New Myrtenal–Adamantane Conjugates Alleviate Alzheimer’s-Type Dementia in Rat Model
Abstract
:1. Introduction
2. Results
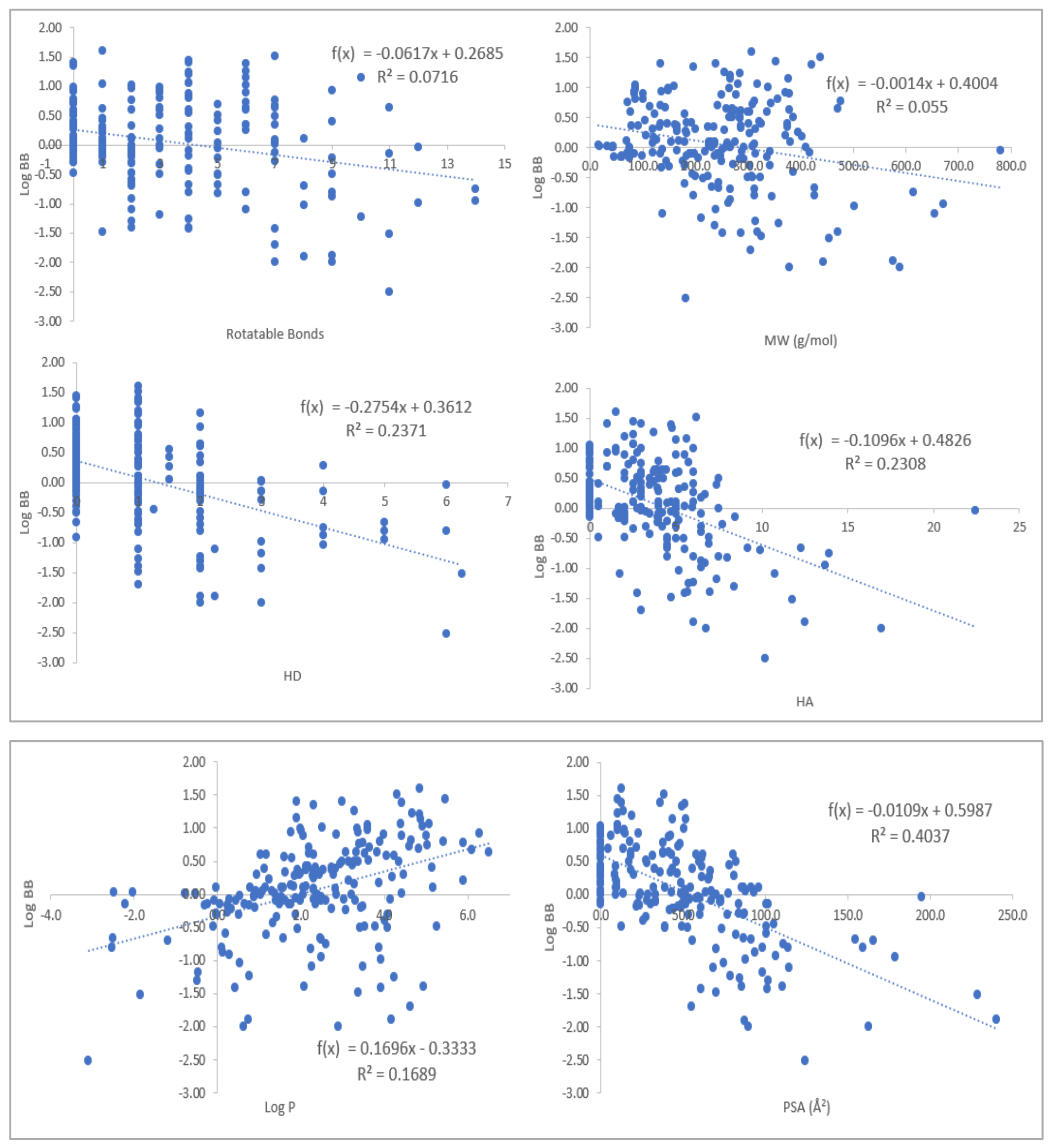
2.1. Physicochemical Propertied Affecting Blood-Brain Barrier Diffusion
2.2. Effects of MACs on Scopolamine-Impaired Memory in Rats
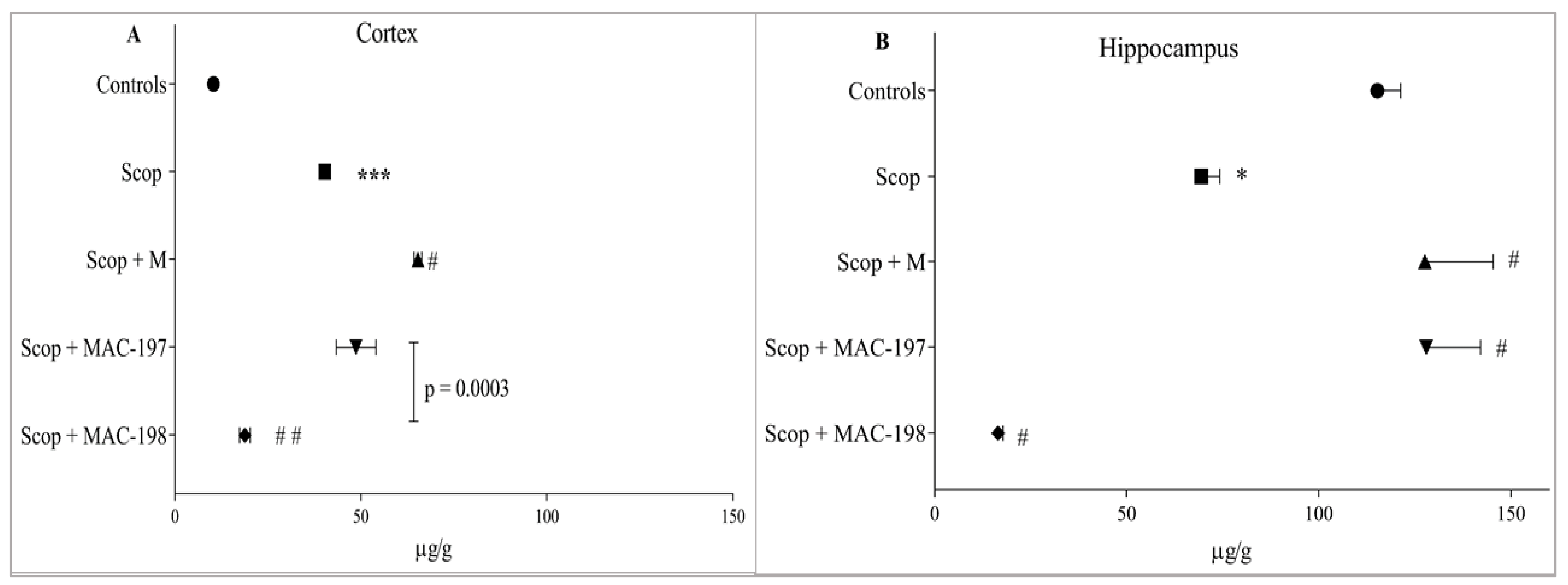
2.3. Effects of MACs on Brain AChE Activity
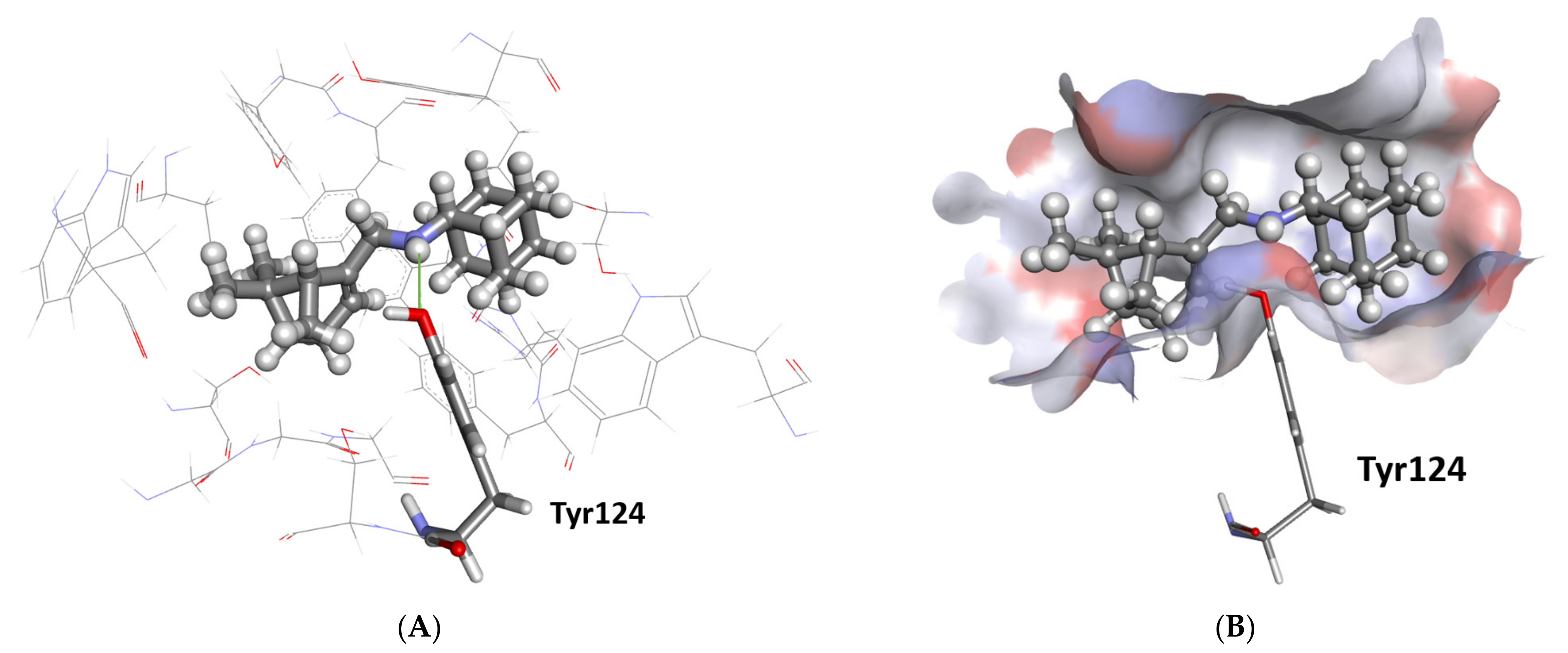
2.3.1. Docking Study
2.3.2. Evaluation of Brain AChE Activity In Vivo
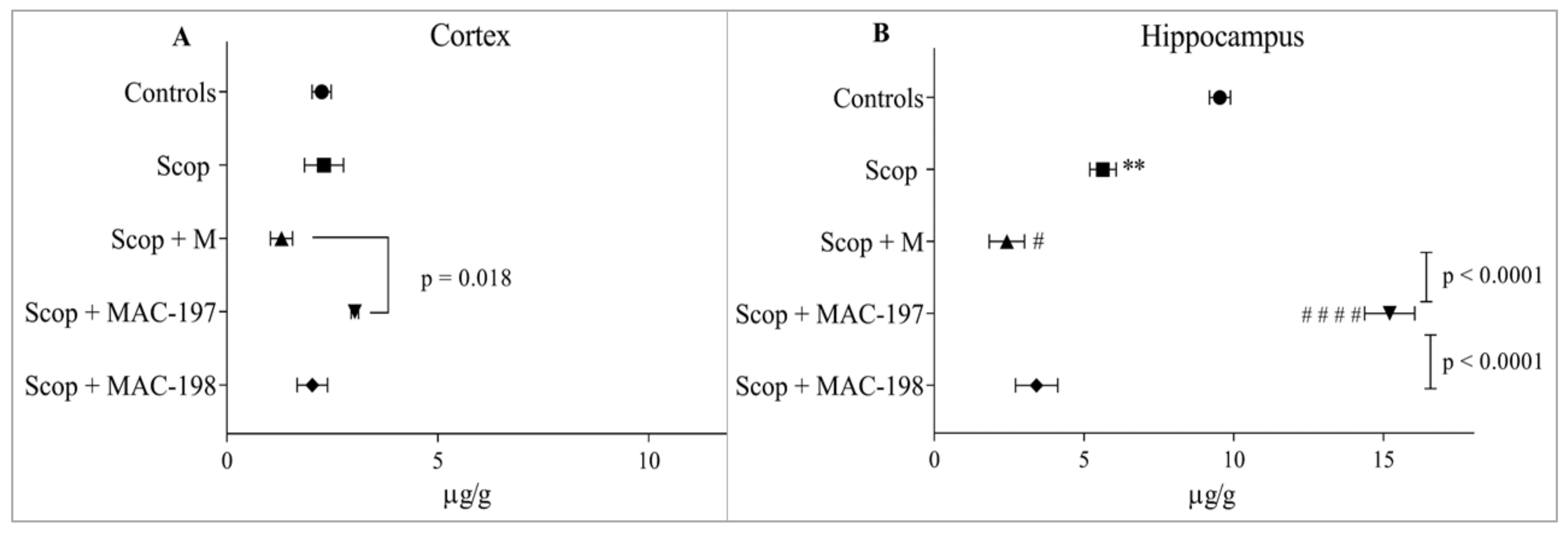
2.4. Effect of MACs on Brain Noradrenaline and Serotonin Levels
2.4.1. Noradrenaline Levels
2.4.2. Serotonin Levels
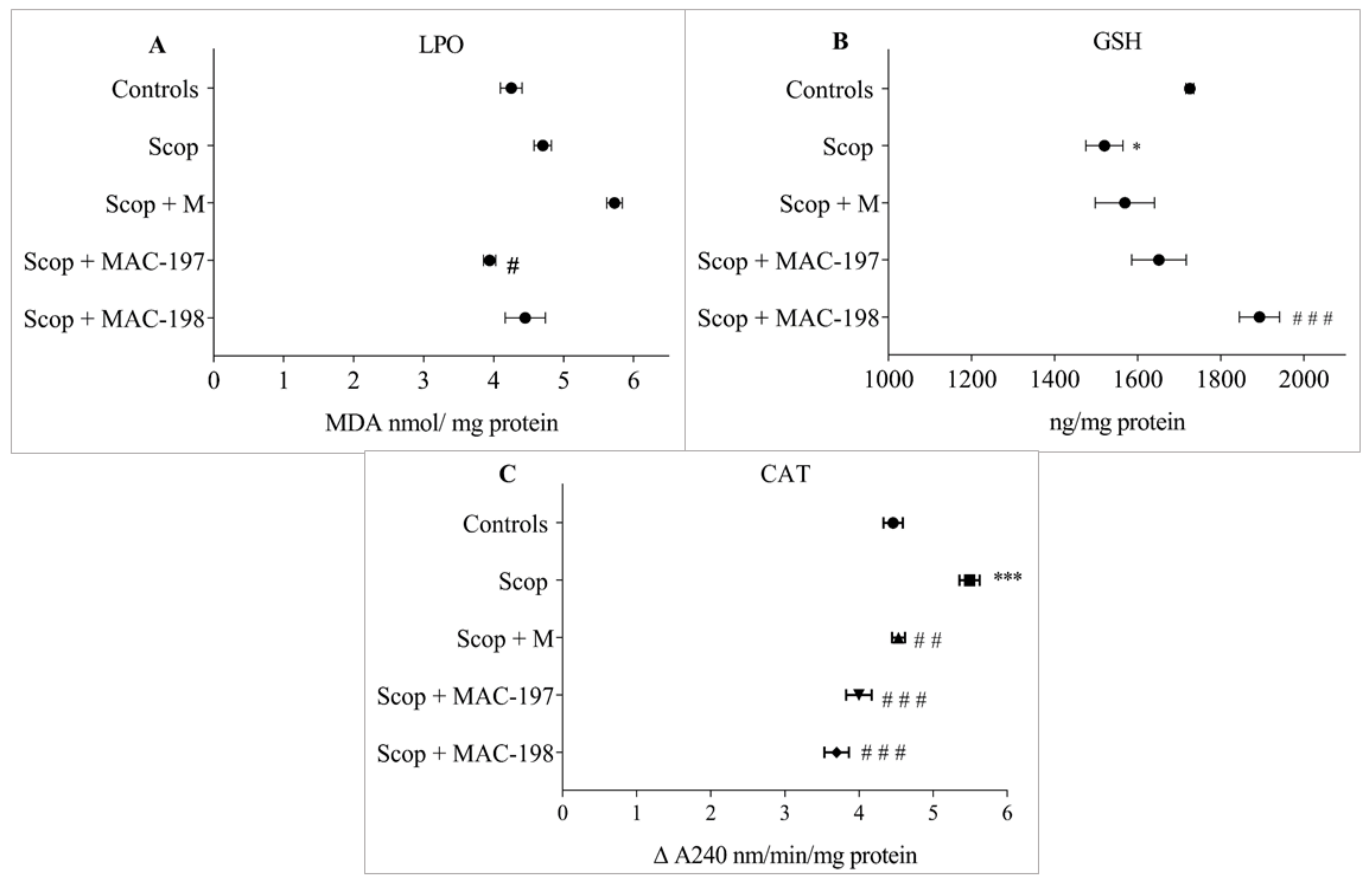
2.5. Antioxidant Activity of MACs in the Brain Cortex
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Modelling and Screening
4.3. Experimental Rodents
4.4. Experimental Design and Drug Treatment
4.5. Behavioral Test
4.6. Biochemical Studies
4.6.1. Evaluation of Brain AChE Activity
4.6.2. Evaluation of Noradrenaline and Serotonin Levels in Cerebral Cortex and HippoCampus
4.6.3. Evaluation of Cerebral Cortex Antioxidant Activity
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Cummings, J.L. The Black Book of Alzheimer’s Disease. Prim. Psychiatry 2008, 15, 66–76. [Google Scholar]
- Wang, J.Y.; Wen, L.L.; Huang, Y.N.; Chen, Y.T.; Ku, M.C. Dual effects of antioxidants in neurodegeneration: Direct neuroprotection against oxidative stress and indirect protection via suppression of glia-mediated inflammation. Curr. Pharm. Des. 2006, 12, 3521–3533. [Google Scholar] [CrossRef] [PubMed]
- Goverdhan, P.; Sravanthi, A.; Mamatha, T. Neuroprotective Effects of Meloxicam and Selegiline in Scopolamine-Induced Cognitive Impairment and Oxidative Stress. Int. J. Alzheimer’s Dis. 2012, 2012, 974013. [Google Scholar] [CrossRef] [PubMed]
- White, N.M.; McDonald, R.J. Multiple Parallel Memory Systems in the Brain of the Rat. Neurobiol. Learn. Mem. 2002, 77, 125–184. [Google Scholar] [CrossRef]
- Garcia-Alloza, M.; Gil-Bea, F.J.; Diez-Ariza, M.; Chen, C.P.L.-H.; Francis, P.T.; Lasheras, B.; Ramirez, M.J. Cholinergic-serotoninergic imbalance contributes to cognitive and behavioral symptoms in Alzheimer’s disease. Neuropsychologia 2005, 43, 442–449. [Google Scholar] [CrossRef]
- Lombardero, L.; Llorente-Ovejero, A.; Manuel, I.; Rodríguez-Puertas, R. Chapter 28—Neurotransmitter receptors in Alzheimer’s disease: From glutamatergic to cholinergic receptors. In Genetics, Neurology, Behavior, and Diet in Dementia: The Neuroscience of Dementia; Martin, C.R., Preedy, V.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 2, pp. 441–456. [Google Scholar] [CrossRef]
- Kaufmann, D.; Dogra, A.K.; Wink, M. Myrtenal inhibits acetylcholinesterase, a known Alzheimer target. J. Pharm. Pharmacol. 2011, 63, 1368–1371. [Google Scholar] [CrossRef]
