compound, S-propargyl-cysteine, prevents STZ-induced diabetic nephropathy. Biochem. Biophys. Res. Commun. 2016, 473, 931–938. [Google Scholar] [CrossRef]
- Wang, D.; Guan, M.-P.; Zheng, Z.-J.; Li, W.-Q.; Lyv, F.-P.; Pang, R.-Y.; Xue, Y.-M. Transcription factor Egr1 is involved in high glucose-induced proliferation and fibrosis in rat glomerular mesangial cells. Cell. Physiol. Biochem. 2015, 36, 2093–2107. [Google Scholar] [CrossRef]
- Mestry, S.N.; Dhodi, J.B.; Kumbhar, S.B.; Juvekar, A.R. Attenuation of diabetic nephropathy in streptozotocin-induced diabetic rats by Punica granatum Linn. leaves extract. J. Tradit. Complement. Med. 2017, 7, 273–280. [Google Scholar] [CrossRef] [Green Version]
- Brownlee, M. The pathobiology of diabetic complications: A unifying mechanism. Diabetes 2005, 54, 1615–1625. [Google Scholar] [CrossRef] [Green Version]
- Steffes, M.W.; Østerby, R.; Chavers, B.; Mauer, S.M. Mesangial expansion as a central mechanism for loss of kidney function in diabetic patients. Diabetes 1989, 38, 1077–1081. [Google Scholar] [CrossRef]
- Kolset, S.; Reinholt, F.; Jenssen, T. Diabetic nephropathy and extracellular matrix. J. Histochem. Cytochem. 2012, 60, 976–986. [Google Scholar] [CrossRef] [Green Version]
- Mason, R.M.; Wahab, N.A. Extracellular matrix metabolism in diabetic nephropathy. J. Am. Soc. Nephrol. 2003, 14, 1358–1373. [Google Scholar] [CrossRef] [Green Version]
- Badid, C.; Desmouliere, A.; Babici, D.; Hadj-Aissa, A.; McGregor, B.; Lefrancois, N.; Touraine, J.L.; Laville, M. Interstitial expression of α-SMA: An early marker of chronic renal allograft dysfunction. Nephrol. Dial. Transplant. 2002, 17, 1993–1998. [Google Scholar] [CrossRef]
- Valcourt, U.; Kowanetz, M.; Niimi, H.; Heldin, C.-H.; Moustakas, A. TGF-β and the Smad signaling pathway support transcriptomic reprogramming during epithelial-mesenchymal cell transition. Mol. Biol. Cell 2005, 16, 1987–2002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brosius, F.C.; Khoury, C.C.; Buller, C.L.; Chen, S. Abnormalities in signaling pathways in diabetic nephropathy. Expert Rev. Endocrinol. Metab. 2010, 5, 51–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dey, P.; Bhakta, T. Evaluation of in vitro anticoagulant activity of Molineria recurpata leaf extract. J. Nat. Prod. Plant Resour. 2012, 2, 685–688. [Google Scholar]
- Dey, P.; Debnath, P.; Bhakta, T. Evaluation of anthelmintic activity of Molineria Recurvata leaf extracts. Int. Res. J. Pharm. Appl. Sci. 2012, 2, 16–20. [Google Scholar]
- Dey, P.; Mukherjee, M.; Bhakta, T.; Ghosh, T.K. Preliminary phytochemical studies of leaf extracts of Molineria recurvata. J. Chem. Pharm. Res. 2012, 4, 3727–3730. [Google Scholar]
- Hostetter, T.H. Hyperfiltration and glomerulosclerosis. In Seminars in Nephrology; Elsevier: Amsterdam, The Netherlands, 2003; pp. 194–199. [Google Scholar]
- Won, A.J.; Kim, S.; Kim, Y.G.; Kim, K.-B.; Choi, W.S.; Kacew, S.; Kim, K.S.; Jung, J.H.; Lee, B.M.; Kim, S. Discovery of urinary metabolomic biomarkers for early detection of acute kidney injury. Mol. BioSyst. 2016, 12, 133–144. [Google Scholar] [CrossRef]
