Non-Oxidative Propane Dehydrogenation on CrOx-ZrO2-SiO2 Catalyst Prepared by One-Pot Template-Assisted Method
Abstract
:1. Introduction
2. Results
2.1. Catalyst Characterization
2.2. Catalytic Tests
3. Discussion
4. Materials and Methods
4.1. Catalyst Preparation
4.2. Catalyst Characterization
4.3. Catalytic Tests
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Sattler, J.J.H.B.; Ruiz-Martinez, J.; Santillan-Jimenez, E.; Weckhuysen, B.M. Catalytic Dehydrogenation of Light Alkanes on Metals and Metal Oxides. Chem. Rev. 2014, 114, 10613–10653. [Google Scholar] [CrossRef] [PubMed]
- Hocking, M.B. 19—Petrochemicals. In Handbook of Chemical Technology and Pollution Control, 3rd ed.; Hocking, M.B., Ed.; Academic Press: San Diego, CA, USA, 2005; pp. 637–668. [Google Scholar] [CrossRef]
- Stangland, E.E. Shale Gas Implications for C2-C3 Olefin Production: Incumbent and Future Technology. Annu. Rev. Chem. Biomol. Eng. 2018, 9, 341–364. [Google Scholar] [CrossRef] [PubMed]
- Otroshchenko, T.; Jiang, G.; Kondratenko, V.A.; Rodemerck, U.; Kondratenko, E.V. Current status and perspectives in oxidative, non-oxidative and CO2-mediated dehydrogenation of propane and isobutane over metal oxide catalysts. Chem. Soc. Rev. 2021, 50, 473–527. [Google Scholar] [CrossRef]
- Siahvashi, A.; Chesterfield, D.; Adesina, A.A. Nonoxidative and Oxidative Propane Dehydrogenation over Bimetallic Mo–Ni/Al2O3 Catalyst. Ind. Eng. Chem. Res. 2013, 52, 4017–4026. [Google Scholar] [CrossRef]
- Atanga, M.A.; Rezaei, F.; Jawad, A.; Fitch, M.; Rownaghi, A.A. Oxidative dehydrogenation of propane to propylene with carbon dioxide. Appl. Catal. B Environ. 2018, 220, 429–445. [Google Scholar] [CrossRef]
- Mitran, G.; Ahmed, R.; Iro, E.; Hajimirzaee, S.; Hodgson, S.; Urdă, A.; Olea, M.; Marcu, I.-C. Propane oxidative dehydrogenation over VOx/SBA-15 catalysts. Catal. Today 2018, 306, 260–267. [Google Scholar] [CrossRef]
- Kondratenko, E.V.; Cherian, M.; Baerns, M.; Su, D.; Schlögl, R.; Wang, X.; Wachs, I.E. Oxidative dehydrogenation of propane over V/MCM-41 catalysts: Comparison of O2 and N2O as oxidants. J. Catal. 2005, 234, 131–142. [Google Scholar] [CrossRef]