- Dragomanova, S. Pharmacological, Toxicological and Neurobiological Studies of Myrtenal—A Bicyclic Monoterpenoid of Natural Origin. Ph.D. Thesis, Bulgarian Academy of Sciences, Sofia, Bulgaria, 2020. [Google Scholar]
- Dragomanova, S.; Pavlov, S.; Marinova, D.; Hodzev, Y.; Petralia, M.C.; Fagone, P.; Nicoletti, F.; Lazarova, M.; Tzvetanova, E.; Alexandrova, A.; et al. Neuroprotective Effects of Myrtenal in an Experimental Model of Dementia Induced in Rats. Antioxidants 2022, 11, 374. [Google Scholar] [CrossRef]
- Tancheva, L.P.; Lazarova, M.I.; Alexandrova, A.V.; Dragomanova, S.T.; Nicoletti, F.; Tzvetanova, E.R.; Hodzhev, Y.K.; Kalfin, R.E.; Miteva, S.A.; Mazzon, E.; et al. Neuroprotective Mechanisms of Three Natural Antioxidants on a Rat Model of Parkinson’s Disease: A Comparative Study. Antioxidants 2020, 9, 49. [Google Scholar] [CrossRef]
- Lamoureux, G.; Artavia, G. Use of the Adamantane Structure in Medicinal Chemistry. Curr. Med. Chem. 2010, 17, 2967–2978. [Google Scholar] [CrossRef]
- Spilovska, K.; Zemek, F.; Korabecny, J.; Nepovimova, E.; Soukup, O.; Windisch, M.; Kuca, K. Adamantane—A Lead Structure for Drugs in Clinical Practice. Curr. Med. Chem. 2016, 23, 3245–3266. [Google Scholar] [CrossRef]
- Teplov, G.V.; Suslov, E.; Zarubaev, V.V.; Shtro, A.A.; Karpinskaya, L.A.; Rogachev, A.; Korchagina, D.V.; Volcho, K.; Salakhutdinov, N.F.; Kiselev, O.I. Synthesis of New Compounds Combining Adamantanamine and Monoterpene Fragments and their Antiviral Activity Against Influenza Virus A(H1N1)pdm09. Lett. Drug Des. Discov. 2013, 10, 477–485. [Google Scholar] [CrossRef]
- Kapitsa, I.G.; Suslov, E.V.; Teplov, G.V.; Korchagina, D.V.; Komarova, N.I.; Volcho, K.; Voronina, T.A.; Shevela, A.I.; Salakhutdinov, N.F. Synthesis and anxiolytic activity of 2-aminoadamantane derivatives containing monoterpene fragments. Pharm. Chem. J. 2012, 46, 263–265. [Google Scholar] [CrossRef]
- Zhu, F.; Logan, G.; Reynisson, J. Wine Compounds as a Source for HTS Screening Collections. A Feasibility Study. Mol. Inform. 2012, 31, 847–855. [Google Scholar] [CrossRef]
- Usansky, H.H.; Sinko, P.J. Computation of log BB values for compounds transported through carrier-mediated mechanisms using in vitro permeability data from brain microvessel endothelial cell (BMEC) monolayers. Pharm. Res. 2003, 20, 390–396. [Google Scholar] [CrossRef]
- Garg, P.; Verma, J. In Silico Prediction of Blood Brain Barrier Permeability: An Artificial Neural Network Model. J. Chem. Inf. Model. 2005, 46, 289–297. [Google Scholar] [CrossRef]
- Guerra, A.; Páez, J.A.; Campillo, N.E. Artificial Neural Networks in ADMET Modeling: Prediction of Blood-Brain Barrier Permeation. QSAR Comb. Sci. 2008, 27, 586–594. [Google Scholar] [CrossRef]
