Atomic and Molecular Layer Deposition as Surface Engineering Techniques for Emerging Alkali Metal Rechargeable Batteries
Abstract
:1. Introduction
2. Mechanisms and Characteristics of ALD and MLD
3. Surface Modifications on Alkali Metals via ALD
3.1. ALD Coatings on Li Metal
3.1.1. Oxide Coatings
3.1.2. Fluoride Coatings
3.1.3. Phosphate Coatings
3.1.4. Sulfide Coatings
3.2. ALD Coatings on Na and K Metal
4. Surface Modifications on Alkali Metals via MLD
4.1. MLD Coatings on Li Metal
4.1.1. Alucone Coatings
4.1.2. Zircone Coatings
4.1.3. Lithicone Coatings
4.1.4. Purely Organic Coatings
4.2. MLD Coatings on Na Metal
5. Dual Layered Coatings on Li Metal via ALD and MLD
6. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yoshino, A. The birth of the lithium-ion battery. Angew. Chem. Int. Ed. 2012, 51, 5798–5800. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Nuwayhid, R.B.; Wu, T.; Lei, Y.; Amine, K.; Lu, J. Atomic layer deposition for Lithium-based batteries. Adv. Mater. Interfaces 2016, 3, 1600564. [Google Scholar] [CrossRef]
- Padbury, R.; Zhang, X. Lithium-oxygen batteries-limiting factors that affect performance. J. Power Sources 2011, 196, 4436–4444. [Google Scholar] [CrossRef]
- Wu, Y. Lithium-ion batteries: Fundamentals and applications; CRC Press: Boca Raton, FL, USA, 2015; Volume 4. [Google Scholar]
- Bi, C.-X.; Zhao, M.; Hou, L.-P.; Chen, Z.-X.; Zhang, X.-Q.; Li, B.-Q.; Yuan, H.; Huang, J.-Q. Anode material options toward 500 Wh kg−1 lithium-sulfur batteries. Adv. Sci. 2022, 9, 2103910. [Google Scholar] [CrossRef]
- Van Noorden, R. The rechargeable revolution: A better battery. Nature 2014, 507, 26–28. [Google Scholar] [CrossRef]
- Wang, H.; Yu, Z.; Kong, X.; Kim, S.C.; Boyle, D.T.; Qin, J.; Bao, Z.; Cui, Y. Liquid electrolyte: The nexus of practical lithium metal batteries. Joule 2022, 6, 588–616. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, L.; Liu, J.; Adair, K.; Zhao, F.; Sun, Y.; Wu, T.; Bi, X.; Amine, K.; Lu, J. Atomic/molecular layer deposition for energy storage and conversion. Chem. Soc. Rev. 2021, 50, 3889–3956. [Google Scholar] [CrossRef]
- Xu, W.; Wang, J.; Ding, F.; Chen, X.; Nasybulin, E.; Zhang, Y.; Zhang, J.-G. Lithium metal anodes for rechargeable batteries. Energy Environ. Sci. 2014, 7, 513–537. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, J.-G.; Xu, W. Advancing lithium metal batteries. Joule 2018, 2, 833–845. [Google Scholar] [CrossRef]
- Lin, D.; Liu, Y.; Cui, Y. Reviving the lithium metal anode for high-energy batteries. Nat. Nanotechnol. 2017, 12, 194–206. [Google Scholar] [CrossRef]
- Tarascon, J.M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. In Materials for Sustainable Energy; Macmillan Publishers Ltd.: London, UK, 2010; pp. 171–179. [Google Scholar]
- Xu, K. Nonaqueous liquid electrolytes for Lithium-based rechargeable batteries. Chem. Rev. 2004, 104, 4303–4418. [Google Scholar] [CrossRef]
- Bruce, P.G.; Freunberger, S.A.; Hardwick, L.J.; Tarascon, J.-M. Li-O2 and Li-S batteries with high energy storage. Nat. Mater. 2012, 11, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.-B.; Zhang, R.; Zhao, C.-Z.; Zhang, Q. Toward safe Lithium metal anode in rechargeable batteries: A review. Chem. Rev. 2017, 117, 10403–10473. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Ma, L.; Zhou, Y.; Wang, L.; Han, N.; Zhao, F.; Deng, J.; Wu, T.; Li, Y.; Lu, J. Amorphous MoS3 as the sulfur-equivalent cathode material for room-temperature Li-S and Na-S batteries. Proc. Natl. Acad. Sci. USA 2017, 114, 13091–13096. [Google Scholar] [CrossRef] [PubMed]
- Schafzahl, L.; Mahne, N.; Schafzahl, B.; Wilkening, M.; Slugovc, C.; Borisov, S.M.; Freunberger, S.A. Singlet oxygen during cycling of the aprotic sodium-O2 battery. Angew. Chem. Int. Ed. 2017, 56, 15728–15732. [Google Scholar] [CrossRef]
