Dynamic Processes of Rhenium Polyhydride Complexes
Abstract
:1. Introduction
2. Catalytic Transformations with Rhenium Polyhydride Precatalysts
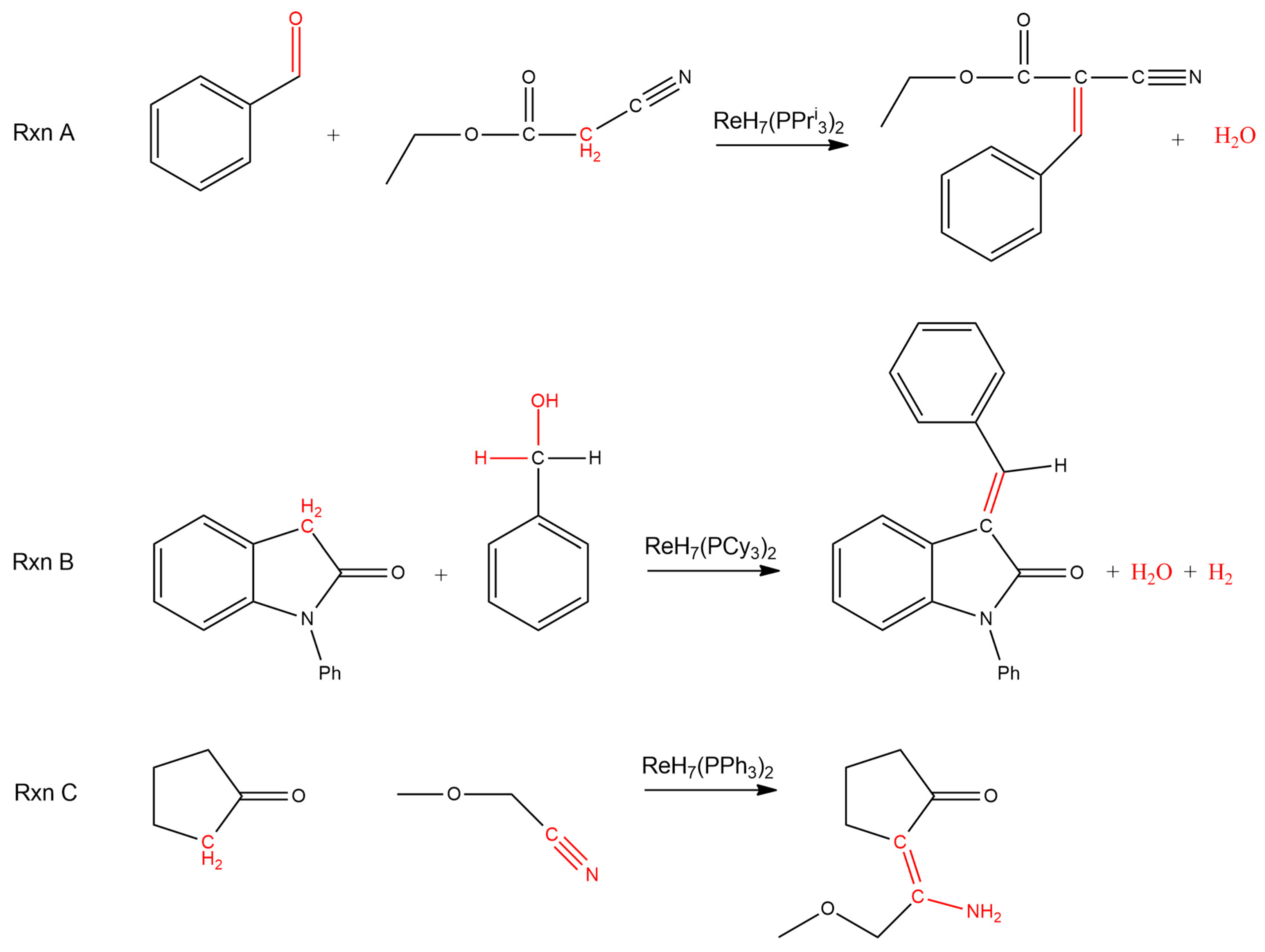
2.1. Catalytic Hydrogenation and Hydrogen Transfer Reactions
2.2. Catalytic Carbon-Hydrogen Bond Activation Where the Hydrogen Atoms Lie Alpha to a Carbonyl Group
2.3. Amide, Ester, or Secondary Amine Formation Catalyzed by Rhenium Polyhydride Precatalysts
2.4. Miscellaneous Reactions Catalyzed by Rhenium Polyhydride Precatalysts
3. Lowest-Energy Structures of Rhenium Polyhydride Complexes
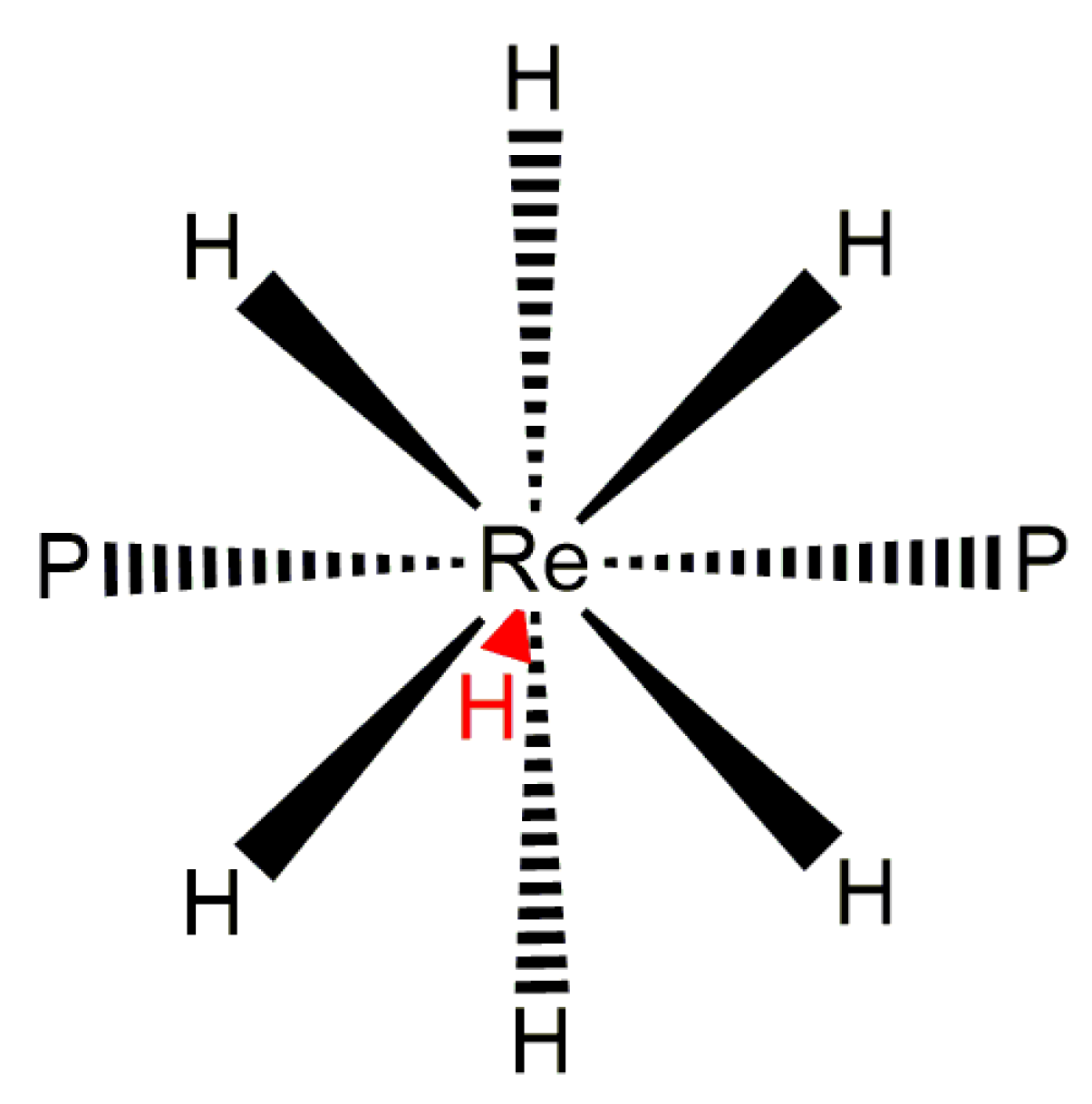
3.1. Nine-Coordinate Rhenium(VII) Complexes
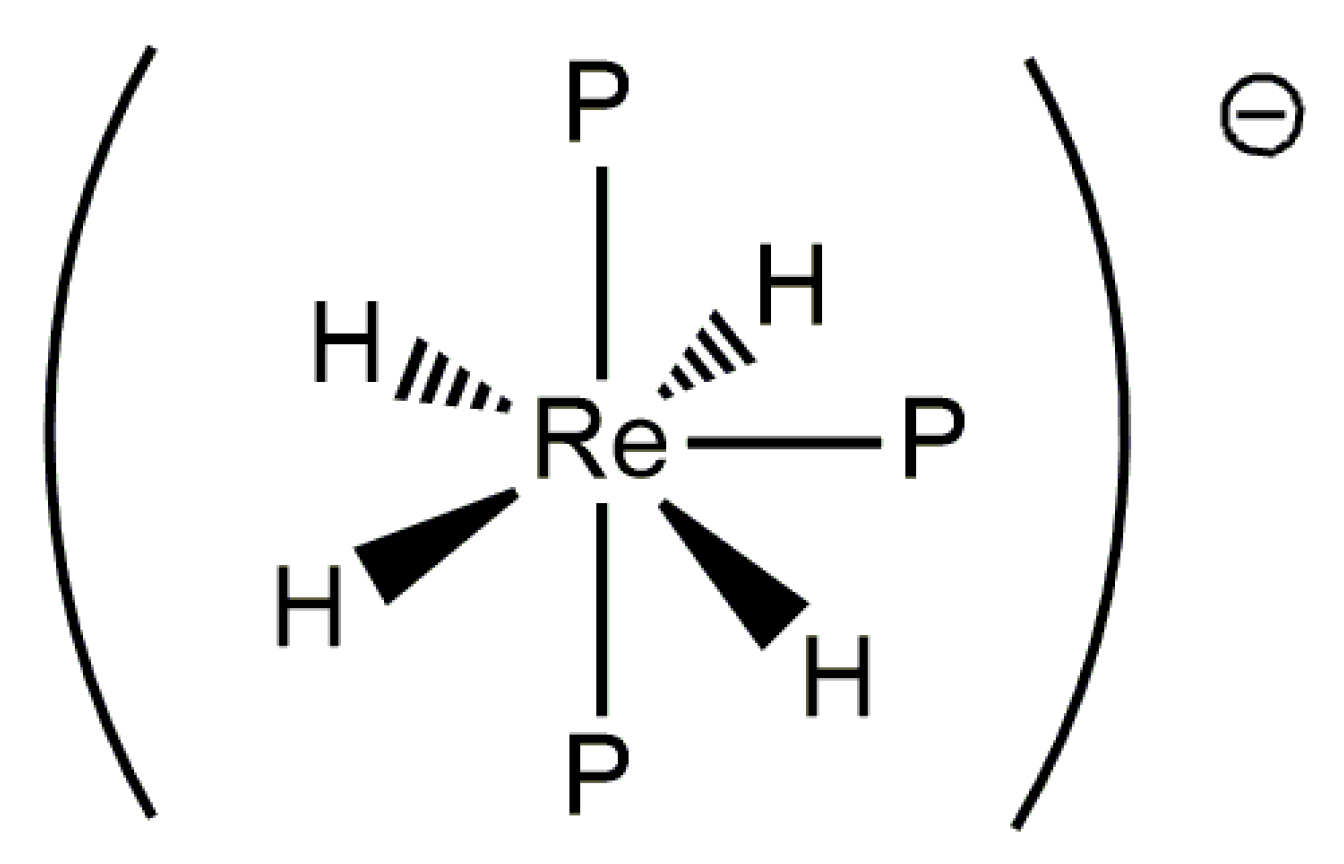
3.2. Eight-Coordinate Rhenium(V) Complexes
3.3. Seven-Coordinate Rhenium(III) Complexes
4. Characterization of Dynamic Processes at Rhenium Polyhydride Complexes
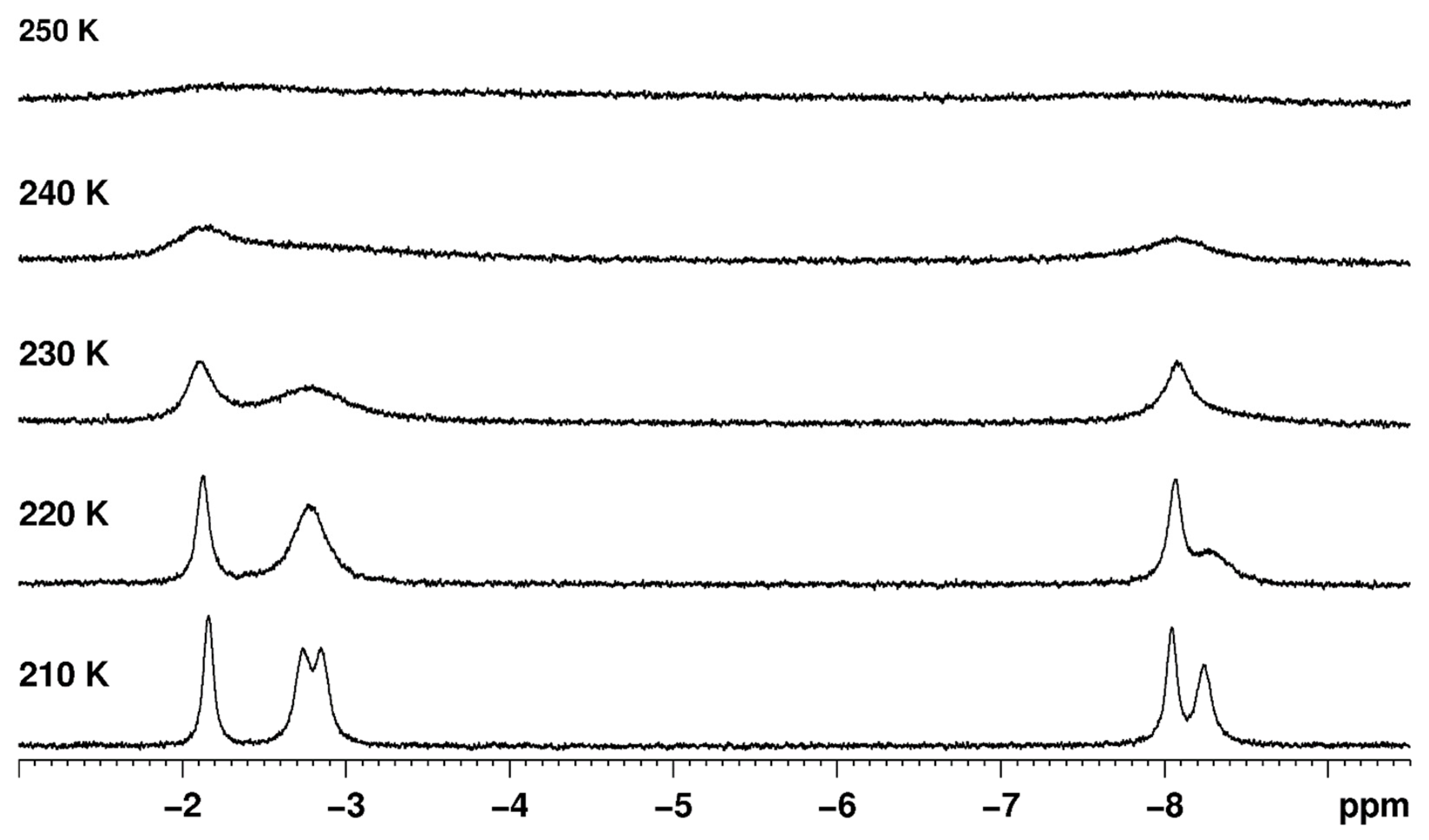
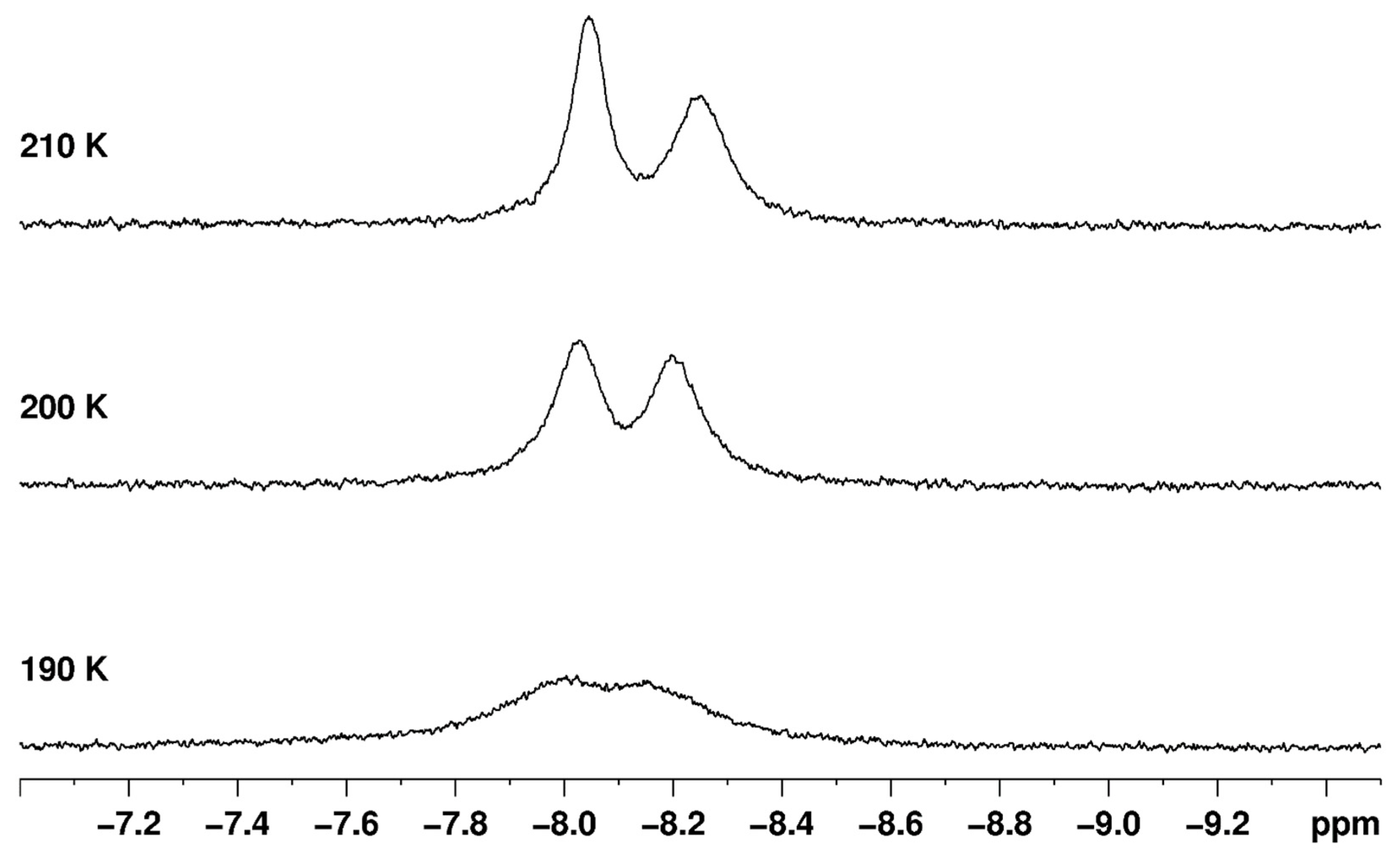
Aspects of Line Shape Fitting of Dynamic NMR Spectra of Rhenium Polyhydride Complexes
5. Dynamic Properties That Occur at Rhenium Polyhydride Complexes
5.1. Intramolecular Processes
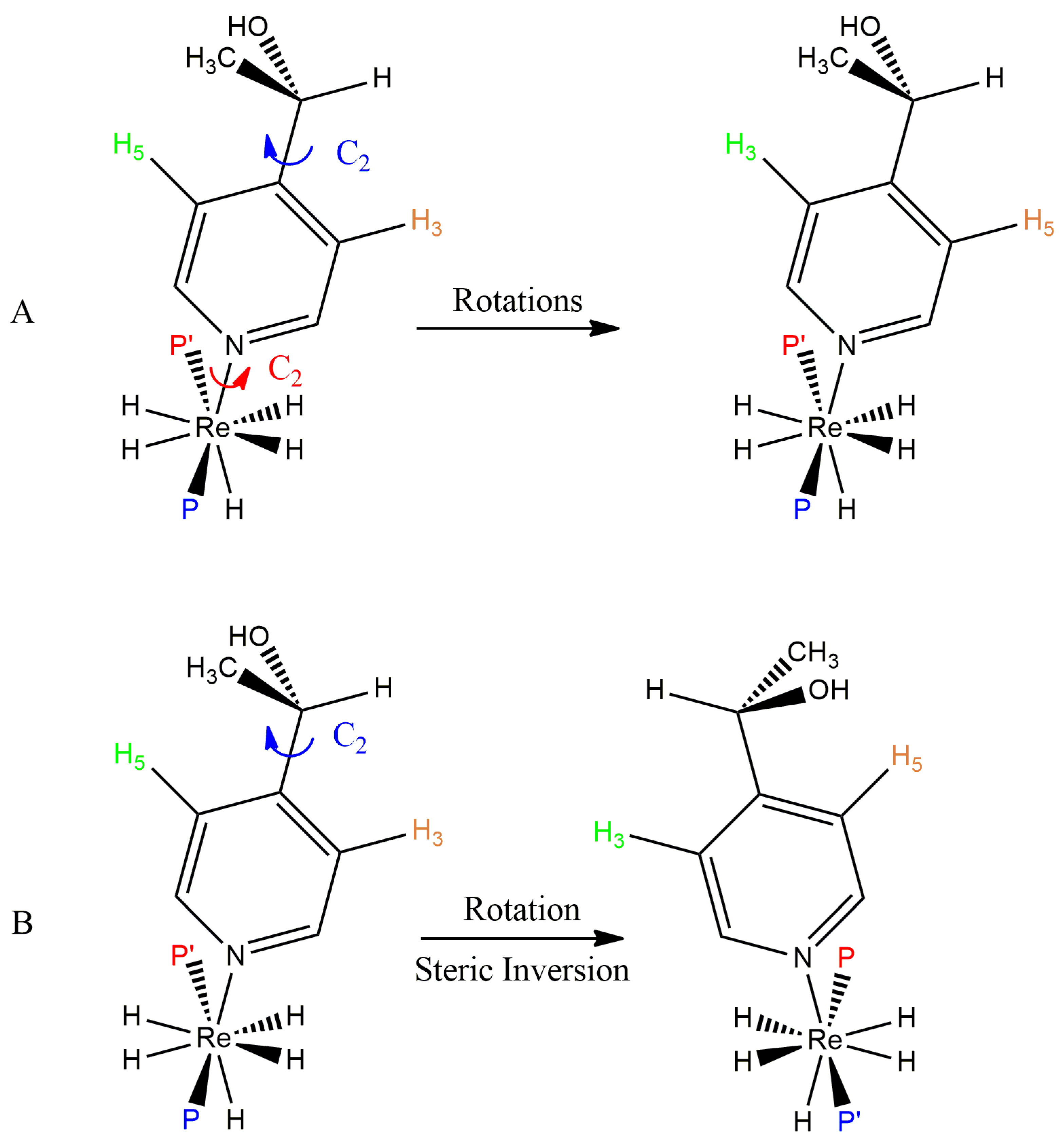
5.1.1. Dynamic Observations Associated with an Amine Ligand Bound to a Rhenium(V) Center
Coalescence of Pyridine Ligand Protons
Interconversion of E and Z Isomers
Coalescence of 31P-{1H} Resonances for Diastereotopic Phosphorus Centers
5.1.2. Dynamic Observations Associated with Phosphorus Atoms
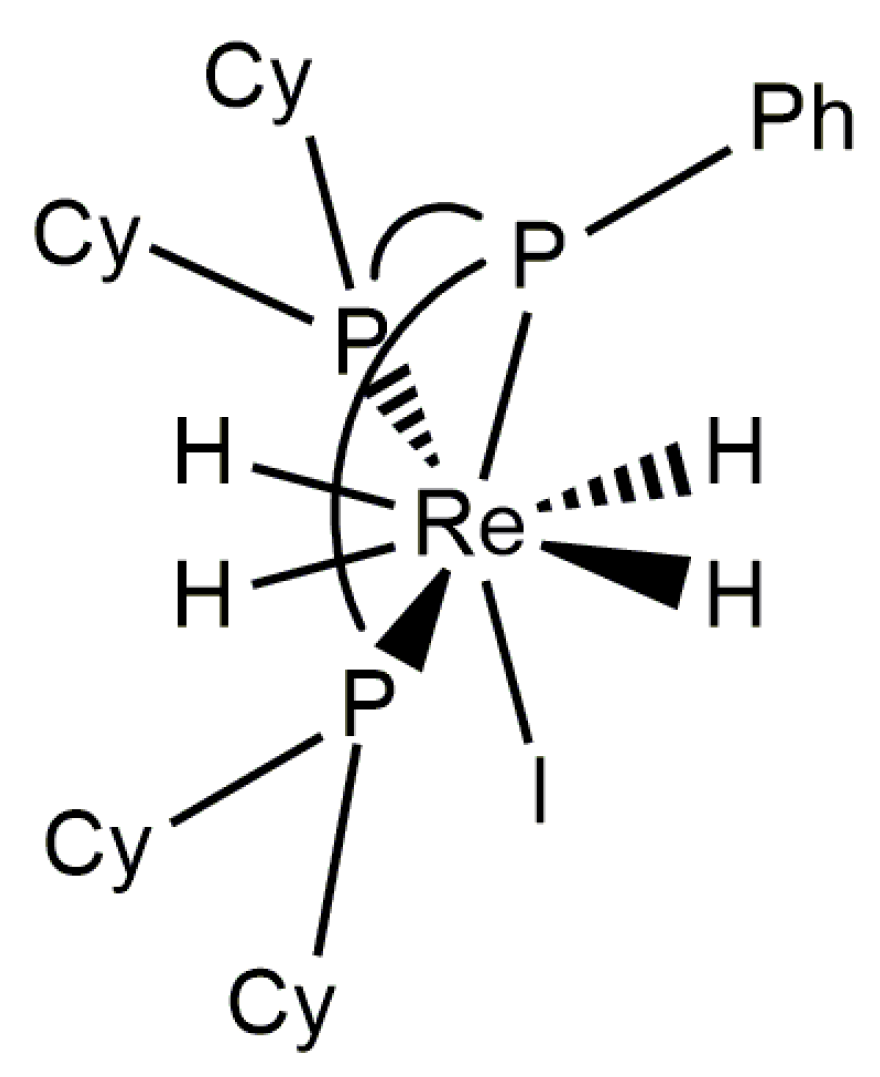
Dynamic Observations Associated with Three Phosphorus Atoms That Participate in Intramolecular Exchange
Dynamic 31P-{1H} Observations That Occur for Certain Rhenium(V) Complexes Ascribed to Tautomers
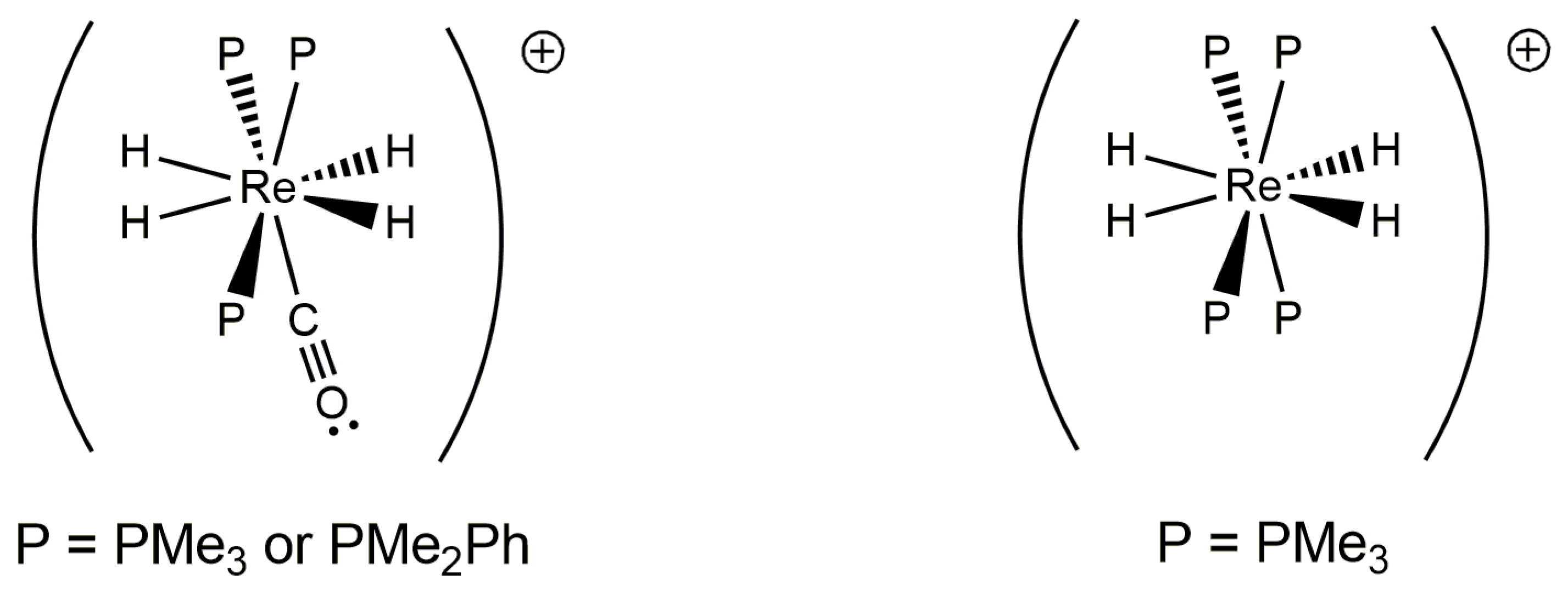
Lack of Observations of Phosphorus Atom Rearrangements for Rhenium(VII) Complexes
Observation of 31P NMR Resonances from Diastereotopic Phosphorus Atoms in a Room-Temperature Solution of a Rhenium(V) Tetrahydride Complex
5.2. Dynamic Processes Associated with Hydride Ligands
5.2.1. Intramolecular Dynamic Processes of Hydride Ligands
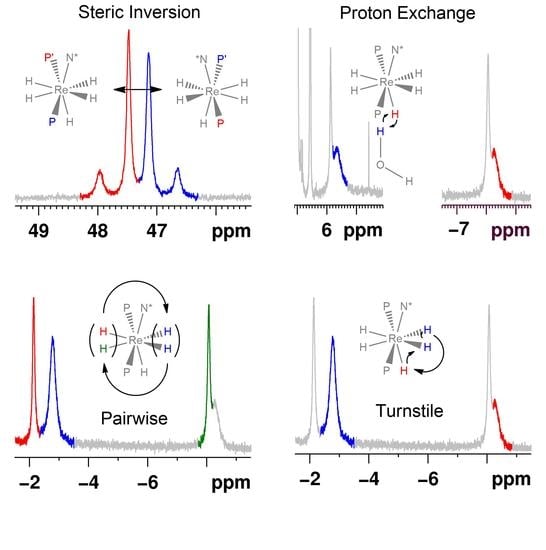
Pairwise Exchange of Adjacent Hydride Ligands
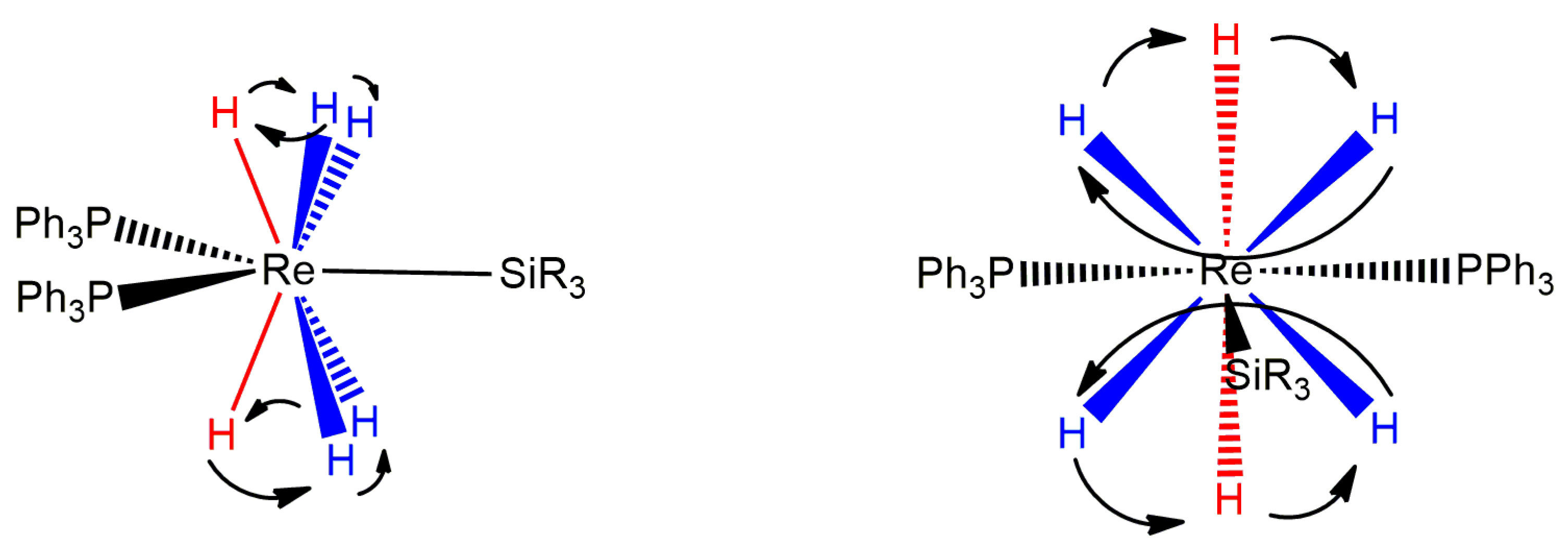
Turnstile Exchange of Three Adjacent Hydride Ligands
Pairwise Exchange of Four A Site Hydride Ligands at Rhenium(V) Centers
Circle Dance Exchange of Five Hydride Ligands
Exchange of Hydride Ligands with a Dihydrogen Ligand
Tautomeric Exchange of a Dihydrogen Ligand with a Pair of Hydride Ligands
5.2.2. Intermolecular Dynamic Exchange of Inner Sphere Hydride Ligands with Hydrogen from Beyond the Inner Coordination sphere
Proton Exchange between Hydride Ligands and Exchangeable Protons of Small Molecules in Solution
Exchange of Hydride Ligands with Free Dihydrogen
Exchange of Hydride Ligands with Hydrogen Bound to Carbon
Direct Exchange of Hydride Ligands between the Inner Coordination Spheres of Two Complexes
6. Thermodynamic Characterizations of Dynamic Processes
6.1. Exchange of Protons between Water and a Unique B Site Hydride Ligand
6.2. Psuedorotation or Pseudorotation-like Rearrangement of Rhenium Polyhydride Complexes
6.3. Turnstile Exchange of Rhenium(V) Polyhydride Complexes
6.4. Pairwise Exchange of Adjacent Hydride Ligands
7. Map of the Hydride Ligand Chemical Properties with Respect to the Coordination Site
8. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chatt, J.; Coffey, R.S. Hydrido-complexes of rhenium-containing tertiary phosphines. J. Chem. Soc. A 1969, 1963–1972. [Google Scholar] [CrossRef]
- Green, M.A.; Huffman, J.C.; Caulton, K.G.; Rybak, W.K.; Ziolkowski, J.J. Ligand scavenging and catalytic utilization of the phototransient ReH5(PMe2Ph)2. J. Organomet. Chem. 1981, 218, C39. [Google Scholar] [CrossRef]