- Huš, M.; Kopač, D.; Likozar, B. Kinetics of non-oxidative propane dehydrogenation on Cr2O3 and the nature of catalyst deactivation from first-principles simulations. J. Catal. 2020, 386, 126–138. [Google Scholar] [CrossRef]
- Rodemerck, U.; Stoyanova, M.; Kondratenko, E.V.; Linke, D. Influence of the kind of VOx structures in VOx/MCM-41 on activity, selectivity and stability in dehydrogenation of propane and isobutane. J. Catal. 2017, 352, 256–263. [Google Scholar] [CrossRef]
- Jeon, N.; Choe, H.; Jeong, B.; Yun, Y. Propane dehydrogenation over vanadium-doped zirconium oxide catalysts. Catal. Today 2020, 352, 337–344. [Google Scholar] [CrossRef]
- Dai, Y.; Gu, J.; Tian, S.; Wu, Y.; Chen, J.; Li, F.; Du, Y.; Peng, L.; Ding, W.; Yang, Y. γ-Al2O3 sheet-stabilized isolate Co2+ for catalytic propane dehydrogenation. J. Catal. 2020, 381, 482–492. [Google Scholar] [CrossRef]
- Zhao, Y.; Sohn, H.; Hu, B.; Niklas, J.; Poluektov, O.G.; Tian, J.; Delferro, M.; Hock, A.S. Zirconium Modification Promotes Catalytic Activity of a Single-Site Cobalt Heterogeneous Catalyst for Propane Dehydrogenation. ACS Omega 2018, 3, 11117–11127. [Google Scholar] [CrossRef]
- Matam, S.K.; Moffat, C.; Hellier, P.; Bowker, M.; Silverwood, I.P.; Catlow, C.R.A.; Jackson, S.D.; Craswell, J.; Wells, P.P.; Parker, S.F.; et al. Investigation of MoOx/Al2O3 under Cyclic Operation for Oxidative and Non-Oxidative Dehydrogenation of Propane. Catalysts 2020, 10, 1370. [Google Scholar] [CrossRef]
- Perechodjuk, A.; Zhang, Y.; Kondratenko, V.A.; Rodemerck, U.; Linke, D.; Bartling, S.; Kreyenschulte, C.R.; Jiang, G.; Kondratenko, E.V. The effect of supported Rh, Ru, Pt or Ir nanoparticles on activity and selectivity of ZrO2-based catalysts in non-oxidative dehydrogenation of propane. Appl. Catal. A Gen. 2020, 602, 117731. [Google Scholar] [CrossRef]
- Natarajan, P.; Khan, H.A.; Jaleel, A.; Park, D.S.; Kang, D.-C.; Yoon, S.; Jung, K.-D. The pronounced effect of Sn on RhSn catalysts for propane dehydrogenation. J. Catal. 2020, 392, 8–20. [Google Scholar] [CrossRef]
