Quantitative Proteomics Explore the Potential Targets and Action Mechanisms of Hydroxychloroquine
Abstract
:1. Introduction
2. Results
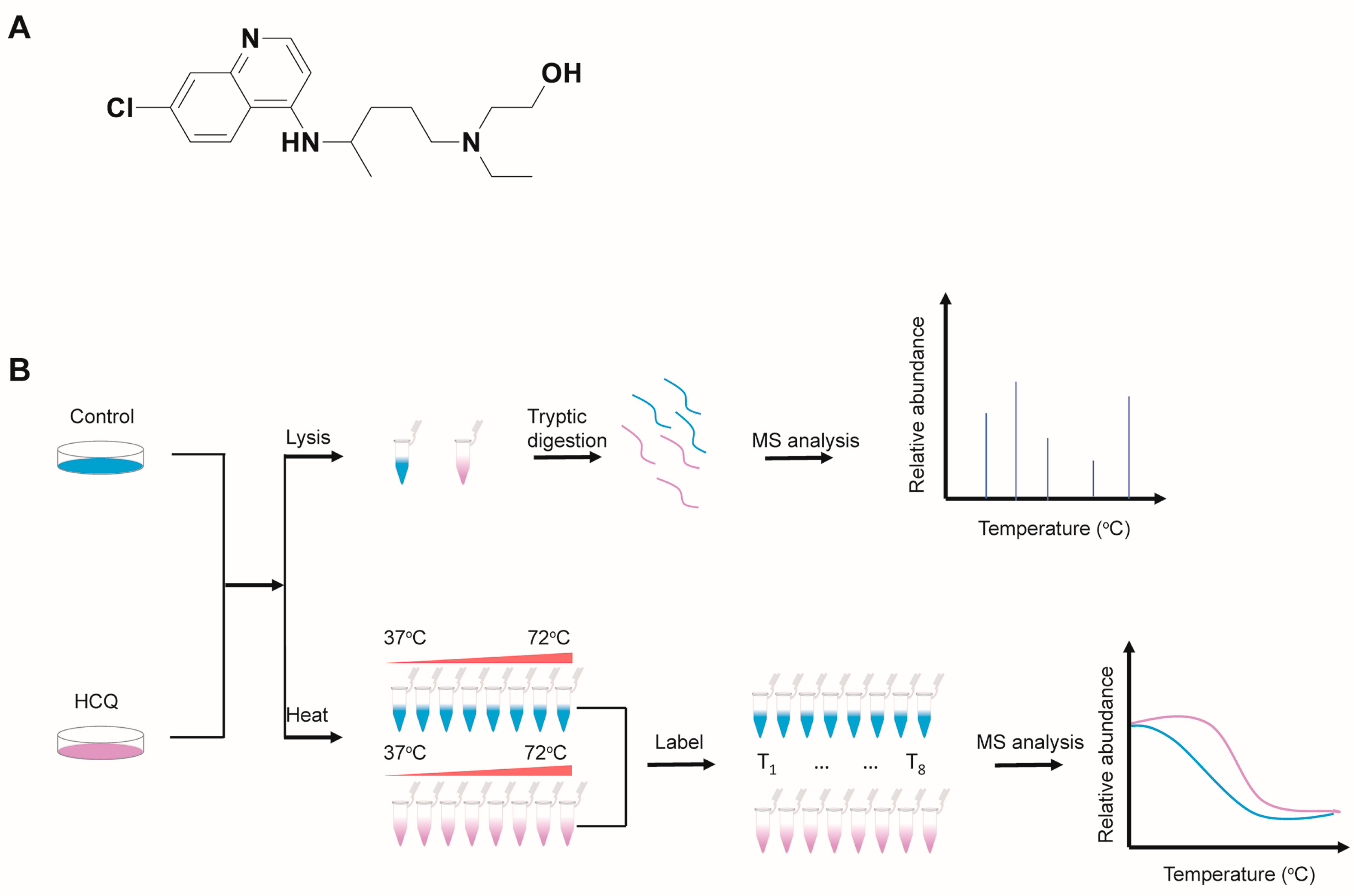
2.1. Overview of the Quantitative Proteomics and Thermal Proteome Profiling Studies
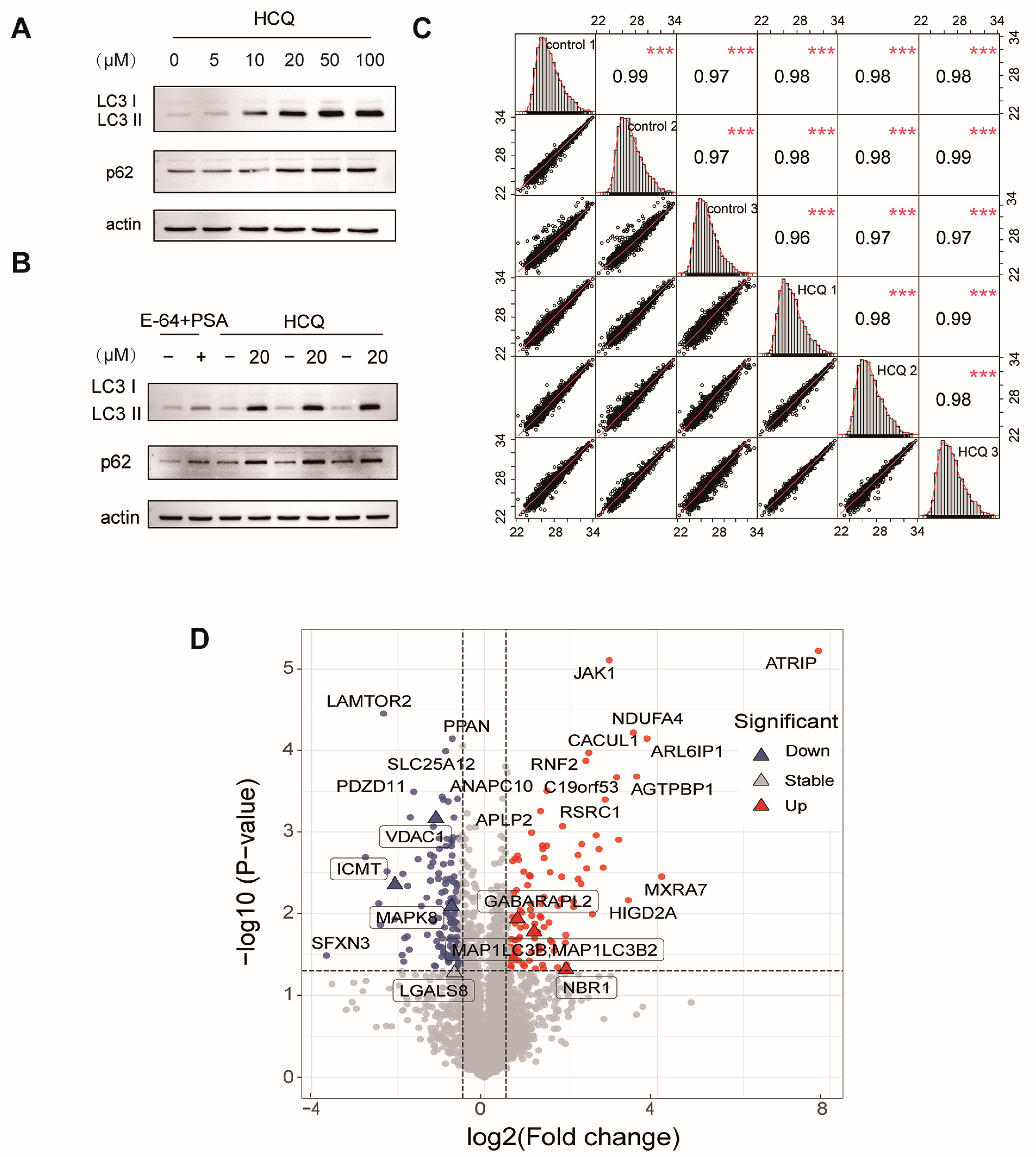
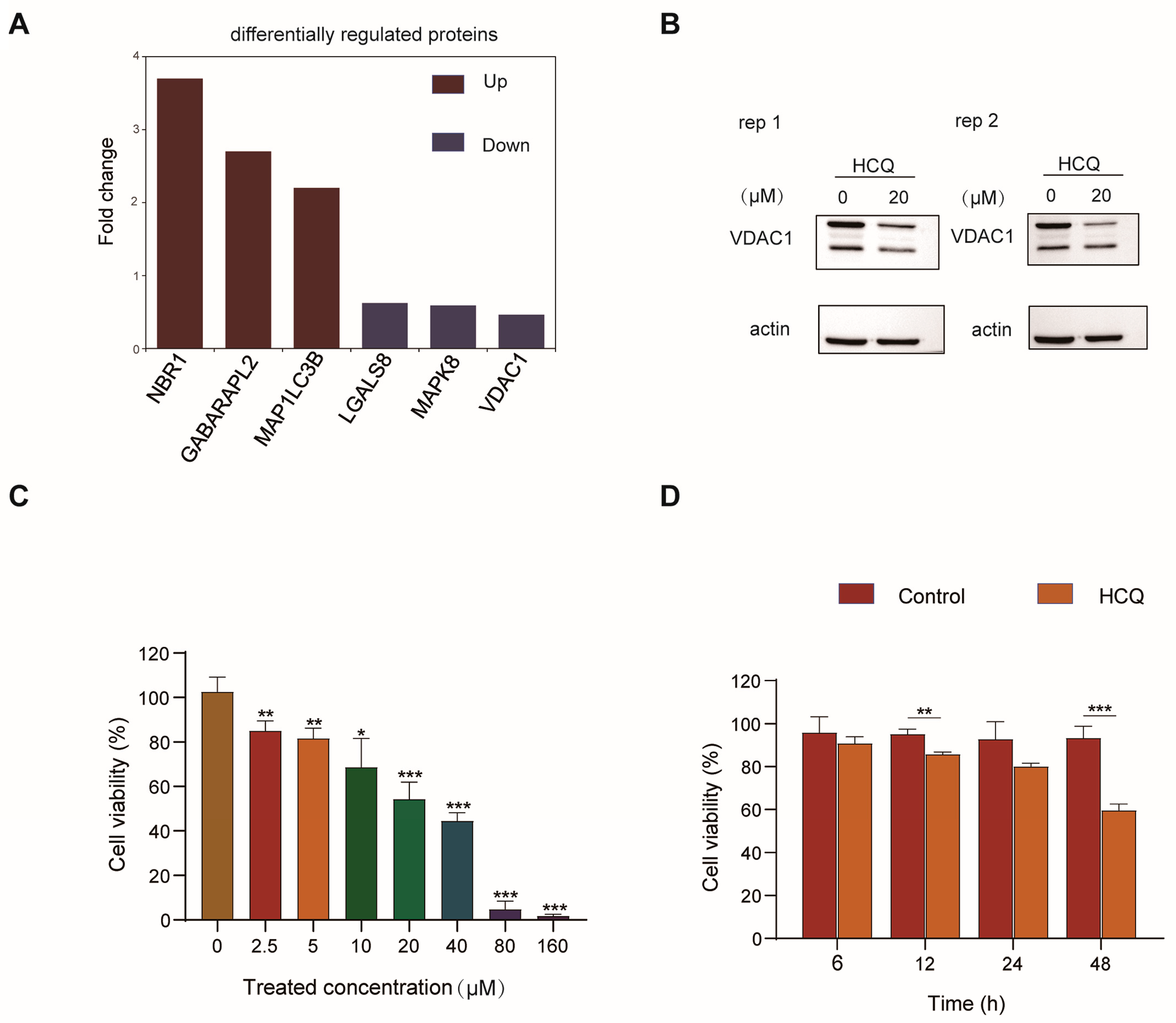
2.2. Dynamics of the Proteome Induced by HCQ
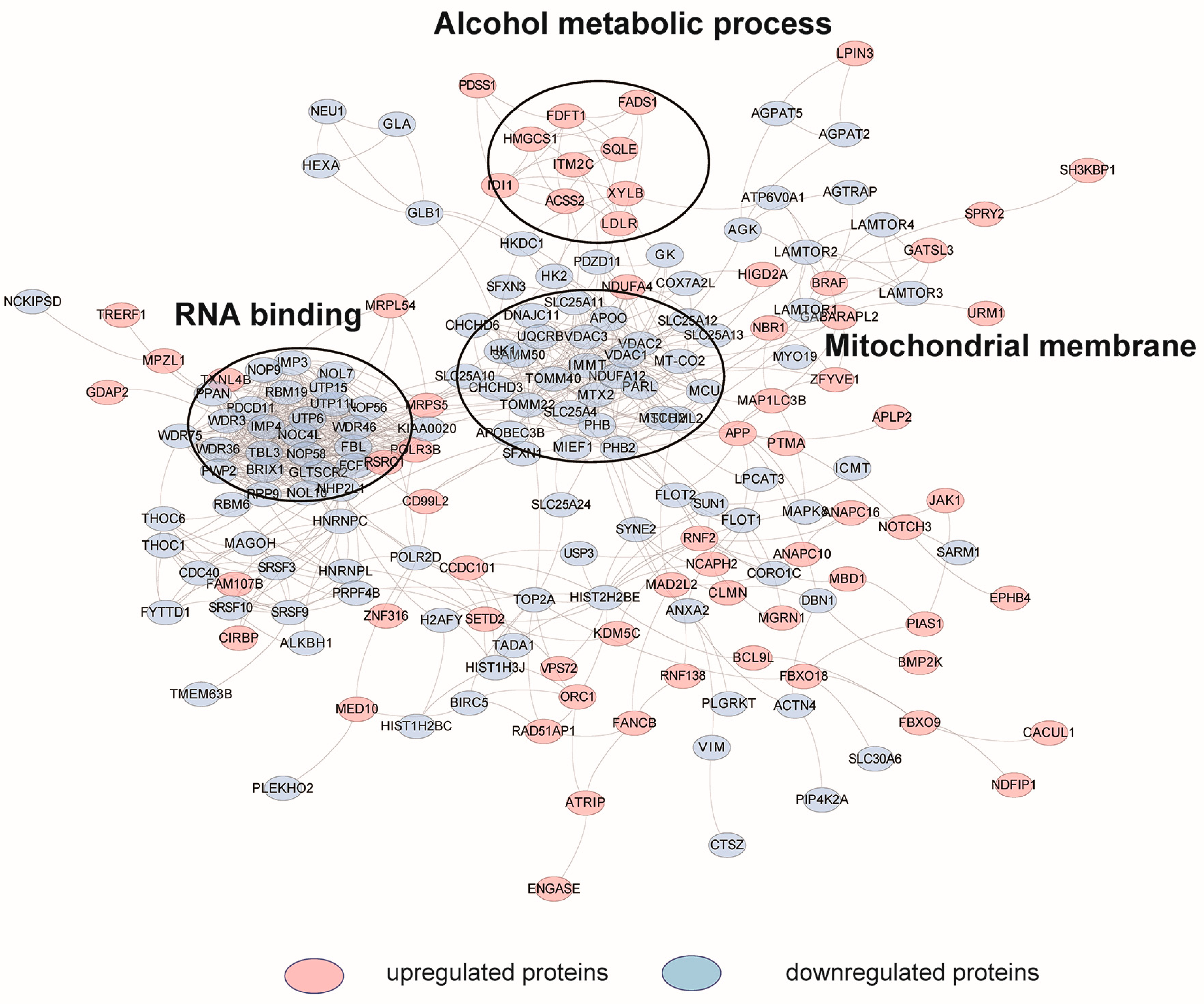
2.3. Cellular Pathway Analysis of the Dynamic Proteins Induced by HCQ
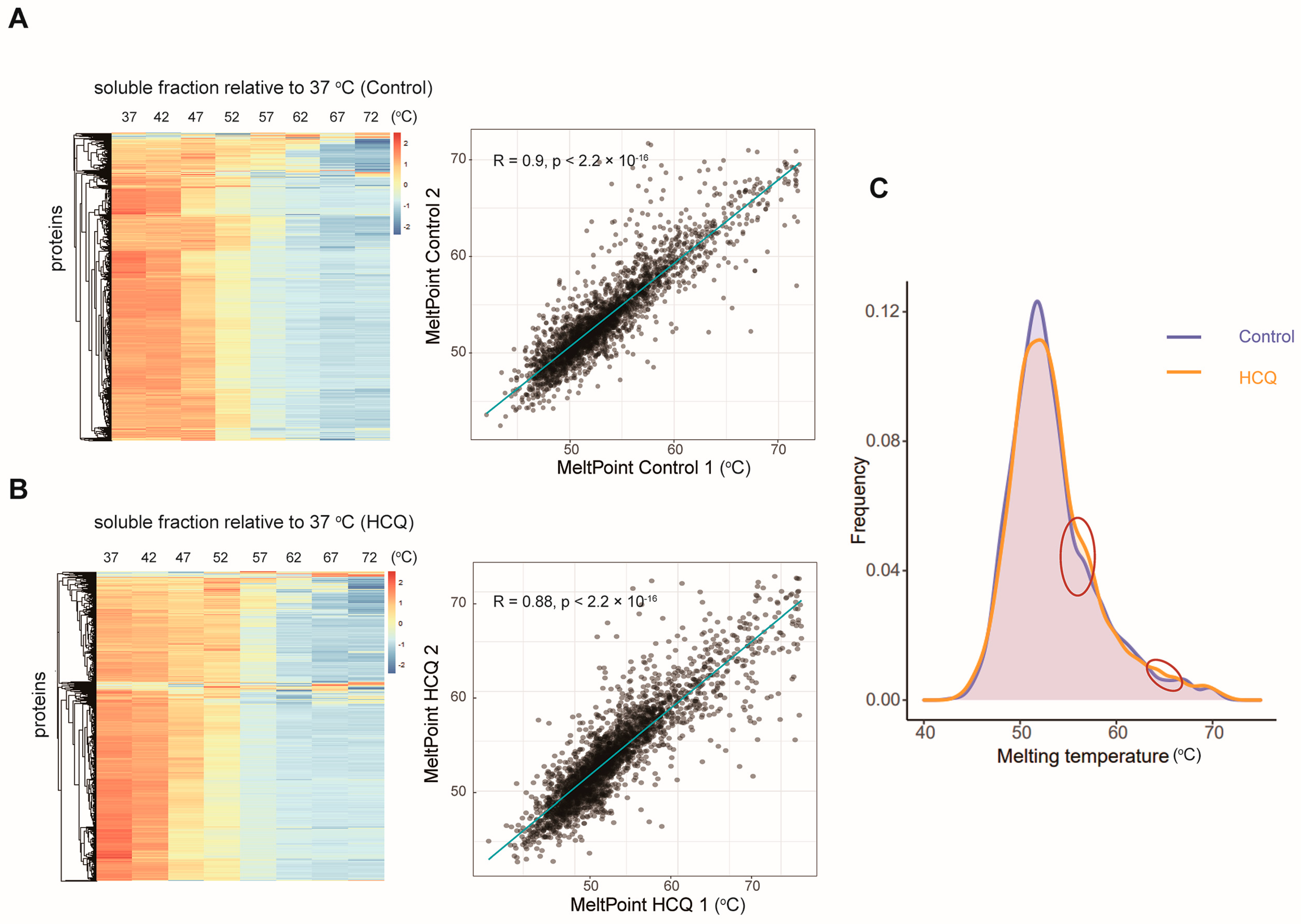
2.4. Thermal Proteome Profiling of MIA PaCa-2 Cells Treated with HCQ
2.5. Identification of the Potential Binding Proteins of HCQ
3. Discussion
4. Materials and Methods
4.1. Cell Lines, Antibody, and TMT Agent
4.2. Cell Culture
4.3. Thermal Proteome Profiling
4.4. TMT Labeling
4.5. Proteomics Sample Analysis
4.6. LC-MS/MS Analysis
4.7. Data Analysis
4.8. Bioinformatic Analysis
4.9. Cell Viability Assay
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Koranda, F.C. Antimalarials. J. Am. Acad. Dermatol. 1981, 4, 650–655. [Google Scholar] [CrossRef]
- Al-Bari, M.A. Chloroquine analogues in drug discovery: New directions of uses, mechanisms of actions and toxic manifestations from malaria to multifarious diseases. J. Antimicrob. Chemother. 2015, 70, 1608–1621. [Google Scholar] [CrossRef]
- Schrezenmeier, E.; Dörner, T. Mechanisms of action of hydroxychloroquine and chloroquine: Implications for rheumatology. Nat. Rev. Rheumatol. 2020, 16, 155–166. [Google Scholar] [CrossRef]