- Forbes, J.M.; Thallas, V.; Thomas, M.C.; Founds, H.W.; Burns, W.C.; Jerums, G.; Cooper, M.E. The breakdown of pre-existing advanced glycation end products is associated with reduced renal fibrosis in experimental diabetes. FASEB J. 2003, 17, 1762–1764. [Google Scholar] [CrossRef]
- Price, N.L.; Gomes, A.P.; Ling, A.J.; Duarte, F.V.; Martin-Montalvo, A.; North, B.J.; Agarwal, B.; Ye, L.; Ramadori, G.; Teodoro, J.S. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab. 2012, 15, 675–690. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Nie, L.; Yin, Y.-G.; Tang, J.-L.; Zhou, J.-Y.; Li, D.-D.; Zhou, S.-W. Resveratrol protects against hyperglycemia-induced oxidative damage to mitochondria by activating SIRT1 in rat mesangial cells. Toxicol. Appl. Pharmacol. 2012, 259, 395–401. [Google Scholar] [CrossRef]
- Dey, P.; Bardalai, D.; Kumar, N.R.; Subramani, C.; Mukherjee, M.; Bhakta, T. Ethnomedicinal knowledge about various medicinal plants used by the tribes of Tripura. Res. J. Pharmacogn. Phytochem. 2012, 4, 297–302. [Google Scholar]
- Dey, P.; Kundu, A.; Chakraborty, H.J.; Kar, B.; Choi, W.S.; Lee, B.M.; Bhakta, T.; Atanasov, A.G.; Kim, H.S. Therapeutic value of steroidal alkaloids in cancer: Current trends and future perspectives. Int. J. Cancer 2019, 145, 1731–1744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dey, P.; Karuna, D.; Bhakta, T. Medicinal plants used as anti-acne agents by tribal and non-tribal people of Tripura, India. Am. J. Phytomed. Clin. Ther. 2014, 2, 556–570. [Google Scholar]
- Dey, P.; Kundu, A.; Kumar, A.; Gupta, M.; Lee, B.M.; Bhakta, T.; Dash, S.; Kim, H.S. Analysis of alkaloids (indole alkaloids, isoquinoline alkaloids, tropane alkaloids). In Recent Advances in Natural Products Analysis; Elsevier: Amsterdam, The Netherlands, 2020; pp. 505–567. [Google Scholar]
- Garg, A.; Sharma, R.; Dey, P.; Kundu, A.; Kim, H.S.; Bhakta, T.; Kumar, A. Analysis of triterpenes and triterpenoids. In Recent Advances in Natural Products Analysis; Elsevier: Amsterdam, The Netherlands, 2020; pp. 393–426. [Google Scholar]
- Dey, P.; Debnath, P.; Bhakta, T. Evaluation of anthelmintic activity of pineapple fruit extract using Indian earthworm (Pheritima posthuma). Mintage J. Pharm. Med. Sci. 2013, 2, 26–27. [Google Scholar]
- Dey, P.; Kundu, A.; Kar, B.; Bhakta, A.; Vishal, V.; Keerthana, S.; Kumar, A.; Bhakta, T.; Dash, S.; Kim, H.S. Bioactive Natural Leads Targeting Cancer Cell Metabolism. In Evidence Based Validation of Traditional Medicines: A Comprehensive Approach; Springer: Singapore, 2021; pp. 29–75. [Google Scholar]
- Karuna, D.; Dey, P.; Das, S.; Kundu, A.; Bhakta, T. In vitro antioxidant activities of root extract of Asparagus racemosus Linn. J. Tradit. Complement. Med. 2018, 8, 60–65. [Google Scholar] [CrossRef]
- Karuna, D.; Dey, P.; Kundu, A.; Vishal, V.; Bhakta, T. Evaluation of in vitro antioxidant potential of Aconitum napellus Linn. root extract. Int. J. Pharmacogn. Chin. Med. 2018, 2, 125–129. [Google Scholar]
