The Emergence of Edible and Food-Application Coatings for Food Packaging: A Review
Abstract
:1. Introduction
2. Current Food Packaging Trends
2.1. Conventional Packaging
2.1.1. Petroleum-Based Plastics
2.1.2. Paper and Cardboard
2.1.3. Metal
2.1.4. Glass
2.2. Biodegradable Packaging
2.2.1. Proteins
2.2.2. Polysaccharides
2.2.3. Lipids
| Food Products | Biopolymeric Matrix | Coating Techniques | Results | References |
|---|---|---|---|---|
| - | Gelatin + papaya peel powder | Biodegradable film |
| [50] |
| Fruits and vegetables | Shellac + gelatin | Biodegradable film |
| [51] |
| - | Cashew gum + gelatin | Biodegradable film |
| [52] |
| - | Cellulose nanocrystal (CNC) + polyvinyl alcohol (PVA) + carboxymethyl cellulose (CMC) | Biodegradable film |
| [67] |
| Guava | Acetylated cassava starch (ACS) + hydroxyethyl cellulose (HEC) | Biodegradable film |
| [70] |
| Fruits and vegetables | Gum cordia + lipids + beeswax + glycerol monosterate | Biodegradable film |
| [84] |
2.3. Edible Packaging
2.3.1. Polysaccharide-Based Edible Films
2.3.2. Protein-Based Edible Films
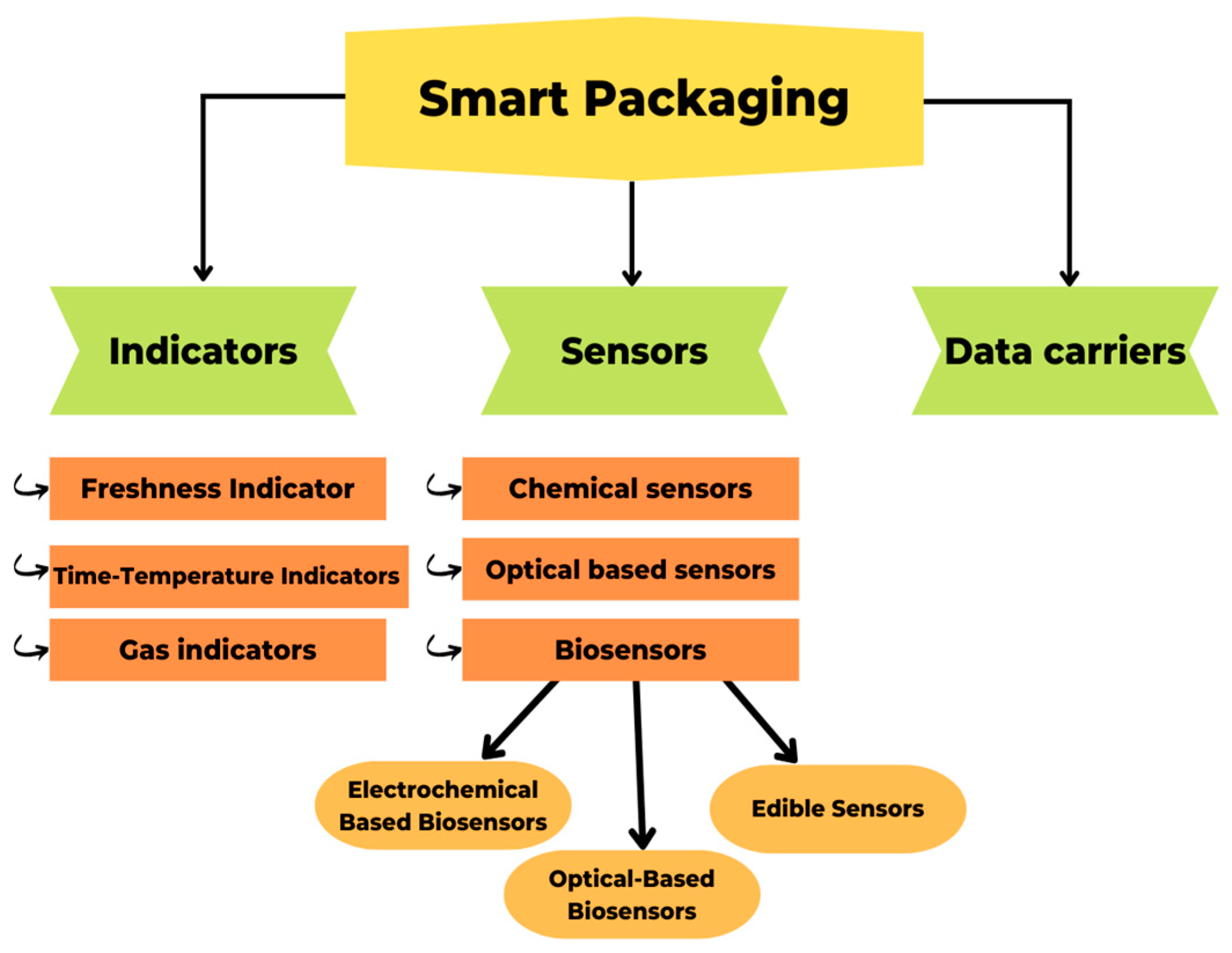
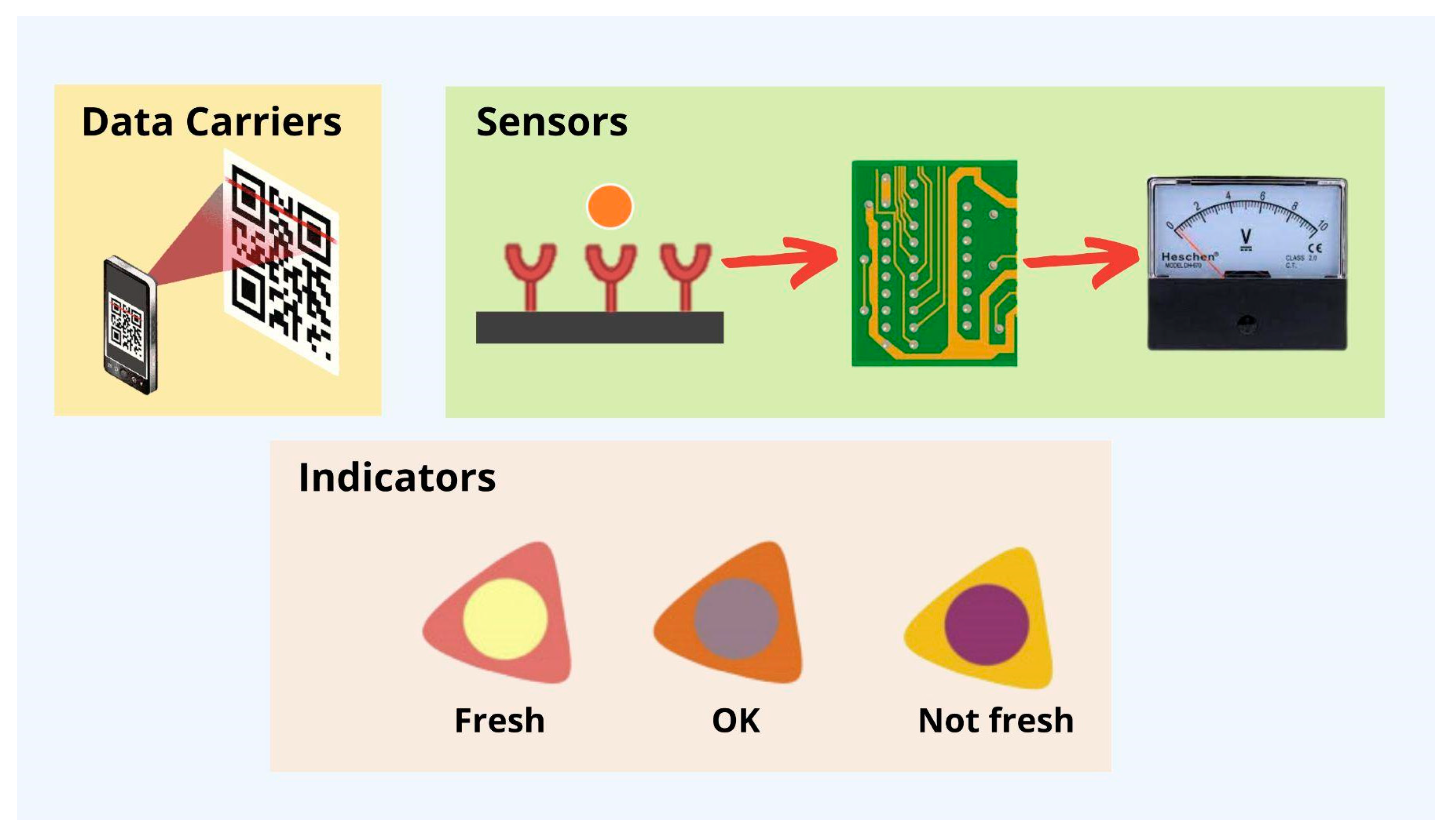
2.4. Smart Packaging
Indicators
- Critical temperature indicators (CTI), which tell you if food has been heated above or cooled below a specific temperature during its lifetime;
- Critical temperature/time integrators (CTTI), which tell you if food has been heated above or cooled below a specific temperature for a more extended period; and
- Temperature sensors describe whether the food has been heated above or cooled below.