- Mekinian, A.; Lazzaroni, M.G.; Kuzenko, A.; Alijotas-Reig, J.; Ruffatti, A.; Levy, P.; Canti, V.; Bremme, K.; Bezanahary, H.; Bertero, T.; et al. The efficacy of hydroxychloroquine for obstetrical outcome in anti-phospholipid syndrome: Data from a European multicenter retrospective study. Autoimmun. Rev. 2015, 14, 498–502. [Google Scholar] [CrossRef]
- Yoon, Y.H.; Cho, K.S.; Hwang, J.J.; Lee, S.J.; Choi, J.A.; Koh, J.Y. Induction of lysosomal dilatation, arrested autophagy, and cell death by chloroquine in cultured ARPE-19 cells. Investig. Ophthalmol. Vis. Sci. 2010, 51, 6030–6037. [Google Scholar] [CrossRef]
- Kersh, G.J. Antimicrobial therapies for Q fever. Expert Rev. Anti-Infect. Ther. 2013, 11, 1207–1214. [Google Scholar] [CrossRef]
- Keshavarzi, F. Fungistatic effect of hydroxychloroquine, lessons from a case. Med. Mycol. Case Rep. 2016, 13, 17–18. [Google Scholar] [CrossRef]
- Wang, L.F.; Lin, Y.S.; Huang, N.C.; Yu, C.Y.; Tsai, W.L.; Chen, J.J.; Kubota, T.; Matsuoka, M.; Chen, S.R.; Yang, C.S.; et al. Hydroxychloroquine-inhibited dengue virus is associated with host defense machinery. J. Interferon Cytokine Res. 2015, 35, 143–156. [Google Scholar] [CrossRef]
- Lagier, J.C.; Fenollar, F.; Lepidi, H.; Giorgi, R.; Million, M.; Raoult, D. Treatment of classic Whipple’s disease: From in vitro results to clinical outcome. J. Antimicrob. Chemother. 2014, 69, 219–227. [Google Scholar] [CrossRef]
- Verbaanderd, C.; Maes, H.; Schaaf, M.B.; Sukhatme, V.P.; Pantziarka, P.; Sukhatme, V.; Agostinis, P.; Bouche, G. Repurposing Drugs in Oncology (ReDO)-chloroquine and hydroxychloroquine as anti-cancer agents. Ecancermedicalscience 2017, 11, 781. [Google Scholar] [CrossRef]