- Debnath, P.; Dey, P.; Chanda, A.; Bhakta, T. A Survey on Pineapple and its medicinal value. Sch. Acad. J. Pharm. 2012, 1, 24–29. [Google Scholar]
- Islam, M.T.; Ali, E.S.; Uddin, S.J.; Shaw, S.; Islam, M.A.; Ahmed, M.I.; Shill, M.C.; Karmakar, U.K.; Yarla, N.S.; Khan, I.N. Phytol: A review of biomedical activities. Food Chem. Toxicol. 2018, 121, 82–94. [Google Scholar] [CrossRef]
- Lenzen, S. The mechanisms of alloxan-and streptozotocin-induced diabetes. Diabetologia 2008, 51, 216–226. [Google Scholar] [CrossRef] [Green Version]
- Zafar, M.; Naqvi, S.N.-u.-H. Effects of STZ-Induced Diabetes on the Relative Weights of Kidney, Liver and Pancreas in Albino Rats: A Comparative Study. Int. J. Morphol. 2010, 28, 135–142. [Google Scholar] [CrossRef] [Green Version]
- Kishore, L.; Kaur, N.; Singh, R. Nephroprotective effect of Paeonia emodi via inhibition of advanced glycation end products and oxidative stress in streptozotocin–nicotinamide induced diabetic nephropathy. J. Food Drug Anal. 2017, 25, 576–588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saravanan, S.; Pari, L. Protective effect of thymol on high fat diet induced diabetic nephropathy in C57BL/6J mice. Chem.-Biol. Interact. 2016, 245, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.; Lei, J.; Li, X.; Zhang, R. Trigonella foenum graecum seed extract protects kidney function and morphology in diabetic rats via its antioxidant activity. Nutr. Res. 2011, 31, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Cheon, J.H.; Kim, S.Y.; Son, J.Y.; Kang, Y.R.; An, J.H.; Kwon, J.H.; Song, H.S.; Moon, A.; Lee, B.M.; Kim, H.S. Pyruvate kinase M2: A novel biomarker for the early detection of acute kidney injury. Toxicol. Res. 2016, 32, 47–56. [Google Scholar] [CrossRef] [Green Version]
- Fiseha, T. Urinary biomarkers for early diabetic nephropathy in type 2 diabetic patients. Biomark. Res. 2015, 3, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.S.; Yang, H.Y.; Song, H.; Kang, Y.R.; Kwon, J.; An, J.; Son, J.Y.; Kwack, S.J.; Kim, Y.-M.; Bae, O.-N. Identification of a sensitive urinary biomarker, selenium-binding protein 1, for early detection of acute kidney injury. J. Toxicol. Environ. Health Part A 2017, 80, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.K.; Shin, Y.-J.; Park, E.Y.; Kim, N.D.; Moon, A.; Kwack, S.J.; Son, J.Y.; Kacew, S.; Lee, B.M.; Bae, O.-N. Selenium-binding protein 1: A sensitive urinary biomarker to detect heavy metal-induced nephrotoxicity. Arch. Toxicol. 2017, 91, 1635–1648. [Google Scholar] [CrossRef]
- Parikh, C.R.; Edelstein, C.L.; Devarajan, P.; Cantley, L. Biomarkers of acute kidney injury: Early diagnosis, pathogenesis, and recovery. J. Investig. Med. 2007, 55, 333–340. [Google Scholar] [CrossRef]
- Forbes, J.M.; Coughlan, M.T.; Cooper, M.E. Oxidative stress as a major culprit in kidney disease in diabetes. Diabetes 2008, 57, 1446–1454. [Google Scholar] [CrossRef] [Green Version]