- Muehlbacher, M.; Spitzer, G.M.; Liedl, K.R.; Kornhuber, J. Qualitative prediction of blood–brain barrier permeability on a large and refined dataset. J. Comput. Aided Mol. Des. 2011, 25, 1095–1106. [Google Scholar] [CrossRef]
- Eurtivong, C.; Reynisson, J. The Development of a Weighted Index to Optimise Compound Libraries for High Throughput Screening. Mol. Inform. 2018, 38, e1800068. [Google Scholar] [CrossRef]
- Ferreira, I.L.; Resende, R.; Ferreiro, E.; Rego, A.C.; Pereira, C.F. Multiple defects in energy metabolism in Alzheimer’s disease. Curr. Drug Targets 2010, 11, 1193–1206. [Google Scholar] [CrossRef]
- Bolognesi, M.L. Polypharmacology in a single drug: Multi-target drugs. Curr. Med. Chem. 2013, 20, 1639–1645. [Google Scholar] [CrossRef]
- Ji, H.-F.; Zhang, H.Y. Multipotent natural agents to combat Alzheimer’s disease. Functional spectrum and structural features 1. Acta Pharmacol. Sin. 2008, 29, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, L.; Yang, C.-C. Multi-Target Strategy and Experimental Studies of Traditional Chinese Medicine for Alzheimer’s Disease Therapy. Curr. Top. Med. Chem. 2015, 16, 537–548. [Google Scholar] [CrossRef] [PubMed]
- Dey, A.; Bhattacharya, R.; Mukherjee, A.; Pandey, D.K. Natural products against Alzheimer’s disease: Pharmaco-therapeutics and biotechnological interventions. Biotechnol. Adv. 2017, 35, 178–216. [Google Scholar] [CrossRef] [PubMed]
- Wanka, L.; Iqbal, K.; Schreiner, P.R. The Lipophilic Bullet Hits the Targets: Medicinal Chemistry of Adamantane Derivatives. Chem. Rev. 2013, 113, 3516–3604. [Google Scholar] [CrossRef]
- Neganova, M.; Aleksandrova, Y.; Suslov, E.; Mozhaitsev, E.; Munkuev, A.; Tsypyshev, D.; Chicheva, M.; Rogachev, A.; Sukocheva, O.; Volcho, K.; et al. Novel Multitarget Hydroxamic Acids with a Natural Origin CAP Group against Alzheimer’s Disease: Synthesis, Docking and Biological Evaluation. Pharmaceutics 2021, 13, 1893. [Google Scholar] [CrossRef]
- Sacksteder, K.A.; Protopopova, M.; Barry, C.E.; Andries, K.; Nacy, C.A. Discovery and development of SQ109: A new antitubercular drug with a novel mechanism of action. Future Microbiol. 2012, 7, 823–837. [Google Scholar] [CrossRef]
- Chepanova, A.A.; Mozhaitsev, E.S.; Munkuev, A.A.; Suslov, E.V.; Korchagina, D.V.; Zakharova, O.D.; Zakharenko, A.L.; Patel, J.; Ayine-Tora, D.M.; Reynisson, J.; et al. The Development of Tyrosyl-DNA Phosphodiesterase 1 Inhibitors. Combination of Monoterpene and Adamantine Moieties via Amide or Thioamide Bridges. Appl. Sci. 2019, 9, 2767. [Google Scholar] [CrossRef]
- Suslov, E.; Ponomarev, K.; Rogachev, A.; Pokrovsky, M.; Pokrovsky, A.; Pykhtina, M.; Beklemishev, A.; Korchagina, D.; Volcho, K.; Salakhutdinov, N. Compounds Combining Aminoadamantane and Monoterpene Moieties: Cytotoxicity and Mutagenic Effects. Med. Chem. 2015, 11, 629–635. [Google Scholar] [CrossRef]