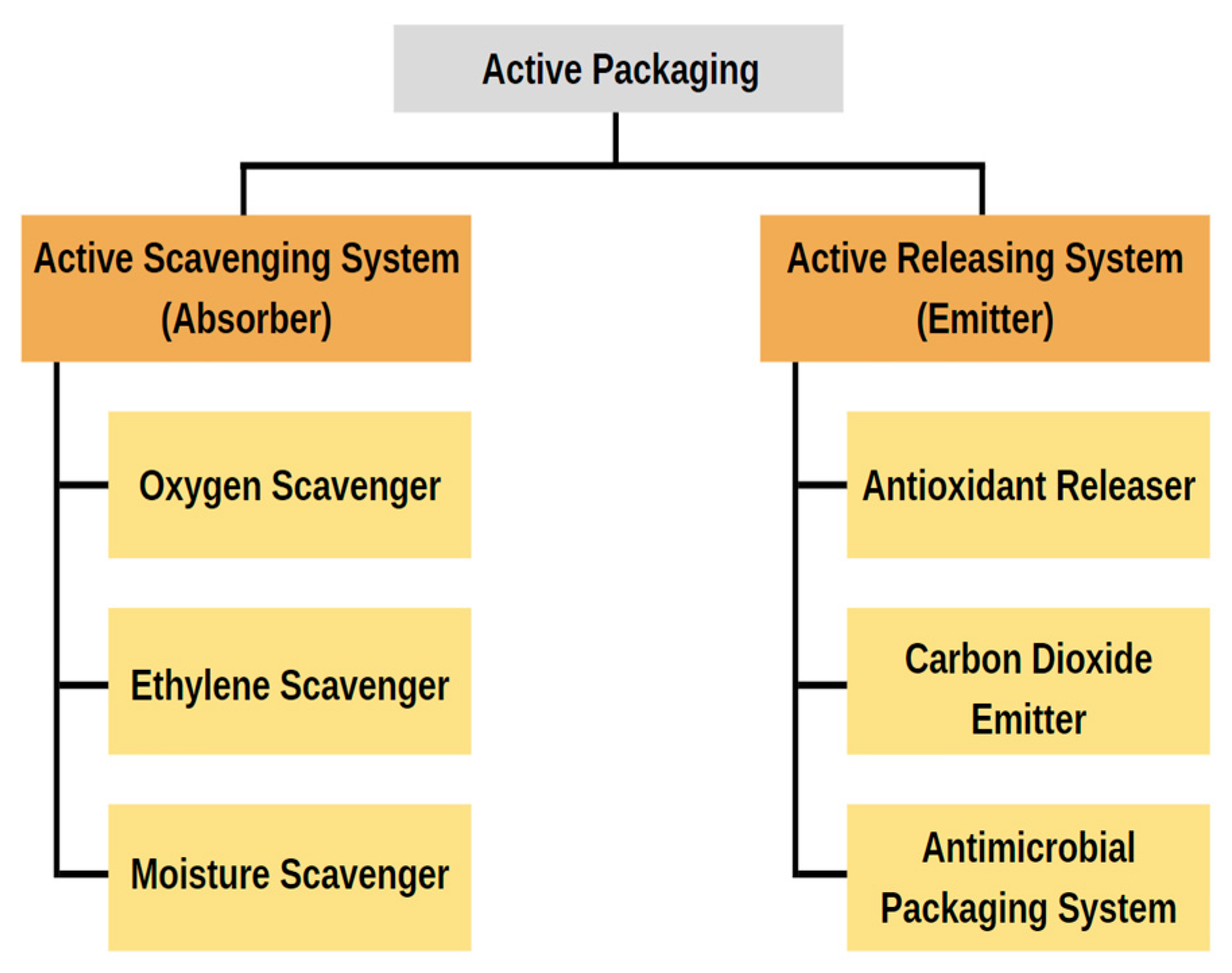
2.5. Active Packaging
2.5.1. Active Scavenging System (Absorber)
2.5.2. Active Releasing System (Emitter)
3. Food Safety Issues in Food Packaging
4. Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raheem, D. Application of plastics and paper as food packaging materials—An overview. Emir. J. Food Agric. 2013, 25, 177–188. [Google Scholar] [CrossRef]
- Marsh, K.; Bugusu, B. Food packaging—Roles, materials, and environmental issues. J. Food Sci. 2007, 72, R39–R55. [Google Scholar] [CrossRef] [PubMed]
- Coles, R. Introduction. In Food Packaging Technology; Coles, R., McDowell, D., Kirwan, M.J., Eds.; Blackwell Publishing: London, UK, 2003; pp. 1–31. [Google Scholar]
- Ramos, Ó.L.; Reinas, I.; Silva, S.I.; Fernandes, J.C.; Cerqueira, M.A.; Pereira, R.N.; Vicente, A.A.; Poças, M.F.; Pintado, M.E.; Malcata, F.X. Effect of whey protein purity and glycerol content upon physical properties of edible films manufactured therefrom. Food Hydrocoll. 2013, 30, 110–122. [Google Scholar] [CrossRef]
- Gontard, N.; Guilbert, S. Bio-packaging: Technology and properties of edible and/or biodegradable material of agricultural origin. In Food Packaging and Preservation; Mathlouthi, M., Ed.; Blackie Academic and Professional: Glasgow, UK, 1994; pp. 159–181. [Google Scholar]
- Akelah, A. Polymers in food packaging and protection. In Functionalized Polymeric Materials in Agriculture and the Food Industry; Springer: Boston, MA, USA, 2013; pp. 293–347. [Google Scholar] [CrossRef]
- Murmu, S.B.; Mishra, H.N. The effect of edible coating based on arabic gum, sodium caseinate and essential oil of cinnamon and lemon grass on guava. Food Chem. 2018, 245, 820–828. [Google Scholar] [CrossRef]
- Dehghani, S.; Hosseini, S.V.; Regenstein, J.M. Edible films and coatings in seafood preservation: A review. Food Chem. 2018, 240, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Montes, E.; Castro-Muñoz, R. Edible films and coatings as food-quality preservers: An overview. Foods 2021, 10, 249. [Google Scholar] [CrossRef]
- Ncube, L.K.; Ude, A.U.; Ogunmuyiwa, E.N.; Zulkifli, R.; Beas, I.N. Environmental Impact of Food Packaging Materials: A Review of Contemporary Development from Conventional Plastics to Polylactic Acid Based Materials. Materials 2020, 13, 4994. [Google Scholar] [CrossRef]
- Abdelwahab, N.A.; Abd El-Ghaffar, M.A. Preparation, Characterization, and Evaluation of Poly(p-azidoaniline) as Thermal Stabilizer for Low Density Polyethylene Films. Polym. Plast. Technol. Eng. 2015, 55, 158–170. [Google Scholar] [CrossRef]
- Dong, Z.; Xu, F.; Ahmed, I.; Li, Z.; Lin, H. Characterization and preservation performance of active polyethylene films containing rosemary and cinnamon essential oils for Pacific white shrimp packaging. Food Control 2018, 92, 37–46. [Google Scholar] [CrossRef]
- Vidács, A.; Kerekes, E.; Rajkó, R.; Petkovits, T.; Alharbi, N.S.; Khaled, J.M.; Vágvölgyi, C.; Krisch, J. Optimization of essential oil-based natural disinfectants against Listeria monocytogenes and Escherichia coli biofilms formed on polypropylene surfaces. J. Mol. Liq. 2018, 255, 257–262. [Google Scholar] [CrossRef]
- Llana-Ruiz-Cabello, M.; Pichardo, S.; Bermudez, J.M.; Baños, A.; Ariza, J.J.; Guillamón, E.; Aucejo, S.; Cameán, A.M. Characterisation and antimicrobial activity of active polypropylene films containing oregano essential oil and Allium extract to be used in packaging for meat products. Food Addit. Contam. 2018, 35, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Khalaj, M.J.; Ahmadi, H.; Lesankhosh, R.; Khalaj, G. Study of physical and mechanical properties of polypropylene nanocomposites for food packaging application: Nano-clay modified with iron nanoparticles. Trends Food Sci. Technol. 2016, 51, 41–48. [Google Scholar] [CrossRef]
- Kouloumpis, V.; Pell, R.; Correa-Cano, M.; Yan, X. Potential trade-offs between eliminating plastics and mitigating climate change: An LCA perspective on Polyethylene Terephthalate (PET) bottles in Cornwall. Sci. Total Environ. 2020, 727, 138681. [Google Scholar] [CrossRef] [PubMed]
- Gisario, A.; Veniali, F.; Barletta, M.; Tagliaferri, V.; Vesco, S. Laser transmission welding of poly(ethylene terephthalate) and biodegradable poly(ethylene terephthalate)—Based blends. Opt. Lasers Eng. 2017, 90, 110–118. [Google Scholar] [CrossRef]
- Kik, K.; Bukowska, B.; Sicińska, P. Polystyrene nanoparticles: Sources, occurrence in the environment, distribution in tissues, accumulation and toxicity to various organisms. Environ. Pollut. 2020, 262, 114297. [Google Scholar] [CrossRef]
- Abolghasemi, F.L.; Ghanbarzadeh, B.; Dehghannya, J.; Abbasi, F.; Ranjbar, H. Optimization of mechanical and color properties of polystyrene/nanoclay/nano ZnO based nanocomposite packaging sheet using response surface methodology. Food Packag. 2018, 17, 11–24. [Google Scholar] [CrossRef]
- Ho, B.T.; Roberts, T.K.; Lucas, S. An overview on biodegradation of polystyrene and modified polystyrene: The microbial approach. Crit. Rev. Biotechnol. 2017, 38, 308–320. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, J.; Wu, W.M.; Zhao, J.; Song, Y.; Gao, L.; Yang, R.; Jiang, L. Biodegradation and Mineralization of Polystyrene by Plastic-Eating Mealworms: Part 2. Role of Gut Microorganisms. Environ. Sci. Technol. 2015, 49, 12087–12093. [Google Scholar] [CrossRef]
- Deshwal, G.K.; Panjagari, N.R.; Alam, T. An overview of paper and paper based food packaging materials: Health safety and environmental concerns. J. Food Sci. Technol. 2019, 56, 4391–4403. [Google Scholar] [CrossRef]
- Deshwal, G.K.; Panjagari, N.R. Review on metal packaging: Materials, forms, food applications, safety and recyclability. J. Food Sci. Technol. 2020, 57, 2377–2392. [Google Scholar] [CrossRef]
- Ahmet, Y.; Sezgin, A. Food Packaging: Glass And Plastic. In Researches on Science and Art in 21st Century Turkey; Gece Kitapligi: Ankara, Turkey, 2017; pp. 735–740. [Google Scholar]
- Mangaraj, S.; Yadav, A.; Bal, L.M.; Dash, S.K.; Mahanti, N.K. Application of biodegradable polymers in food packaging industry: A comprehensive review. J. Packag. Technol. Res. 2019, 3, 77–96. [Google Scholar] [CrossRef]
- Schmid, M.; Müller, K. Whey protein-based packaging films and coatings. In Whey Proteins; Academic Press: Cambridge, MA, USA, 2019; pp. 407–437. [Google Scholar] [CrossRef]
- Kaewprachu, P.; Osako, K.; Benjakul, S.; Tongdeesoontorn, W.; Rawdkuen, S. Biodegradable protein-based films and their properties: A comparative study. Packag. Technol. Sci. 2016, 29, 77–90. [Google Scholar] [CrossRef]
- Zhang, H.; Mittal, G. Biodegradable protein-based films from plant resources: A review. Environ. Prog. Sustain. Energy 2010, 29, 203–220. [Google Scholar] [CrossRef]
- Pérez, L.M.; Piccirilli, G.N.; Delorenzi, N.J.; Verdini, R.A. Effect of different combinations of glycerol and/or trehalose on physical and structural properties of whey protein concentrate-based edible films. Food Hydrocoll. 2016, 56, 352–359. [Google Scholar] [CrossRef]
- Zavareze, E.D.R.; Halal, S.L.M.E.; Marques e Silva, R.; Dias, A.R.G.; Prentice-Hernández, C. Mechanical, Barrier and Morphological Properties of Biodegradable Films Based on Muscle and Waste Proteins from the Whitemouth Croaker (Micropogonias furnieri). J. Food Process. Preserv. 2014, 38, 1973–1981. [Google Scholar] [CrossRef]
- Shendurse, A.M.; Gopikrishna, G.; Patel, A.C.; Pandya, A.J. Milk protein based edible films and coatings–preparation, properties and food applications. J. Nutr. Health Food Eng. 2018, 8, 219–226. [Google Scholar] [CrossRef]
- Qiu, Y.T.; Wang, B.J.; Weng, Y.M. Preparation and characterization of genipin cross-linked and lysozyme incorporated antimicrobial sodium caseinate edible films. Food Packag. Shelf Life 2020, 26, 100601. [Google Scholar] [CrossRef]