- Kosanovich, A.J.; Reibenspies, J.H.; Ozerov, O.V. Complexes of high-valent rhenium supported by the PCP pincer. Organometallics 2016, 35, 513–519. [Google Scholar] [CrossRef]
- Grieco, G.; Blacque, O. Solution and Solid-State Structure of the First NHC-Substituted Rhenium Heptahydrides. Eur. J. Inorg. Chem. 2019, 34, 3810–3819. [Google Scholar] [CrossRef]
- Abrahams, S.C.; Ginsberg, A.P.; Knox, K. Transition metal-hydrogen compounds. II. The crystal and molecular structure of potassium rhenium hydride, K2ReH9. Inorg. Chem. 1964, 3, 558–567. [Google Scholar] [CrossRef]
- Fontaine, X.L.R.; Fowles, E.H.; Shaw, B.L. The reversible protonation of rhenium(VII) heptahydrides of type [ReH7(PR3)2] to give η2-dihydrogen complexes. J. Chem. Soc. Chem. Commun. 1988, 482–483. [Google Scholar] [CrossRef]
- Luo, X.L.; Crabtree, R.H. Solution equilibrium between classical and nonclassical polyhydride tautomers [ReH4(CO)L3]+ and ReH2(η2-H2)(CO)L3]+ (L = PMe2Ph). Equilibrium isotope effects and an intermediate trihydrogen complex in intramolecular site exchange of dihydrogen and hydride ligands. J. Am. Chem. Soc. 1990, 112, 6912–6918. [Google Scholar] [CrossRef]
- Kim, Y.; Deng, H.; Gallucci, J.C.; Wojcicki, A. Rhenium Polyhydride Complexes Containing PhP(CH2CH2CH2PCy2)2 (Cyttp): Protonation, Insertion, and Ligand Substitution Reactions of ReH5(Cyttp) and Structural Characterization of ReH5(Cyttp) and [ReH4(η2-H2)(Cyttp)]SbF6. Inorg. Chem. 1996, 35, 7166–7173. [Google Scholar] [CrossRef]
- Gusev, D.G.; Nietlispach, D.; Eremenko, I.L.; Berke, H. Structure and solution behavior of a series of classical and nonclassical rhenium hydride complexes. Inorg. Chem. 1993, 32, 3628–3636. [Google Scholar] [CrossRef]
- Bolano, S.; Bravo, J.; Garcia-Fontan, A. Mono- and Dinuclear Rhenium Polyhydride Complexes Bearing the Chelating Ligand 1,2-Bis(dicyclohexylphosphanyloxy)ethane. Eur. J. Inor. Chem. 2004, 2004, 4812–4819. [Google Scholar] [CrossRef]
- Hamilton, D.G.; Crabtree, R.H. An NMR method for distinguishing classical from nonclassical structures in transition-metal polyhydrides. J. Am. Chem. Soc. 1988, 110, 4126–4133. [Google Scholar] [CrossRef]
- Costello, M.T.; Walton, R.A. Electrochemical redox behavior of the mononuclear rhenium heptahydride complexes ReH7(PR3)2: Evidence for the (η2-dihydrogen) ligand in this class of complex. Inorg. Chem. 1988, 27, 2563–2564. [Google Scholar] [CrossRef]
- Howard, J.A.K.; Mason, S.A.; Johnson, O.; Diamond, I.C.; Crennell, S.; Keller, P.A.; Spencer, J.L. Classical versus non-classical polyhydride complexes: Single crystal neutron diffraction study of 1,2-bis(diphenylphosphino)ethaneheptahydridorhenium(VII)[ReH7(dppe)] using a position-sensitive detector. J. Chem. Soc. Chem. Commun. 1988, 1502–1503. [Google Scholar] [CrossRef]
- Cotton, F.A.; Luck, R.L. X-ray crystal structure of ReH5(PPh3)3 and variable-temperature T1 studies of ReH5(PPh3)3 and ReH5(PMe2Ph)3 in various solvents; are T1 measurements reliable in predicting whether polyhydride complexes contain molecular hydrogen ligands? J. Am. Chem. Soc. 1989, 111, 5757–5761. [Google Scholar] [CrossRef]
- Haynes, G.R.; Martin, R.L.; Hay, P.J. Theoretical investigations of classical and nonclassical structures of MH7L2 polyhydride complexes of rhenium and technetium. J. Am. Chem. Soc. 1992, 114, 28–36. [Google Scholar] [CrossRef]
- Beringhelli, T.; D’Alfonso, G.; Freni, M.; Minoja, A.P. Scalar and dipolar contributions of rhenium to the relaxation properties of coordinated ligands. Inorg. Chem. 1992, 31, 848–852. [Google Scholar] [CrossRef]
- Heinekey, D.M.; Schomber, B.M.; Radzewich, C.E. Cationic hydrogen complexes of rhenium. J. Am. Chem. Soc. 1994, 116, 4515–4516. [Google Scholar] [CrossRef]