- Wang, P.; Yao, J.; Jiang, Q.; Gao, X.; Lin, D.; Yang, H.; Wu, L.; Tang, Y.; Tan, L. Stabilizing the isolated Pt sites on PtGa/Al2O3 catalyst via silica coating layers for propane dehydrogenation at low temperature. Appl. Catal. B Environ. 2022, 300, 120731. [Google Scholar] [CrossRef]
- Jeon, N.; Seo, O.; Oh, J.; Park, J.; Chung, I.; Kim, J.; Sakata, O.; Tayal, A.; Yun, Y. Non-oxidative propane dehydrogenation over alumina-supported Co-V oxide catalysts. Appl. Catal. A Gen. 2021, 614, 118036. [Google Scholar] [CrossRef]
- Michorczyk, P.; Pietrzyk, P.; Ogonowski, J. Preparation and characterization of SBA-1–supported chromium oxide catalysts for CO2 assisted dehydrogenation of propane. Microporous Mesoporous Mater. 2012, 161, 56–66. [Google Scholar] [CrossRef]
- Otroshchenko, T.P.; Rodemerck, U.; Linke, D.; Kondratenko, E.V. Synergy effect between Zr and Cr active sites in binary CrZrOx or supported CrOx/LaZrOx: Consequences for catalyst activity, selectivity and durability in non-oxidative propane dehydrogenation. J. Catal. 2017, 356, 197–205. [Google Scholar] [CrossRef]
- Otroshchenko, T.; Kondratenko, V.A.; Rodemerck, U.; Linke, D.; Kondratenko, E.V. ZrO2-based unconventional catalysts for non-oxidative propane dehydrogenation: Factors determining catalytic activity. J. Catal. 2017, 348, 282–290. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Y.; Otroshchenko, T.; Lund, H.; Pohl, M.-M.; Rodemerck, U.; Linke, D.; Jiao, H.; Jiang, G.; Kondratenko, E.V. Control of coordinatively unsaturated Zr sites in ZrO2 for efficient C–H bond activation. Nat. Commun. 2018, 9, 3794. [Google Scholar] [CrossRef]
- Zubkov, A.; Bugrova, T.; Salaev, M.; Mamontov, G. Influence of Cr/Zr Ratio on Activity of Cr-Zr Oxide Catalysts in Non-Oxidative Propane Dehydrogenation. Crystals 2021, 11, 1435. [Google Scholar] [CrossRef]
- Han, S.; Otroshchenko, T.; Zhao, D.; Lund, H.; Rockstroh, N.; Vuong, T.H.; Rabeah, J.; Rodemerck, U.; Linke, D.; Gao, M.; et al. The effect of ZrO2 crystallinity in CrZrOx/SiO2 on non-oxidative propane dehydrogenation. Appl. Catal. A Gen. 2020, 590, 117350. [Google Scholar] [CrossRef]