- Bigelsen, S. Evidence-based complementary treatment of pancreatic cancer: A review of adjunct therapies including paricalcitol, hydroxychloroquine, intravenous vitamin C, statins, metformin, curcumin, and aspirin. Cancer Manag. Res. 2018, 10, 2003–2018. [Google Scholar] [CrossRef]
- Goldberg, S.B.; Supko, J.G.; Neal, J.W.; Muzikansky, A.; Digumarthy, S.; Fidias, P.; Temel, J.S.; Heist, R.S.; Shaw, A.T.; McCarthy, P.O.; et al. A phase I study of erlotinib and hydroxychloroquine in advanced non-small-cell lung cancer. J. Thorac. Oncol. 2012, 7, 1602–1608. [Google Scholar] [CrossRef]
- Vogl, D.T.; Stadtmauer, E.A.; Tan, K.S.; Heitjan, D.F.; Davis, L.E.; Pontiggia, L.; Rangwala, R.; Piao, S.; Chang, Y.C.; Scott, E.C.; et al. Combined autophagy and proteasome inhibition: A phase 1 trial of hydroxychloroquine and bortezomib in patients with relapsed/refractory myeloma. Autophagy 2014, 10, 1380–1390. [Google Scholar] [CrossRef]
- Onorati, A.V.; Dyczynski, M.; Ojha, R.; Amaravadi, R.K. Targeting autophagy in cancer. Cancer 2018, 124, 3307–3318. [Google Scholar] [CrossRef]
- Lin, Y.C.; Lin, J.F.; Wen, S.I.; Yang, S.C.; Tsai, T.F.; Chen, H.E.; Chou, K.Y.; Hwang, T.I. Chloroquine and hydroxychloroquine inhibit bladder cancer cell growth by targeting basal autophagy and enhancing apoptosis. Kaohsiung J. Med. Sci. 2017, 33, 215–223. [Google Scholar] [CrossRef]
- Yang, S.; Wang, X.; Contino, G.; Liesa, M.; Sahin, E.; Ying, H.; Bause, A.; Li, Y.; Stommel, J.M.; Dell’antonio, G.; et al. Pancreatic cancers require autophagy for tumor growth. Genes Dev. 2011, 25, 717–729. [Google Scholar] [CrossRef]