- Ha, H.; Hwang, I.-A.; Park, J.H.; Lee, H.B. Role of reactive oxygen species in the pathogenesis of diabetic nephropathy. Diabetes Res. Clin. Pract. 2008, 82, S42–S45. [Google Scholar] [CrossRef]
- Bolati, D.; Shimizu, H.; Yisireyili, M.; Nishijima, F.; Niwa, T. Indoxyl sulfate, a uremic toxin, downregulates renal expression of Nrf2 through activation of NF-κB. BMC Nephrol. 2013, 14, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, V.-C.; Young, G.-H.; Huang, P.-H.; Lo, S.-C.; Wang, K.-C.; Sun, C.-Y.; Liang, C.-J.; Huang, T.-M.; Chen, J.-H.; Chang, F.-C. In acute kidney injury, indoxyl sulfate impairs human endothelial progenitor cells: Modulation by statin. Angiogenesis 2013, 16, 609–624. [Google Scholar] [CrossRef] [PubMed]
- Yabuuchi, N.; Sagata, M.; Saigo, C.; Yoneda, G.; Yamamoto, Y.; Nomura, Y.; Nishi, K.; Fujino, R.; Jono, H.; Saito, H. Indoxyl sulfate as a mediator involved in dysregulation of pulmonary aquaporin-5 in acute lung injury caused by acute kidney injury. Int. J. Mol. Sci. 2017, 18, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Owada, S.; Goto, S.; Bannai, K.; Hayashi, H.; Nishijima, F.; Niwa, T. Indoxyl sulfate reduces superoxide scavenging activity in the kidneys of normal and uremic rats. Am. J. Nephrol. 2008, 28, 446–454. [Google Scholar] [CrossRef]
- Sohn, E.; Kim, J.; Kim, C.-S.; Lee, Y.M.; Jo, K.; Shin, S.D.; Kim, J.H.; Kim, J.S. The extract of Litsea japonica reduced the development of diabetic nephropathy via the inhibition of advanced glycation end products accumulation in db/db mice. Evid.-Based Complement. Altern. Med. 2013, 2013, 769416. [Google Scholar] [CrossRef] [Green Version]
- Niedowicz, D.M.; Daleke, D.L. The role of oxidative stress in diabetic complications. Cell Biochem. Biophys. 2005, 43, 289–330. [Google Scholar] [CrossRef]
- Fakhruddin, S.; Alanazi, W.; Jackson, K.E. Diabetes-induced reactive oxygen species: Mechanism of their generation and role in renal injury. J. Diabetes Res. 2017, 2017, 8379327. [Google Scholar] [CrossRef] [Green Version]
- Volpe, C.M.O.; Villar-Delfino, P.H.; dos Anjos, P.M.F.; Nogueira-Machado, J.A. Cellular death, reactive oxygen species (ROS) and diabetic complications. Cell Death Dis. 2018, 9, 119. [Google Scholar] [CrossRef]
- Horie, K.; Miyata, T.; Maeda, K.; Miyata, S.; Sugiyama, S.; Sakai, H.; de Strihou, C.v.Y.; Monnier, V.M.; Witztum, J.L.; Kurokawa, K. Immunohistochemical colocalization of glycoxidation products and lipid peroxidation products in diabetic renal glomerular lesions. Implication for glycoxidative stress in the pathogenesis of diabetic nephropathy. J. Clin. Investig. 1997, 100, 2995–3004. [Google Scholar] [CrossRef] [Green Version]
- Soetikno, V.; Suzuki, K.; Veeraveedu, P.T.; Arumugam, S.; Lakshmanan, A.P.; Sone, H.; Watanabe, K. Molecular understanding of curcumin in diabetic nephropathy. Drug Discov. Today 2013, 18, 756–763. [Google Scholar] [CrossRef]