- Rahman, N.J.A.; Ramli, A.; Jumbri, K.; Uemura, Y. Tailoring the surface area and the acid–base properties of ZrO2 for biodiesel production from Nannochloropsis sp. Sci. Rep. 2019, 9, 16223. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wang, Y.; Wu, W.; Zhang, J.; Cao, Y.; Huang, K.; Jiang, L. Slurry-Phase Hydrocracking of a Decalin–Phenanthrene Mixture by MoS2/SiO2–ZrO2 Bifunctional Catalysts. Ind. Eng. Chem. Res. 2021, 60, 230–242. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, C.; Zhu, M.; Yu, F.; Dai, B. Highly Active and Stable ZrO2-SiO2-Supported Cu–Catalysts for the Hydrogenation of Dimethyl Oxalate to Methyl Glycolate. ChemistrySelect 2017, 2, 4823–4829. [Google Scholar] [CrossRef]
- Mariyah Ulfa, S.; Prihartini, D.; Taufiq, A. Structural Characterization of Ni/ZrO2/SiO2 Nanocomposites Prepared by Wet Impregnation Route. IOP Conf. Ser. Mater. Sci. Eng. 2019, 515, 012014. [Google Scholar] [CrossRef]
- Kongwudthiti, S.; Praserthdam, P.; Tanakulrungsank, W.; Inoue, M. The influence of Si–O–Zr bonds on the crystal-growth inhibition of zirconia prepared by the glycothermal method. J. Mater. Process. Technol. 2003, 136, 186–189. [Google Scholar] [CrossRef]
- Luizon Filho, R.A.; Possato, L.G.; Santisteban, O.A.N.; de Vasconcellos, A.; da Silva, D.A.; Lima, M.F.; Martins, L.; Nery, J.G. Synthesis and characterization of chromium silicate catalyst and its application in the gas phase glycerol transformation into acetaldehyde. Inorg. Chem. Commun. 2020, 112, 107710. [Google Scholar] [CrossRef]
- Camposeco, R.; Castillo, S.; Nava, N.; Zanella, R. Boosting of Soot Combustion on Alkaline Mn/ZrO2 Nanostructures. Top. Catal. 2020, 63, 481–491. [Google Scholar] [CrossRef]
- Hoang, D.L.; Lieske, H. Temperature-programmed reduction study of chromium oxide supported on zirconia and lanthana–zirconia. Thermochim. Acta 2000, 345, 93–99. [Google Scholar] [CrossRef]