- Lagalwar, S.; Bordayo, E.Z.; Hoffmann, K.L.; Fawcett, J.R.; Frey, W.H. Anandamides Inhibit Binding to the Muscarinic Acetylcholine Receptor. J. Mol. Neurosci. 1999, 13, 55–62. [Google Scholar] [CrossRef]
- Lott, I.T. Neurological phenotypes for Down syndrome across the life span. Prog. Brain Res. 2012, 197, 101–121. [Google Scholar] [CrossRef] [Green Version]
- Qin, M.; Zeidler, Z.; Moulton, K.; Krych, L.; Xia, Z.; Smith, C.B. Endocannabinoid-mediated improvement on a test of aversive memory in a mouse model of fragile X syndrome. Behav. Brain Res. 2015, 291, 164–171. [Google Scholar] [CrossRef]
- More, S.V.; Kumar, H.; Cho, D.-Y.; Yun, Y.-S.; Choi, D.-K. Toxin-Induced Experimental Models of Learning and Memory Impairment. Int. J. Mol. Sci. 2016, 17, 1447. [Google Scholar] [CrossRef]
- Tang, K.S. The cellular and molecular processes associated with scopolamine-induced memory deficit: A model of Alzheimer’s biomarkers. Life Sci. 2019, 233, 116695. [Google Scholar] [CrossRef]
- Tancheva, L.; Lazarova, M.; Velkova, L.; Dolashki, A.; Uzunova, D.; Minchev, B.; Petkova-Kirova, P.; Hassanova, Y.; Gavrilova, P.; Tasheva, K.; et al. Beneficial Effects of Snail Helix aspersa Extract in an Experimental Model of Alzheimer’s Type Dementia. J. Alzheimer’s Dis. 2022, 88, 155–175. [Google Scholar] [CrossRef]
- Staykov, H.; Lazarova, M.; Hassanova, Y.; Stefanova, M.; Tancheva, L.; Nikolov, R. Neuromodulatory Mechanisms of a Memory Loss-Preventive Effect of Alpha-Lipoic Acid in an Experimental Rat Model of Dementia. J. Mol. Neurosci. 2022, 72, 1018–1025. [Google Scholar] [CrossRef]
- D’Amelio, M.; Rossini, P.M. Brain excitability and connectivity of neuronal assemblies in Alzheimer’s disease: From animal models to human findings. Prog. Neurobiol. 2012, 99, 42–60. [Google Scholar] [CrossRef]
- Roy, D.S.; Arons, A.; Mitchell, T.I.; Pignatelli, M.; Ryan, T.; Tonegawa, S. Memory retrieval by activating engram cells in mouse models of early Alzheimer’s disease. Nature 2016, 531, 508–512. [Google Scholar] [CrossRef]
- Scheff, S.W.; Price, D.A.; Schmitt, F.A.; Mufson, E.J. Hippocampal synaptic loss in early Alzheimer’s disease and mild cognitive impairment. Neurobiol. Aging 2006, 27, 1372–1384. [Google Scholar] [CrossRef]
- Burns, J.M.; Galvin, J.E.; Roe, C.M.; Morris, J.C.; McKeel, D.W. The pathology of the substantia nigra in Alzheimer disease with extrapyramidal signs. Neurology 2005, 64, 1397–1403. [Google Scholar] [CrossRef]
- Drinenberg, A.C. Alzheimer’s disease: More than a “cholinergic disorder”-evidence that cholinergic-monoaminergic interactions contribute to EEG slowing and dementia. Behav. Brain Res. 2000, 115, 235–249. [Google Scholar] [CrossRef]