- Cheng, D.; Liang, B.; Li, Y. Antihyperglycemic effect of Ginkgo biloba extract in streptozotocin-induced diabetes in rats. BioMed Res. Int. 2013, 2013, 162724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gujjala, S.; Putakala, M.; Nukala, S.; Bangeppagari, M.; Ramaswamy, R.; Desireddy, S. Renoprotective effect of Caralluma fimbriata against high-fat diet-induced oxidative stress in Wistar rats. J. Food Drug Anal. 2016, 24, 586–593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakai, K.; Fujii, H.; Kono, K.; Goto, S.; Kitazawa, R.; Kitazawa, S.; Hirata, M.; Shinohara, M.; Fukagawa, M.; Nishi, S. Vitamin D activates the Nrf2-Keap1 antioxidant pathway and ameliorates nephropathy in diabetic rats. Am. J. Hypertens. 2014, 27, 586–595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Đorđević, M.; Mihailović, M.; Jovanović, J.A.; Grdović, N.; Uskoković, A.; Tolić, A.; Sinadinović, M.; Rajić, J.; Mišić, D.; Šiler, B. Centaurium erythraea methanol extract protects red blood cells from oxidative damage in streptozotocin-induced diabetic rats. J. Ethnopharmacol. 2017, 202, 172–183. [Google Scholar] [CrossRef]
- Giribabu, N.; Karim, K.; Kilari, E.K.; Salleh, N. Phyllanthus niruri leaves aqueous extract improves kidney functions, ameliorates kidney oxidative stress, inflammation, fibrosis and apoptosis and enhances kidney cell proliferation in adult male rats with diabetes mellitus. J. Ethnopharmacol. 2017, 205, 123–137. [Google Scholar] [CrossRef]
- Achi, N.; Ohaeri, O.; Ijeh, I.; Eleazu, C. Modulation of the lipid profile and insulin levels of streptozotocin induced diabetic rats by ethanol extract of Cnidoscolus aconitifolius leaves and some fractions: Effect on the oral glucose tolerance of normoglycemic rats. Biomed. Pharmacother. 2017, 86, 562–569. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, T.; Gong, Y.; Dong, X.; Zhang, W.; Sun, S.; Wang, H.; Gu, Y.; Lu, X.; Yan, M. Attenuation of diabetic nephropathy by Chaihuang-Yishen granule through anti-inflammatory mechanism in streptozotocin-induced rat model of diabetics. J. Ethnopharmacol. 2014, 151, 556–564. [Google Scholar] [CrossRef]
- Hinokio, Y.; Suzuki, S.; Hirai, M.; Suzuki, C.; Suzuki, M.; Toyota, T. Urinary excretion of 8-oxo-7, 8-dihydro-2′-deoxyguanosine as a predictor of the development of diabetic nephropathy. Diabetologia 2002, 45, 877–882. [Google Scholar] [CrossRef] [Green Version]
- Ying, C.; Mao, Y.; Chen, L.; Wang, S.; Ling, H.; Li, W.; Zhou, X. Bamboo leaf extract ameliorates diabetic nephropathy through activating the AKT signaling pathway in rats. Int. J. Biol. Macromol. 2017, 105, 1587–1594. [Google Scholar] [CrossRef]
- Navarro-González, J.F.; Jarque, A.; Muros, M.; Mora, C.; García, J. Tumor necrosis factor-α as a therapeutic target for diabetic nephropathy. Cytokine Growth Factor Rev. 2009, 20, 165–173. [Google Scholar] [CrossRef]