- Yamamoto, K.; Venida, A.; Yano, J.; Biancur, D.E.; Kakiuchi, M.; Gupta, S.; Sohn, A.S.W.; Mukhopadhyay, S.; Lin, E.Y.; Parker, S.J.; et al. Autophagy promotes immune evasion of pancreatic cancer by degrading MHC-I. Nature 2020, 581, 100–105. [Google Scholar] [CrossRef]
- Savitski, M.M.; Reinhard, F.B.; Franken, H.; Werner, T.; Savitski, M.F.; Eberhard, D.; Martinez Molina, D.; Jafari, R.; Dovega, R.B.; Klaeger, S.; et al. Tracking cancer drugs in living cells by thermal profiling of the proteome. Science 2014, 346, 1255784. [Google Scholar] [CrossRef]
- Kirkin, V.; Lamark, T.; Sou, Y.S.; Bjørkøy, G.; Nunn, J.L.; Bruun, J.A.; Shvets, E.; McEwan, D.G.; Clausen, T.H.; Wild, P.; et al. A role for NBR1 in autophagosomal degradation of ubiquitinated substrates. Mol. Cell 2009, 33, 505–516. [Google Scholar] [CrossRef]
- Weidberg, H.; Shvets, E.; Shpilka, T.; Shimron, F.; Shinder, V.; Elazar, Z. LC3 and GATE-16/GABARAP subfamilies are both essential yet act differently in autophagosome biogenesis. EMBO J. 2010, 29, 1792–1802. [Google Scholar] [CrossRef]
- Thurston, T.L.; Wandel, M.P.; von Muhlinen, N.; Foeglein, A.; Randow, F. Galectin 8 targets damaged vesicles for autophagy to defend cells against bacterial invasion. Nature 2012, 482, 414–418. [Google Scholar] [CrossRef]
- Jia, J.; Abudu, Y.P.; Claude-Taupin, A.; Gu, Y.; Kumar, S.; Choi, S.W.; Peters, R.; Mudd, M.H.; Allers, L.; Salemi, M.; et al. Galectins control MTOR and AMPK in response to lysosomal damage to induce autophagy. Autophagy 2019, 15, 169–171. [Google Scholar] [CrossRef]