- Hu, X.; Sun, J.; Li, Z.; Zhao, Q.; Chen, C.; Chen, J. Rechargeable room-temperature Na-CO2 batteries. Angew. Chem. Int. Ed. 2016, 55, 6482–6486. [Google Scholar] [CrossRef]
- Hu, X.; Li, Z.; Zhao, Y.; Sun, J.; Zhao, Q.; Wang, J.; Tao, Z.; Chen, J. Quasi–solid state rechargeable Na-CO2 batteries with reduced graphene oxide Na anodes. Sci. Adv. 2017, 3, e1602396. [Google Scholar] [CrossRef]
- Wang, H.; Yu, D.; Kuang, C.; Cheng, L.; Li, W.; Feng, X.; Zhang, Z.; Zhang, X.; Zhang, Y. Alkali metal anodes for rechargeable batteries. Chem 2019, 5, 313–338. [Google Scholar] [CrossRef]
- Peled, E. The electrochemical behavior of alkali and alkaline earth metals in nonaqueous battery systems—the solid electrolyte interphase model. J. Electrochem. Soc. 1979, 126, 2047. [Google Scholar] [CrossRef]
- Peled, E.; Menkin, S. SEI: Past, present and future. J. Electrochem. Soc. 2017, 164, A1703. [Google Scholar] [CrossRef]
- Gu, Y.; Wang, W.-W.; Li, Y.-J.; Wu, Q.-H.; Tang, S.; Yan, J.-W.; Zheng, M.-S.; Wu, D.-Y.; Fan, C.-H.; Hu, W.-Q. Designable ultra-smooth ultra-thin solid-electrolyte interphases of three alkali metal anodes. Nat. Commun. 2018, 9, 1339. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-Q.; Cheng, X.-B.; Zhang, Q. Advances in interfaces between Li metal anode and electrolyte. Adv. Mater. Interfaces 2018, 5, 1701097. [Google Scholar] [CrossRef]
- Zheng, G.; Lee, S.W.; Liang, Z.; Lee, H.-W.; Yan, K.; Yao, H.; Wang, H.; Li, W.; Chu, S.; Cui, Y. Interconnected hollow carbon nanospheres for stable lithium metal anodes. Nat. Nanotechnol. 2014, 9, 618–623. [Google Scholar] [CrossRef]
- Sun, F.; Manke, I. Differentiating and quantifying dead Lithium. ChemElectroChem 2019, 6, 5787–5789. [Google Scholar] [CrossRef]
- Zheng, X.; Li, P.; Cao, Z.; Luo, W.; Sun, F.; Wang, Z.; Ding, B.; Wang, G.; Huang, Y. Boosting the reversibility of Sodium metal anode via heteroatom-doped hollow carbon fibers. Small 2019, 15, 1902688. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Wang, Y.; Hao, H.; Basu, S.; Feng, X.; Xu, Y.; Boscoboinik, J.A.; Nanda, J.; Watt, J.; Mitlin, D. Stable Potassium metal anodes with an all-aluminum current collector through improved electrolyte wetting. Adv. Mater. 2020, 32, 2002908. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.B.; Zhang, R.; Zhao, C.Z.; Wei, F.; Zhang, J.G.; Zhang, Q. A review of solid electrolyte interphases on lithium metal anode. Adv. Sci. 2016, 3, 1500213. [Google Scholar] [CrossRef] [PubMed]
- Kozen, A.C.; Lin, C.-F.; Pearse, A.J.; Schroeder, M.A.; Han, X.; Hu, L.; Lee, S.-B.; Rubloff, G.W.; Noked, M. Next-generation lithium metal anode engineering via atomic layer deposition. ACS Nano 2015, 9, 5884–5892. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, A.; Liu, X.; Luo, J. Dendrites in lithium metal anodes: Suppression, regulation, and elimination. Acc. Chem. Res. 2019, 52, 3223–3232. [Google Scholar] [CrossRef]
- Zhang, J.-G.; Xu, W.; Henderson, W.A. Lithium Metal Anodes and Rechargeable Lithium Metal Batteries; Springer: Cham, Switzerland, 2017; Volume 249. [Google Scholar]
- Lee, B.; Paek, E.; Mitlin, D.; Lee, S.W. Sodium metal anodes: Emerging solutions to dendrite growth. Chem. Rev. 2019, 119, 5416–5460. [Google Scholar] [CrossRef]
- Aslam, M.K.; Niu, Y.; Hussain, T.; Tabassum, H.; Tang, W.; Xu, M.; Ahuja, R. How to avoid dendrite formation in metal batteries: Innovative strategies for dendrite suppression. Nano Energy 2021, 86, 106142. [Google Scholar] [CrossRef]
- Khurana, R.; Schaefer, J.L.; Archer, L.A.; Coates, G.W. Suppression of lithium dendrite growth using cross-linked polyethylene/poly (ethylene oxide) electrolytes: A new approach for practical lithium-metal polymer batteries. J. Am. Chem. Soc. 2014, 136, 7395–7402. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Kotobuki, M.; Zheng, F.; Xu, C.; Savilov, S.V.; Hu, N.; Lu, L.; Wang, Y.; Li, W.D.Z. A hybrid polymer/oxide/ionic-liquid solid electrolyte for Na-metal batteries. J. Mater. Chem. A 2017, 5, 6424–6431. [Google Scholar] [CrossRef]