- Braak, H.; Braak, E. Pathology of Alzheimer diseas. In Neurodegenerative Diseases; Calne, D.B., Ed.; W.B. Saunders: Philadelphia, PA, USA, 1994; pp. 585–613. [Google Scholar]
- Kolar, D. Scopolamine as a potential treatment option in major depressive disorder—A literature review. Isr. J. Psychiatry 2021, 58, 48–53. [Google Scholar]
- Selles, M.; Oliveira, M.M.; Ferreira, S.T. Brain inflammation connects cognitive and non-cognitive symptoms in Alzheimer’s disease. J. Alz. Dis. 2018, 64, S313–S327. [Google Scholar] [CrossRef]
- Gutiérrez, I.L.; Russo, C.D.; Novellino, F.; Caso, J.R.; García-Bueno, B.; Leza, J.C.; Madrigal, J.L.M. Noradrenaline in Alzheimer’s Disease: A New Potential Therapeutic Target. Int. J. Mol. Sci. 2022, 23, 6143. [Google Scholar] [CrossRef]
- Falsafi, S.K.; Deli, A.; Höger, H.; Pollak, A.; Lubec, G. Scopolamine Administration Modulates Muscarinic, Nicotinic and NMDA Receptor Systems. PLoS ONE 2012, 7, e32082. [Google Scholar] [CrossRef]
- Meltzer, C.C.; Smith, G.; DeKosky, S.; Pollock, B.G.; Mathis, C.A.; Moore, R.Y.; Kupfer, D.J.; Iii, C.F.R. Serotonin in Aging, Late-Life Depression, and Alzheimer’s Disease: The Emerging Role of Functional Imaging. Neuropsychopharmacology 1998, 18, 407–430. [Google Scholar] [CrossRef]
- Mesulam, M. The cholinergic lesion of Alzheimer’s disease: A pivotal factor or side show? Learn. Mem. 2004, 11, 43–49. [Google Scholar] [CrossRef]
- Kovacs, G.G.; Klöppel, S.; Fischer, I.; Dorner, S.; Lindeck-Pozza, E.; Birner, P.; Bötefür, I.C.; Pilz, P.; Volk, B.; Budka, H. Nucleus-specific alteration of raphe neurons in human neurodegenerative disorders. NeuroReport 2003, 14, 73–76. [Google Scholar] [CrossRef]
- Bert, B.; Fink, H.; Sohr, R.; Rex, A. Different effects of diazepam in Fischer rats and two stocks of Wistar rats in tests of anxiety. Pharmacol. Biochem. Behav. 2001, 70, 411–420. [Google Scholar] [CrossRef]
- Markesbery, W.R. Oxidative stress hypothesis in Alzheimer’s disease. Free Radic. Biol. Med. 1997, 23, 134–147. [Google Scholar] [CrossRef]
- Markesbery, W.R.; Carney, J.M. Oxidative alterations in Alzheimer’s disease. Brain Pathol. 1999, 9, 133–146. [Google Scholar] [CrossRef]
- Markesbery, W.R. The Role of Oxidative Stress in Alzheimer Disease. Arch. Neurol. 1999, 56, 1449–1452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pratico, D.; Delanty, N. Oxidative injury in diseases of the central nervous system: Focus on Alzheimer’s disease. Am. J. Med. 2000, 109, 577–585. [Google Scholar] [CrossRef]
- Floyd, R.A.; Hensley, K. Oxidative stress in brain aging: Implications for therapeutics of neurodegenerative diseases. Neurobiol. Aging 2002, 23, 795–807. [Google Scholar] [CrossRef]