- Skupinski, W.; Huffman, J.C.; Bruno, J.W.; Caulton, K.G. Dinuclear elimination from rhenium hydrides and trimethylaluminum: Rhenium/aluminum polyhydrides. J. Am. Chem. Soc. 1984, 106, 8128–8136. [Google Scholar] [CrossRef]
- Moehring, G.A.; Fanwick, P.E.; Walton, R.A. Mixed Polyhydride-Phosphine Clusters That Contain the Re2Au and Re2Au2 Cores: Reversible Protonation and Auration of the Dirhenium Polyhydride Complex Re2(μ-H)4H4(PPh3)4. Inorg. Chem. 1987, 26, 1861–1866. [Google Scholar] [CrossRef]
- Baker, R.T.; Glassman, T.E.; Ovenall, D.W.; Calabrese, J.C. Unsaturated Bis(phosphido)-bridged Heterobimetallic Polyhydrides via Dihydrogen Activation. Isr. J. Chem. 1991, 31, 33–53. [Google Scholar] [CrossRef]
- He, Z.; Nefedov, S.; Lugan, N.; Neibecker, D.; Mathieau, R. Synthesis and reactivity of a new unsaturated heterobimetallic polyhydride complex (CO)(PPh3)2HRe(μ-H)3RuH(PPh3)2. Organometallics 1993, 12, 3837–3845. [Google Scholar] [CrossRef]
- Lin, Z.; Hall, M.B. Tansition metal polyhydride complexes. 7. Classical and nonclassical structures of {ReH4(CO)(PR3)3]+. J. Am. Chem. Soc. 1994, 116, 4446–4448. [Google Scholar] [CrossRef]
- Lee, J.C., Jr.; Yao, W.; Crabtree, R.H.; Regger, H. Fluxionality in [ReH5(PPh3)2(pyridine)]. Inorg. Chem. 1996, 35, 695–699. [Google Scholar] [CrossRef]
- Patel, B.P.; Kavallieratos, K.; Crabtree, R.H. Effects of dihydrogen bonding on fluxionality in ReH5(PPh3)2L. J. Organometal. Chem. 1997, 528, 205–207. [Google Scholar] [CrossRef]
- Rabie, U.M.; Patel, B.P.; Crabtree, R.H. A new charge transfer complex involving ReH5(PPh3)3. Inorg. Chem. 1997, 36, 2236–2238. [Google Scholar] [CrossRef]
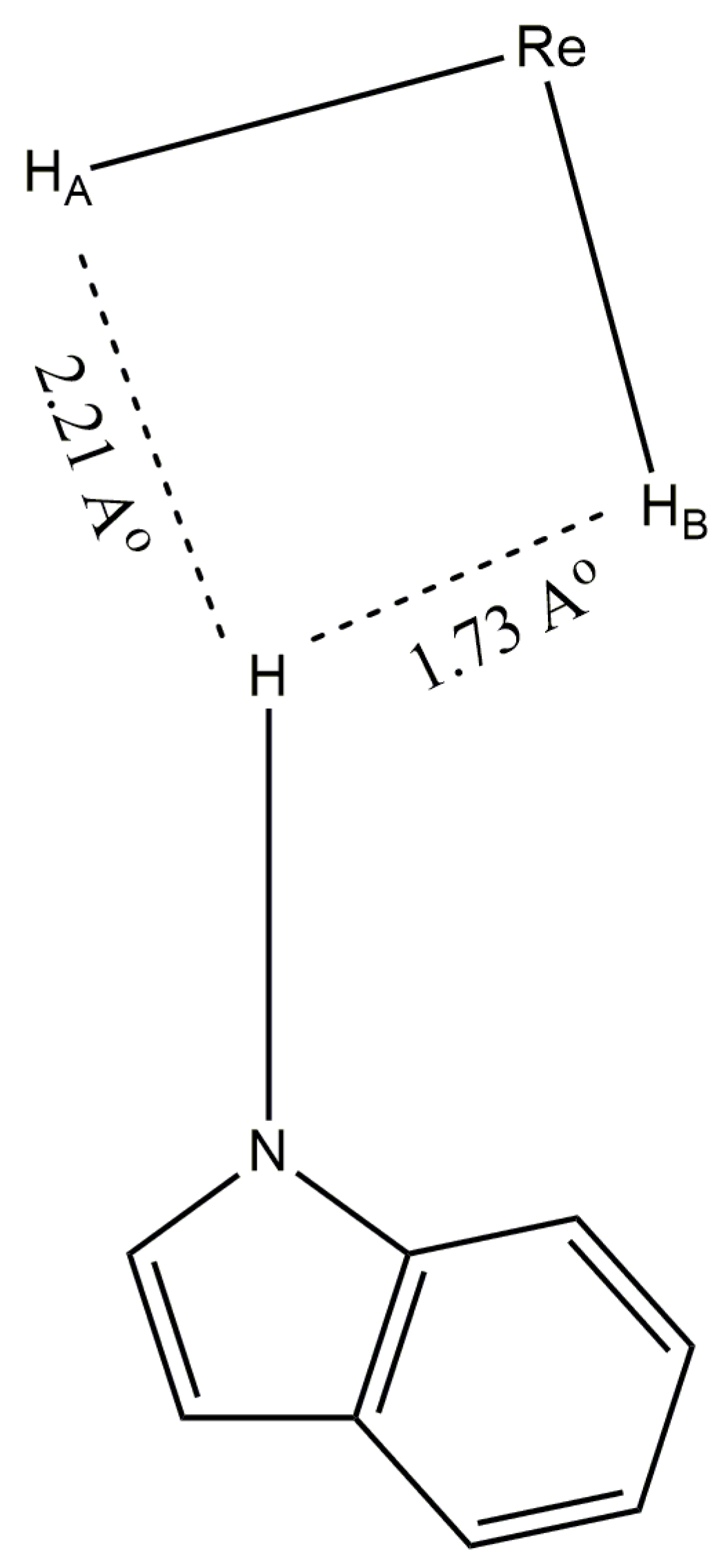
- Patel, B.P.; Wessel, J.; Yao, W.; Lee, J.C., Jr.; Peris, E.; Koetzle, T.F.; Yap, G.P.A.; Fortin, J.B.; Ricci, J.S.; Sini, G.; et al. Intermolecular N-H…H-Re interactions involving rhenium polyhydrides. New J. Chem. 1997, 21, 413–421. [Google Scholar]
- Bosque, R.; Maseras, F.; Eisenstein, O.; Patel, B.P.; Yao, W.; Crabtree, R.H. Site Preference Energetics, Fluxionality, and Intramolecular M−H···H−N Hydrogen Bonding in a Dodecahedral Transition Metal Polyhydride. Inorg. Chem. 1997, 36, 5505–5511. [Google Scholar] [CrossRef]
- Sini, G.; Eisenstein, O.; Yao, Y.; Crabtree, R.H. Intermolecular Re-H…H-X hydrogen bonding (X=N, C) involving ReH5(PPh3)3. Inorg. Chem. Acta 1998, 280, 26–29. [Google Scholar] [CrossRef]
- Wessel, J.; Lee, J.C., Jr.; Peris, E.; Yap, G.P.A.; Fortin, J.B.; Ricci, J.S.; Sini, G.; Albinati, A.; Koetzle, T.F.; Eisenstein, O.; et al. An unconventional intermolecular three-center N-H...H2Re hydrogen bond in crystalline [ReH5(PPh3)3].indole.C6H6. Angew. Chem. Int. Ed. 1995, 34, 2507–2509. [Google Scholar] [CrossRef]
- Hinman, J.G.; Lough, A.J.; Morris, R.H. Properties of the polyhydride anions [WH5(PMe2Ph)3]- and [ReH4(PMePh2)3]- and periodic trends in the acidity of polyhydride complexes. Inorg. Chem. 2007, 46, 4392–4401. [Google Scholar] [CrossRef]
- Wazio, J.A.; Jimenez, V.; Soparawalla, S.; John, S.; Moehring, G.A. Hydrogen exchange of rhenium(VII) heptahydridobis(triphenylphosphine) with water, aniline, methanol, and itself. Inorg. Chim. Acta 2009, 362, 159–165. [Google Scholar] [CrossRef]
- Jimenez, Y.; Strepka, A.M.; Borgohain, M.D.; Hinojosa, P.A.; Moehring, G.A. Ortho-metalation, rotational isomerization, and hydride–hydride coupling at rhenium (V) polyhydride complexes stabilized by aromatic amine ligands. Inorg. Chim. Acta 2009, 362, 3259–3266. [Google Scholar] [CrossRef]