- Lei, D.; He, Y.-B.; Huang, H.; Yuan, Y.; Zhong, G.; Zhao, Q.; Hao, X.; Zhang, D.; Lai, C.; Zhang, S.; et al. Cross-linked beta alumina nanowires with compact gel polymer electrolyte coating for ultra-stable sodium metal battery. Nat. Commun. 2019, 10, 4244. [Google Scholar] [CrossRef]
- Zhang, Y.; Bahi, A.; Ko, F.; Liu, J. Polyacrylonitrile-reinforced composite gel polymer electrolytes for stable potassium metal anodes. Small 2022, 18, 2107186. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, P.; Zhu, K.; Zhang, Z.; Si, Y.; Wang, Y.; Jiao, L. Solid-state electrolytes for sodium metal batteries. Energy Fuels 2021, 35, 9063–9079. [Google Scholar] [CrossRef]
- Zhang, Y.; Qiu, P.; Zheng, J.; Chen, X.; Chen, X.-M.; Li, S.; Ji, C.; Wu, Y.; Chen, X. KB3H8·NH3B3H7 complex as a potential solid-state electrolyte with excellent stability against K metal. ACS Appl. Mater. Interfaces 2022, 14, 17378–17387. [Google Scholar] [CrossRef]
- Qian, J.; Henderson, W.A.; Xu, W.; Bhattacharya, P.; Engelhard, M.; Borodin, O.; Zhang, J.-G. High rate and stable cycling of lithium metal anode. Nat. Commun. 2015, 6, 6362. [Google Scholar] [CrossRef]
- Zheng, J.; Chen, S.; Zhao, W.; Song, J.; Engelhard, M.H.; Zhang, J.-G. Extremely stable sodium metal batteries enabled by localized high-concentration electrolytes. ACS Energy Lett. 2018, 3, 315–321. [Google Scholar] [CrossRef]
- Xu, W.; Wang, H.; Hu, J.; Zhang, H.; Zhang, B.; Kang, F.; Zhai, D. A highly concentrated electrolyte for high-efficiency potassium metal batteries. Chem. Commun. 2021, 57, 1034–1037. [Google Scholar] [CrossRef]
- Basile, A.; Bhatt, A.; O’Mullane, A. Stabilizing lithium metal using ionic liquids for long-lived batteries. Nat. Commun. 2016, 7, 11794. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Zhu, G.; Xu, X.; Liao, M.; Li, Y.-Y.; Angell, M.; Gu, M.; Zhu, Y.; Hung, W.H.; Li, J. A safe and non-flammable sodium metal battery based on an ionic liquid electrolyte. Nat. Commun. 2019, 10, 3302. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Liang, P.; Zhu, G.; Hung, W.H.; Li, Y.-Y.; Tai, H.-C.; Huang, C.-L.; Li, J.; Meng, Y.; Angell, M. A high-performance potassium metal battery using safe ionic liquid electrolyte. Proc. Natl. Acad. Sci. USA 2020, 117, 27847–27853. [Google Scholar] [CrossRef]
- Tu, Z.; Nath, P.; Lu, Y.; Tikekar, M.D.; Archer, L.A. Nanostructured electrolytes for stable lithium electrodeposition in secondary batteries. Acc. Chem. Res. 2015, 48, 2947–2956. [Google Scholar] [CrossRef]
- Sun, B.; Xiong, P.; Maitra, U.; Langsdorf, D.; Yan, K.; Wang, C.; Janek, J.; Schröder, D.; Wang, G. Design strategies to enable the efficient use of sodium metal anodes in high-energy batteries. Adv. Mater. 2020, 32, 1903891. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Engelhard, M.H.; Mei, D.; Jiao, S.; Polzin, B.J.; Zhang, J.-G.; Xu, W. Electrolyte additive enabled fast charging and stable cycling lithium metal batteries. Nat. Energy 2017, 2, 17012. [Google Scholar] [CrossRef]
- Wei, C.; Tao, Y.; Fei, H.; An, Y.; Tian, Y.; Feng, J.; Qian, Y. Recent advances and perspectives in stable and dendrite-free potassium metal anodes. Energy Storage Mater. 2020, 30, 206–227. [Google Scholar] [CrossRef]
- Hao, H.; Hutter, T.; Boyce, B.L.; Watt, J.; Liu, P.; Mitlin, D. Review of multifunctional separators: Stabilizing the cathode and the anode for alkali (Li, Na, and K) metal–sulfur and selenium batteries. Chem. Rev. 2022, 122, 8053–8125. [Google Scholar] [CrossRef]
- Seh, Z.W.; Sun, J.; Sun, Y.; Cui, Y. A highly reversible room-temperature sodium metal anode. ACS Cent. Sci. 2015, 1, 449–455. [Google Scholar] [CrossRef]