- Kamat, P.K.; Kalani, A.; Rai, S.; Swarnkar, S.; Tota, S.; Nath, C.; Tyagi, N. Mechanism of Oxidative Stress and Synapse Dysfunction in the Pathogenesis of Alzheimer’s Disease: Understanding the Therapeutics Strategies. Mol. Neurobiol. 2016, 53, 648–661. [Google Scholar] [CrossRef] [PubMed]
- Giridharan, V.V.; Thandavarayan, R.A.; Sato, S.; Ko, K.M.; Konishi, T. Prevention of scopolamine-induced memory deficits by schisandrin B, an antioxidant lignan from Schisandra chinensis in mice. Free Radic. Res. 2011, 45, 950–958. [Google Scholar] [CrossRef] [PubMed]
- Nade, V.S.; Kanhere, S.V.; Kawale, L.A.; Yadav, A.V. Cognitive enhancing and antioxidant activity of ethyl acetate soluble fraction of the methanol extract of Hibiscus rosa sinensis in scopolamine-induced amnesia. Indian J. Pharmacol. 2011, 43, 137–142. [Google Scholar] [CrossRef]
- Budzynska, B.; Boguszewska-Czubara, A.; Kruk-Slomka, M.; Skalicka-Woźniak, K.; Michalak, A.; Musik, I.; Biala, G. Effects of imperatorin on scopolamine-induced cognitive impairment and oxidative stress in mice. Psychopharmacology 2014, 232, 931–942. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, C.; Qi, Y.; Fang, L.; Luo, J.; Bi, K.; Jia, Y. Timosaponin B-II ameliorates scopolamine-induced cognition deficits by attenuating acetylcholinesterase activity and brain oxidative damage in mice. Metab. Brain Dis. 2016, 31, 1455–1461. [Google Scholar] [CrossRef]
- El-Sherbiny, D.A.; Khalifa, A.E.; Attia, A.S.; Eldenshary, E.E.-D.S. Hypericum perforatum extract demonstrates antioxidant properties against elevated rat brain oxidative status induced by amnestic dose of scopolamine. Pharmacol. Biochem. Behav. 2003, 76, 525–533. [Google Scholar] [CrossRef]
- Fan, Y.; Hu, J.; Li, J.; Yang, Z.; Xin, X.; Wang, J.; Ding, J.; Geng, M. Effect of acidic oligosaccharide sugar chain on scopolamine-induced memory impairment in rats and its related mechanisms. Neurosci. Lett. 2005, 374, 222–226. [Google Scholar] [CrossRef]
- Jeong, E.J.; Lee, K.Y.; Kim, S.H.; Sung, S.H.; Kim, Y.C. Cognitive-enhancing and antioxidant activities of iridoid glycosides from Scrophularia buergeriana in scopolamine-treated mice. Eur. J. Pharmacol. 2008, 588, 78–84. [Google Scholar] [CrossRef]
- Jeong, E.J.; Ma, C.J.; Lee, K.Y.; Kim, S.H.; Sung, S.H.; Kim, Y.C. KD-501, a standardized extract of Scrophularia buergeriana has both cognitive-enhancing and antioxidant activities in mice given scopolamine. J. Ethnopharmacol. 2009, 121, 98–105. [Google Scholar] [CrossRef]
- Babu, L.H.; Perumal, S.; Balasubramanian, M.P. Myrtenal attenuates diethylnitrosamine-induced hepatocellular carcinoma in rats by stabilizing intrinsic antioxidants and modulating apoptotic and anti-apoptotic cascades. Cell. Oncol. 2012, 35, 269–283. [Google Scholar] [CrossRef]