- Wang, F.; Fan, J.-L.; Zhao, Y.; Zhang, W.-X.; Liang, Y.; Lu, J.-Q.; Luo, M.-F.; Wang, Y.-J. Effects of yttrium-doping on the performance of Cr2O3 catalysts for vapor phase fluorination of 1,1,2,3-tetrachloropropene. J. Fluor. Chem. 2014, 166, 78–83. [Google Scholar] [CrossRef]
- Bai, P.T.; Manokaran, V.; Saiprasad, P.S.; Srinath, S. Studies on Heat and Mass Transfer Limitations in Oxidative Dehydrogenation of Ethane Over Cr2O3 /Al2O3 Catalyst. Procedia Eng. 2015, 127, 1338–1345. [Google Scholar] [CrossRef]
- Zhong, L.; Yu, Y.; Cai, W.; Geng, X.; Zhong, Q. Structure–activity relationship of Cr/Ti-PILC catalysts using a pre-modification method for NO oxidation and their surface species study. Phys. Chem. Chem. Phys. 2015, 17, 15036–15045. [Google Scholar] [CrossRef]
- Hosseini, S.A.; Alvarez-Galvan, M.C. Study of physical–chemical properties and catalytic activities of ZnCr2O4 spinel nano oxides obtained from different methods—Modeling the synthesis process by response surface methodology and optimization by genetic algorithm. J. Taiwan Inst. Chem. Eng. 2016, 61, 261–269. [Google Scholar] [CrossRef]
- Dines, T.J.; Inglis, S. Raman spectroscopic study of supported chromium (VI) oxide catalysts. Phys. Chem. Chem. Phys. 2003, 5, 1320–1328. [Google Scholar] [CrossRef]
- Vaccaro, G.; Agnello, S.; Buscarino, G.; Cannas, M.; Vaccaro, L. Structural and luminescence properties of amorphous SiO2 nanoparticles. J. Non-Cryst. Solids 2011, 357, 1941–1944. [Google Scholar] [CrossRef]
- Basahel, S.N.; Ali, T.T.; Mokhtar, M.; Narasimharao, K. Influence of crystal structure of nanosized ZrO2 on photocatalytic degradation of methyl orange. Nanoscale Res. Lett. 2015, 10, 73. [Google Scholar] [CrossRef]
- Gao, X.; Fierro, J.L.G.; Wachs, I.E. Structural Characteristics and Catalytic Properties of Highly Dispersed ZrO2/SiO2 and V2O5/ZrO2/SiO2 Catalysts. Langmuir 1999, 15, 3169–3178. [Google Scholar] [CrossRef]
- Basha, S.J.; Sesha Reddy, A.S.; Kostrzewa, M.; Ingram, A.; Venkatramaiah, N.; Kityk, I.V.; Kumar, V.R.; Veeraiah, N. Positron annihilation spectroscopy and third harmonic generation studies on MnO mixed lead zirconium silicate glass ceramics. Opt. Mater. X 2019, 1, 100024. [Google Scholar] [CrossRef]