- Shikano, M.; Sobajima, H.; Yoshikawa, H.; Toba, T.; Kushimoto, H.; Katsumata, H.; Tomita, M.; Kawashima, S. Usefulness of a highly sensitive urinary and serum IL-6 assay in patients with diabetic nephropathy. Nephron 2000, 85, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, A.; Shikata, K.; Hiramatsu, M.; Nakatou, T.; Kitamura, T.; Wada, J.; Itoshima, T.; Makino, H. Serum interleukin-18 levels are associated with nephropathy and atherosclerosis in Japanese patients with type 2 diabetes. Diabetes Care 2005, 28, 2890–2895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahzad, K.; Bock, F.; Dong, W.; Wang, H.; Kopf, S.; Kohli, S.; Ranjan, S.; Wolter, J.; Wacker, C.; Biemann, R. Nlrp3-inflammasome activation in non-myeloid-derived cells aggravates diabetic nephropathy. Kidney Int. 2015, 87, 74–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, A.K.; Tesch, G.H. Inflammation in diabetic nephropathy. Mediat. Inflamm. 2012, 2012, 146154. [Google Scholar] [CrossRef]
- Lu, Q.; Zuo, W.-Z.; Ji, X.-J.; Zhou, Y.-X.; Liu, Y.-Q.; Yao, X.-Q.; Zhou, X.-Y.; Liu, Y.-W.; Zhang, F.; Yin, X.-X. Ethanolic Ginkgo biloba leaf extract prevents renal fibrosis through Akt/mTOR signaling in diabetic nephropathy. Phytomedicine 2015, 22, 1071–1078. [Google Scholar] [CrossRef] [PubMed]
- Ponnusamy, M.; Zhou, X.; Yan, Y.; Tang, J.; Tolbert, E.; Zhao, T.C.; Gong, R.; Zhuang, S. Blocking sirtuin 1 and 2 inhibits renal interstitial fibroblast activation and attenuates renal interstitial fibrosis in obstructive nephropathy. J. Pharmacol. Exp. Ther. 2014, 350, 243–256. [Google Scholar] [CrossRef] [Green Version]
- Hinz, B.; Celetta, G.; Tomasek, J.J.; Gabbiani, G.; Chaponnier, C. Alpha-smooth muscle actin expression upregulates fibroblast contractile activity. Mol. Biol. Cell 2001, 12, 2730–2741. [Google Scholar] [CrossRef] [Green Version]
- Zhong, Y.; Lee, K.; He, J.C. SIRT1 is a potential drug target for treatment of diabetic kidney disease. Front. Endocrinol. 2018, 9, 624. [Google Scholar] [CrossRef]
- Kitada, M.; Takeda, A.; Nagai, T.; Ito, H.; Kanasaki, K.; Koya, D. Dietary restriction ameliorates diabetic nephropathy through anti-inflammatory effects and regulation of the autophagy via restoration of Sirt1 in diabetic Wistar fatty (fa/fa) rats: A model of type 2 diabetes. Exp. Diabetes Res. 2011, 2011, 908185. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Cai, F.; Yang, Y.; Zhao, X.; Wang, C.; Li, J.; Jia, Y.; Tang, J. Tetrahydroxystilbene glucoside ameliorates diabetic nephropathy in rats: Involvement of SIRT1 and TGF-β1 pathway. Eur. J. Pharmacol. 2010, 649, 382–389. [Google Scholar] [CrossRef]
- Hasegawa, K.; Wakino, S.; Simic, P.; Sakamaki, Y.; Minakuchi, H.; Fujimura, K.; Hosoya, K.; Komatsu, M.; Kaneko, Y.; Kanda, T. Renal tubular Sirt1 attenuates diabetic albuminuria by epigenetically suppressing Claudin-1 overexpression in podocytes. Nat. Med. 2013, 19, 1496–1504. [Google Scholar] [CrossRef] [Green Version]