- Streisel, D.J.; Petrou, A.L.; Scorzelli, A.G.; Macalush, B.E.; Siebert, H.M.; Torres, G.S.; Joswick, C.M.; Moehring, G.A. Fluxionality, substitution, and hydrogen exchange at eight-coordinate rhenium(V) polyhydride centers. Inorg. Chim. Acta 2019, 496, 119028. [Google Scholar] [CrossRef]
- Scorzelli, A.G.; Macalush, B.E.; Naik, D.V.; Moehring, G.A. Comparative study of fluxional processes at two different classes of eight-coordinate rhenium(V) polyhydride complexes. Inorg. Chim. Acta 2021, 516, 120120. [Google Scholar] [CrossRef]
- Tao, Y.; Sou, W.; Luo, G.-G.; Kraka, E. Describing Polytopal Rearrangement Processes of Octacoordinate Structures. I. Renewed Insights into Fluxionality of the Rhenium Polyhydride Complex ReH5(PPh3)2(Pyridine). Inorg. Chem. 2021, 60, 2492–2502. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Wang, X.; Zou, W.; Luo, G.-G.; Kraka, E. Unusual intramolecular motion of ReH92- in K2ReH9 crystal: Circle dance and three-arm turnstile mechanisms revealed by computational studies. Inorg. Chem. 2022, 61, 1041–1050. [Google Scholar] [CrossRef]
- Geetha, B.; Petrou, A.L.; Mansour, M.; Tadros, S.M.; Naik, D.V.; Moehring, G.A. Chiral amine ligands at rhenium(V) pentahydride complexes allow for characterization of an energetically accessible and reversible steric inversion of diastereotopic phosphorus atoms. Inorg. Chim. Acta 2022, 531, 120741. [Google Scholar] [CrossRef]
- Baudry, D.; Ephritikhine, M.; Felkin, H.; Holmes-Smith, R. The selective catalytic conversion of cycloalkanes into cycloalkenes using a soluble rhenium polyhydride system. J. Chem. Soc. Chem. Commun. 1983, 788–789. [Google Scholar] [CrossRef]
- Loza, M.L.; de Gala, S.R.; Crabtree, R.H. Steric crowding in a rhenium polyhydride induced by a chelating disilyl ligand: Synthesis, characterization, and reactivity of ReH5(disil)(PPh3)2 (disil = 1,2-bis(dimethylsilyl)benzene and 1,2-bis(dimethylsilyl)ethane). Inorg. Chem. 1994, 33, 5073–5078. [Google Scholar] [CrossRef]
- Mejia, E.; Togni, A. Rhenium Complexes Containing the Chiral Tridentate Ferrocenyl Ligand Pigiphos. Organometallics 2011, 30, 4765–4770. [Google Scholar] [CrossRef]
- Lin, Y.; Zhu, X.; Xiang, M. Transition metal polyhydrides-catalyzed addition of activated nitriles to aldehydes and ketones via Knoevenagel condensation. J. Organomet. Chem. 1993, 448, 215–218. [Google Scholar] [CrossRef]
- Takaya, H.; Ito, M.; Murahashi, S.-I. Rhenium-Catalyzed Addition of Carbonyl Compounds to the Carbon−Nitrogen Triple Bonds of Nitriles: α-C−H Activation of Carbonyl Compounds. J. Am. Chem. Soc. 2009, 131, 10824–10825. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Xie, J.; Pan, C.; Zhu, Z.; Cheng, Y.; Zhu, C. Rhenium-Catalyzed Acceptorless Dehydrogenative Coupling via Dual Activation of Alcohols and Carbonyl Compounds. ACS Catal. 2013, 3, 2195–2198. [Google Scholar] [CrossRef]
- Schleker, P.P.M.; Honeker, R.; Klankermayer, J.; Leitner, W. Catalytic Dehydrogenative Amide and Ester Formation with Rhenium–Triphos Complexes. ChemCatChem 2013, 5, 1762–1764. [Google Scholar] [CrossRef]
- Abdukader, A.; Jin, H.; Cheng, Y.; Zhu, C. Rhenium-catalyzed amination of alcohols by hydrogen transfer process. Tetrahedron Lett. 2014, 55, 4172–4174. [Google Scholar] [CrossRef]
- Komiya, S.; Chigira, T.; Suzuki, T.; Hirano, M. Polymerization of alkyl methacrylate catalyzed by hydrideorhenium complexes. Chem. Lett. 1999, 28, 347–348. [Google Scholar] [CrossRef]
- Jin, H.; Zhu, Z.; Jin, N.; Xie, J.; Cheng, Y.; Zhu, C. CO-enabled rhenium hydride catalyst for directed C(sp2)–H bond alkylation with olefins. Org. Chem. Front. 2015, 2, 378–382. [Google Scholar] [CrossRef]
- Donnelly, L.J.; Faber, T.; Morrison, C.A.; Nichol, G.S.; Thomas, S.P.; Love, J.B. C–H Borylation Catalysis of Heteroaromatics by a Rhenium Boryl Polyhydride. ACS Catal. 2021, 11, 7394–7400. [Google Scholar] [CrossRef]