- Ciszak, C.; Mermoux, M.; Gutierrez, G.; Leprêtre, F.; Duriez, C.; Popa, I.; Fayette, L.; Chevalier, S. Raman spectra analysis of ZrO2 thermally grown on Zircaloy substrates irradiated with heavy ion: Effects of oxygen isotopic substitution. J. Raman Spectrosc. 2019, 50, 425–435. [Google Scholar] [CrossRef]
- Bai, Y.; Qu, S.; Jia, Z.; Zhang, L.; Zhu, G.; Feng, A.; Wu, G.; Wu, H. Cr2O3 nanocrystal anode materials with improved cyclic stability for lithium ion batteries. J. Mater. Sci. Mater. Electron. 2018, 29, 11795–11800. [Google Scholar] [CrossRef]
- Marinković Stanojević, Z.V.; Romčević, N.; Stojanović, B. Spectroscopic study of spinel ZnCr2O4 obtained from mechanically activated ZnO–Cr2O3 mixtures. J. Eur. Ceram. Soc. 2007, 27, 903–907. [Google Scholar] [CrossRef]
- Qi, J.; Walton, J.; Thompson, G.E.; Albu, S.P.; Carr, J. Spectroscopic Studies of Chromium VI Formed in the Trivalent Chromium Conversion Coatings on Aluminum. J. Electrochem. Soc. 2016, 163, C357–C363. [Google Scholar] [CrossRef]
- Malleswara Rao, T.V.; Deo, G.; Jehng, J.-M.; Wachs, I.E. In Situ UV−Vis−NIR Diffuse Reflectance and Raman Spectroscopy and Catalytic Activity Studies of Propane Oxidative Dehydrogenation over Supported CrO3/ZrO2 Catalysts. Langmuir 2004, 20, 7159–7165. [Google Scholar] [CrossRef]
- Chakrabarti, A.; Gierada, M.; Handzlik, J.; Wachs, I.E. Operando Molecular Spectroscopy During Ethylene Polymerization by Supported CrOx/SiO2 Catalysts: Active Sites, Reaction Intermediates, and Structure-Activity Relationship. Top. Catal. 2016, 59, 725–739. [Google Scholar] [CrossRef]
- Weckhuysen, B.M.; Jehng, J.-M.; Wachs, I.E. In Situ Raman Spectroscopy of Supported Transition Metal Oxide Catalysts: 18O2−16O2 Isotopic Labeling Studies. J. Phys. Chem. B 2000, 104, 7382–7387. [Google Scholar] [CrossRef]
- Weckhuysen, B.M.; Wachs, I.E. In Situ Raman Spectroscopy of Supported Chromium Oxide Catalysts: 18O2−16O2 Isotopic Labeling Studies. J. Phys. Chem. B 1997, 101, 2793–2796. [Google Scholar] [CrossRef]