- Allinger, N.L.; Yuh, Y.H.; Lii, J.H. Molecular mechanics. The MM3 force field for hydrocarbons. 1. J. Am. Chem. Soc. 1989, 111, 8551–8566. [Google Scholar] [CrossRef]
- Lii, J.H.; Allinger, N.L. Molecular mechanics. The MM3 force field for hydrocarbons. 2. Vibrational frequencies and thermodynamics. J. Am. Chem. Soc. 1989, 111, 8566–8575. [Google Scholar] [CrossRef]
- Lii, J.H.; Allinger, N.L. Molecular mechanics. The MM3 force field for hydrocarbons. 3. The van der Waals’ potentials and crystal data for aliphatic and aromatic hydrocarbons. J. Am. Chem. Soc. 1989, 111, 8576–8582. [Google Scholar] [CrossRef]
- Gotō, H.; Ōsawa, E. An efficient algorithm for searching low-energy conformers of cyclic and acyclic molecules. J. Chem. Soc. Perkin Trans. 2 1993, 2, 187–198. [Google Scholar] [CrossRef]
- Franklin, M.C.; Rudolph, M.J.; Ginter, C.; Cassidy, M.S.; Cheung, J. Structures of paraoxon-inhibited human acetylcholinesterase reveal perturbations of the acyl loop and the dimer interface. Proteins Struct. Funct. Bioinform. 2016, 84, 1246–1256. [Google Scholar] [CrossRef]
- Jones, G.; Willett, P.; Glen, R.C.; Leach, A.R.; Taylor, R. Development and validation of a genetic algorithm for flexible docking. J. Mol. Biol. 1997, 267, 727–748. [Google Scholar] [CrossRef]
- Eldridge, M.D.; Murray, C.W.; Auton, T.R.; Paolini, G.V.; Mee, R.P. Empirical scoring functions: I. The development of a fast empirical scoring function to estimate the binding affinity of ligands in receptor complexes. J. Comput. Aided Mol. Des. 1997, 11, 425–445. [Google Scholar] [CrossRef]
- Verdonk, M.L.; Cole, J.C.; Hartshorn, M.J.; Murray, C.W.; Taylor, R.D. Improved protein-ligand docking using GOLD. Proteins Struct. Funct. Bioinform. 2003, 52, 609–623. [Google Scholar] [CrossRef]
- Korb, O.; Stutzle, T.; Exner, T.E. Empirical Scoring Functions for Advanced Protein–Ligand Docking with PLANTS. J. Chem. Inf. Model. 2009, 49, 84–96. [Google Scholar] [CrossRef]
- Mooij, W.T.M.; Verdonk, M.L. General and targeted statistical potentials for protein-ligand interactions. Proteins Struct. Funct. Bioinform. 2005, 61, 272–287. [Google Scholar] [CrossRef]
- Ioakimidis, L.; Thoukydidis, L.; Mirza, A.; Naeem, S.; Reynisson, J. Benchmarking the Reliability of QikProp. Correlation between Experimental and Predicted Values. QSAR Comb. Sci. 2008, 27, 445–456. [Google Scholar] [CrossRef]
- Jarvik, M.E.; Kopp, R. An Improved One-Trial Passive Avoidance Learning Situation. Psychol. Rep. 1967, 21, 221–224. [Google Scholar] [CrossRef]
- Venault, P.; Chapouthier, G.; de Carvalho, L.P.; Simiand, J.; Morre, M.; Dodd, R.H.; Rossier, J. Benzodiazepine impairs and β-carboline enhances performance in learning and memory tasks. Nature 1986, 321, 864–866. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Jacobowitz, D.M.; Richardson, J. Method for the rapid determination of norepinephrine, dopamine, and serotonin in the same brain region. Pharmacol. Biochem. Behav. 1978, 8, 515–519. [Google Scholar] [CrossRef]