- Ginsberg, A.P.; Abrahams, S.C.; Jamieson, P.B. Nonrigid stereochemistry in eight-coordinate pentahydridorhenium complexes. J. Am. Chem. Soc. 1973, 95, 4751–4752. [Google Scholar] [CrossRef]
- Bau, R.; Carroll, W.E.; Hart, D.W.; Teller, R.G.; Koetzle, T.F. Cystallographic investigations on polyhydride metal complexes. Adv. Chem. Ser. 1978, 167, 73–92. [Google Scholar]
- Emge, T.J.; Koetzle, T.F.; Bruno, J.W.; Caulton, K.G. Pentahydridorhenium: Crystal and molecular structure of ReH5(PMePh2)3. Inorg. Chem. 1984, 23, 4012–4017. [Google Scholar] [CrossRef]
- Lunder, D.M.; Green, M.A.; Streib, W.E.; Caulton, K.G. Structure and reactivity of tetrakis(dimethylphenylphosphine)tetrahydridorhenium(1+). Inorg. Chem. 1989, 28, 4527–4531. [Google Scholar] [CrossRef]
- Alvarez, D., Jr.; Lundquist, E.G.; Ziller, J.W.; Evans, W.J.; Caulton, K.G. Synthesis, structure, and applications of transition-metal polyhydride anions. J. Am. Chem. Soc. 1989, 111, 9392–9398. [Google Scholar] [CrossRef]
- Abdur-Rashid, K.; Lough, A.J.; Morris, R.H. Intra- and inter-ion-pair protonic-hydridic bonding in polyhydridobis(phosphine)rhenates. Can. J. Chem. 2001, 79, 964–976. [Google Scholar] [CrossRef]
- Moehring, G.A.; Williams, C.C.; Buford, J.; Kaviani, M.; Sulko, J.; Fanwick, P.E. Structural Determination and Acidolysis Reactions of Ortho-Metalated Rhenium Tetrahydride Compounds Prepared from Reactions of ReH7(PPh3)2 with Benzylic Imines. Inorg. Chem. 1998, 37, 3848–3852. [Google Scholar] [CrossRef]
- Baudry, D.; Boydell, P.; Ephritikhine, M.; Felkin, H.; Guilhem, J.; Pascard, C.; Dau, E.T.H. Preparation and crystal structure of an η-allyl transition metal tetrahydride, tetrahydrido [1,2-bis(diphenylphosphino)ethane](η3-2-isopropylallyl)rhenium. J. Chem. Soc. Chem. Commun. 1985, 670–671. [Google Scholar] [CrossRef]
- Leeaphon, M.K.; Rohl, T.R.J.; Fanwick, P.E.; Walton, R.A. Reactions of the polyhydride complex ReH7(PPh3)2 with quinoline, 2-hydroxyquinoline, and 2-mercaptoquinoline. The preparation and characterization of hydrido complexes of rhenium(V) and chloro complexes of rhenium(III). Inorg. Chem. 1993, 32, 5562–5568. [Google Scholar] [CrossRef]
- Bolano, S.; Bravo, J.; Garcia-Fontan, S.; Castro, J. Rhenium pentahydride complexes: Characterization and protonation reactions. Crystal structure of ReH5L1L2 (L1 = Ph2PO(CH2)2OPPh2; L2 = P(OCH3)3, P(OCH2CH3)3). J. Organomet. Chem. 2003, 667, 103–111. [Google Scholar] [CrossRef]
- Albinati, A.; Bakhmutov, V.I.; Belkova, N.V.; Bianchini, C.; De los Rios, I.; Epstein, L.; Gutsul, E.I.; Marvelli, L.; Peruzzini, M.; Rossi, R.; et al. Synthesis, Characterization, and Interconversion of the Rhenium Polyhydrides [ReH3(η4-NP3)] and [ReH4(η4-NP3)]+ {NP3 = tris [2-(diphenylphosphanyl)ethyl]amine}. Euro J. Inorg. Chem. 2002, 1530–1539. [Google Scholar] [CrossRef]
- Carr, S.W.; Fowles, E.H.; Fontaine, X.L.R.; Shaw, B.L. Multihydride complexes of rhenium, osmium or iridium containing monodentate ditertiary phosphine ligands: Selective hydrogen-deuterium exchanges of the rhenium multihydrides. J. Chem. Soc. Dalton Trans. 1990, 573–579. [Google Scholar] [CrossRef]
- Kakizawa, T.; Kawano, Y.; Shimoi, M. H-D exchange reaction of borane-lewis base adducts by rhenium polyhydride complexes. Chem. Lett. 1989, 28, 869–870. [Google Scholar] [CrossRef]
- Bianchini, C.; Peruzzini, M.; Zanobini, F.; Magon, L.; Marvelli, L.; Rossi, R. Synthesis and characterization of rhenium polyhydrides stabilized by the tripodal ligand MeC(CH2PPh2)3. J. Organomet. Chem. 1993, 451, 97–106. [Google Scholar] [CrossRef]
- Crabtree, R.H. Dihydrogen complexes: Some structural and chemical studies. Acc. Chem. Res. 1990, 23, 95–101. [Google Scholar] [CrossRef]