- Azevedo, V.M.; de Oliveira, A.C.S.; Borges, S.V.; Raguzzoni, J.C.; Dias, M.V.; Costa, A.L.R. Pea protein isolate nanocomposite films for packaging applications: Effect of starch nanocrystals on the structural, morphological, thermal, mechanical and barrier properties. Emir. J. Food Agric. 2020, 32, 495–504. [Google Scholar] [CrossRef]
- Schmid, M.; Proels, S.; Kainz, D.M.; Hammann, F. Effect of thermally induced denaturation on molecular interaction-response relationships of whey protein isolate based films and coatings. Prog. Org. Coat. 2017, 104, 161–172. [Google Scholar] [CrossRef]
- Akhtar, M.J.; Aïder, M. Study of the barrier and mechanical properties of packaging edible films fabricated with hydroxypropyl methylcellulose (HPMC) combined with electro-activated whey. J. Packag. Technol. Res. 2018, 2, 169–180. [Google Scholar] [CrossRef]
- Chalermthai, B.; Chan, W.Y.; Bastidas-Oyanedel, J.R.; Taher, H.; Olsen, B.D.; Schmidt, J.E. Preparation and characterization of whey protein-based polymers produced from residual dairy streams. Polymers 2019, 11, 722. [Google Scholar] [CrossRef] [PubMed]
- Brady, J.W. Introductory Food Chemistry; Comstock Publishing Associates: Ithaca, NY, USA, 2013. [Google Scholar]
- Rakhmanova, A.; Khan, Z.A.; Sharif, R.; Lv, X. Meeting the requirements of halal gelatin: A mini review. MOJ Food Proc. Technol. 2018, 6, 477–482. [Google Scholar] [CrossRef]
- Karim, A.A.; Bhat, R. Fish gelatin: Properties, challenges, and prospects as an alternative to mammalian gelatins. Food Hydrocoll. 2009, 23, 563–576. [Google Scholar] [CrossRef]
- Gornall, J.L.; Terentjev, E.M. Helix–coil transition of gelatin: Helical morphology and stability. Soft Matter 2008, 4, 544–549. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, S.A.; El-Sakhawy, M.; Nashy, E.S.H.; Othman, A.M. Novel natural composite films as packaging materials with enhanced properties. Int. J. Biol. Macromol. 2019, 136, 774–784. [Google Scholar] [CrossRef]
- Reddy, N.; Yang, Y. Thermoplastic films from plant proteins. J. Appl. Polym. Sci. 2013, 130, 729–738. [Google Scholar] [CrossRef]
- Vahedikia, N.; Garavand, F.; Tajeddin, B.; Cacciotti, I.; Jafari, S.M.; Omidi, T.; Zahedi, Z. Biodegradable zein film composites reinforced with chitosan nanoparticles and cinnamon essential oil: Physical, mechanical, structural and antimicrobial attributes. Colloids Surf. B Biointerfaces 2019, 177, 25–32. [Google Scholar] [CrossRef]
- Ünalan, İ.U.; Korel, F.; Yemenicioğlu, A. Active packaging of ground beef patties by edible zein films incorporated with partially purified lysozyme and Na2EDTA. Int. J. Food Sci. 2011, 46, 1289–1295. [Google Scholar] [CrossRef] [Green Version]
- Visakh, P.M. Biomonomers for Green Polymers: Introduction. In Bio Monomers for Green Polymeric Composite Materials; Wiley: Hoboken, NJ, USA, 2019; pp. 1–24. [Google Scholar] [CrossRef]
- dos Santos Paglione, I.; Galindo, M.V.; de Souza, K.C.; Yamashita, F.; Grosso, C.R.F.; Sakanaka, L.S.; Shirai, M.A. Optimization of the conditions for producing soy protein isolate films. Emir. J. Food Agric. 2019, 31, 297–303. [Google Scholar] [CrossRef]
- Ortiz, C.M.; de Moraes, J.O.; Vicente, A.R.; Laurindo, J.B.; Mauri, A.N. Scale-up of the production of soy (Glycine max L.) protein films using tape casting: Formulation of film-forming suspension and drying conditions. Food Hydrocoll. 2017, 66, 110–117. [Google Scholar] [CrossRef]
- Lodha, P.; Netravali, A.N. Thermal and mechanical properties of environment-friendly ‘green’plastics from stearic acid modified-soy protein isolate. Ind. Crops Prod. 2005, 21, 49–64. [Google Scholar] [CrossRef]
- Mohareb, E.; Mittal, G.S. Formulation and process conditions for biodegradable/edible soy-based packaging trays. Packag. Technol. Sci. 2007, 20, 1–15. [Google Scholar] [CrossRef]
- de Moraes Crizel, T.; de Oliveira Rios, A.; Alves, V.D.; Bandarra, N.; Moldão-Martins, M.; Hickmann Flôres, S. Biodegradable films based on gelatin and papaya peel microparticles with antioxidant properties. Food Bioproc. Tech. 2018, 11, 536–550. [Google Scholar] [CrossRef]
- Soradech, S.; Nunthanid, J.; Limmatvapirat, S.; Luangtana-anan, M. Utilization of shellac and gelatin composite film for coating to extend the shelf life of banana. Food Control. 2017, 73, 1310–1317. [Google Scholar] [CrossRef]
- Oliveira, M.A.; Furtado, R.F.; Bastos, M.S.R.; Leitão, R.C.; Benevides, S.D.; Muniz, C.R.; Biswas, A. Performance evaluation of cashew gum and gelatin blend for food packaging. Food Packag. Shelf Life 2018, 17, 57–64. [Google Scholar] [CrossRef]
- Huang, T.; Fang, Z.; Zhao, H.; Xu, D.; Yang, W.; Yu, W.; Zhang, J. Physical properties and release kinetics of electron beam irradiated fish gelatin films with antioxidants of bamboo leaves. Food Biosci. 2020, 36, 100597. [Google Scholar] [CrossRef]
- Zhu, F. Polysaccharide based films and coatings for food packaging: Effect of added polyphenols. Food Chem. 2021, 359, 129871. [Google Scholar] [CrossRef]
- Gao, H.X.; He, Z.; Sun, Q.; He, Q.; Zeng, W.C. A functional polysaccharide film forming by pectin, chitosan, and tea polyphenols. Carbohydr. Polym. 2018, 215, 1–7. [Google Scholar] [CrossRef]
- Zhu, F. Dietary fiber polysaccharides of amaranth, buckwheat and quinoa grains: A review of chemical structure, biological functions and food uses. Carbohydr. Polym. 2020, 248, 116819. [Google Scholar] [CrossRef]
- Mostafavi, F.S.; Zaeim, D. Agar-based edible films for food packaging applications-A review. Int. J. Biol. Macromol. 2020, 159, 1165–1176. [Google Scholar] [CrossRef]
- Alizadeh-Sani, M.; Ehsani, A.; Moghaddas Kia, E.; Khezerlou, A. Microbial gums: Introducing a novel functional component of edible coatings and packaging. Appl. Microbiol. Biotechnol. 2019, 103, 6853–6866. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Chen, L.; Jin, Z.; Jiao, A. Research progress of starch-based biodegradable materials: A review. J. Mater. Sci. 2021, 56, 11187–11208. [Google Scholar] [CrossRef]
- Shirazani, M.T.; Bakhshi, H.; Rashidi, A.; Taghizadeh, M. Starch-based activated carbon micro-spheres for adsorption of methane with superior performance in ANG technology. J. Environ. Chem. Eng. 2020, 8, 103910. [Google Scholar] [CrossRef]
- Ilyas, R.A.; Sapuan, S.M.; Ishak, M.R.; Zainudin, E.S. Sugar palm nanocrystalline cellulose reinforced sugar palm starch composite: Degradation and water-barrier properties. IOP Conf. Ser. Mater. Sci. Eng. 2018, 368, 012006. [Google Scholar] [CrossRef]
- Palma-Rodríguez, H.M.; Aguirre-Álvarez, G.; Chavarría-Hernández, N.; Rodríguez-Hernández, A.I.; Bello-Pérez, L.A.; Vargas-Torres, A. Oxidized banana starch–polyvinyl alcohol film: Partial characterization. Starch-Stärke 2012, 64, 882–889. [Google Scholar] [CrossRef]
- Klemm, D.; Kramer, F.; Moritz, S.; Lindström, T.; Ankerfors, M.; Gray, D.; Dorris, A. Nanocelluloses: A new family of nature-based materials. Angew. Chem. Int. Ed. 2011, 50, 5438–5466. [Google Scholar] [CrossRef]
- Moghimi, R.; Aliahmadi, A.; Rafati, H. Antibacterial hydroxypropyl methyl cellulose edible films containing nanoemulsions of Thymus daenensis essential oil for food packaging. Carbohydr. Polym. 2017, 175, 241–248. [Google Scholar] [CrossRef]
- Tabari, M. Investigation of carboxymethyl cellulose (CMC) on mechanical properties of cold water fish gelatin biodegradable edible films. Foods 2017, 6, 41. [Google Scholar] [CrossRef]
- Shanmugam, K.; Doosthosseini, H.; Varanasi, S.; Garnier, G.; Batchelor, W. Nanocellulose films as air and water vapour barriers: A recyclable and biodegradable alternative to polyolefin packaging. Sustain. Mater. Technol. 2019, 22, e00115. [Google Scholar] [CrossRef]