- Kume, S.; Haneda, M.; Kanasaki, K.; Sugimoto, T.; Araki, S.-i.; Isono, M.; Isshiki, K.; Uzu, T.; Kashiwagi, A.; Koya, D. Silent information regulator 2 (SIRT1) attenuates oxidative stress-induced mesangial cell apoptosis via p53 deacetylation. Free Radic. Biol. Med. 2006, 40, 2175–2182. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Anjaneyulu, M.; Kulkarni, S.; Chopra, K. Resveratrol, a polyphenolic phytoalexin, attenuates diabetic nephropathy in rats. Pharmacology 2006, 76, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.-Y.; Zhang, L.; Sui, M.-X.; Zhu, Y.-H.; Zeng, L. Protective effects of sirtuin 3 in a murine model of sepsis-induced acute kidney injury. Sci. Rep. 2016, 6, 33201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salminen, A.; Kaarniranta, K.; Kauppinen, A. Crosstalk between oxidative stress and SIRT1: Impact on the aging process. Int. J. Mol. Sci. 2013, 14, 3834–3859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lombard, D.B.; Alt, F.W.; Cheng, H.-L.; Bunkenborg, J.; Streeper, R.S.; Mostoslavsky, R.; Kim, J.; Yancopoulos, G.; Valenzuela, D.; Murphy, A. Mammalian Sir2 homolog SIRT3 regulates global mitochondrial lysine acetylation. Mol. Cell. Biol. 2007, 27, 8807–8814. [Google Scholar] [CrossRef] [Green Version]
- Hebert, A.S.; Dittenhafer-Reed, K.E.; Yu, W.; Bailey, D.J.; Selen, E.S.; Boersma, M.D.; Carson, J.J.; Tonelli, M.; Balloon, A.J.; Higbee, A.J. Calorie restriction and SIRT3 trigger global reprogramming of the mitochondrial protein acetylome. Mol. Cell 2013, 49, 186–199. [Google Scholar] [CrossRef] [Green Version]
- Haigis, M.C.; Mostoslavsky, R.; Haigis, K.M.; Fahie, K.; Christodoulou, D.C.; Murphy, A.J.; Valenzuela, D.M.; Yancopoulos, G.D.; Karow, M.; Blander, G. SIRT4 inhibits glutamate dehydrogenase and opposes the effects of calorie restriction in pancreatic β cells. Cell 2006, 126, 941–954. [Google Scholar] [CrossRef] [Green Version]
- Mahlknecht, U.; Voelter-Mahlknecht, S. Fluorescence in situ hybridization and chromosomal organization of the sirtuin 4 gene (Sirt4) in the mouse. Biochem. Biophys. Res. Commun. 2009, 382, 685–690. [Google Scholar] [CrossRef]
- Chen, Y.-R.; Fang, S.-R.; Fu, Y.-C.; Zhou, X.-H.; Xu, M.-Y.; Xu, W.-C. Calorie restriction on insulin resistance and expression of SIRT1 and SIRT4 in rats. Biochem. Cell Biol. 2010, 88, 715–722. [Google Scholar] [CrossRef]
- Pacholec, M.; Bleasdale, J.E.; Chrunyk, B.; Cunningham, D.; Flynn, D.; Garofalo, R.S.; Griffith, D.; Griffor, M.; Loulakis, P.; Pabst, B. SRT1720, SRT2183, SRT1460, and Resveratrol Are Not Direct Activators of SIRT1. J. Biol. Chem. 2010, 285, 8340–8351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeung, F.; Hoberg, J.E.; Ramsey, C.S.; Keller, M.D.; Jones, D.R.; Frye, R.A.; Mayo, M.W. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004, 23, 2369–2380. [Google Scholar] [CrossRef] [Green Version]
- Lee, I.H.; Cao, L.; Mostoslavsky, R.; Lombard, D.B.; Liu, J.; Bruns, N.E.; Tsokos, M.; Alt, F.W.; Finkel, T. A role for the NAD-dependent deacetylase Sirt1 in the regulation of autophagy. Proc. Natl. Acad. Sci. USA 2008, 105, 3374–3379. [Google Scholar] [CrossRef] [Green Version]
- Kundu, A.; Richa, S.; Dey, P.; Kim, K.S.; Son, J.Y.; Kim, H.R.; Lee, S.-Y.; Lee, B.-H.; Lee, K.Y.; Kacew, S. Protective effect of EX-527 against high-fat diet-induced diabetic nephropathy in Zucker rats. Toxicol. Appl. Pharmacol. 2020, 390, 114899. [Google Scholar] [CrossRef] [PubMed]