- Shanan-Atidi, H.; Bar-Eli, K.H. A convenient method for obtaining free energies of activation by the coalescence temperature of an unequal doublet. J. Phys. Chem. 1970, 74, 961–963. [Google Scholar] [CrossRef]
- Tadros, S.M.; Mansour, M.; Naik, D.V.; Moehring, G.A. Line Shape Analysis of Dynamic NMR Spectra for Characterizing Coordination Sphere Rearrangements at a Chiral Rhenium Polyhydride Complex. J. Vis. Exp. 2022; accepted for publication. [Google Scholar]
- Moehring, G.A.; Walton, R.A. Reactivity of the polyhydride complexes [ReH5(PPh3)3], [ReH3(dppe)2] (dppe = Ph2PCH2CH2PPh2), [ReH3(PPh3)3L], and [ReH4(PPh3)3L]PF6 (L = MeCN or ButNC) towards electrophiles and nucleophiles. J. Chem. Soc. Dalton Trans. 1987, 715–720. [Google Scholar] [CrossRef]
- Costello, M.T.; Fanwick, P.E.; Green, M.A.; Walton, R.A. Rhenium polyhydride complexes that contain the tripodal phosphine CH3C(CH2PPh2)3 (triphos). Inorg. Chem. 1992, 31, 2359–2365. [Google Scholar] [CrossRef]
- Chiu, K.W.; Howard, C.G.; Rzepa, H.S.; Sheppard, R.N.; Wilkinson, G.; Gala, A.M.R.; Hursthouse, M.B. Trimethyl and diethylphenylphosphine complexes of rhenium(I, III, IV, V) and their reaction. X-ray crystal structures of a bis(η5-cyclopentadienyl)-ethanebridged dirhenium(I) complex obtained from phenylacetylene, tetrakis(diethylphenylphosphine) (dinitrogen) hydridorhenium(I), tetrakis(trimethylphosphine) (η2-dimethylphosphinomethyl)rhenium(I) and tetrakis(trimethylphosphine) (iodo)methyl rhenium(III) iodide-tetramethylphosphonium iodide. Polyhedron 1982, 1, 441–451. [Google Scholar] [CrossRef]
- Bolano, S.; Gonsalvi, L.; Barbaro, P.; Albinati, A.; Rizzato, S.; Gutsul, E.; Belkova, N.; Epstein, L.; Shubina, E.; Peruzzini, M.J. Synthesis, characterization, protonation studies and X-ray crystal structure of ReH5(PPh3)2(PTA) (PTA = 1,3,5-triaza-7-phosphaadamantane). J. Organomet. Chem. 2006, 691, 629–637. [Google Scholar] [CrossRef]
- Luo, X.L.; Baudry, D.; Boydell, P.; Charpin, P.; Nierlich, M.; Ephritikhine, M.; Crabtree, R.H. Reaction of heptahydridobis(triphenylphosphine)rhenium with silanes: Preparation and characterization of the first silyl polyhydride complexes, ReH6(SiR3)(PPh3)2 (SiR3 = SiPh3, SiEt3, SiHEt2). Inorg. Chem. 1990, 29, 1511–1517. [Google Scholar] [CrossRef]
- Fontaine, X.L.R.; Layzell, T.P.; Shaw, B.L. New multihydride complexes of rhenium containing diphosphine ligands: Low-temperature protonation of [ReH7(Ph2PCH=CHPPh2-PP′)]. J. Chem. Soc. Dalton Trans. 1994, 917–924. [Google Scholar] [CrossRef]
- Ginsberg, A.P. Nine-co-ordinate octahydrido(tertiary phosphine)rhenate complex anions. Chem. Commun. 1968, 857–858. [Google Scholar] [CrossRef]
- Perez-Acosta, C.; Antunes, N.A.; Gilford, J.C.; Chin, J.A.; Parr, M.L. Rhenium(VII) polyhydrides supported by chelating bis-phosphine ligands: DPEphos, xantphos, and biphep. J. Coord. Chem. 2009, 62, 1051–1057. [Google Scholar] [CrossRef]
- Fanwick, P.E.; Root, D.R.; Walton, R.A. Reactions of the dirhenium(II) complexes Re2Cl4(PR3)4 with lithium aluminum hydride. A convenient synthetic route to the dirhenium octahydride complexes Re2H8(PR3)4. Inorg. Chem. 1989, 28, 3203–3209. [Google Scholar] [CrossRef]
- Patel, B.P.; Yao, W.; Yap, G.P.A.; Rheingold, A.L.; Crabtree, R.H. Re–H⋯H–N interactions in the second-coordination sphere of crystalline [Re(PPh3)2(imidazole)]. Chem. Commun. 1996, 991–992. [Google Scholar] [CrossRef]
- Peris, E.; Wessel, J.; Patel, B.P.; Crabtree, R.H. d0 and d2 polyhydrides as unconventional proton acceptors in intermolecular hydrogen bonding. J. Chem. Soc. Chem. Commun. 1995, 2175–2176. [Google Scholar] [CrossRef]