- Tietze, F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: Applications to mammalian blood and other tissues. Anal. Biochem. 1969, 27, 502–522. [Google Scholar] [CrossRef]
- Hunter, F.E.; Gebicki, J.; Hoffsten, P.; Weinstein, J.; Scott, A. Swelling and Lysis of Rat Liver Mitochondria Induced by Ferrous Ions. J. Biol. Chem. 1963, 238, 828–835. [Google Scholar] [CrossRef]
- Aebi, H. [13] Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [PubMed]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Günzler, W.A.; Vergin, H.; Müller, I.; Flohé, L. Glutathion-Peroxidase, VI. Die Reaktion der Glutathion-Peroxidase mit verschiedenen Hydroperoxiden [Glutathione peroxidase VI: The reaction of glutahione peroxidase with various hydroperox-ides]. Hoppe Seylers Z Physiol. Chem. 1972, 353, 1001–1004. (In German) [Google Scholar] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]


| RB | MW (g/mol) | HD | HA | Log p | PSA (Å2) | KDI2a | KDI2b | |
|---|---|---|---|---|---|---|---|---|
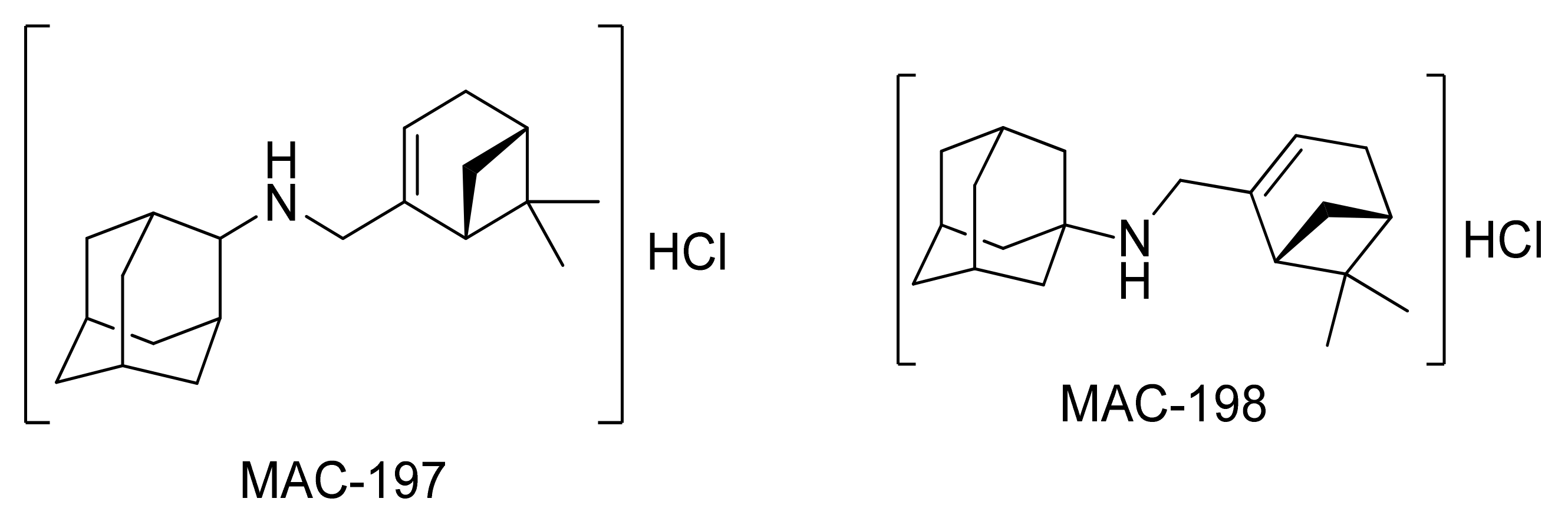
| MAC-197 | 3 | 285.5 | 1 | 1.5 | 4.5 | 9.6 | 4.05 | 0.07 |
| MAC-198 | 3 | 285.5 | 1 | 1 | 4.9 | 10.7 | 3.89 | 0.04 |
| Myrtenal | 1 | 150.2 | 0 | 2 | 1.8 | 36.6 | 3.38 | 0.01 |
| ASP | ChemPLP | CS | GS | |
|---|---|---|---|---|
| MAC-197 | 34.9 | 68.4 | 40.9 | 47.3 |
| MAC-198 | 35.0 | 64.2 | 40.3 | 45.1 |
| Myrtenal | 23.8 | 44.0 | 28.5 | 35.1 |
| HI6 | 48.1 | 85.6 | 29.1 | 62.4 |
| Acetylcholine | 24.1 | 43.1 | 18.2 | 36.6 |
| Donepezil | 59.1 | 83.2 | 43.3 | 56.9 |
| Tacrine | 42.1 | 66.0 | 35.1 | 57.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dragomanova, S.; Lazarova, M.; Munkuev, A.; Suslov, E.; Volcho, K.; Salakhutdinov, N.; Bibi, A.; Reynisson, J.; Tzvetanova, E.; Alexandrova, A.; et al. New Myrtenal–Adamantane Conjugates Alleviate Alzheimer’s-Type Dementia in Rat Model. Molecules 2022, 27, 5456. https://doi.org/10.3390/molecules27175456
Dragomanova S, Lazarova M, Munkuev A, Suslov E, Volcho K, Salakhutdinov N, Bibi A, Reynisson J, Tzvetanova E, Alexandrova A, et al. New Myrtenal–Adamantane Conjugates Alleviate Alzheimer’s-Type Dementia in Rat Model. Molecules. 2022; 27(17):5456. https://doi.org/10.3390/molecules27175456
Chicago/Turabian StyleDragomanova, Stela, Maria Lazarova, Aldar Munkuev, Evgeniy Suslov, Konstantin Volcho, Nariman Salakhutdinov, Amina Bibi, Jóhannes Reynisson, Elina Tzvetanova, Albena Alexandrova, and et al. 2022. "New Myrtenal–Adamantane Conjugates Alleviate Alzheimer’s-Type Dementia in Rat Model" Molecules 27, no. 17: 5456. https://doi.org/10.3390/molecules27175456