- El Achaby, M.; El Miri, N.; Aboulkas, A.; Zahouily, M.; Bilal, E.; Barakat, A.; Solhy, A. Processing and properties of eco-friendly bio-nanocomposite films filled with cellulose nanocrystals from sugarcane bagasse. Int. J. Biol. Macromol. 2017, 96, 340–352. [Google Scholar] [CrossRef]
- Venkatachalam, K.; Lekjing, S. A chitosan-based edible film with clove essential oil and nisin for improving the quality and shelf life of pork patties in cold storage. RSC Adv. 2017, 10, 17777–17786. [Google Scholar] [CrossRef] [PubMed]
- Sani, M.A.; Tavassoli, M.; Hamishehkar, H.; McClements, D.J. Carbohydrate-based films containing pH-sensitive red barberry anthocyanins: Application as biodegradable smart food packaging materials. Carbohydr. Polym. 2021, 255, 117488. [Google Scholar] [CrossRef] [PubMed]
- Francisco, C.B.; Pellá, M.G.; Silva, O.A.; Raimundo, K.F.; Caetano, J.; Linde, G.A.; Colauto, N.B.; Dragunski, D.C. Shelf-life of guavas coated with biodegradable starch and cellulose-based films. Int. J. Biol. Macromol. 2020, 152, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Ngo, T.M.P.; Nguyen, T.H.; Dang, T.M.Q.; Tran, T.X.; Rachtanapun, P. Characteristics and antimicrobial properties of active edible films based on pectin and nanochitosan. Int. J. Mol. Sci. 2020, 21, 2224. [Google Scholar] [CrossRef]
- Rai, S.K.; Chaturvedi, K.; Yadav, S.K. Evaluation of structural integr.ty and functionality of commercial pectin based edible films incorporated with corn flour, beetroot, orange peel, muesli and rice flour. Food Hydrocoll. 2019, 91, 127–135. [Google Scholar] [CrossRef]
- Bermúdez-Oria, A.; Rodríguez-Gutiérrez, G.; Vioque, B.; Rubio-Senent, F.; Fernández-Bolaños, J. Physical and functional properties of pectin-fish gelatin films containing the olive phenols hydroxytyrosol and 3, 4-dihydroxyphenylglycol. Carbohydr. Polym. 2017, 178, 368–377. [Google Scholar] [CrossRef]
- Stephen, A.M.; Phillips, G.O. Food Polysaccharides and Their Applications; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Phillips, G.O.; Williams, P.A. Handbook of Hydrocolloids; Elsevier: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Hassan, B.; Chatha, S.A.S.; Hussain, A.I.; Zia, K.M.; Akhtar, N. Recent advances on polysaccharides, lipids and protein based edible films and coatings: A review. Int. J. Biol. Macromol. 2018, 109, 1095–1107. [Google Scholar] [CrossRef]
- Akoh, C.C. Food Lipids: Chemistry, Nutrition, and Biotechnology; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Galus, S.; Arik Kibar, E.A.; Gniewosz, M.; Kraśniewska, K. Novel materials in the preparation of edible films and coatings—A review. Coatings 2020, 10, 674. [Google Scholar] [CrossRef]
- Mohamed, S.A.; El-Sakhawy, M.; El-Sakhawy, M.A.M. Polysaccharides, protein and lipid-based natural edible films in food packaging: A review. Carbohydr. Polym. 2020, 238, 116178. [Google Scholar] [CrossRef]
- Rodrigues, D.C.; Cunha, A.P.; Brito, E.S.; Azeredo, H.M.; Gallao, M.I. Mesquite seed gum and palm fruit oil emulsion edible films: Influence of oil content and sonication. Food Hydrocoll. 2016, 56, 227–235. [Google Scholar] [CrossRef]
- Vargas, M.; Albors, A.; Chiralt, A. Application of chitosan-sunflower oil edible films to pork meat hamburgers. Procedia Food Sci. 2011, 1, 39–43. [Google Scholar] [CrossRef]
- Randazzo, W.; Jiménez-Belenguer, A.; Settanni, L.; Perdones, A.; Moschetti, M.; Palazzolo, E.; Moschetti, G. Antilisterial effect of citrus essential oils and their performance in edible film formulations. Food Control. 2016, 59, 750–758. [Google Scholar] [CrossRef]
- Syafiq, R.; Sapuan, S.M.; Zuhri, M.Y.M.; Ilyas, R.A.; Nazrin, A.; Sherwani, S.F.K.; Khalina, A. Antimicrobial activities of starch-based biopolymers and biocomposites incorporated with plant essential oils: A review. Polymers 2020, 12, 2403. [Google Scholar] [CrossRef] [PubMed]
- Haq, M.A.; Hasnain, A.; Jafri, F.A.; Akbar, M.F.; Khan, A. Characterization of edible gum cordia film: Effects of beeswax. LWT Food Sci. Technol. 2016, 68, 674–680. [Google Scholar] [CrossRef]
- Bizymis, A.P.; Tzia, C. Edible films and coatings: Properties for the selection of the components, evolution through composites and nanomaterials, and safety issues. Crit. Rev. Food Sci. Nutr. 2021, 8, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Tavassoli-Kafrani, E.; Shekarchizadeh, H.; Masoudpour-Behabadi, M. Development of edible films and coatings from alginates and carrageenans. Carbohydr. Polym. 2016, 137, 360–374. [Google Scholar] [CrossRef] [PubMed]
- Murrieta-Martínez, C.L.; Soto-Valdez, H.; Pacheco-Aguilar, R.; Torres-Arreola, W.; Rodríguez-Felix, F.; Márquez Ríos, E. Edible protein films: Sources and behavior. Packag. Technol. Sci. 2018, 31, 113–122. [Google Scholar] [CrossRef]
- Imre, B.; García, L.; Puglia, D.; Vilaplana, F. Reactive compatibilization of plant polysaccharides and biobased polymers: Review on current strategies, expectations and reality. Carbohydr. Polym. 2019, 209, 20–37. [Google Scholar] [CrossRef]
- Cazón, P.; Velazquez, G.; Ramírez, J.A.; Vázquez, M. Polysaccharide-based films and coatings for food packaging: A review. Food Hydrocoll. 2017, 68, 136–148. [Google Scholar] [CrossRef]
- Kumar, A.; Hasan, M.; Mangaraj, S.; Pravitha, M.; Verma, D.K.; Srivastav, P.P. Trends in Edible Packaging Films and its Prospective Future in Food: A Review. Appl. Food Biotechnol. 2022, 2, 100118. [Google Scholar] [CrossRef]
- Hon, D.N.S. Cellulose and its derivatives: Structures, reactions, and medical uses. In Polysaccharides in Medicinal Applications; Routledge: Abingdon-on-Thames, UK, 2017; pp. 87–105. [Google Scholar]
- Ngatirah, N.; Ruswanto, A.; Sunardi, S. Effect of Hydroxypropyl methylcellulose from oil palm empty fruit bunch on oil uptake and physical properties of French fries. Food Sci. Technol. 2022, 42, e110421. [Google Scholar] [CrossRef]
- Fagundes, C.; Palou, L.; Monteiro, A.R.; Pérez-Gago, M.B. Hydroxypropyl methylcellulose-beeswax edible coatings formulated with antifungal food additives to reduce alternaria black spot and maintain postharvest quality of cold-stored cherry tomatoes. Sci. Hortic. 2015, 193, 249–257. [Google Scholar] [CrossRef]
- Askari, F.; Sadeghi, E.; Mohammadi, R.; Rouhi, M.; Taghizadeh, M.; Hosein Shirgardoun, M.; Kariminejad, M. The physicochemical and structural properties of psyllium gum/modified starch composite edible film. J. Food Process. Preserv. 2018, 42, e13715. [Google Scholar] [CrossRef]
- Ceballos, R.L.; Ochoa-Yepes, O.; Goyanes, S.; Bernal, C.; Famá, L. Effect of yerba mate extract on the performance of starch films obtained by extrusion and compression molding as active and smart packaging. Carbohydr. Polym. 2020, 244, 116495. [Google Scholar] [CrossRef]
- Krishna, M.; Nindo, C.I.; Min, S.C. Development of fish gelatin edible films using extrusion and compression molding. J. Food Eng. 2012, 108, 337–344. [Google Scholar] [CrossRef]
- Morales-Contreras, B.E.; Wicker, L.; Rosas-Flores, W.; Contreras-Esquivel, J.C.; Gallegos-Infante, J.A.; Reyes-Jaquez, D.; Morales-Castro, J. Apple pomace from variety “Blanca de Asturias” as sustainable source of pectin: Composition, rheological, and thermal properties. LWT 2020, 117, 108641. [Google Scholar] [CrossRef]
- Espitia, P.J.; Batista, R.A.; Azeredo, H.M.; Otoni, C.G. Probiotics and their potential applications in active edible films and coatings. Int. Food Res. J. 2016, 90, 42–52. [Google Scholar] [CrossRef] [Green Version]
- Chodijah, S.; Husaini, A.; Zaman, M. Extraction of pectin from banana peels (musa paradiasica fomatypica) for biodegradable plastic films. J. Phys. Conf. Ser. 2019, 1167, 012061, IOP Publishing. [Google Scholar] [CrossRef]
- Gorrasi, G.; Bugatti, V. Edible bio-nano-hybrid coatings for food protection based on pectins and LDH-salicylate: Preparation and analysis of physical properties. LWT Food Sci. Technol. 2016, 69, 139–145. [Google Scholar] [CrossRef]