- Desmurs, P.; Kavallieratos, K.; Yao, W.; Crabtree, R.H. Intermolecular Re-H···H-N and Re-H···base hydrogen bonding estimated in solution by a UV-VIS spectroscopic method. New J. Chem. 1999, 23, 1111. [Google Scholar] [CrossRef]
- Lee, Y.-J.; Lee, J.-D.; Kim, S.-J.; Ko, J.; Suh, I.-H.; Cheong, M.; Kang, S.O. Steric influence on the reactivity of silyl-o-carboranes: Oxidative-addition reactions involving Si-H and B-H activation. Organometallics 2004, 23, 135–143. [Google Scholar] [CrossRef]
- Ozerov, O.V.; Watson, L.A.; Pink, M.; Caulton, K.G. Transformation of acyclic alkenes to hydrido carbynes by (PNPR)Re complexes. J. Am. Chem. Soc. 2004, 126, 6363–6378. [Google Scholar] [CrossRef]
- Ozerov, O.V.; Watson, L.A.; Pink, M.; Baik, M.-H.; Caulton, K.G. Terminal acetylenes react to increase unsaturation in [(tBu2PCH2SiMe2)2N]Re(H)4. Organometallics 2004, 23, 4934–4943. [Google Scholar] [CrossRef]
- Ozerov, O.V.; Huffman, J.C.; Watson, L.A.; Caulton, K.G. Conversion of ethylene to hydride and ethylidyne by an amido rhenium polyhydride. Organometallics 2003, 22, 2539–2541. [Google Scholar] [CrossRef]
- Ozerov, O.V.; Watson, L.A.; Pink, M.; Caulton, K.G. Operationally unsaturated pincer/rhenium complexes form metal carbenes from cycloalkenes and metal carbynes from alkanes. J. Am. Chem. Soc. 2007, 129, 6003–6016. [Google Scholar] [CrossRef] [PubMed]
- Ozerov, O.V.; Watson, L.A.; Pink, M.; Caulton, K.G. A π-basic rhenium center that effects cyclohexene isomerization to a β-agostic carbene ligand. J. Am. Chem. Soc. 2003, 125, 9604–9605. [Google Scholar] [CrossRef] [PubMed]
- Ozerov, O.V.; Pink, M.; Watson, L.A.; Caulton, K.G. Aromatic vs. aliphatic C-H cleavage of alkyl-substituted pyridines by (PNPiPr)Re compounds. J. Am. Chem. Soc. 2004, 126, 2105–2113. [Google Scholar] [CrossRef]
- Baudry, D.; Ephritikhine, M.; Felkin, H.; Zakrzewski, J. Activation of C-H bonds in saturated hydrocarbons. The formation of bis-(triphenylphosphine)(π-alkadiene)rhenium trihydrides from n-alkanes, and their selective conversion into the corresponding 1-alkenes. Tetrahedron Lett. 1984, 25, 1283–1286. [Google Scholar] [CrossRef]
- Trimarchi, M.C.L.; Green, M.A.; Huffman, J.C.; Caulton, K.G. Photoinitiated intramolecular hydrogen transfer from rhenium polyhydrides to C8 cyclopolyolefins. Organometallics 1985, 4, 514. [Google Scholar] [CrossRef]
- Leeaphon, M.; Ondracek, A.L.; Thomas, R.J.; Fanwick, P.E.; Walton, R.A. Reactions of rhenium polyhydrides with internal and terminal alkynes as a route to a new class of hydrido-alkylidyne complexes. J. Am. Chem. Soc. 1995, 117, 9715–9724. [Google Scholar] [CrossRef]
- He, G.; Fan, T.; Chen, J.; Sung, H.H.Y.; Williams, E.D.; Lin, Z.; Jia, G. Effects of substituents on the formation of rhenium carbyne and η2-vinyl complexes from the reactions of ReH5(PMe2Ph)3 with terminal alkynes. New J. Chem. 2013, 37, 1823–1932. [Google Scholar] [CrossRef]
- Chen, J.; Shi, C.; Sung, H.H.Y.; Williams, I.D.; Lin, Z.; Jia, G. Synthesis and characterization of rhenabenzyne complexes. Eur. J. Chem. 2012, 18, 14128–14139. [Google Scholar] [CrossRef]
- Chen, J.; He, G.; Sung, H.H.Y.; Williams, I.D.; Lin, Z.; Jia, G. Rhenium carbyne and η2-vinyl complexes from one-pot reactions of ReH5(PMe2Ph)3 with terminal alkynes. Organometallics 2010, 29, 2693–2701. [Google Scholar] [CrossRef]
- He, G.; Chen, J.; Sung, H.H.-Y.; Williams, I.D.; Jia, G. Substituent effect on reactions of ReH5(PMe2Ph)3 with propargyl alcohols. Inorg. Chim. Acta 2021, 518, 120239. [Google Scholar] [CrossRef]
- Kelle Zeiher, E.H.; DeWit, D.G.; Caulton, K.G. Mechanistic features of C-H activation by ReH7{P(C6H11)3]2. J. Am. Chem. Soc. 1984, 106, 7006–7011. [Google Scholar] [CrossRef]