- Wang, T.; Hua, Y.; Xu, Z.; Yu, J.S. Recent advanced development of artificial interphase engineering for stable sodium metal anodes. Small 2022, 18, 2102250. [Google Scholar] [CrossRef]
- Meng, X. Atomic-scale surface modifications and novel electrode designs for high-performance sodium-ion batteries via atomic layer deposition. J. Mater. Chem. A 2017, 5, 10127–10149. [Google Scholar] [CrossRef]
- Meng, X. An overview of molecular layer deposition for organic and organic–inorganic hybrid materials: Mechanisms, growth characteristics, and promising applications. J. Mater. Chem. A 2017, 5, 18326–18378. [Google Scholar] [CrossRef]
- Han, Z.; Zhang, C.; Lin, Q.; Zhang, Y.; Deng, Y.; Han, J.; Wu, D.; Kang, F.; Yang, Q.H.; Lv, W. A Protective layer for lithium metal anode: Why and how. Small Methods 2021, 5, 2001035. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Lau, K.C.; Zhou, H.; Ghosh, S.K.; Benamara, M.; Zou, M. Molecular layer deposition of crosslinked polymeric lithicone for superior lithium metal anodes. Energy Mater. Adv. 2021, 2021, 9786201. [Google Scholar] [CrossRef]
- Puurunen, R.L. A short history of atomic layer deposition: Tuomo suntola’s atomic layer epitaxy. Chem. Vap. Depos. 2014, 20, 332–344. [Google Scholar] [CrossRef]
- Zhu, C.; Han, K.; Geng, D.; Ye, H.; Meng, X. Achieving high-performance silicon anodes of lithium-ion batteries via atomic and molecular layer deposited surface coatings: An overview. Electrochim. Acta 2017, 251, 710–728. [Google Scholar] [CrossRef]
- Elam, J.W.; Dasgupta, N.P.; Prinz, F.B. ALD for clean energy conversion, utilization, and storage. MRS Bull. 2011, 36, 899–906. [Google Scholar] [CrossRef]
- Sønsteby, H.H.; Yanguas-Gil, A.; Elam, J.W. Consistency and reproducibility in atomic layer deposition. J. Vac. Sci. Technol. A 2020, 38, 020804. [Google Scholar] [CrossRef]
- Zhao, Y.; Zheng, K.; Sun, X. Addressing interfacial issues in liquid-based and solid-state batteries by atomic and molecular layer deposition. Joule 2018, 2, 2583–2604. [Google Scholar] [CrossRef]
- Li, X.; Liang, J.; Yang, X.; Adair, K.R.; Wang, C.; Zhao, F.; Sun, X. Progress and perspectives on halide lithium conductors for all-solid-state lithium batteries. Energy Environ. Sci. 2020, 13, 1429–1461. [Google Scholar] [CrossRef]
- Cao, Y.; Meng, X.; Li, A. Atomic layer deposition of high-capacity anodes for next-generation Lithium-ion batteries and beyond. Energy Environ. Mater. 2021, 4, 363–391. [Google Scholar] [CrossRef]
- Meng, X. Atomic and molecular layer deposition in pursuing better batteries. J. Mater. Res. 2021, 36, 2–25. [Google Scholar] [CrossRef]
- Meng, X. Atomic layer deposition of solid-state electrolytes for next-generation lithium-ion batteries and beyond: Opportunities and challenges. Energy Storage Mater. 2020, 30, 296–328. [Google Scholar] [CrossRef]
- Meng, X.; Wang, X.; Geng, D.; Ozgit-Akgun, C.; Schneider, N.; Elam, J.W. Atomic layer deposition for nanomaterial synthesis and functionalization in energy technology. Mater. Horiz. 2017, 4, 133–154. [Google Scholar] [CrossRef]
- Groner, M.; Fabreguette, F.; Elam, J.; George, S. Low-temperature Al2O3 atomic layer deposition. Chem. Mater. 2004, 16, 639–645. [Google Scholar] [CrossRef]
- Cai, J.; Sun, Q.; Meng, X. Novel nanostructured materials by atomic and molecular layer deposition. AIMS Mater. Sci. 2018, 5, 957–999. [Google Scholar] [CrossRef]
- George, S.M.; Yoon, B.; Dameron, A.A. Surface Chemistry for Molecular Layer Deposition of Organic and Hybrid Organic−Inorganic Polymers. Acc. Chem. Res. 2009, 42, 498–508. [Google Scholar] [CrossRef]
- Meng, X.; Liu, Y.; Libera, J.A.; Zavadil, K.R.; Elam, J.W. Approaching high-performance Li-Sulfur batteries by surface modification using atomic layer deposition. In ECS Meeting Abstracts; IOP Publishing: Bristol, UK, 2016. [Google Scholar]