- Wei, Y.; Pattingre, S.; Sinha, S.; Bassik, M.; Levine, B. JNK1-mediated phosphorylation of Bcl-2 regulates starvation-induced autophagy. Mol. Cell 2008, 30, 678–688. [Google Scholar] [CrossRef]
- Yang, X.; Zhou, Y.; Liang, H.; Meng, Y.; Liu, H.; Zhou, Y.; Huang, C.; An, B.; Mao, H.; Liao, Z. VDAC1 promotes cardiomyocyte autophagy in anoxia/reoxygenation injury via the PINK1/Parkin pathway. Cell Biol. Int. 2021, 45, 1448–1458. [Google Scholar] [CrossRef]
- Cui, L.; Nakano, K.; Obchoei, S.; Setoguchi, K.; Matsumoto, M.; Yamamoto, T.; Obika, S.; Shimada, K.; Hiraoka, N. Small Nucleolar Noncoding RNA SNORA23, Up-Regulated in Human Pancreatic Ductal Adenocarcinoma, Regulates Expression of Spectrin Repeat-Containing Nuclear Envelope 2 to Promote Growth and Metastasis of Xenograft Tumors in Mice. Gastroenterology 2017, 153, 292–306.e292. [Google Scholar] [CrossRef]
- Manu, K.A.; Chai, T.F.; Teh, J.T.; Zhu, W.L.; Casey, P.J.; Wang, M. Inhibition of Isoprenylcysteine Carboxylmethyltransferase Induces Cell-Cycle Arrest and Apoptosis through p21 and p21-Regulated BNIP3 Induction in Pancreatic Cancer. Mol. Cancer Ther. 2017, 16, 914–923. [Google Scholar] [CrossRef]
- Kitada, M.; Koya, D. Autophagy in metabolic disease and ageing. Nat. Rev. Endocrinol. 2021, 17, 647–661. [Google Scholar] [CrossRef]
- Zhao, X.; Su, L.; He, X.; Zhao, B.; Miao, J. Long noncoding RNA CA7-4 promotes autophagy and apoptosis via sponging MIR877-3P and MIR5680 in high glucose-induced vascular endothelial cells. Autophagy 2020, 16, 70–85. [Google Scholar] [CrossRef]
- Wauson, E.M.; Dbouk, H.A.; Ghosh, A.B.; Cobb, M.H. G protein-coupled receptors and the regulation of autophagy. Trends Endocrinol. Metab. TEM 2014, 25, 274–282. [Google Scholar] [CrossRef]