- Madsen, M.; Westh, P.; Khan, S.; Ipsen, R.; Almdal, K.; Aachmann, F.L.; Svensson, B. Impact of Alginate Mannuronic-Guluronic Acid Contents and pH on Protein Binding Capacity and Complex Size. Biomacromolecules 2021, 22, 649–660. [Google Scholar] [CrossRef]
- Senturk, P.T.; Müller, K.; Schmid, M. Alginate-based edible films and coatings for food packaging applications. Foods 2018, 7, 170. [Google Scholar] [CrossRef] [PubMed]
- Mahcene, Z.; Khelil, A.; Hasni, S.; Bozkurt, F.; Goudjil, M.B.; Tornuk, F. Home-made cheese preservation using sodium alginate based on edible film incorporating essential oils. J. Food Sci. Technol. 2021, 58, 2406–2419. [Google Scholar] [CrossRef] [PubMed]
- Coltelli, M.B.; Danti, S.; De Clerck, K.; Lazzeri, A.; Morganti, P. Pullulan for advanced sustainable body-and skin-contact applications. J. Funct. Biomater. 2020, 11, 20. [Google Scholar] [CrossRef] [PubMed]
- Omar-Aziz, M.; Khodaiyan, F.; Yarmand, M.S.; Mousavi, M.; Gharaghani, M.; Kennedy, J.F.; Hosseini, S.S. Combined effects of octenylsuccination and beeswax on pullulan films: Water-resistant and mechanical properties. Carbohydr. Polym. 2021, 255, 117471. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Mukherjee, A.; Dutta, J. Chitosan based nanocomposite films and coatings: Emerging antimicrobial food packaging alternatives. Trends Food Sci. Technol. 2020, 97, 196–209. [Google Scholar] [CrossRef]
- Li, Y.; Cao, C.; Pei, Y.; Liu, X.; Tang, K. Preparation and properties of microfibrillated chitin/gelatin composites. Int. J. Biol. Macromol. 2019, 130, 715–719. [Google Scholar] [CrossRef]
- Ribeiro, A.M.; Estevinho, B.N.; Rocha, F. Preparation and incorporation of functional ingredients in edible films and coatings. Food Bioproc. Tech. 2021, 14, 209–231. [Google Scholar] [CrossRef]
- Nguyen, A.T.; Parker, L.; Brennan, L.; Lockrey, S. A consumer definition of eco-friendly packaging. J. Clean. Prod. 2020, 252, 119792. [Google Scholar] [CrossRef]
- Mellinas, C.; Ramos, M.; Jiménez, A.; Garrigós, M.C. Recent trends in the use of pectin from agro-waste residues as a natural-based biopolymer for food packaging applications. Materials 2020, 13, 673. [Google Scholar] [CrossRef]
- Galus, S.; Kadzińska, J. Whey protein edible films modified with almond and walnut oils. Food Hydrocoll. 2016, 52, 78–86. [Google Scholar] [CrossRef]
- Kumari, M.; Mahajan, H.; Joshi, R.; Gupta, M. Development and structural characterization of edible films for improving fruit quality. Food Packag. Shelf Life 2017, 12, 42–50. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, H. Preparation and properties of zein–rutin composite nanoparticle/corn starch films. Carbohydr. Polym. 2017, 169, 385–392. [Google Scholar] [CrossRef]
- Wittaya, T. Protein-based edible films: Characteristics and improvement of properties. Struct. Funct. Food Eng. 2012, 3, 44–70. [Google Scholar] [CrossRef]
- Bourtoom, T. Edible protein films: Properties enhancement. Int. Food Res. J. 2009, 16, 1–9. [Google Scholar]
- Wagh, Y.R.; Pushpadass, H.A.; Emerald, F.; Nath, B.S. Preparation and characterization of milk protein films and their application for packaging of Cheddar cheese. J. Food Sci. Technol. 2014, 51, 3767–3775. [Google Scholar] [CrossRef] [PubMed]
- Çakmak, H.; Özselek, Y.; Turan, O.Y.; Fıratlıgil, E.; Karbancioğlu-Güler, F. Whey protein isolate edible films incorporated with essential oils: Antimicrobial activity and barrier properties. Polym. Degrad. Stab. 2020, 179, 109285. [Google Scholar] [CrossRef]
- Seydim, A.C.; Sarikus-Tutal, G.; Sogut, E. Effect of whey protein edible films containing plant essential oils on microbial inactivation of sliced Kasar cheese. Food Packag. Shelf Life 2020, 26, 100567. [Google Scholar] [CrossRef]
- Fernandes, L.M.; Guimarães, J.T.; Pimentel, T.C.; Esmerino, E.A.; Freitas, M.Q.; Carvalho, C.W.P.; Silva, M.C. Edible whey protein films and coatings added with prebiotic ingredients. In Agri-Food Industry Strategies for Healthy Diets and Sustainability; Academic Press: Cambridge, MA, USA, 2020; pp. 177–193. [Google Scholar] [CrossRef]
- Zoghi, A.; Khosravi-Darani, K.; Mohammadi, R. Application of edible films containing probiotics in food products. J. Food Prot. 2020, 15, 307–320. [Google Scholar] [CrossRef]
- Yam, K.L.; Takhistov, P.T.; Miltz, J. Intelligent packaging: Concepts and applications. J. Food Sci. 2005, 70, R1–R10. [Google Scholar] [CrossRef]
- Vanderroost, M.; Ragaert, P.; Devlieghere, F.; De Meulenaer, B. Intelligent food packaging: The next generation. Trends Food Sci. Technol. 2014, 39, 47–62. [Google Scholar] [CrossRef]
- Siracusa, V.; Lotti, N. Intelligent packaging to improve shelf life. In Food Quality and Shelf Life; Academic Press: Cambridge, MA, USA, 2019; pp. 261–279. [Google Scholar]
- Ghaani, M.; Cozzolino, C.A.; Castelli, G.; Farris, S. An overview of the intelligent packaging technologies in the food sector. Trends Food Sci. Technol. 2016, 51, 1–11. [Google Scholar] [CrossRef]
- Han, J.W.; Ruiz-Garcia, L.; Qian, J.P.; Yang, X.T. Food packaging: A comprehensive review and future trends. Compr. Rev. Food Sci. Food Saf. 2018, 17, 860–877. [Google Scholar] [CrossRef] [PubMed]
- Drago, E.; Campardelli, R.; Pettinato, M.; Perego, P. Innovations in smart packaging concepts for food: An extensive review. Foods 2020, 9, 1628. [Google Scholar] [CrossRef] [PubMed]
- Yam, K.L. Intelligent packaging to enhance food safety and quality. In Emerging Food Packaging Technologies; Woodhead Publishing: Sawston, UK, 2012; pp. 137–152. [Google Scholar]
- Kalpana, S.; Priyadarshini, S.R.; Leena, M.M.; Moses, J.A.; Anandharamakrishnan, C. Intelligent packaging: Trends and applications in food systems. Trends Food Sci. Technol. 2019, 93, 145–157. [Google Scholar] [CrossRef]
- Poyatos-Racionero, E.; Ros-Lis, J.V.; Vivancos, J.L.; Martinez-Manez, R. Recent advances on intelligent packaging as tools to reduce food waste. J. Clean. Prod. 2018, 172, 3398–3409. [Google Scholar] [CrossRef]
- Jiang, G.; Hou, X.; Zeng, X.; Zhang, C.; Wu, H.; Shen, G.; Li, S.; Luo, Q.; Li, M.; Liu, X.; et al. Preparation and characterization of indicator films from carboxymethyl-cellulose/starch and purple sweet potato (Ipomoea batatas (L.) lam) anthocyanins for monitoring fish freshness. Int. J. Biol. Macromol. 2020, 143, 359–372. [Google Scholar] [CrossRef]
- Amogne, N.Y.; Ayele, D.W.; Tsigie, Y.A. Recent advances in anthocyanin dyes extracted from plants for dye sensitized solar cell. Mater. Renew. Sustain. Energy 2020, 9, 23. [Google Scholar] [CrossRef]
- Park, K.J.; Lee, J.S.; Jo, H.J.; Kim, E.S.; Lee, H.G. Antimicrobial and indicator properties of edible film containing clove bud oil-loaded chitosan capsules and red cabbage for fish preservation. Int. J. Biol. Macromol. 2022, 196, 163–171. [Google Scholar] [CrossRef]
- Erna, K.H.; Felicia, W.X.L.; Vonnie, J.M.; Rovina, K.; Yin, K.W.; Nur’Aqilah, M.N. Synthesis and Physicochemical Characterization of Polymer Film-Based Anthocyanin and Starch. Biosensors 2022, 12, 211. [Google Scholar] [CrossRef]
- Erna, K.H.; Felicia, W.X.L.; Rovina, K.; Vonnie, J.M.; Huda, N. Development of curcumin/rice starch films for sensitive detection of hypoxanthine in chicken and fish meat. Carbohydr. Polym. Techn. App. 2022, 3, 100189. [Google Scholar] [CrossRef]
- He, F.; Kong, Q.; Jin, Z.; Mou, H. Developing a unidirectionally permeable edible film based on ĸ-carrageenan and gelatin for visually detecting the freshness of grass carp fillets. Carbohydr. Polym. 2020, 241, 116336. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Yang, Y.; Liu, R.; Liu, X.; Ma, J.; Wu, M.; Wang, S. Preparation of sugarcane bagasse nanocellulose hydrogel as a colourimetric freshness indicator for intelligent food packaging. Carbohydr. Polym. 2020, 249, 116831. [Google Scholar] [CrossRef] [PubMed]