- George, S.M. Atomic layer deposition: An overview. Chem. Rev. 2010, 110, 111–131. [Google Scholar] [CrossRef]
- Nazarov, D.; Bobrysheva, N.; Osmolovskaya, O.; Osmolovsky, M.; Smirnov, V. Atomic layer deposition of tin dioxide nanofilms: A review. Rev. Adv. Mater. Sci. 2015, 40, 262–275. [Google Scholar]
- Dasgupta, N.P.; Meng, X.; Elam, J.W.; Martinson, A.B. Atomic layer deposition of metal sulfide materials. Acc. Chem. Res. 2015, 48, 341–348. [Google Scholar] [CrossRef]
- Niinistö, L.; Nieminen, M.; Päiväsaari, J.; Niinistö, J.; Putkonen, M.; Nieminen, M. Advanced electronic and optoelectronic materials by Atomic Layer Deposition: An overview with special emphasis on recent progress in processing of high-k dielectrics and other oxide materials. Phys. Status Solidi 2004, 201, 1443–1452. [Google Scholar] [CrossRef]
- Kim, H. Atomic layer deposition of metal and nitride thin films: Current research efforts and applications for semiconductor device processing. J. Vac. Sci. Technol. B Microelectron. Nanometer Struct. Process. Meas. Phenom. 2003, 21, 2231–2261. [Google Scholar] [CrossRef]
- Ylilammi, M.; Ranta-aho, T. Metal fluoride thin films prepared by atomic layer deposition. J. Electrochem. Soc. 1994, 141, 1278. [Google Scholar] [CrossRef]
- Liu, J.; Banis, M.N.; Li, X.; Lushington, A.; Cai, M.; Li, R.; Sham, T.-K.; Sun, X. Atomic layer deposition of lithium tantalate solid-state electrolytes. J. Phys. Chem. C 2013, 117, 20260–20267. [Google Scholar] [CrossRef]
- McDaniel, M.D.; Ngo, T.Q.; Hu, S.; Posadas, A.; Demkov, A.A.; Ekerdt, J.G. Atomic layer deposition of perovskite oxides and their epitaxial integration with Si, Ge, and other semiconductors. Appl. Phys. Rev. 2015, 2, 041301. [Google Scholar] [CrossRef]
- Aaltonen, T.; Nilsen, O.; Magrasó, A.; Fjellvag, H. Atomic layer deposition of Li2O–Al2O3 thin films. Chem. Mater. 2011, 23, 4669–4675. [Google Scholar] [CrossRef]
- Thimsen, E.; Riha, S.C.; Baryshev, S.V.; Martinson, A.B.; Elam, J.W.; Pellin, M.J. Atomic layer deposition of the quaternary chalcogenide Cu2ZnSnS4. Chem. Mater. 2012, 24, 3188–3196. [Google Scholar] [CrossRef]
- Meng, X.; Liu, J.; Li, X.; Banis, M.N.; Yang, J.; Li, R.; Sun, X. Atomic layer deposited Li4Ti5O12 on nitrogen-doped carbon nanotubes. RSC Adv. 2013, 3, 7285–7288. [Google Scholar] [CrossRef]
- Yoshimura, T.; Tatsuura, S.; Sotoyama, W. Polymer-films formed with monolayer growth steps by molecular layer deposition. Appl. Phys. Lett. 1991, 59, 482–484. [Google Scholar] [CrossRef]
- de Paula, C.; Richey, N.E.; Zeng, L.; Bent, S.F. Mechanistic study of nucleation enhancement in atomic layer deposition by pre-Treatment with small organometallic molecules. Chem. Mater. 2019, 32, 315–325. [Google Scholar] [CrossRef]
- Kazyak, E.; Wood, K.N.; Dasgupta, N.P. Improved cycle life and stability of lithium metal anodes through ultrathin atomic layer deposition surface treatments. Chem. Mater. 2015, 27, 6457–6462. [Google Scholar] [CrossRef]
- Cao, Y.; Meng, X.; Elam, J.W. Atomic layer deposition of LixAlyS solid-state electrolytes for stabilizing Lithium-metal anodes. ChemElectroChem 2016, 3, 858–863. [Google Scholar] [CrossRef]
- Chen, L.; Connell, J.G.; Nie, A.; Huang, Z.; Zavadil, K.R.; Klavetter, K.C.; Yuan, Y.; Sharifi-Asl, S.; Shahbazian-Yassar, R.; Libera, J.A. Lithium metal protected by atomic layer deposition metal oxide for high performance anodes. J. Mater. Chem. A 2017, 5, 12297–12309. [Google Scholar] [CrossRef]