- Doncheva, N.T.; Morris, J.H.; Gorodkin, J.; Jensen, L.J. Cytoscape StringApp: Network Analysis and Visualization of Proteomics Data. J. Proteome Res. 2019, 18, 623–632. [Google Scholar] [CrossRef]
- Jing, J.; Wang, B.; Liu, P. The Functional Role of SEC23 in Vesicle Transportation, Autophagy and Cancer. Int. J. Biol. Sci. 2019, 15, 2419–2426. [Google Scholar] [CrossRef]
- Dammermann, A.; Merdes, A. Assembly of centrosomal proteins and microtubule organization depends on PCM-1. J. Cell Biol. 2002, 159, 255–266. [Google Scholar] [CrossRef]
- Holdgaard, S.G.; Cianfanelli, V.; Pupo, E.; Lambrughi, M.; Lubas, M.; Nielsen, J.C.; Eibes, S.; Maiani, E.; Harder, L.M.; Wesch, N.; et al. Selective autophagy maintains centrosome integrity and accurate mitosis by turnover of centriolar satellites. Nat. Commun. 2019, 10, 4176. [Google Scholar] [CrossRef]
- Evans, A.S.; Lennemann, N.J.; Coyne, C.B. BPIFB3 interacts with ARFGAP1 and TMED9 to regulate non-canonical autophagy and RNA virus infection. J. Cell Sci. 2021, 134, jcs251835. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, Y.; Li, N.; Liu, W.T.; Liang, J.Z.; Sun, Y.; Zhang, W.X.; Fang, R.D.; Huang, S.L.; Sun, Z.H.; et al. Curcumol Overcomes TRAIL Resistance of Non-Small Cell Lung Cancer by Targeting NRH:Quinone Oxidoreductase 2 (NQO2). Adv. Sci. 2020, 7, 2002306. [Google Scholar] [CrossRef]
- Saxton, R.A.; Sabatini, D.M. mTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 168, 960–976. [Google Scholar] [CrossRef]