- Niture, N.T.; Patil, D.G.; Somani, R.S.; Sahane, R.S. Effect of rutin on early diabetic neuropathy in experimental animals. J. Nat. Prod. Plant Resour. 2014, 4, 1–9. [Google Scholar]
- Quaile, M.P.; Melich, D.H.; Jordan, H.L.; Nold, J.B.; Chism, J.P.; Polli, J.W.; Smith, G.A.; Rhodes, M.C. Toxicity and toxicokinetics of metformin in rats. Toxicol. Appl. Pharmacol. 2010, 243, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N.; Kishore, L.; Singh, R. Dillenia indica L. attenuates diabetic nephropathy via inhibition of advanced glycation end products accumulation in STZ-nicotinamide induced diabetic rats. J. Tradit. Complement. Med. 2018, 8, 226–238. [Google Scholar] [CrossRef]
- Dey, P.; Son, J.Y.; Kundu, A.; Kim, K.S.; Lee, Y.; Yoon, K.; Yoon, S.; Lee, B.M.; Nam, K.T.; Kim, H.S. Knockdown of Pyruvate Kinase M2 Inhibits Cell Proliferation, Metabolism, and Migration in Renal Cell Carcinoma. Int. J. Mol. Sci. 2019, 20, 5622. [Google Scholar] [CrossRef] [Green Version]
- Dey, P.; Kundu, A.; Sachan, R.; Park, J.; Ahn, M.Y.; Yoon, K.; Lee, J.; Kim, N.D.; Kim, I.S.; Lee, B.M. PKM2 Knockdown Induces Autophagic Cell Death via the AKT/mTOR Pathway in Human Prostate Cancer Cells. Cell. Physiol. Biochem. 2019, 52, 1535–1552. [Google Scholar]
- Kundu, A.; Dey, P.; Sarkar, P.; Karmakar, S.; Tae, I.H.; Kim, K.S.; Park, J.H.; Lee, S.H.; Lee, B.M.; Renthlei, L. Protective effects of Croton hookeri on streptozotocin-induced diabetic nephropathy. Food Chem. Toxicol. 2020, 135, 110873. [Google Scholar] [CrossRef]
- Sachan, R.; Kundu, A.; Dey, P.; Son, J.Y.; Kim, K.S.; Lee, D.E.; Kim, H.R.; Park, J.H.; Lee, S.H.; Kim, J.-H. Dendropanax morbifera Protects against Renal Fibrosis in Streptozotocin-Induced Diabetic Rats. Antioxidants 2020, 9, 84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Phytochemicals | Methanolic Extract |
|---|---|
| Alkaloid | +++ |
| Carbohydrate | + |
| Saponin | − |
| Steroid | ++ |
| Sulphate | − |
| Tannin | − |
| Flavonoid | ++ |
| Hydroxy-anthraquinone glycoside | + |
| Starch | − |
| Dextrin | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dey, P.; Kundu, A.; Lee, H.E.; Kar, B.; Vishal, V.; Dash, S.; Kim, I.S.; Bhakta, T.; Kim, H.S. Molineria recurvata Ameliorates Streptozotocin-Induced Diabetic Nephropathy through Antioxidant and Anti-Inflammatory Pathways. Molecules 2022, 27, 4985. https://doi.org/10.3390/molecules27154985
Dey P, Kundu A, Lee HE, Kar B, Vishal V, Dash S, Kim IS, Bhakta T, Kim HS. Molineria recurvata Ameliorates Streptozotocin-Induced Diabetic Nephropathy through Antioxidant and Anti-Inflammatory Pathways. Molecules. 2022; 27(15):4985. https://doi.org/10.3390/molecules27154985
Chicago/Turabian StyleDey, Prasanta, Amit Kundu, Ha Eun Lee, Babli Kar, Vineet Vishal, Suvakanta Dash, In Su Kim, Tejendra Bhakta, and Hyung Sik Kim. 2022. "Molineria recurvata Ameliorates Streptozotocin-Induced Diabetic Nephropathy through Antioxidant and Anti-Inflammatory Pathways" Molecules 27, no. 15: 4985. https://doi.org/10.3390/molecules27154985