- Alaboina, P.K.; Rodrigues, S.; Rottmayer, M.; Cho, S.-J. In situ dendrite suppression study of nanolayer encapsulated Li metal enabled by zirconia atomic layer deposition. ACS Appl. Mater. Interfaces 2018, 10, 32801–32808. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Chen, K.-S.; Chen, X.; Ramirez, G.; Huang, Z.; Geise, N.R.; Steinrück, H.-G.; Fisher, B.L.; Shahbazian-Yassar, R.; Toney, M.F. Novel ALD chemistry enabled low-temperature synthesis of lithium fluoride coatings for durable lithium anodes. ACS Appl. Mater. Interfaces 2018, 10, 26972–26981. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.-P.; Hennessy, J.; Billings, K.J.; Krause, F.C.; Pasalic, J.; Bugga, R.V. Communication-atomic layer deposition of aluminum fluoride for lithium metal anodes. J. Electrochem. Soc. 2020, 167, 060502. [Google Scholar] [CrossRef]
- Niu, J.; Wang, M.; Cao, T.; Cheng, X.; Wu, R.; Liu, H.; Zhang, Y.; Liu, X. Li metal coated with Li3PO4 film via atomic layer deposition as battery anode. Ionics 2021, 27, 2445–2454. [Google Scholar] [CrossRef]
- Wang, M.; Cheng, X.; Cao, T.; Niu, J.; Wu, R.; Liu, X.; Zhang, Y. Constructing ultrathin TiO2 protection layers via atomic layer deposition for stable lithium metal anode cycling. J. Alloys Compd. 2021, 865, 158748. [Google Scholar] [CrossRef]
- Jin, E.; Tantratian, K.; Zhao, C.; Codirenzi, A.; Goncharova, L.V.; Wang, C.; Yang, F.; Wang, Y.; Pirayesh, P.; Guo, J. Ionic conductive and highly-stable interface for alkali metal anodes. Small 2022, 18, 2203045. [Google Scholar] [CrossRef]
- Luo, W.; Lin, C.-F.; Zhao, O.; Noked, M.; Zhang, Y.; Rubloff, G.W.; Hu, L. Ultrathin surface coating enables the stable sodium metal anode. Adv. Energy Mater. 2017, 7, 1601526. [Google Scholar] [CrossRef]
- Zhao, Y.; Goncharova, L.V.; Lushington, A.; Sun, Q.; Yadegari, H.; Wang, B.; Xiao, W.; Li, R.; Sun, X. Superior stable and long life sodium metal anodes achieved by atomic layer deposition. Adv. Mater. 2017, 29, 1606663. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-F.; Kozen, A.C.; Noked, M.; Liu, C.; Rubloff, G.W. ALD protection of Li-metal anode surfaces-quantifying and preventing chemical and electrochemical corrosion in organic solvent. Adv. Mater. Interfaces 2016, 3, 1600426. [Google Scholar] [CrossRef]
- Harrop, P.; Wanklyn, J. The dielectric constant of zirconia. Br. J. Appl. Phys. 1967, 18, 739. [Google Scholar] [CrossRef]
- Shamala, K.; Murthy, L.; Rao, K.N. Studies on optical and dielectric properties of Al2O3 thin films prepared by electron beam evaporation and spray pyrolysis method. Mater. Sci. Eng. B 2004, 106, 269–274. [Google Scholar] [CrossRef]
- Boehler, R. Melting temperature, adiabats, and Grüneisen parameter of lithium, sodium and potassium versus pressure. Phys. Rev. B 1983, 27, 6754. [Google Scholar] [CrossRef]
- Yang, J.; Wang, Y.; Li, W.; Wang, L.; Fan, Y.; Jiang, W.; Luo, W.; Wang, Y.; Kong, B.; Selomulya, C. Amorphous TiO2 shells: A vital elastic buffering layer on silicon nanoparticles for high-performance and safe lithium storage. Adv. Mater. 2017, 29, 1700523. [Google Scholar]
- Cheng, X.; Li, Y.; Sang, L.; Ma, J.; Shi, H.; Liu, X.; Lu, J.; Zhang, Y. Boosting the electrochemical performance of MoO3 anode for long-life lithium ion batteries: Dominated by an ultrathin TiO2 passivation layer. Electrochim. Acta 2018, 269, 241–249. [Google Scholar] [CrossRef]
- Peled, E.; Tow, D.B.; Merson, A.; Gladkich, A.; Burstein, L.; Golodnitsky, D. Composition, depth profiles and lateral distribution of materials in the SEI built on HOPG-TOF SIMS and XPS studies. J. Power Sources 2001, 97, 52–57. [Google Scholar] [CrossRef]
- Richards, W.D.; Miara, L.J.; Wang, Y.; Kim, J.C.; Ceder, G. Interface stability in solid-state batteries. Chem. Mater. 2016, 28, 266–273. [Google Scholar] [CrossRef]
- Monroe, C.; Newman, J. Dendrite growth in lithium/polymer systems: A propagation model for liquid electrolytes under galvanostatic conditions. J. Electrochem. Soc. 2003, 150, A1377. [Google Scholar] [CrossRef]