- Ezati, P.; Priyadarshi, R.; Bang, Y.J.; Rhim, J.W. CMC and CNF-based intelligent pH-responsive color indicator films integrated with shikonin to monitor fish freshness. Food Cont. 2021, 126, 108046. [Google Scholar] [CrossRef]
- Baek, S.; Maruthupandy, M.; Lee, K.; Kim, D.; Seo, J. Freshness indicator for monitoring changes in quality of packaged kimchi during storage. Food Packag. Shelf Life 2020, 25, 100528. [Google Scholar] [CrossRef]
- Kuswandi, B.; Asih, N.P.; Pratoko, D.K.; Kristiningrum, N.; Moradi, M. Edible pH sensor based on immobilized red cabbage anthocyanins into bacterial cellulose membrane for intelligent food packaging. Packag. Technol. Sci. 2020, 33, 321–332. [Google Scholar] [CrossRef]
- Taghinia, P.; Abdolshahi, A.; Sedaghati, S.; Shokrollahi, B. Smart edible films based on mucilage of lallemantia iberica seed incorporated with curcumin for freshness monitoring. Food Sci. Nutr. 2021, 9, 1222–1231. [Google Scholar] [CrossRef] [PubMed]
- Mariah, M.A.A.; Vonnie, J.M.; Erna, K.H.; Nur’Aqilah, N.M.; Huda, N.; Abdul Wahab, R.; Rovina, K. The Emergence and Impact of Ethylene Scavengers Techniques in Delaying the Ripening of Fruits and Vegetables. Membranes 2022, 12, 117. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh-Sani, M.; Mohammadian, E.; McClements, D.J. Eco-friendly active packaging consisting of nanostructured biopolymer matrix reinforced with TiO2 and essential oil: Application for preservation of refrigerated meat. Food Chem. 2020, 322, 126782. [Google Scholar] [CrossRef]
- Lamba, A.; Garg, V. Recent innovations in food packaging: A review. Int. J. Food Sci. Nutr. Int. 2019, 4, 123–129. [Google Scholar]
- Roy, S.; Rhim, J.W. Anthocyanin food colorant and its application in pH-responsive color change indicator films. Crit. Rev. Food Sci. Nutr. 2021, 61, 2297–2325. [Google Scholar] [CrossRef]
- Sohail, M.; Sun, D.W.; Zhu, Z. Recent developments in intelligent packaging for enhancing food quality and safety. Crit. Rev. Food Sci. Nutr. 2018, 58, 2650–2662. [Google Scholar] [CrossRef] [PubMed]
- Won, K.; Jang, N.Y.; Jeon, J. A natural component-based oxygen indicator with in-pack activation for intelligent food packaging. J. Agric. Food Chem. 2016, 64, 9675–9679. [Google Scholar] [CrossRef] [PubMed]
- Jang, N.Y.; Won, K. New pressure-activated compartmented oxygen indicator for intelligent food packaging. J. Food Sci. Technol. 2014, 49, 650–654. [Google Scholar] [CrossRef]
- Chen, H.Z.; Zhang, M.; Bhandari, B.; Guo, Z. Applicability of a colorimetric indicator label for monitoring freshness of fresh-cut green bell pepper. Postharvest Biol. Technol. 2018, 140, 85–92. [Google Scholar] [CrossRef]
- Abu-Hani, A.F.; Greish, Y.E.; Mahmoud, S.T.; Awwad, F.; Ayesh, A.I. Low-temperature and fast response H2S gas sensor using semiconducting chitosan film. Sens. Actuators B Chem. 2017, 253, 677–684. [Google Scholar] [CrossRef]
- Ko, G.J.; Han, S.D.; Kim, J.K.; Zhu, J.; Han, W.B.; Chung, J.; Yang, S.M.; Cheng, H.; Kim, D.H.; Kang, C.Y.; et al. Biodegradable, flexible silicon nanomembrane-based NOx gas sensor system with record-high performance for transient environmental monitors and medical implants. NPG Asia Mater. 2020, 12, 71. [Google Scholar] [CrossRef]
- Zhai, X.; Shi, J.; Zou, X.; Wang, S.; Jiang, C.; Zhang, J.; Holmes, M. Novel colorimetric films based on starch/polyvinyl alcohol incorporated with roselle anthocyanins for fish freshness monitoring. Food Hydrocoll. 2017, 69, 308–317. [Google Scholar] [CrossRef]
- Ma, Q.; Du, L.; Wang, L. Tara gum/polyvinyl alcohol-based colorimetric NH3 indicator films incorporating curcumin for intelligent packaging. Sens. Actuators B Chem. 2017, 244, 759–766. [Google Scholar] [CrossRef]
- Alizadeh-Sani, M.; Mohammadian, E.; Rhim, J.W.; Jafari, S.M. pH-sensitive (halochromic) smart packaging films based on natural food colorants for the monitoring of food quality and safety. Trends Food Sci. Technol. 2020, 105, 93–144. [Google Scholar] [CrossRef]
- Yousefi, H.; Su, H.M.; Imani, S.M.; Alkhaldi, K.M.; Filipe, C.D.; Didar, T.F. Intelligent food packaging: A review of smart sensing technologies for monitoring food quality. ACS Sens. 2019, 4, 808–821. [Google Scholar] [CrossRef]
- Taoukis, P.; Labuza, T.P. Applicability of time-temperature indicators as shelf life monitors of food products. J. Food Sci. 1989, 54, 783–788. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, G.; Xiao, X.; Liu, Y.; Zheng, X. Application of microbial TTIs as smart label for food quality: Response mechanism, application and research trends. Trends Food Sci. Technol. 2016, 51, 12–23. [Google Scholar] [CrossRef]
- Göransson, M.; Nilsson, F. Jevinger Temperature performance and food shelf-life accuracy in cold food supply chains—Insights from multiple field studies. Food Cont. 2018, 86, 332–341. [Google Scholar] [CrossRef]
- Maciel, V.B.; Yoshida, C.M.; Franco, T.T. Development of a prototype of a colourimetric temperature indicator for monitoring food quality. J. Food Eng. 2012, 111, 21–27. [Google Scholar] [CrossRef]
- Saenjaiban, A.; Singtisan, T.; Suppakul, P.; Jantanasakulwong, K.; Punyodom, W.; Rachtanapun, P. Novel color change film as a time–temperature indicator using polydiacetylene/silver nanoparticles embedded in carboxymethyl cellulose. Polymers 2020, 12, 2306. [Google Scholar] [CrossRef] [PubMed]
- Priyadarshi, R.; Kumar, B.; Negi, Y.S. Chitosan film incorporated with citric acid and glycerol as an active packaging material for extension of green chilli shelf life. Carbohydr. Polym. 2018, 195, 329–338. [Google Scholar] [CrossRef]
- European Commission. EU Guidance to the Commission Regulation (EC) No 450/2009 of 29 May 2009 on Active and Intelligent Materials and Articles Intended to Come into the Contact with Food (Version 1.0). 2009. Available online: https://ec.europa.eu/food/safety/chemical_safety/food_contact_materials_en (accessed on 1 July 2022).
- Yildirim, S.; Röcker, B.; Pettersen, M.K.; Nilsen-Nygaard, J.; Ayhan, Z.; Rutkaite, R.; Coma, V. Active packaging applications for food. Compr. Rev. Food Sci. Food Saf. 2018, 17, 165–199. [Google Scholar] [CrossRef]
- Qian, M.; Liu, D.; Zhang, X.; Yin, Z.; Ismail, B.B.; Ye, X.; Guo, M. A review of active packaging in bakery products: Applications and future trends. Trends Food Sci. Technol. 2021, 114, 459–471. [Google Scholar] [CrossRef]
- Gaikwad, K.K.; Singh, S.; Negi, Y.S. Ethylene scavengers for active packaging of fresh food produce. Environ. Chem. Lett. 2020, 18, 269–284. [Google Scholar] [CrossRef]
- Arfat, Y.A.; Ejaz, M.; Jacob, H.; Ahmed, J. Deciphering the potential of guar gum/Ag-Cu nanocomposite films as an active food packaging material. Carbohydr. Polym. 2017, 157, 65–71. [Google Scholar] [CrossRef]
- Valdivieso-Ugarte, M.; Gomez-Llorente, C.; Plaza-Díaz, J.; Gil, Á. Antimicrobial, antioxidant, and immunomodulatory properties of essential oils: A systematic review. Nutrients 2019, 11, 2786. [Google Scholar] [CrossRef]
- Brockgreitens, J.; Abbas, A. Responsive food packaging: Recent progress and technological prospects. Compr. Rev. Food Sci. Food Saf. 2016, 15, 3–15. [Google Scholar] [CrossRef]
- Alves, J.; Gaspar, P.D.; Lima, T.M.; Silva, P.D. What is the role of active packaging in the future of food sustainability? A systematic review. J. Sci. Food Agric. 2022. [Google Scholar] [CrossRef] [PubMed]
- Gaikwad, K.K.; Singh, S.; Lee, Y.S. Oxygen scavenging films in food packaging. Environ. Chem. Lett. 2018, 16, 523–538. [Google Scholar] [CrossRef]
- Juan-Polo, A.; Maestre Pérez, S.E.; Monedero Prieto, M.; Tone, A.M.; Sánchez Reig, C.; Beltrán Sanahuja, A. Double-function oxygen scavenger and aromatic food packaging films based on LDPE/polybutadiene and peanut aroma. Polymers 2021, 13, 1310. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.S.; Wang, H.J.; Jaisan, C.; An, D.S. Active food packaging to control carbon dioxide. Packag. Technol. Sci. 2022, 35, 213–227. [Google Scholar] [CrossRef]