- Ayari, F.; Mhamdi, M.; Álvarez-Rodríguez, J.; Ruiz, A.R.G.; Delahay, G.; Ghorbel, A. Selective catalytic reduction of NO with NH3 over Cr-ZSM-5 catalysts: General characterization and catalysts screening. Appl. Catal. B Environ. 2013, 134–135, 367–380. [Google Scholar] [CrossRef]
- Lee, E.L.; Wachs, I.E. In Situ Spectroscopic Investigation of the Molecular and Electronic Structures of SiO2 Supported Surface Metal Oxides. J. Phys. Chem. C 2007, 111, 14410–14425. [Google Scholar] [CrossRef]
- Joya, M.R.; Fonseca, K.M.; Barba-Ortega, J. Difference in the relative intensities Raman of the perovskite PLT with temperature. AIP Conf. Proc. 2014, 1627, 42–45. [Google Scholar] [CrossRef]
- Dementjev, A.P.; Ivanova, O.P.; Vasilyev, L.A.; Naumkin, A.V.; Nemirovsky, D.M.; Shalaev, D.Y. Altered layer as sensitive initial chemical state indicator. J. Vac. Sci. Technol. A 1994, 12, 423–427. [Google Scholar] [CrossRef]
- Steinberger, R.; Duchoslav, J.; Greunz, T.; Arndt, M.; Stifter, D. Investigation of the chemical stability of different Cr(VI) based compounds during regular X-ray photoelectron spectroscopy measurements. Corros. Sci. 2015, 90, 562–571. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Brown, C.; Mycroft, J.R.; Davidson, R.D.; McIntyre, N.S. X-ray photoelectron spectroscopy studies of chromium compounds. Surf. Interface Anal. 2004, 36, 1550–1563. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Santhosh Kumar, M.; Hammer, N.; Rønning, M.; Holmen, A.; Chen, D.; Walmsley, J.C.; Øye, G. The nature of active chromium species in Cr-catalysts for dehydrogenation of propane: New insights by a comprehensive spectroscopic study. J. Catal. 2009, 261, 116–128. [Google Scholar] [CrossRef]
- Węgrzyniak, A.; Jarczewski, S.; Węgrzynowicz, A.; Michorczyk, B.; Kuśtrowski, P.; Michorczyk, P. Catalytic Behavior of Chromium Oxide Supported on Nanocasting-Prepared Mesoporous Alumina in Dehydrogenation of Propane. Nanomaterials 2017, 7, 249. [Google Scholar] [CrossRef]
- Macnaughtan, M.L.; Soo, H.S.; Frei, H. Binuclear ZrOCo Metal-to-Metal Charge-Transfer Unit in Mesoporous Silica for Light-Driven CO2 Reduction to CO and Formate. J. Phys. Chem. C 2014, 118, 7874–7885. [Google Scholar] [CrossRef]