- Baudry, D.; Daran, J.-C.; Dromzee, Y.; Ephritikhine, M.; Felkin, H.; Jeannin, Y.; Zakrzewski, J. The disruption of furan by bis(triphenylphosphine)rhenium heptahydride: Synthesis and crystal structure of the 1-oxapentadienyl complex Re(η5-C4H5O)(PPh3)2(CO). J. Chem. Soc. Chem. Commun. 1983, 813–814. [Google Scholar] [CrossRef]
- Morris, R.H. Estimating the Acidity of Transition Metal Hydride and Dihydrogen Complexes by Adding Ligand Acidity Constants. J. Am. Chem. Soc. 2014, 136, 1948–1959. [Google Scholar] [CrossRef] [PubMed]
- Abdur-Rashid, K.; Fong, T.P.; Greaves, B.; Gusev, D.G.; Hinman, J.G.; Landau, S.E.; Lough, A.J.; Morris, R.H. An Acidity Scale for Phosphorus-Containing Compounds Including Metal Hydrides and Dihydrogen Complexes in THF: Toward the Unification of Acidity Scales. J. Am. Chem. Soc. 2000, 122, 9155–9171. [Google Scholar] [CrossRef]
- Jones, W.D.; Maguire, J.A. Direct intermolecular transfer of dihydrogen between two transition metal complexes. J. Am. Chem. Soc. 1985, 107, 4544–4546. [Google Scholar] [CrossRef]
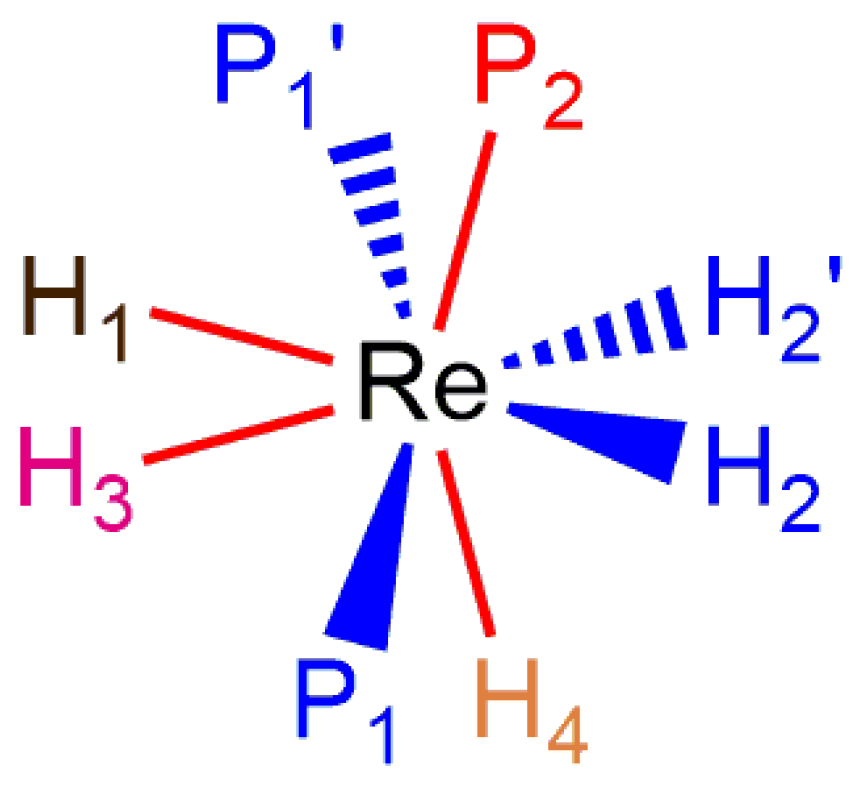

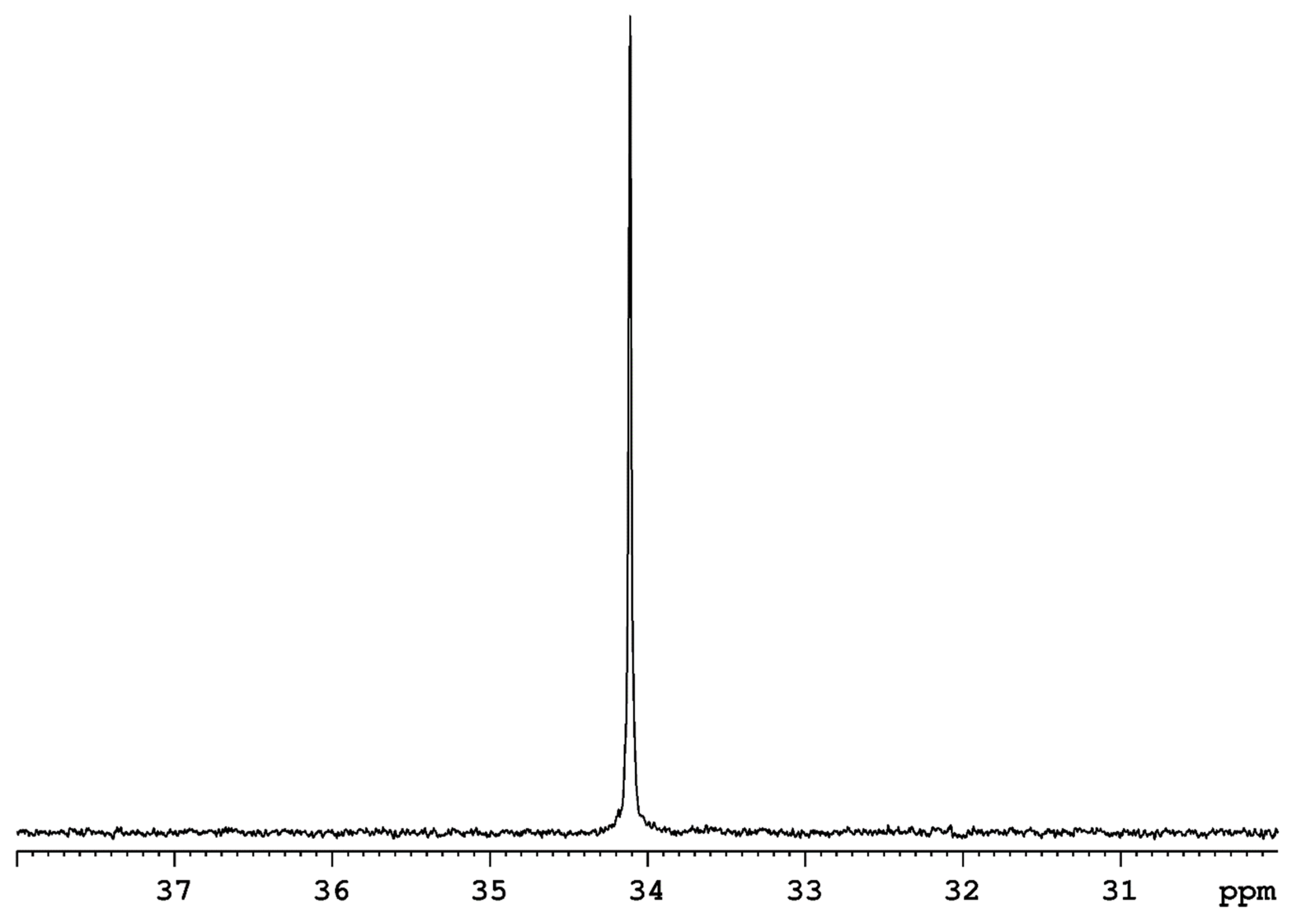
















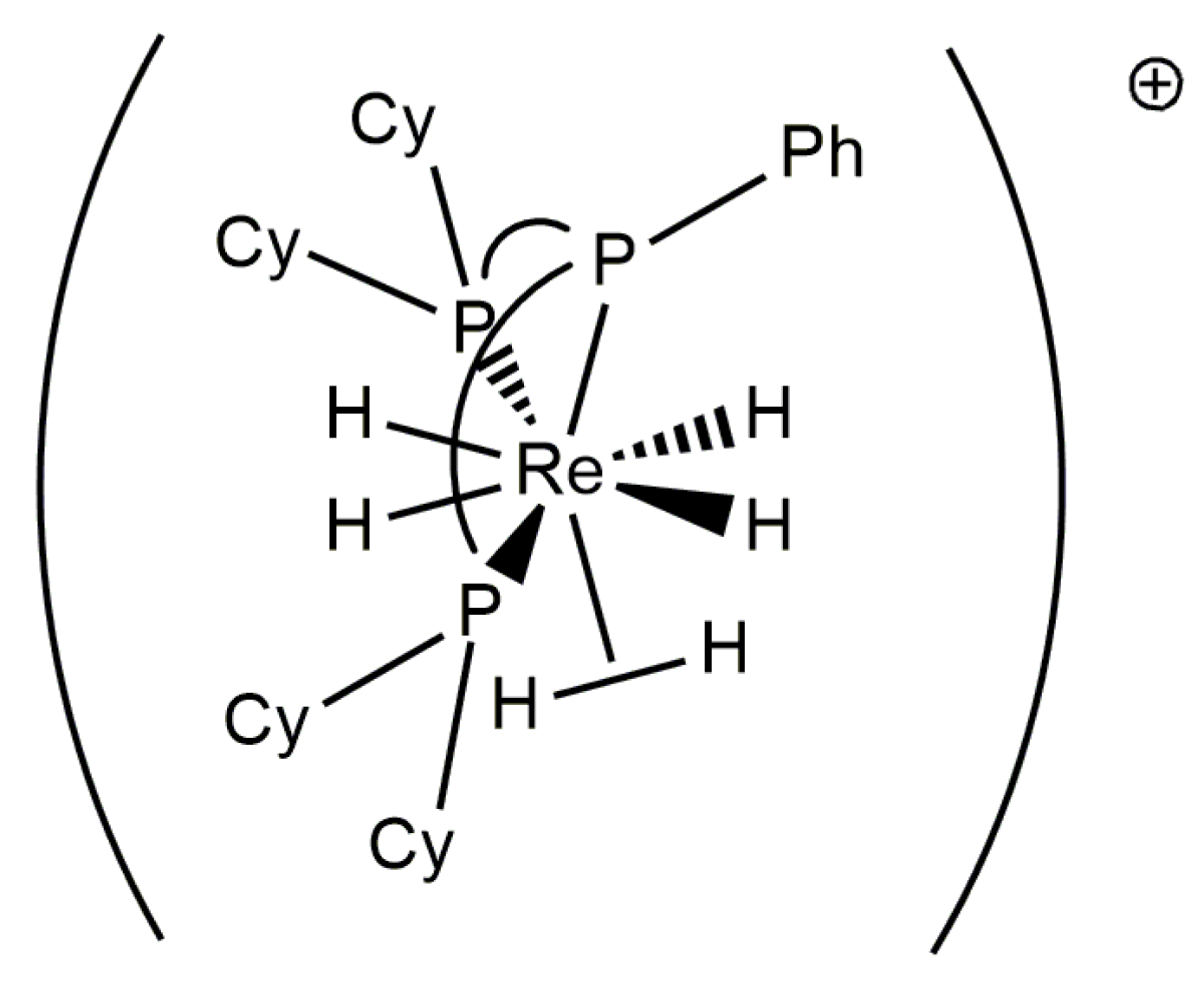

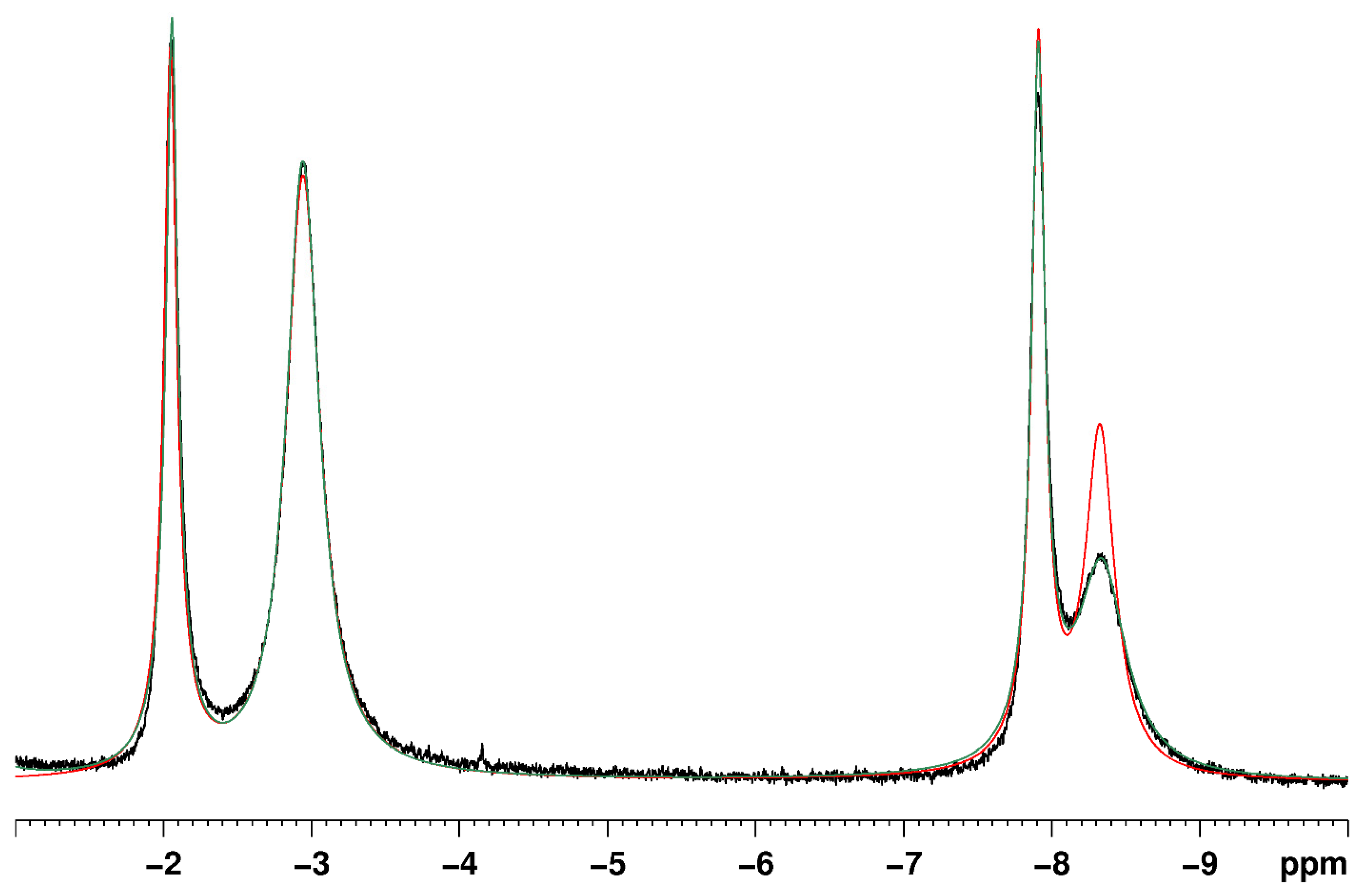
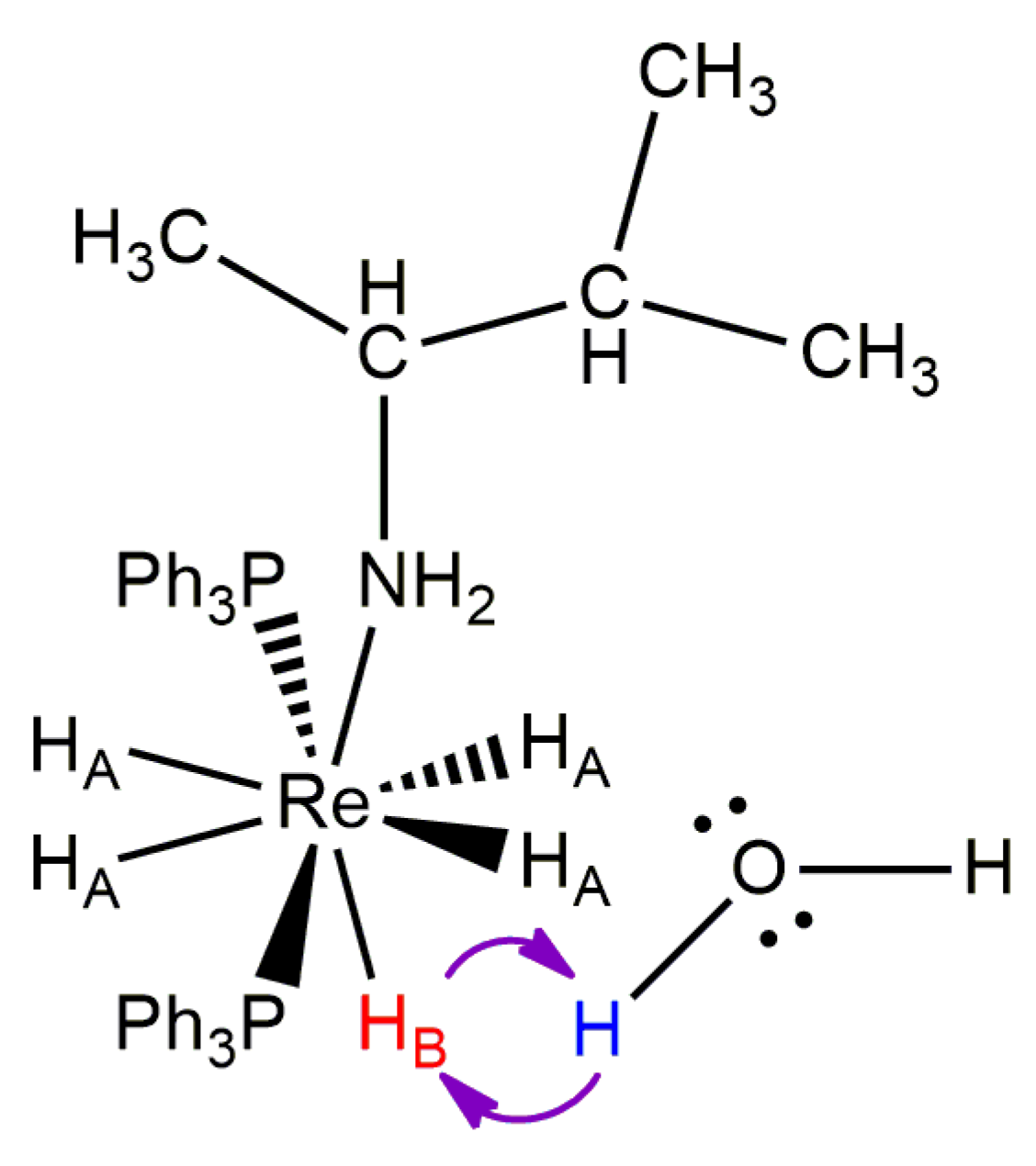
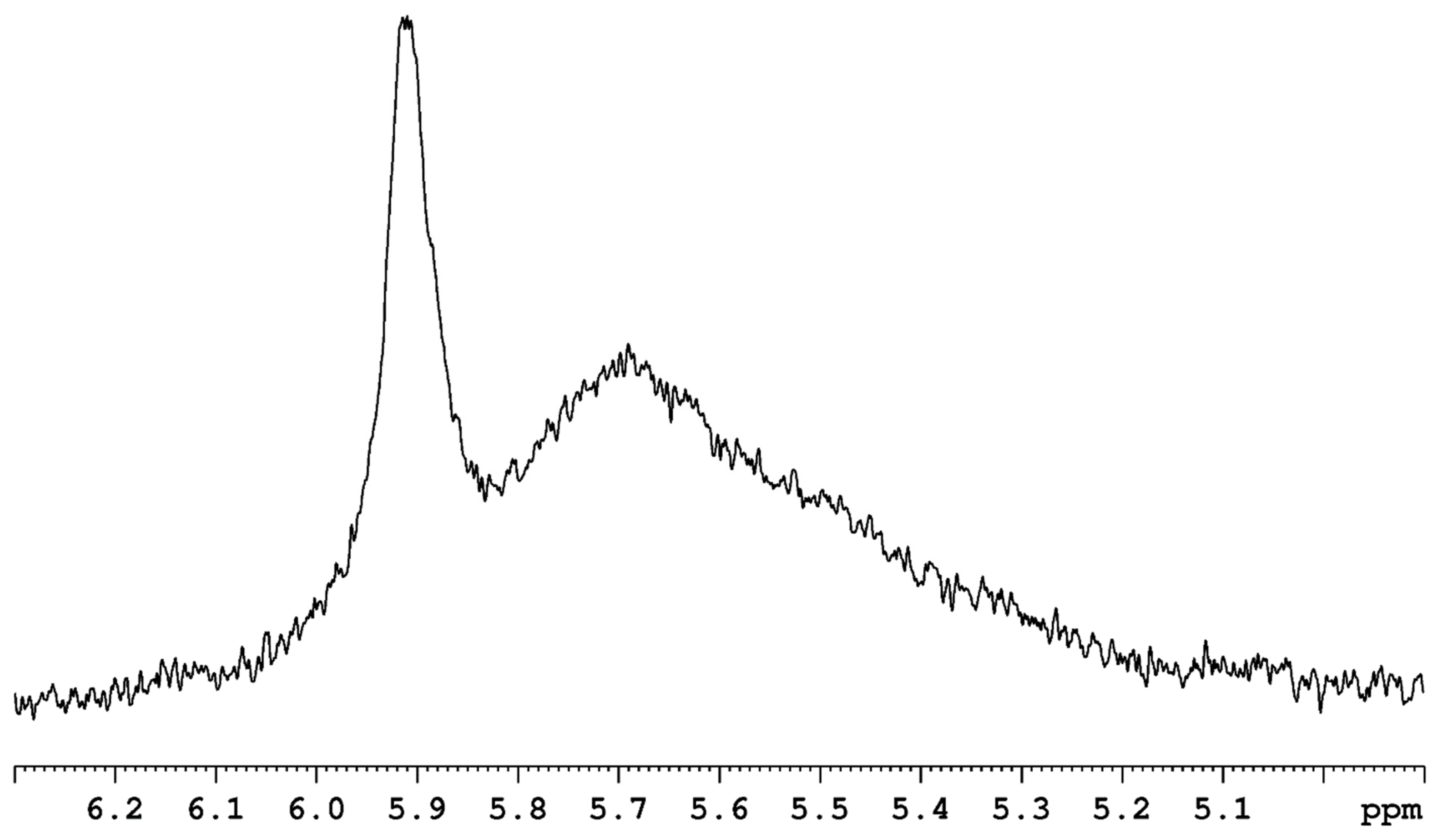

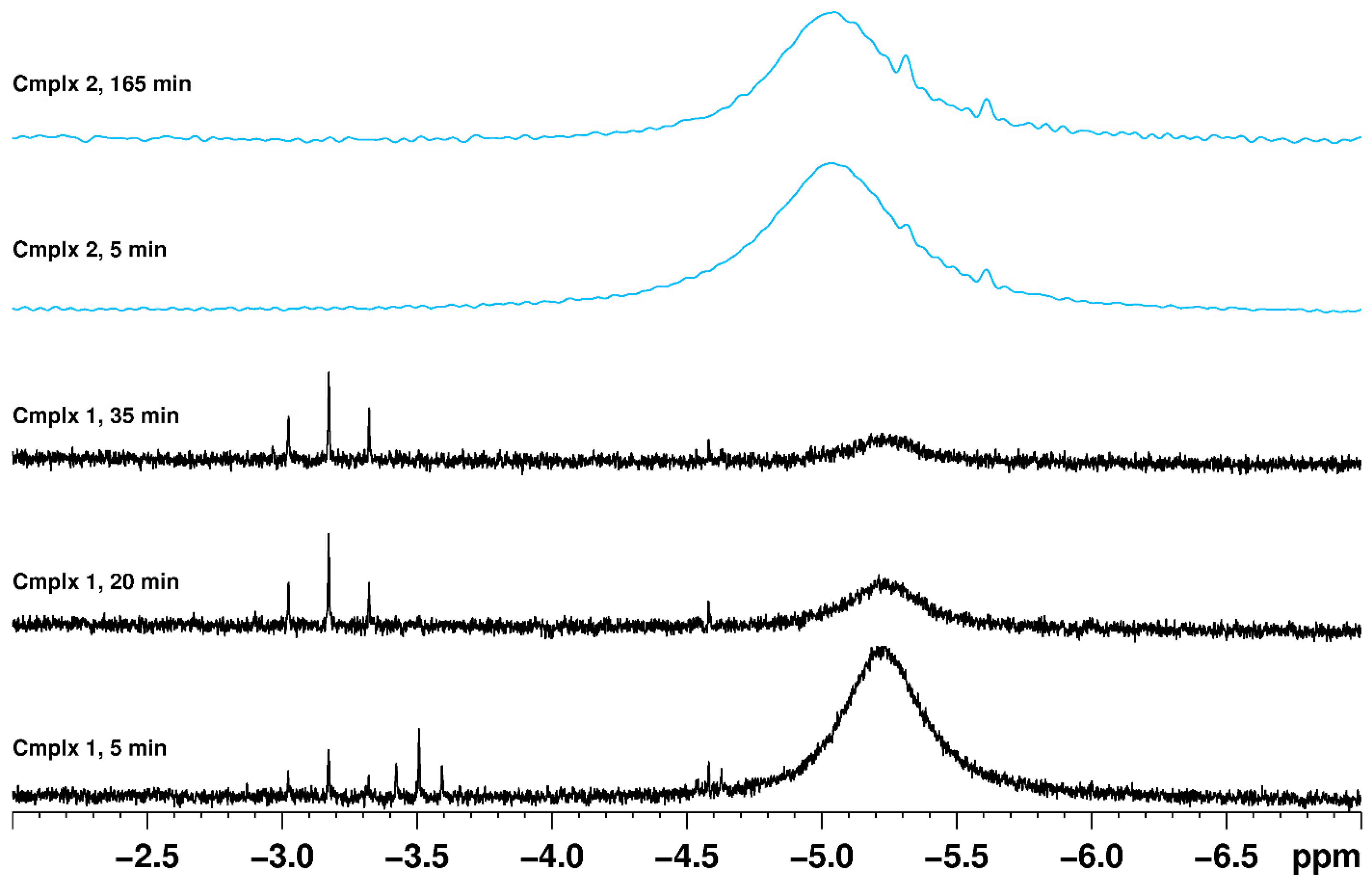
| Complex | ΔG‡260 (kcal/mol) | ΔH‡ (kcal/mol) | ΔS‡ (cal/mol·K) |
|---|---|---|---|
| ReH5(PPh3)2(1,2-ethanolamine) [33] | 10.8 | 14.5 | 18.5 |
| ReH5(PPh3)2(ethylene diamine) [33] | 10.5 | 13.4 | 14.4 |
| ReH5(PPh3)2(1,3-diaminopropane) [33] | 10.4 | 13.4 | 14.9 |
| ReH5(PPh3)2(α,4-dimethylbenzylamine) [37] | 8.4 | 20.7 | 47.3 |
| ReH5(PPh3)2(2-amino-3-methylbutane) [37] | 10.5 | 13.0 | 9.4 |
| ReH5(PPh3)2[4-CH3CH(OH)py] [37] | 9.9 | 11.0 | 4.4 |
| Complex | ΔG‡(260) (kcal/mol) | ΔH‡ (kcal/mol) | ΔS‡ (cal/mol·K) | Resonances Fitted |
|---|---|---|---|---|
| [ReH4(PPh3)3(NCMe)]PF6 [34] | 12 | 8.5 | −13.7 | 1H Hydride |
| 12.9 | 6.5 | −24.8 | 31P | |
| ReH5(PPh3)2(α,4-dimethylbenzylamine) [37] | 11.9 | 6.1 | −22.3 | 1H Hydride |
| 11.8 | 7.6 | −16 | 31P | |
| ReH5(PPh3)2[4-CH3CH(OH)py] [37] | 11.8 | 5.6 | −23.7 | 1H Hydride |
| 11.6 | 6.7 | −18.8 | 1H 3 and 5 | |
| ReH5(PPh3)2(2-amino−3-methylbutane) [37] | 11.5 | 5.7 | −22.3 | 1H Hydride |
| ReH5(PPh3)2(sec-butylamine) [33] | 11.4 | 9 | −9.2 | 1H Hydride |
| 11.5 | 6.2 | −20.5 | 31P | |
| ReH4I(PPh3)3 [34] | 10.9 | 6.3 | −17.6 | 1H Hydride |
| 11.2 | 4.9 | −24.4 | 31P | |
| ReH5(PPh3)2(1,3-diaminopropane) [33] | 11 | 7.5 | −13.6 | 1H Hydride |
| ReH5[Cy2PO(CH2)2OPCy2][P(OEt)3] [10] | 10.9 | 14.1 | 12.5 | 1H Hydride |
| ReH5[Cy2PO(CH2)2OPCy2][PPh(OEt)2] [10] | 10.8 | 13.1 | 8.9 | 1H Hydride |
| ReH5(PPh3)2(1,2-diaminoethane) [33] | 10.8 | 8.9 | −7.3 | 1H Hydride |
| ReH5(PPh3)2(1,2-ethanolamine) [33] | 10.6 | 9.7 | −3.6 | 1H Hydride |
| ReH5(PPh3)2(3-aminopyridine) [33] | 10.3 | 14.4 | 15.8 | 31P |
| ReH5(PPh3)2(3-picoline) [33] | 10.4 | 16.9 | 24.8 | 31P |
| 10.3 | 16 | 22.1 | 1H Methyl | |
| ReH5[Cy2PO(CH2)2OPCy2][PPh2(OEt)] [10] | 9.9 | 12.3 | 9.3 | 1H Hydride |
| ReH5(PPh3)3 [33] | 9.3 | 6.9 | −9.2 | 31P |
| [ReH4(η4-tris{2-(diphenylphosphanyl)ethyl}amine]+ [59] | 9.1 | 8 | −2.9 | 1H Hydride |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naik, D.V.; Moehring, G.A. Dynamic Processes of Rhenium Polyhydride Complexes. Molecules 2022, 27, 5017. https://doi.org/10.3390/molecules27155017
Naik DV, Moehring GA. Dynamic Processes of Rhenium Polyhydride Complexes. Molecules. 2022; 27(15):5017. https://doi.org/10.3390/molecules27155017
Chicago/Turabian StyleNaik, Datta V., and Gregory A. Moehring. 2022. "Dynamic Processes of Rhenium Polyhydride Complexes" Molecules 27, no. 15: 5017. https://doi.org/10.3390/molecules27155017