- Musa, A.A. Effect of different packaging methods on consumers eating quality of beef. J. Food Saf. 2019, 18, 235–245. [Google Scholar]
- Rovera, C.; Ghaani, M.; Farris, S. Nano-inspired oxygen barrier coatings for food packaging applications: An overview. Trends Food Sci. Technol. 2020, 97, 210–220. [Google Scholar] [CrossRef]
- Hasani-Javanmardi, M.; Fallah, A.A.; Abbasvali, M. Effect of safflower oil nanoemulsion and cumin essential oil combined with oxygen absorber packaging on the quality and shelf-life of refrigerated lamb loins. LWT 2021, 147, 111557. [Google Scholar] [CrossRef]
- Syamila, M.; Gedi, M.A.; Briars, R.; Ayed, C.; Gray, D.A. Effect of temperature, oxygen and light on the degradation of β-carotene, lutein and α-tocopherol in spray-dried spinach juice powder during storage. Food Chem. 2019, 284, 188–197. [Google Scholar] [CrossRef]
- Foltynowicz, Z. Nanoiron-based composite oxygen scavengers for food packaging. Compos. Mater. Food Packag. 2018, 1, 209–234. [Google Scholar]
- Restuccia, D.; Spizzirri, U.G.; Parisi, O.I.; Cirillo, G.; Curcio, M.; Iemma, F.; Puoci, F.; Vinci, G.; Picci, N. New EU regulation aspects and global market of active and intelligent packaging for food industry applications. Food Cont. 2010, 21, 1425–1435. [Google Scholar] [CrossRef]
- Alonso, Y.N.; Grafia, A.L.; Castillo, L.A.; Barbosa, S.E. Active packaging films based on polyolefins modified by organic and inorganic nanoparticles. React. Funct. Polym. 2021, 3, 5–28. [Google Scholar] [CrossRef]
- Lee, J.S.; Chang, Y.; Lee, E.S.; Song, H.G.; Chang, P.S.; Han, J. Ascorbic acid-based oxygen scavenger in active food packaging system for raw meatloaf. J. Food Sci. 2018, 83, 682–688. [Google Scholar] [CrossRef] [PubMed]
- Ramakanth, D.; Akhila, K.; Gaikwad, K.K.; Maji, P.K. UV-activated oxygen scavenging system based on natural rubber latex from Hevea brasiliensis for active packaging applications. Ind. Crops Prod. 2022, 178, 114658. [Google Scholar] [CrossRef]
- He, Y.; Hu, X.; Xu, M.; Ng, A.M.C.; Djurišić, A.B. Mesoporous silica nanosphere-based oxygen scavengers. Microporous Mesoporous Mater. 2021, 327, 111426. [Google Scholar] [CrossRef]
- Rashed, M.S.; Sobhy, M.; Pathania, S. Active Packaging of Foods. In Shelf Life and Food Safety; CRC Press: Boca Raton, FL, USA, 2020; pp. 253–284. [Google Scholar]
- Hu, K.D.; Zhang, X.Y.; Yao, G.F.; Rong, Y.L.; Ding, C.; Tang, J.; Yang, F.; Huang, Z.Q.; Xu, Z.M.; Chen, X.Y.; et al. A nuclear-localized cysteine desulfhydrase plays a role in fruit ripening in tomato. Hortic. Res. 2020, 7, 211. [Google Scholar] [CrossRef]
- Wang, C.; Ajji, A. Development of a novel ethylene scavenger made up of pumice and potassium permanganate and its effect on preservation quality of avocados. J. Food Eng. 2022, 330, 111101. [Google Scholar] [CrossRef]
- Pirsa, S. Nanocomposite base on carboxymethylcellulose hydrogel: Simultaneous absorbent of ethylene and humidity to increase the shelf life of banana fruit. Int. J. Biol. Macromol. 2021, 193, 300–310. [Google Scholar] [CrossRef]
- Jaimun, R.; Sangsuwan, J. Efficacy of chitosan-coated paper incorporated with vanillin and ethylene adsorbents on the control of anthracnose and the quality of Nam Dok Mai mango fruit. Packag. Technol. Sci. 2019, 32, 383–394. [Google Scholar] [CrossRef]
- Fontana, A.J., Jr.; Carter, B.P. Measurement of water activity, moisture sorption isotherm, and moisture content of foods. Water Act. Foods Fundam. Appl. 2020, 207–226. [Google Scholar] [CrossRef]
- Hidayati, S.; Sartika, D.; Fudholi, A. Predict the Shelf Life of Instant Chocolate in Vacuum Packing by Using Accelerated Shelf Life Test (ASLT). Math. Model. Eng. Probl. 2022, 9, 443–450. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Dao, U.T.T.; Bui, Q.P.T.; Bach, G.L.; Thuc, C.H.; Thuc, H.H. Enhanced antimicrobial activities and physiochemical properties of edible film based on chitosan incorporated with Sonneratia caseolaris (L.) Engl. leaf extract. Prog. Org. Coat. 2020, 140, 105487. [Google Scholar] [CrossRef]
- Garavito, J.; Mendoza, S.M.; Castellanos, D.A. Configuration of biodegradable equilibrium modified atmosphere packages, including a moisture absorber for fresh cape gooseberry (Physalis peruviana L.) fruits. J. Food Eng. 2022, 314, 110761. [Google Scholar] [CrossRef]
- Hong, E.J.; Park, S.H.; & Kang, D.H. Sequential treatment of hydrogen peroxide, vacuum packaging, and dry heat for inactivating Salmonella Typhimurium on alfalfa seeds without detrimental effect on seeds viability. Food Microbiol. 2019, 77, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Siddique, S.; Majumder, S.; Abdul, M.I.; Rahman, S.A.; Lateef, D.; Dan, S.; Bose, A. A systemic approach on understanding the role of moisture in pharmaceutical product degradation and its prevention: Challenges and perspectives. Biomed. Res. 2018, 29, 3336–3343. [Google Scholar] [CrossRef]
- Chai, S.; Zhao, Y.; Ge, T.; Dai, Y. Experimental study on a fresh air heat pump desiccant dehumidification system using rejected heat. Appl. Therm. Eng. 2020, 179, 115742. [Google Scholar] [CrossRef]
- Bhardwaj, A.; Alam, T.; Talwar, N. Recent advances in active packaging of agri-food products: A review. J. Postharvest Technol. 2019, 7, 33–62. [Google Scholar]
- Singh, A.K.; Ramakanth, D.; Kumar, A.; Lee, Y.S.; Gaikwad, K.K. Active packaging technologies for clean label food products: A review. J. Food Meas. Charact. 2021, 15, 4314–4324. [Google Scholar] [CrossRef]
- Ščetar, M.; Kurek, M. Trends in meat and meat products packaging—A review. Croat. J. Food Sci. Technol. 2010, 2, 32–48. [Google Scholar]
- Mohajer, A.; Sadighara, P.; Mohajer, M.; Farkhondeh, T.; Samarghandian, S. A comparison of antioxidant effects of some selected fruits with butylated hydroxytoluene on egg yolk. Curr. Res. Nutr. Food Sci. 2019, 15, 525–527. [Google Scholar] [CrossRef]
- Pateiro, M.; Domínguez, R.; Bermúdez, R.; Munekata, P.E.; Zhang, W.; Gagaoua, M.; Lorenzo, J.M. Antioxidant active packaging systems to extend the shelf life of sliced cooked ham. Curr. Res. Nutr. Food Sci. 2019, 1, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Wrona, M.; Silva, F.; Salafranca, J.; Nerín, C.; Alfonso, M.J.; Caballero, M.Á. Design of new natural antioxidant active packaging: Screening flowsheet from pure essential oils and vegetable oils to ex vivo testing in meat samples. Food Cont. 2021, 120, 10753. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Garrido, L.; Faba, S.; Guarda, A.; Galotto, M.J.; López de Dicastillo, C. Cucumis metuliferus fruit extract loaded acetate cellulose coatings for antioxidant active packaging. Polymers 2020, 12, 124. [Google Scholar] [CrossRef] [PubMed]
- Ward, C.P.; Armstrong, C.J.; Walsh, A.N.; Jackson, J.H.; Reddy, C.M. Sunlight converts polystyrene to carbon dioxide and dissolved organic carbon. Environ. Sci. Technol. Lett. 2019, 6, 669–674. [Google Scholar] [CrossRef]
- Firouz, M.S.; Mohi-Alden, K.; Omid, M. A critical review on intelligent and active packaging in the food industry: Research and development. Food Res. Int. 2021, 141, 110113. [Google Scholar] [CrossRef]
- Baggio, A.; Marino, M.; Innocente, N.; Celotto, M.; & Maifreni, M. Antimicrobial effect of oxidative technologies in food processing: An overview. Eur. Food Res. Technol. 2020, 246, 669–692. [Google Scholar] [CrossRef]
- Czerwiński, K.; Rydzkowski, T.; Wróblewska-Krepsztul, J.; Thakur, V.K. Towards impact of modified atmosphere packaging (MAP) on shelf-life of polymer-film-packed food products: Challenges and sustainable developments. Coatings 2021, 11, 1504. [Google Scholar] [CrossRef]