- Ha, J.Y.; Kim, J.S.; Kang, Y.H.; Bok, E.; Kim, Y.S.; Son, J.H. Tnfaip8l1/Oxi-β binds to FBXW5, increasing autophagy through activation of TSC2 in a Parkinson’s disease model. J. Neurochem. 2013, 129, 527–538. [Google Scholar] [CrossRef]
- Gan, W.; Zhang, C.; Siu, K.Y.; Satoh, A.; Tanner, J.A.; Yu, S. ULK1 phosphorylates Sec23A and mediates autophagy-induced inhibition of ER-to-Golgi traffic. BMC Cell Biol. 2017, 18, 22. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, P.; Shen, D.; Liu, H.; Xu, L.; Wang, J.; Shen, D.; Sun, H.; Wu, H. FAM172A inhibits EMT in pancreatic cancer via ERK-MAPK signaling. Biol. Open 2020, 9, bio048462. [Google Scholar] [CrossRef]
- Shanks, G.D. Historical Review: Problematic Malaria Prophylaxis with Quinine. Am. J. Trop. Med. Hyg. 2016, 95, 269–272. [Google Scholar] [CrossRef]
- Bozic, B.; Uzelac, T.V.; Kezic, A.; Bajcetic, M. The Role of Quinidine in the Pharmacological Therapy of Ventricular Arrhythmias ‘Quinidine’. Mini Rev. Med. Chem. 2018, 18, 468–475. [Google Scholar] [CrossRef]
- Brocks, D.R.; Mehvar, R. Stereoselectivity in the pharmacodynamics and pharmacokinetics of the chiral antimalarial drugs. Clin. Pharmacokinet. 2003, 42, 1359–1382. [Google Scholar] [CrossRef]
- Jassal, B.; Matthews, L.; Viteri, G.; Gong, C.; Lorente, P.; Fabregat, A.; Sidiropoulos, K.; Cook, J.; Gillespie, M.; Haw, R.; et al. The reactome pathway knowledgebase. Nucleic Acids Res. 2020, 48, D498–D503. [Google Scholar] [CrossRef]


| Gene | △Tm (°C) | Function |
|---|---|---|
| NQO2 | 12 | A quinone reductase |
| GSR | 5.8 | Maintains high levels of reduced glutathione |
| NAMPT | 2.1 | Catalyzes the condensation of nicotinamide |