- Li, N.W.; Yin, Y.X.; Yang, C.P.; Guo, Y.G. An artificial solid electrolyte interphase layer for stable lithium metal anodes. Adv. Mater. 2016, 28, 1853–1858. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Bai, Y.-K.; Shen, X.; Peng, H.-J.; Zhang, Q. Sodiophilicity/potassiophilicity chemistry in sodium/potassium metal anodes. J. Energy Chem. 2020, 51, 1–6. [Google Scholar] [CrossRef]
- Zhao, Y.; Adair, K.R.; Sun, X. Recent developments and insights into the understanding of Na metal anodes for Na-metal batteries. Energy Environ. Sci. 2018, 11, 2673–2695. [Google Scholar] [CrossRef]
- Zhao, L.; Zhao, J.; Hu, Y.S.; Li, H.; Zhou, Z.; Armand, M.; Chen, L. Disodium terephthalate (Na2C8H4O4) as high performance anode material for low-cost room-temperature sodium-ion battery. Adv. Energy Mater. 2012, 2, 962–965. [Google Scholar] [CrossRef]
- Han, X.; Liu, Y.; Jia, Z.; Chen, Y.-C.; Wan, J.; Weadock, N.; Gaskell, K.J.; Li, T.; Hu, L. Atomic-layer-deposition oxide nanoglue for sodium ion batteries. Nano Lett. 2014, 14, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Liu, P.; Mitlin, D. Review of emerging concepts in SEI analysis and artificial SEI membranes for lithium, sodium, and potassium metal battery anodes. Adv. Energy Mater. 2020, 10, 2002297. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, F.; Liu, J.; Cheng, M.; Su, H.; Liu, J.; Xu, Y. Enhanced surface binding energy regulates uniform potassium deposition for stable potassium metal anodes. J. Mater. Chem. A 2020, 8, 5671–5678. [Google Scholar] [CrossRef]
- Zhao, Y.; Goncharova, L.V.; Zhang, Q.; Kaghazchi, P.; Sun, Q.; Lushington, A.; Wang, B.; Li, R.; Sun, X. Inorganic-organic coating via molecular layer deposition enables long life sodium metal anode. Nano Lett. 2017, 17, 5653–5659. [Google Scholar] [CrossRef]
- Chen, L.; Huang, Z.; Shahbazian-Yassar, R.; Libera, J.A.; Klavetter, K.C.; Zavadil, K.R.; Elam, J.W. Directly formed alucone on lithium metal for high-performance Li batteries and Li-S batteries with high sulfur mass loading. ACS Appl. Mater. Interfaces 2018, 10, 7043–7051. [Google Scholar] [CrossRef]
- Zhao, Y.; Goncharova, L.V.; Sun, Q.; Li, X.; Lushington, A.; Wang, B.; Li, R.; Dai, F.; Cai, M.; Sun, X. Robust metallic lithium anode protection by the molecular-layer-deposition technique. Small Methods 2018, 2, 1700417. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, Y.; Sun, Q.; Li, X.; Liu, Y.; Liang, J.; Li, X.; Lin, X.; Li, R.; Adair, K.R. Stabilizing interface between Li10SnP2S12 and Li metal by molecular layer deposition. Nano Energy 2018, 53, 168–174. [Google Scholar] [CrossRef]
- Sun, Y.; Zhao, Y.; Wang, J.; Liang, J.; Wang, C.; Sun, Q.; Lin, X.; Adair, K.R.; Luo, J.; Wang, D. A novel organic “Polyurea” thin film for ultralong-life lithium-metal anodes via molecular-layer deposition. Adv. Mater. 2019, 31, 1806541. [Google Scholar] [CrossRef] [PubMed]
- Adair, K.R.; Zhao, C.; Banis, M.N.; Zhao, Y.; Li, R.; Cai, M.; Sun, X. Highly stable lithium metal anode interface via molecular layer deposition zircone coatings for long life next-generation battery systems. Angew. Chem. 2019, 131, 15944–15949. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, Y.; Zhao, F.; Zhang, L.; Wang, C.; Li, X.; Liang, J.; Li, W.; Sun, Q.; Yu, C. Gradiently sodiated alucone as an interfacial stabilizing strategy for solid-state Na metal batteries. Adv. Funct. Mater. 2020, 30, 2001118. [Google Scholar] [CrossRef]
- Elam, J.W.; Routkevitch, D.; Mardilovich, P.P.; George, S.M. Conformal coating on ultrahigh-aspect-ratio nanopores of anodic alumina by atomic layer deposition. Chem. Mater. 2003, 15, 3507–3517. [Google Scholar] [CrossRef]
- Zhu, H.; Shiraz, M.H.A.; Yao, L.; Adair, K.; Wang, Z.; Tong, H.; Song, X.; Sham, T.-K.; Arjmand, M.; Song, X. Molecular-layer-deposited tincone: A new hybrid organic–inorganic anode material for three-dimensional microbatteries. Chem. Commun. 2020, 56, 13221–13224. [Google Scholar] [CrossRef]