- Pettersen, M.K.; Nilsen-Nygaard, J.; Hansen, A.Å.; Carlehög, M.; Liland, K.H. Effect of Liquid Absorbent Pads and Packaging Parameters on Drip Loss and Quality of Chicken Breast Fillets. Foods 2021, 10, 1340. [Google Scholar] [CrossRef]
- Felicia, W.X.L.; Rovina, K.; Nur’Aqilah, M.N.; Vonnie, J.M.; Erna, K.H.; Misson, M.; Halid, N.F.A. Recent Advancements of Polysaccharides to Enhance Quality and Delay Ripening of Fresh Produce: A Review. Polymers 2022, 14, 1341. [Google Scholar] [CrossRef]
- Al-Moghazy, M.; Mahmoud, M.; Nada, A.A. Fabrication of cellulose-based adhesive composite as an active packaging material to extend the shelf life of cheese. Int. J. Biol. Macromol. 2020, 160, 264–275. [Google Scholar] [CrossRef]
- Ashrafi, A.; Jokar, M.; Nafchi, A.M. Preparation and characterization of biocomposite film based on chitosan and kombucha tea as active food packaging. Int. J. Biol. Macromol. 2018, 108, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Takma, D.K.; Korel, F. Active packaging films as a carrier of black cumin essential oil: Development and effect on quality and shelf-life of chicken breast meat. Food Packag. Shelf Life 2019, 19, 210–217. [Google Scholar] [CrossRef]
- Alizadeh-Sani, M.; Moghaddas Kia, E.; Ghasempour, Z.; Ehsani, A. Preparation of active nanocomposite film consisting of sodium caseinate, ZnO nanoparticles and rosemary essential oil for food packaging applications. J. Polym. Environ. 2021, 29, 588–598. [Google Scholar] [CrossRef]
- Kumar, S.; Boro, J.C.; Ray, D.; Mukherjee, A.; Dutta, J. Bionanocomposite films of agar incorporated with ZnO nanoparticles as an active packaging material for shelf life extension of green grape. Heliyon 2019, 5, e01867. [Google Scholar] [CrossRef] [PubMed]
- Salama, H.E.; Aziz, M.S.A. Optimized carboxymethyl cellulose and guanidinylated chitosan enriched with titanium oxide nanoparticles of improved UV-barrier properties for the active packaging of green bell pepper. Int. J. Biol. Macromol. 2020, 165, 1187–1197. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, A.; Ahmadi, P.; Ehsani, A. Development of an active packaging system containing zinc oxide nanoparticles for the extension of chicken fillet shelf life. Food Sci. Nutr. 2020, 8, 5461–5473. [Google Scholar] [CrossRef] [PubMed]
- Hanif, J.; Khalid, N.; Khan, R.S.; Bhatti, M.F.; Hayat, M.Q.; Ismail, M.; Andleeb, S.; Mansoor, Q.; Khan, F.; Amin, F.; et al. Formulation of active packaging system using Artemisia scoparia for enhancing shelf life of fresh fruits. Mater. Sci. Eng. C 2019, 100, 82–93. [Google Scholar] [CrossRef]
- Lee, K.T. Quality and safety aspects of meat products as affected by various physical manipulations of packaging materials. Meat Sci. 2010, 86, 138–150. [Google Scholar] [CrossRef]
- Alamri, M.S.; Qasem, A.A.; Mohamed, A.A.; Hussain, S.; Ibraheem, M.A.; Shamlan, G.; Alqah, H.A.; Qasha, A.S. Food packaging’s materials: A food safety perspective. Saudi J. Biol. Sci. 2021, 28, 4490–4499. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, B.; Li, C.; Xu, Y.; Luo, Y.; Liang, D.; Huang, C. Comprehensive review of polysaccharide-based materials in edible packaging: A sustainable approach. Foods 2021, 10, 1845. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Chen, F.; Yang, W.; Huang, H. Preparation, deproteinization and comparison of bioactive polysaccharides. Trends Food Sci. Technol. 2021, 109, 564–568. [Google Scholar] [CrossRef]
- Pitkänen, L.; Heinonen, M.; Mikkonen, K.S. Safety considerations of plant polysaccharides for food use: A case study on phenolic-rich softwood galactoglucomannan extract. Food Func. 2018, 9, 1931–1943. [Google Scholar] [CrossRef] [PubMed]
- Gultekin, F.; Doguc, D.K. Allergic and immunologic reactions to food additives. Clin. Rev. Allergy Immunol. 2013, 45, 6–29. [Google Scholar] [CrossRef] [PubMed]
- Skypala, I.J. Food-induced anaphylaxis: Role of hidden allergens and cofactors. Front. Immunol. 2019, 10, 673. [Google Scholar] [CrossRef]
- Milani, J.M.; Tirgarian, B. An overview of edible protein-based packaging: Main sources, advantages, drawbacks, recent progressions and food applications. J. Package Technol. Res. 2020, 4, 103–115. [Google Scholar] [CrossRef]


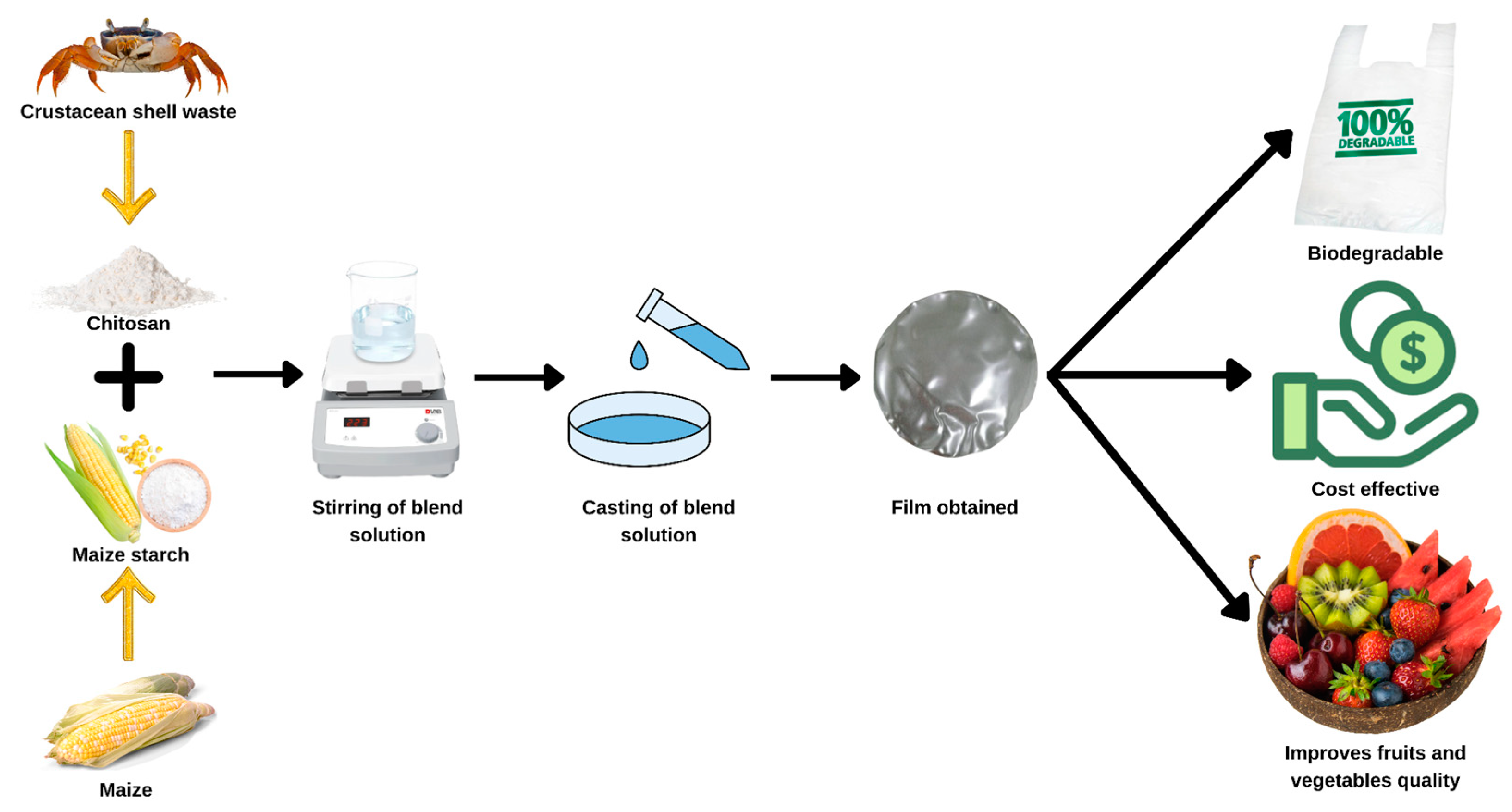
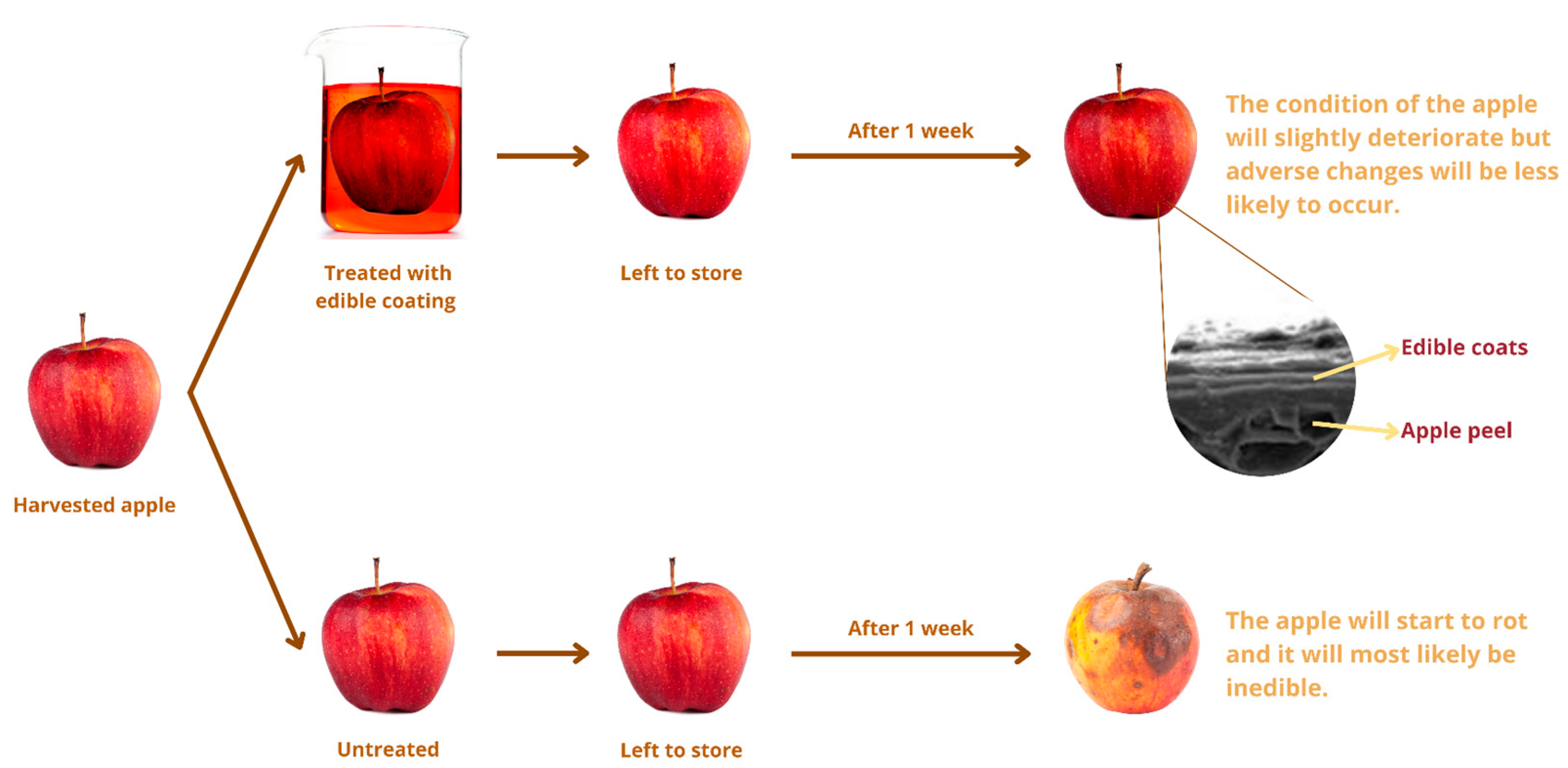

| Food Products | Biopolymeric Matrix | Coating Techniques | Results | References |
|---|---|---|---|---|
| Cherry tomatoes | Hydroxypropyl methylcellulose | Immersion |
| [93] |
| - | Cassava starch | - |
| [95] |
| Apricot | Apples pectin + LDH-salicylate + glycerol | Dipping |
| [100] |
| Homemade cheese | Sodium alginate + essential oil (O. basilicum, L.; R. officinalis, L.; A. herba alba; Asso. M. pulegium, L.) | Immersion |
| [103] |
| Homemade cheese | Sodium alginate + essential oil (O. basilicum, L.; R. officinalis, L.; A. herba alba; Asso. M. pulegium, L.) | Immersion |
| [103] |
| Food Products | Biopolymeric Matrix | Coating Techniques | Results | References |
|---|---|---|---|---|
| Fish | Chitosan + red cabbage (RC) extract + clove bud oil (CBO) | Edible film |
| [132] |
| Chicken breast | Roselle anthocyanin + starch | Edible film |
| [133] |
| Chicken and fish | Curcumin extract + modified rice starch |
| [134] | |
| Grass carp fillets | ĸ-carrageenan + gelatin |
| [135] | |
| Chicken breast | Sugarcane bagasse nanocellulose + poly(ether-block-amide) (PEBA) film | Multilayer films |
| [136] |
| Shrimp | Mucilage of Lallemantia iberica seed gum (LISG) + curcumin |
| [130] | |
| Fish | Carboxymethyl cellulose (CMC) + cellulose nanofibers (CNF) + shikonin extracted from Lithospermum erythrorhizon roots | Multilayer films |
| [137] |
| - | Anthocyanin + chitosan + cellulose matrix |
| [138] |
| Food Products | Biopolymeric Matrix | Coating Techniques | Results | References |
|---|---|---|---|---|
| Cheese | Cellulose-based adhesive gelatin + gelatin electrospun fibers + allyl isothiocyanate (AIC) | Adhesive composite |
| [207] |
| Green chilli | Chitosan + citric acid + glycerol | Film developed as pouches |
| [160] |
| Minced beef | Chitosan + kombucha tea | Biocomposite film |
| [208] |
| Chicken breast meat | Polyester + chitosan + alginate + black cumin oil (BCO) | Multilayer films |
| [209] |
| Lamb meat | Cellulose nanofiber + whey protein + titanium dioxide + rosemary essential oil | Packaging film |
| [210] |
| Sliced cooked ham | Green tea extract + oregano essential oil | Packaging film |
| [198] |
| Green grape | Agar + zinc oxide nanoparticle synthesized from Mimusops elengi fruit extract | Bionanocomposite film |
| [211] |
| Green bell pepper | Chitosan biguanidine hydrochloride + carboxymethyl cellulose (CMC) + titanium oxide nanoparticles | Nanocomposite film |
| [212] |
| Chicken fillet | Gelatin-based nanocomposite + cellulose nanofiber (CNF) + zinc oxide nanoparticles | Nanocomposite film |
| [213] |
| Strawberry, loquats | Calcium alginate + silver nanoparticles | Edible coating |
| [214] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iversen, L.J.L.; Rovina, K.; Vonnie, J.M.; Matanjun, P.; Erna, K.H.; ‘Aqilah, N.M.N.; Felicia, W.X.L.; Funk, A.A. The Emergence of Edible and Food-Application Coatings for Food Packaging: A Review. Molecules 2022, 27, 5604. https://doi.org/10.3390/molecules27175604
Iversen LJL, Rovina K, Vonnie JM, Matanjun P, Erna KH, ‘Aqilah NMN, Felicia WXL, Funk AA. The Emergence of Edible and Food-Application Coatings for Food Packaging: A Review. Molecules. 2022; 27(17):5604. https://doi.org/10.3390/molecules27175604
Chicago/Turabian StyleIversen, Luk Jun Lam, Kobun Rovina, Joseph Merillyn Vonnie, Patricia Matanjun, Kana Husna Erna, Nasir Md Nur ‘Aqilah, Wen Xia Ling Felicia, and Andree Alexander Funk. 2022. "The Emergence of Edible and Food-Application Coatings for Food Packaging: A Review" Molecules 27, no. 17: 5604. https://doi.org/10.3390/molecules27175604