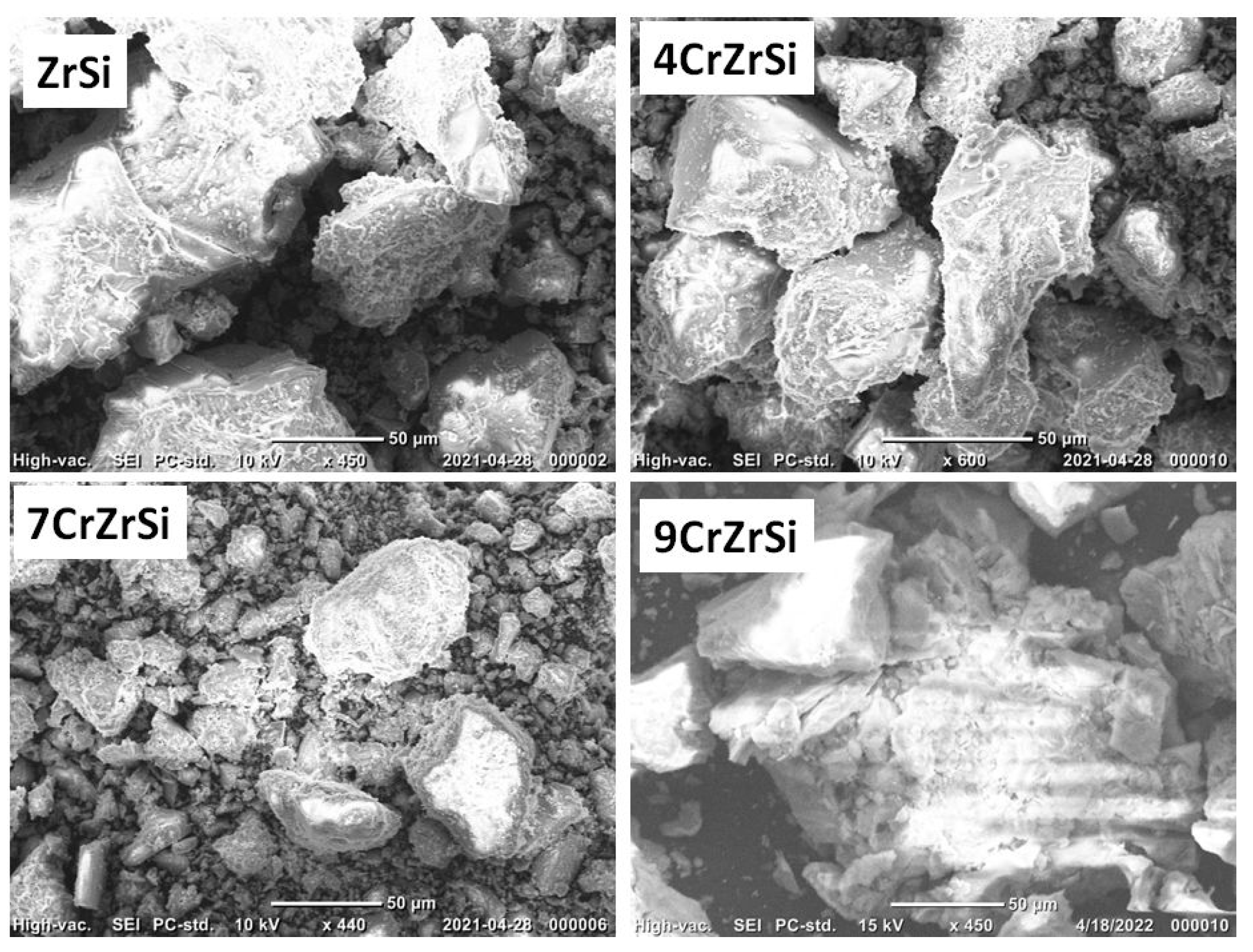
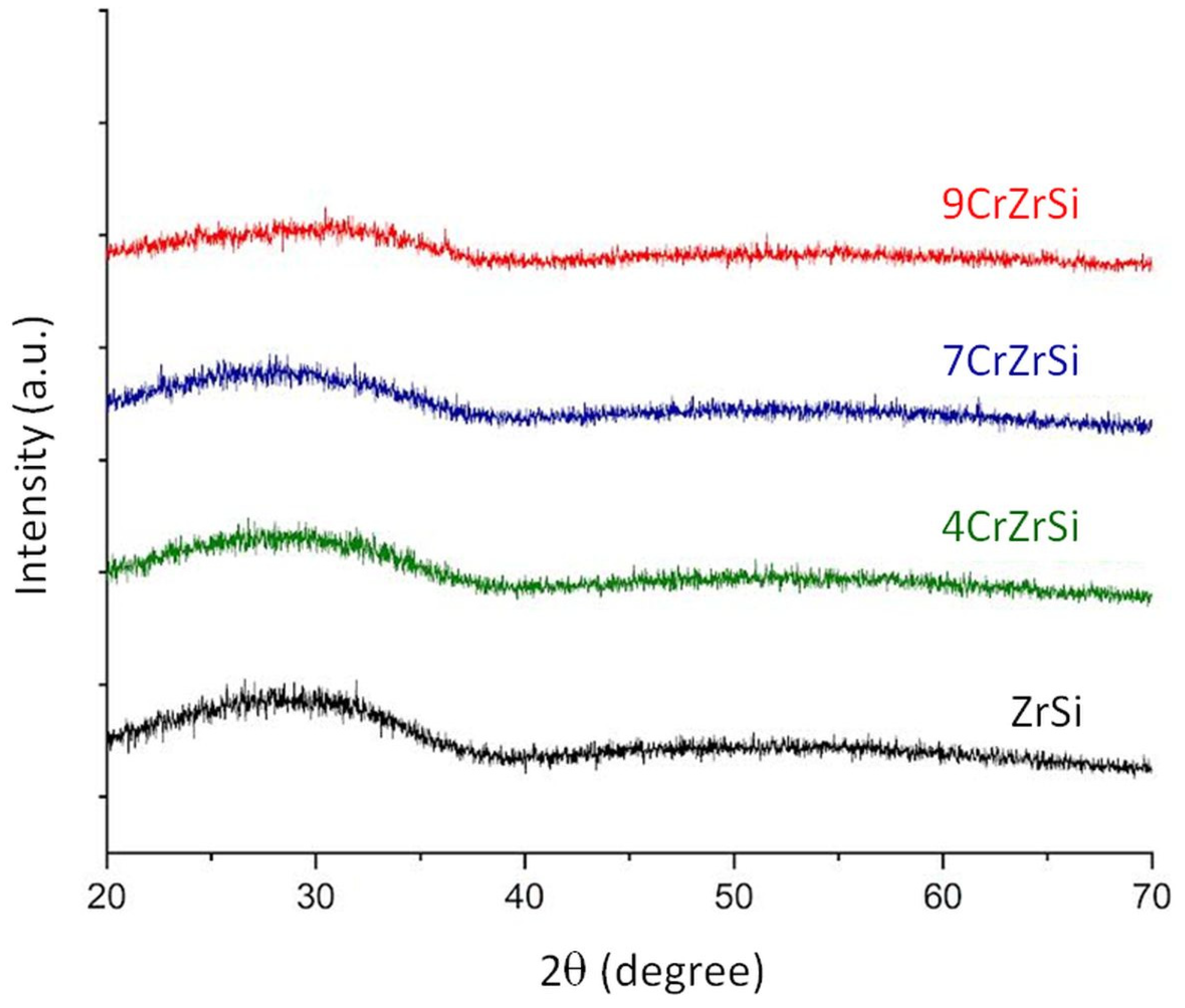
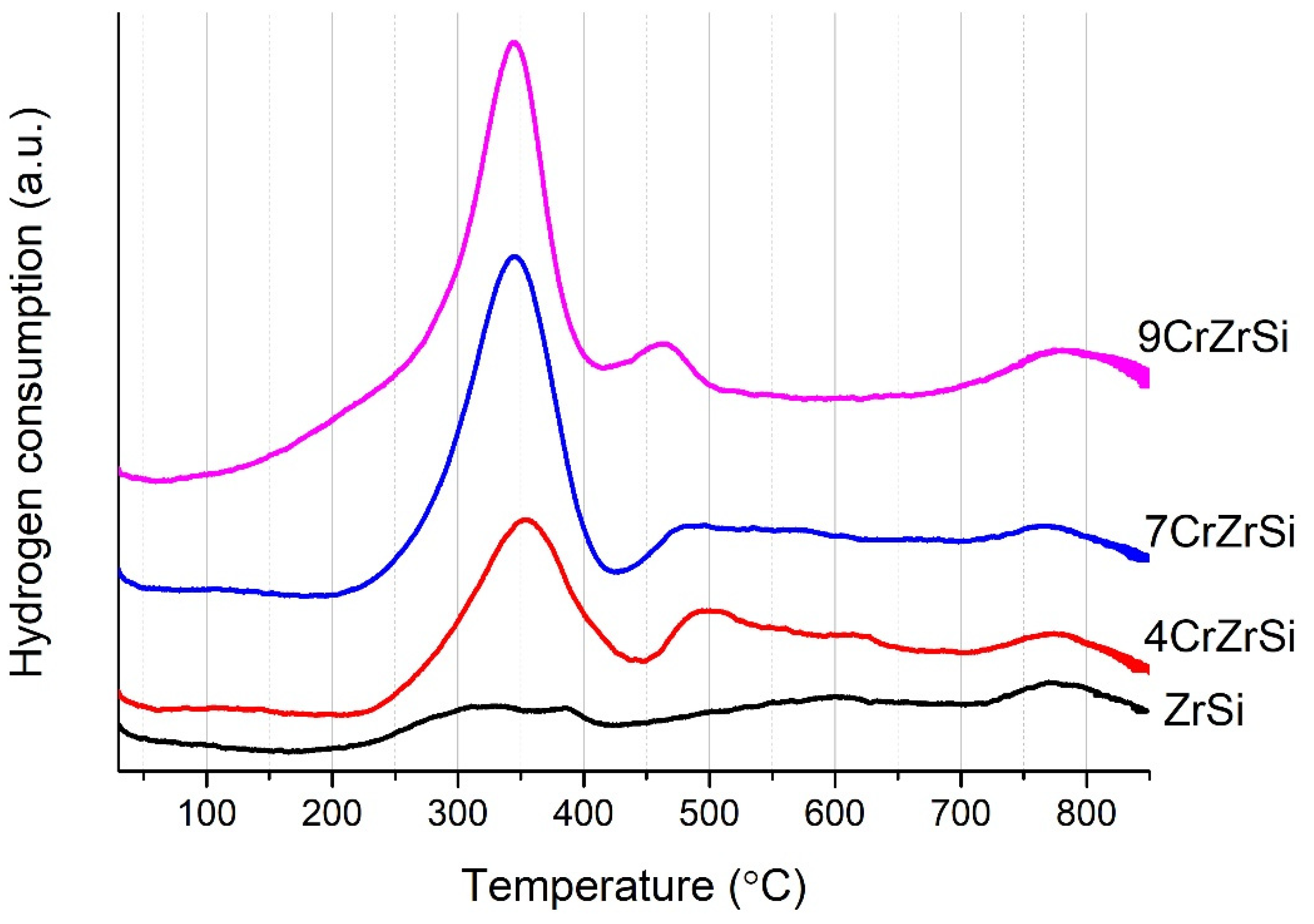




| Sample | Cr2O3, wt.% (AAS) | SBET, m2/g | SEM-EDX | XPS | H2 Uptake 1, μmol/g | |||
|---|---|---|---|---|---|---|---|---|
| Cr, wt.% | Zr:Si | Cr, wt.% | Cr/Zr | Zr:Si | ||||
| ZrSi | – | 464 ± 46 | 0 | 1:2.1 | 0 | – | 1:2.5 | – |
| 4CrZrSi | 4.47 ± 0.03 | 517 ± 52 | 1.4 | 1:2.1 | 3.2 | 0.19 | 1:2.4 | 227 |
| 7CrZrSi | 6.89 ± 0.07 | 518 ± 52 | 2.9 | 1:2.4 | 4.2 | 0.28 | 1:2.7 | 357 |
| 9CrZrSi | 9.28 ± 0.07 | 443 ± 44 | 2.7 | 1:2.3 | 3.8 | 0.28 | 1:3.5 | 381 |
| Sample | Cr3+, % | Cr/Zr | ||
|---|---|---|---|---|
| Fresh | H2 Treated | Fresh | H2 Treated | |
| 4CrZrSi | 69 | 92 | 0.19 | 0.18 |
| 7CrZrSi | 47 | 92 | 0.27 | 0.24 |
| 9CrZrSi | 65 | 94 | 0.28 | 0.26 |
| Scheme 1. | Coke Content in Used Catalyst 1, % | r0(C3H6) (mmol(C3H6)g−1min−1) | 2, % | ||
|---|---|---|---|---|---|
| 500 °C | 550 °C | 600 °C | |||
| ZrSi | – | — | 0.01 | 0.03 | — |
| 4CrZrSi | 6.2 | 0.11 | 0.11 | 0.12 | — |
| 7CrZrSi | 5.9 | 0.11 | 0.24 | 0.29 | 100 |
| 9CrZrSi | 5.1 | 0.31 | 0.50 | 0,47 | 93 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golubina, E.V.; Kaplin, I.Y.; Gorodnova, A.V.; Lokteva, E.S.; Isaikina, O.Y.; Maslakov, K.I. Non-Oxidative Propane Dehydrogenation on CrOx-ZrO2-SiO2 Catalyst Prepared by One-Pot Template-Assisted Method. Molecules 2022, 27, 6095. https://doi.org/10.3390/molecules27186095
Golubina EV, Kaplin IY, Gorodnova AV, Lokteva ES, Isaikina OY, Maslakov KI. Non-Oxidative Propane Dehydrogenation on CrOx-ZrO2-SiO2 Catalyst Prepared by One-Pot Template-Assisted Method. Molecules. 2022; 27(18):6095. https://doi.org/10.3390/molecules27186095
Chicago/Turabian StyleGolubina, Elena V., Igor Yu. Kaplin, Anastasia V. Gorodnova, Ekaterina S. Lokteva, Oksana Ya. Isaikina, and Konstantin I. Maslakov. 2022. "Non-Oxidative Propane Dehydrogenation on CrOx-ZrO2-SiO2 Catalyst Prepared by One-Pot Template-Assisted Method" Molecules 27, no. 18: 6095. https://doi.org/10.3390/molecules27186095