| KIF11 | 2.38 | Motor protein required for establishing a bipolar spindle during mitosis |
| ALDH9A1 | 1.6 | Converts gamma-trimethylaminobutyraldehyde into gamma-butyrobetaine |
| SEC23A | 1.4 | Component of the coat protein complex II (COPII) |
| PUF60 | 1.3 | DNA- and RNA-binding protein |
| PCM1 | 4.7 | Required for centrosome assembly and function |
| NONO | 1.89 | DNA- and RNA binding protein |
| ARFGAP1 | 1.98 | GTPase-activating protein (GAP) for the ADP ribosylation factor 1 (ARF1) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Zhao, Z.; Hou, W.; Jiang, Y.; Liu, G.; Ren, X.; Liu, K.; Liu, H.; Chen, K.; Huang, H. Quantitative Proteomics Explore the Potential Targets and Action Mechanisms of Hydroxychloroquine. Molecules 2022, 27, 5175. https://doi.org/10.3390/molecules27165175
Zhao J, Zhao Z, Hou W, Jiang Y, Liu G, Ren X, Liu K, Liu H, Chen K, Huang H. Quantitative Proteomics Explore the Potential Targets and Action Mechanisms of Hydroxychloroquine. Molecules. 2022; 27(16):5175. https://doi.org/10.3390/molecules27165175
Chicago/Turabian StyleZhao, Jingxiang, Zhiqiang Zhao, Wanting Hou, Yue Jiang, Guobin Liu, Xuelian Ren, Kun Liu, Hong Liu, Kaixian Chen, and He Huang. 2022. "Quantitative Proteomics Explore the Potential Targets and Action Mechanisms of Hydroxychloroquine" Molecules 27, no. 16: 5175. https://doi.org/10.3390/molecules27165175
APA StyleZhao, J., Zhao, Z., Hou, W., Jiang, Y., Liu, G., Ren, X., Liu, K., Liu, H., Chen, K., & Huang, H. (2022). Quantitative Proteomics Explore the Potential Targets and Action Mechanisms of Hydroxychloroquine. Molecules, 27(16), 5175. https://doi.org/10.3390/molecules27165175