- Dameron, A.; Seghete, D.; Burton, B.; Davidson, S.; Cavanagh, A.; Bertrand, J.; George, S. Molecular layer deposition of alucone polymer films using trimethylaluminum and ethylene glycol. Chem. Mater. 2008, 20, 3315–3326. [Google Scholar] [CrossRef]
- Piper, D.M.; Travis, J.J.; Young, M.; Son, S.B.; Kim, S.C.; Oh, K.H.; George, S.M.; Ban, C.; Lee, S.H. Reversible high-capacity Si nanocomposite anodes for lithium-ion batteries enabled by molecular layer deposition. Adv. Mater. 2014, 26, 1596–1601. [Google Scholar] [CrossRef]
- George, S.M.; Lee, B.H.; Yoon, B.; Abdulagatov, A.I.; Hall, R.A. Metalcones: Hybrid organic–inorganic films fabricated using atomic and molecular layer deposition techniques. J. Nanosci. Nanotechnol. 2011, 11, 7948–7955. [Google Scholar] [CrossRef]
- Lee, B.H.; Anderson, V.R.; George, S.M. Metalcone and metalcone/metal oxide alloys grown using atomic & molecular layer deposition techniques. In Electrochemical Society Meeting Abstracts; The Electrochemical Society, Inc.: Pennington, NJ, USA, 2011. [Google Scholar]
- Wang, H.; Gregorczyk, K.E.; Lee, S.B.; Rubloff, G.W.; Lin, C.-F. Li-containing organic thin film-structure of lithium propane dioxide via molecular layer deposition. J. Phys. Chem. C 2020, 124, 6830–6837. [Google Scholar] [CrossRef]
- Kazyak, E.; Shin, M.; LePage, W.S.; Cho, T.H.; Dasgupta, N.P. Molecular layer deposition of Li-ion conducting “Lithicone” solid electrolytes. Chem. Commun. 2020, 56, 15537–15540. [Google Scholar] [CrossRef] [PubMed]
- Loscutoff, P.W.; Zhou, H.; Clendenning, S.B.; Bent, S.F. Formation of organic nanoscale laminates and blends by molecular layer deposition. ACS Nano 2010, 4, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Zhuang, H.L.; Zhang, W.; Fu, Y.; Liao, Z.; Lu, Y. Stable Lithium Electrodeposition at Ultra-High Current Densities Enabled by 3D PMF/Li Composite Anode. Adv. Energy Mater. 2018, 8, 1703360. [Google Scholar] [CrossRef]
- Li, X.; Lushington, A.; Liu, J.; Li, R.; Sun, X. Superior stable sulfur cathodes of Li–S batteries enabled by molecular layer deposition. Chem. Commun. 2014, 50, 9757–9760. [Google Scholar] [CrossRef]
- Li, X.; Lushington, A.; Sun, Q.; Xiao, W.; Liu, J.; Wang, B.; Ye, Y.; Nie, K.; Hu, Y.; Xiao, Q. Safe and durable high-temperature lithium–sulfur batteries via molecular layer deposited coating. Nano Lett. 2016, 16, 3545–3549. [Google Scholar] [CrossRef]
- Zhao, Y.; Amirmaleki, M.; Sun, Q.; Zhao, C.; Codirenzi, A.; Goncharova, L.V.; Wang, C.; Adair, K.; Li, X.; Yang, X. Natural SEI-inspired dual-protective layers via atomic/molecular layer deposition for long-life metallic lithium anode. Matter 2019, 1, 1215–1231. [Google Scholar] [CrossRef]
- Sun, Y.; Zhao, C.; Adair, K.R.; Zhao, Y.; Goncharova, L.V.; Liang, J.; Wang, C.; Li, J.; Li, R.; Cai, M. Regulated lithium plating and stripping by a nano-scale gradient inorganic–organic coating for stable lithium metal anodes. Energy Environ. Sci. 2021, 14, 4085–4094. [Google Scholar] [CrossRef]
- Zhao, Q.; Stalin, S.; Archer, L.A. Stabilizing metal battery anodes through the design of solid electrolyte interphases. Joule 2021, 5, 1119–1142. [Google Scholar] [CrossRef]
- Narayanan, S.; Gibson, J.S.; Aspinall, J.; Weatherup, R.S.; Pasta, M. In situ and operando characterisation of Li metal–Solid electrolyte interfaces. Curr. Opin. Solid State Mater. Sci. 2022, 26, 100978. [Google Scholar] [CrossRef]



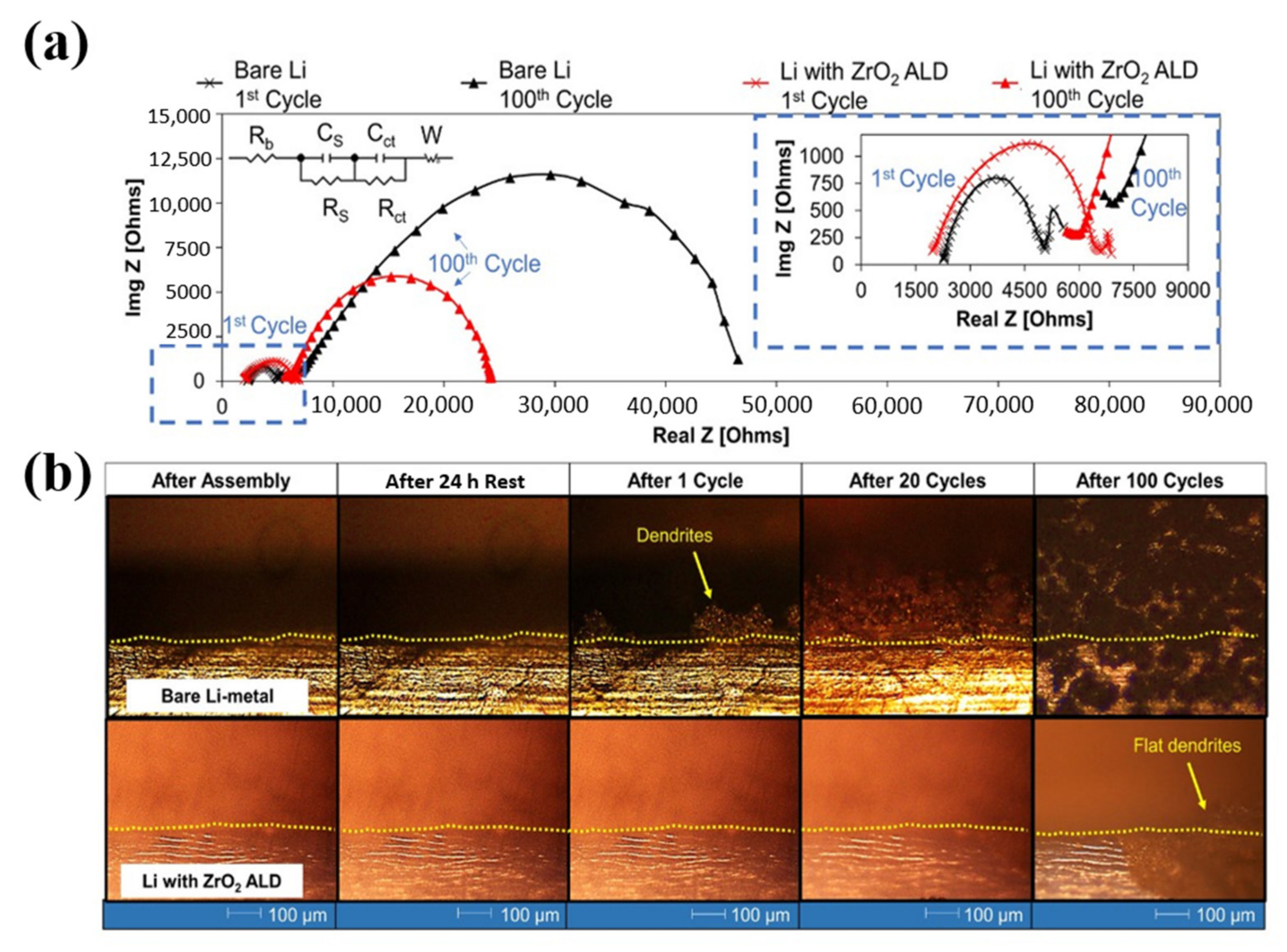
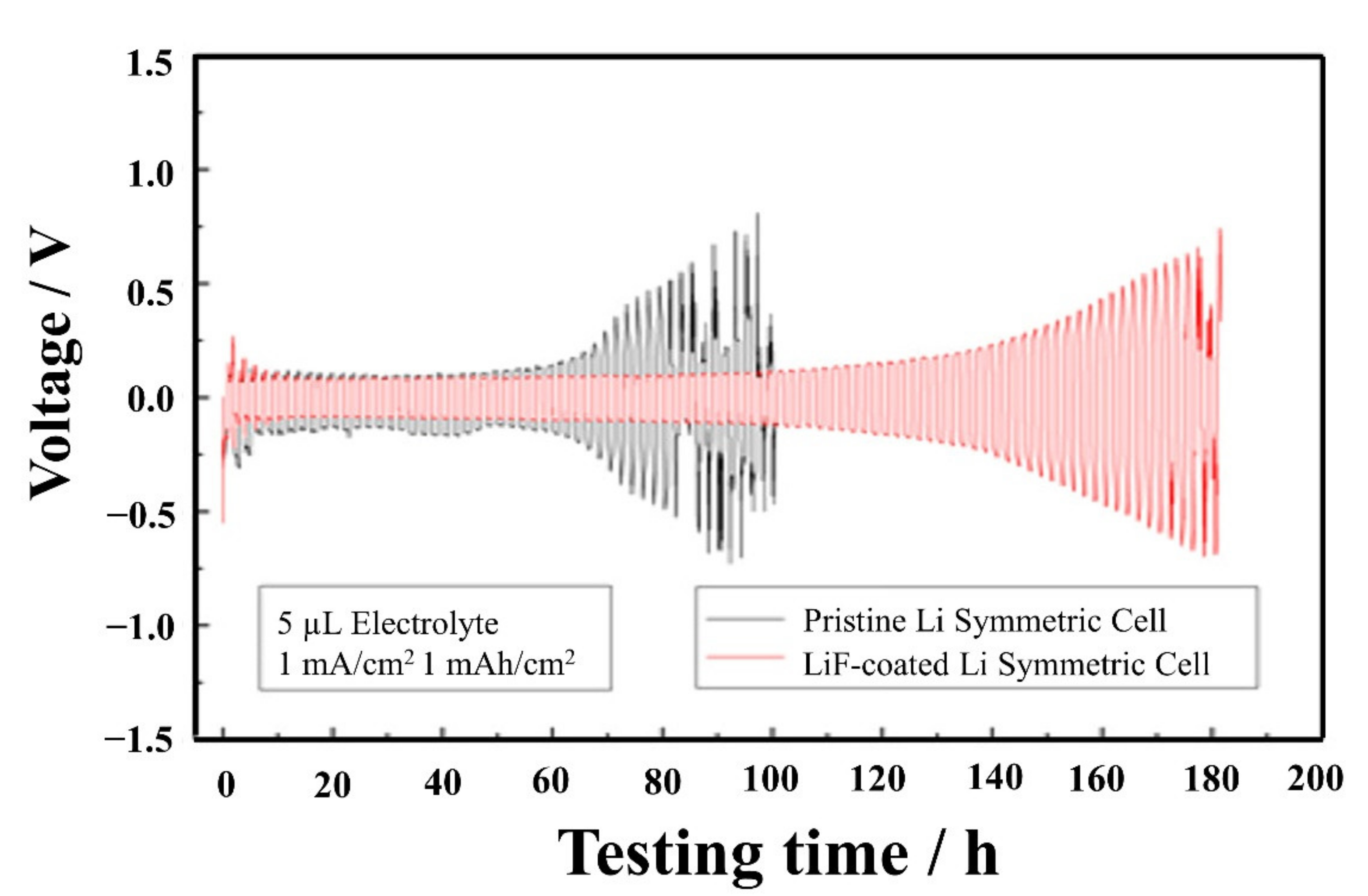
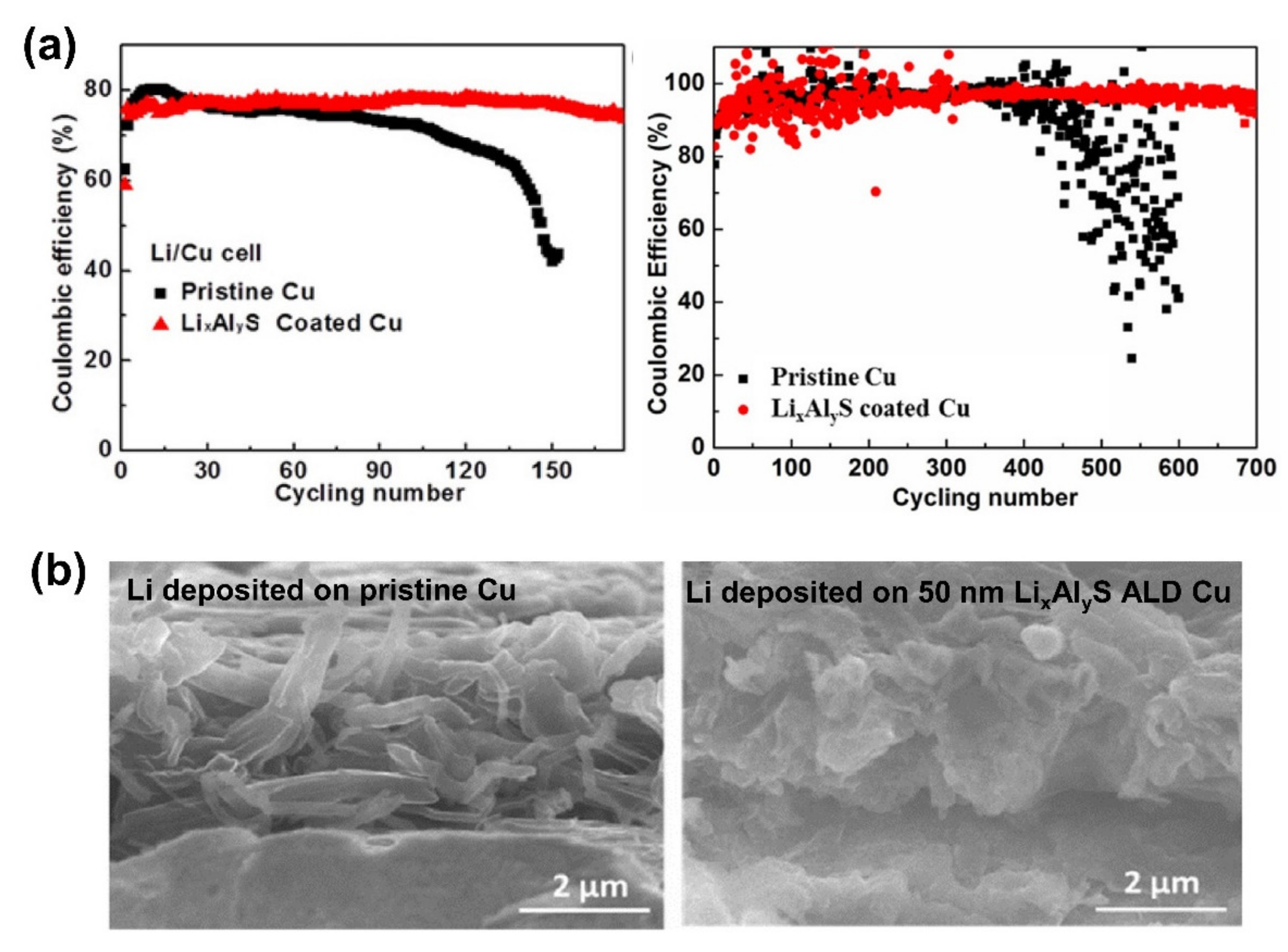
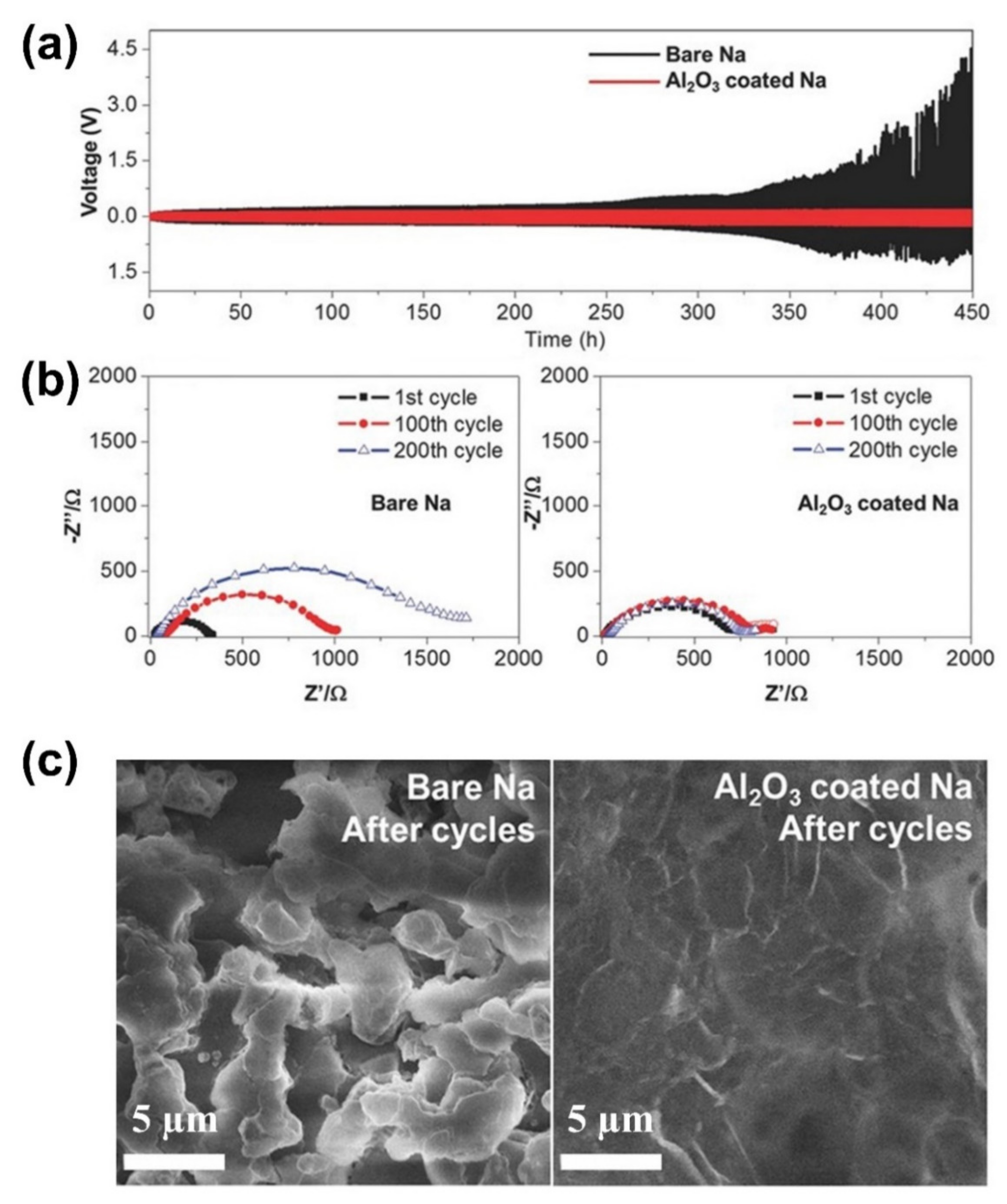
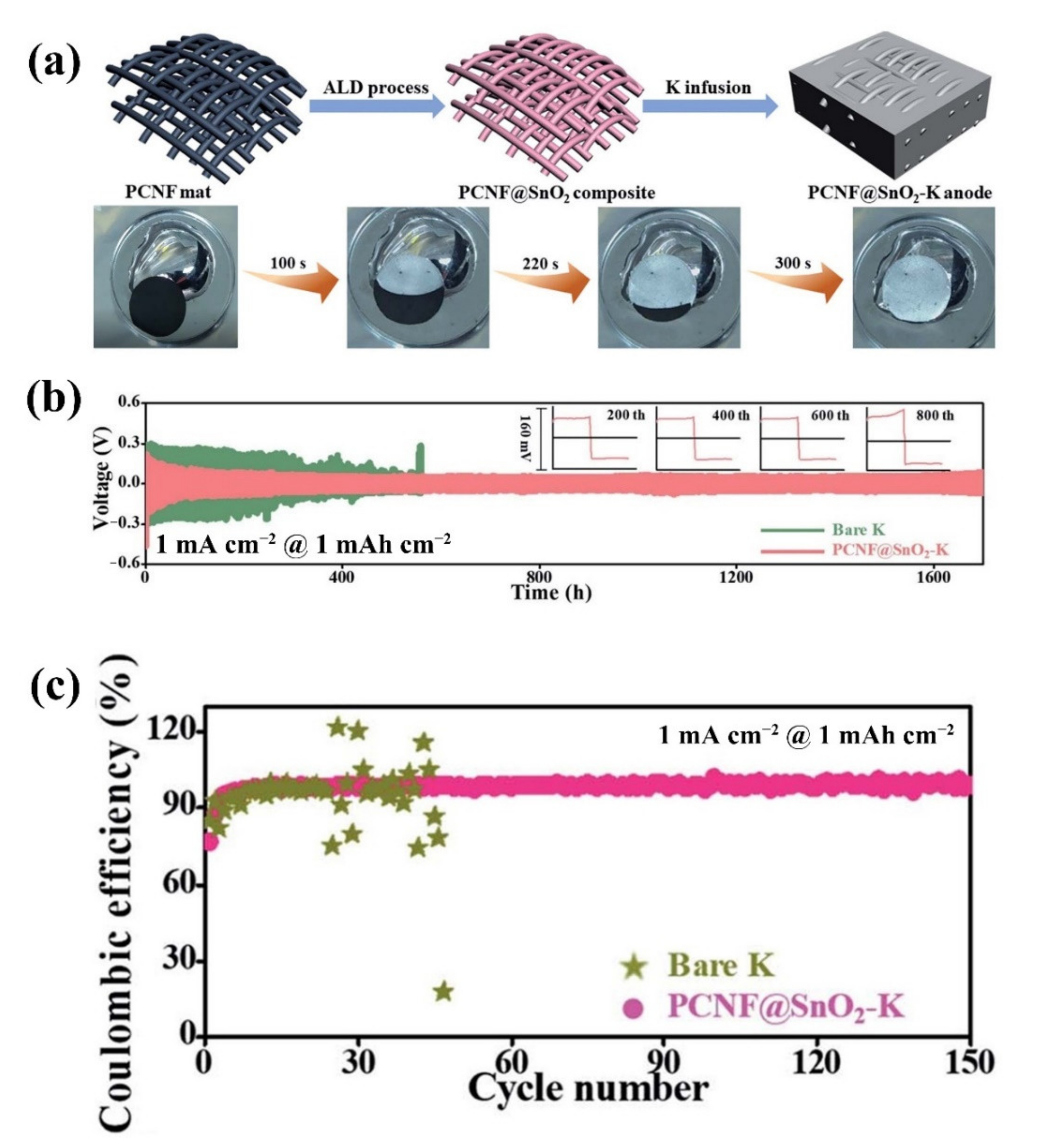
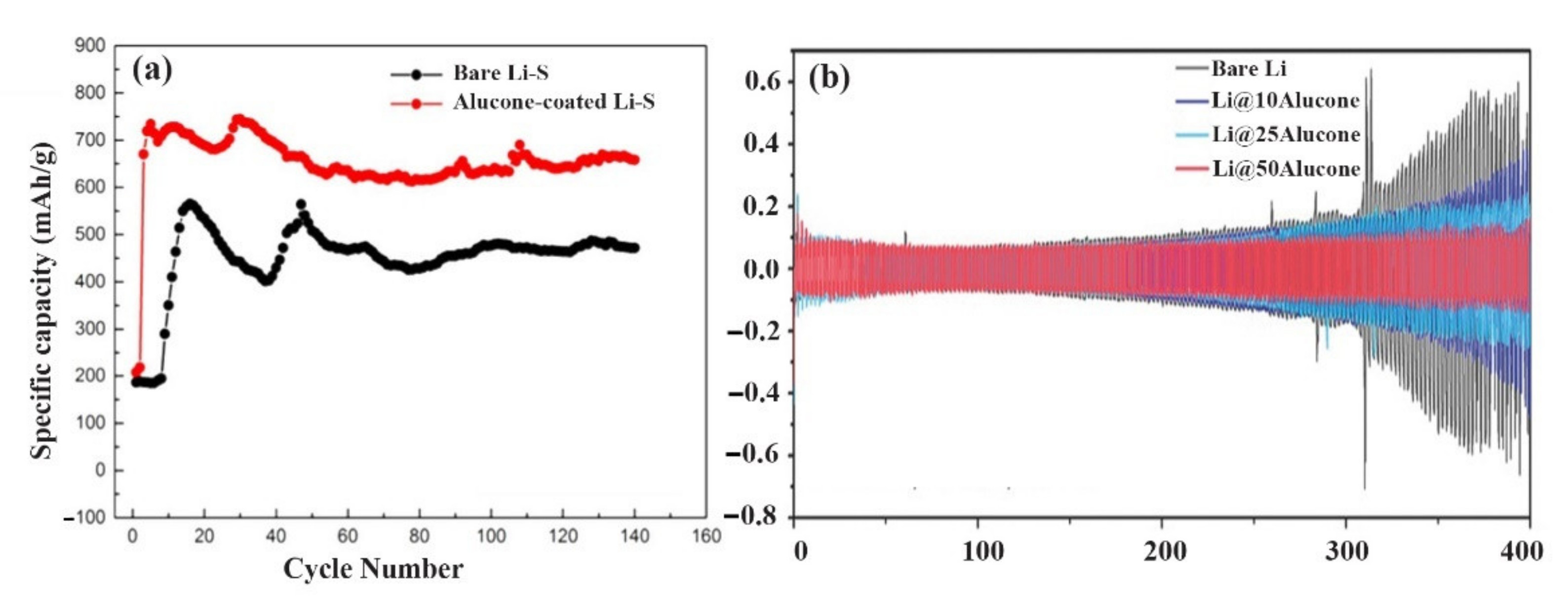
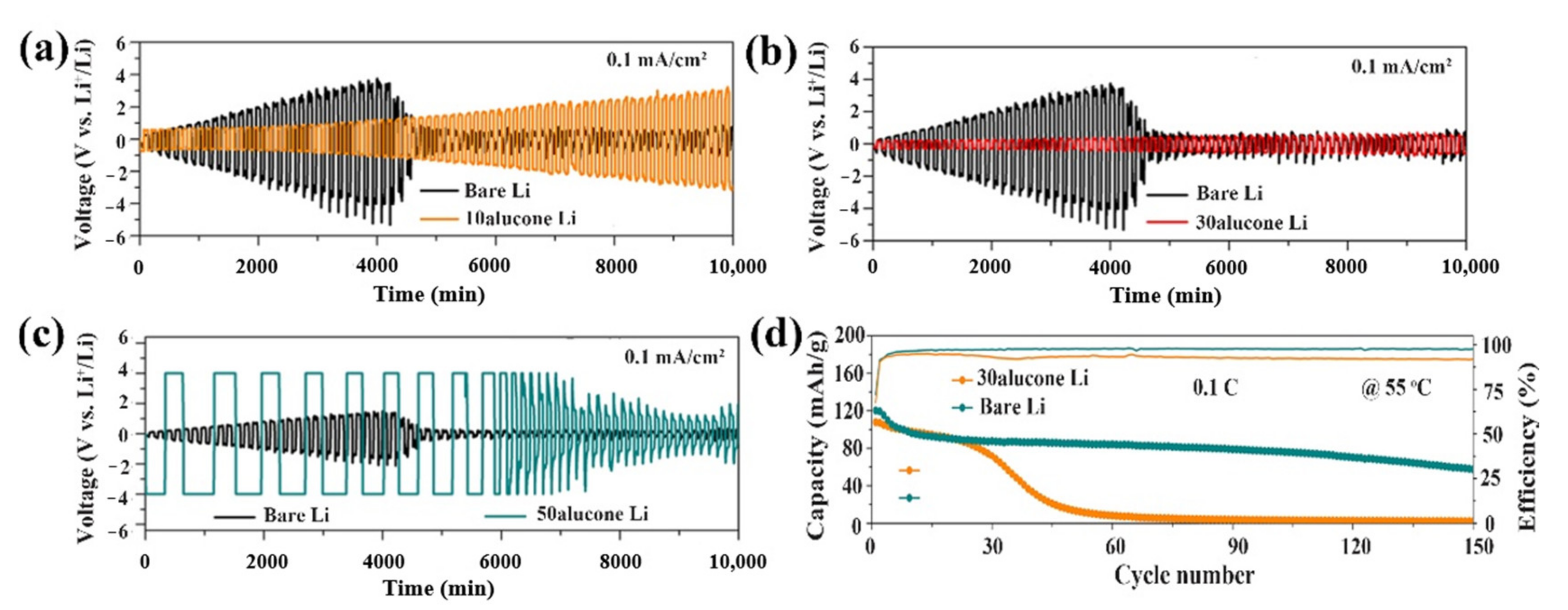

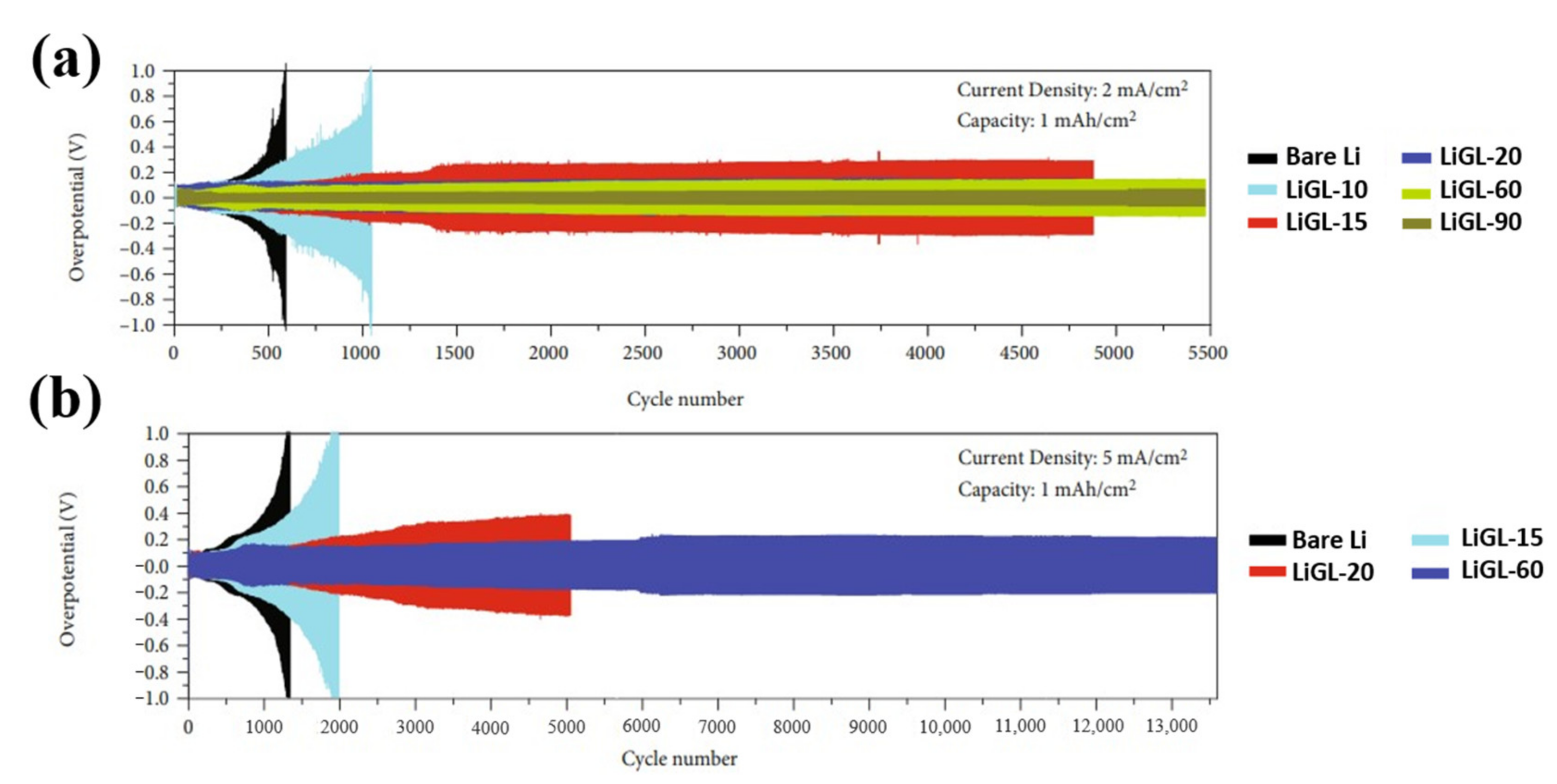
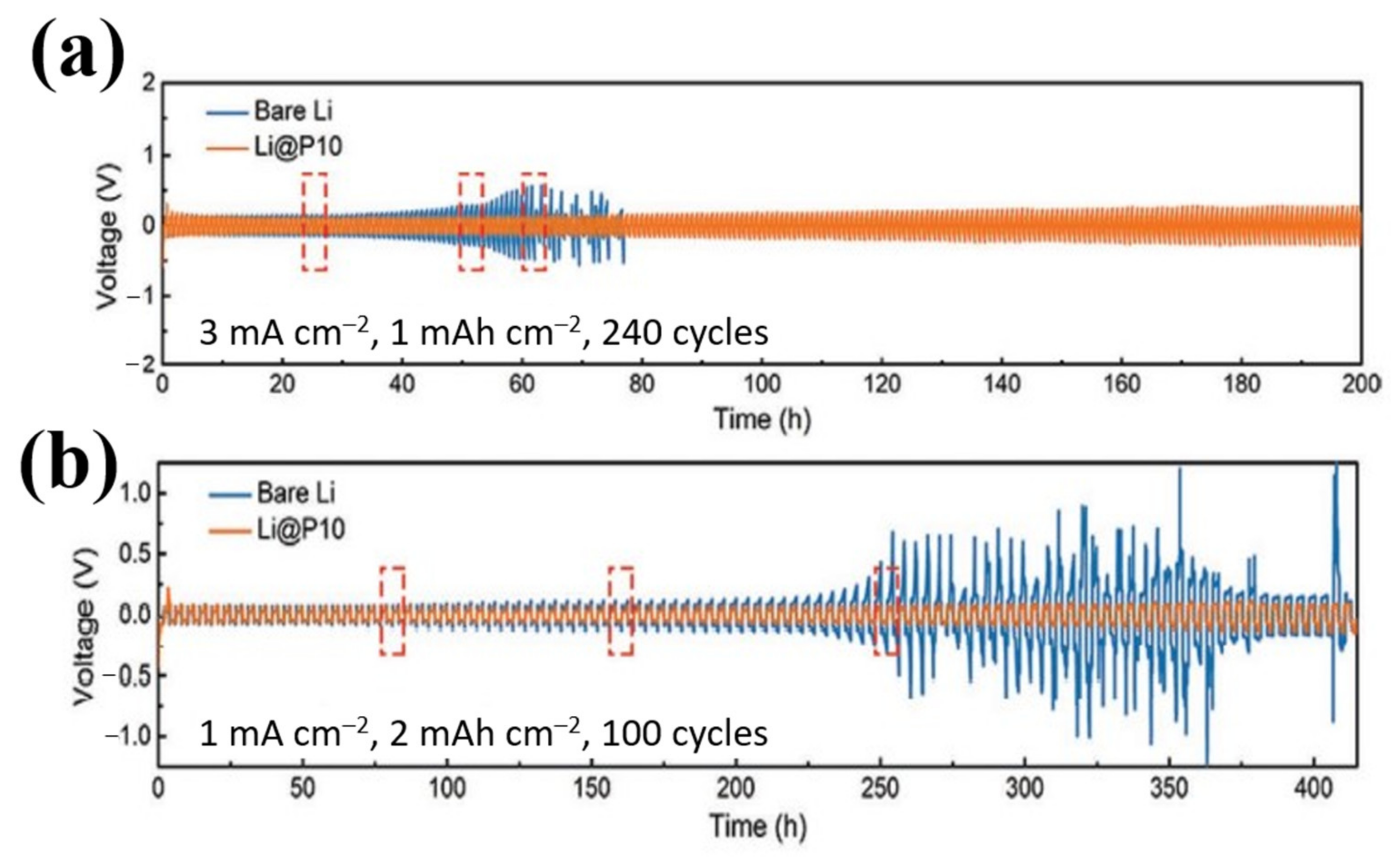
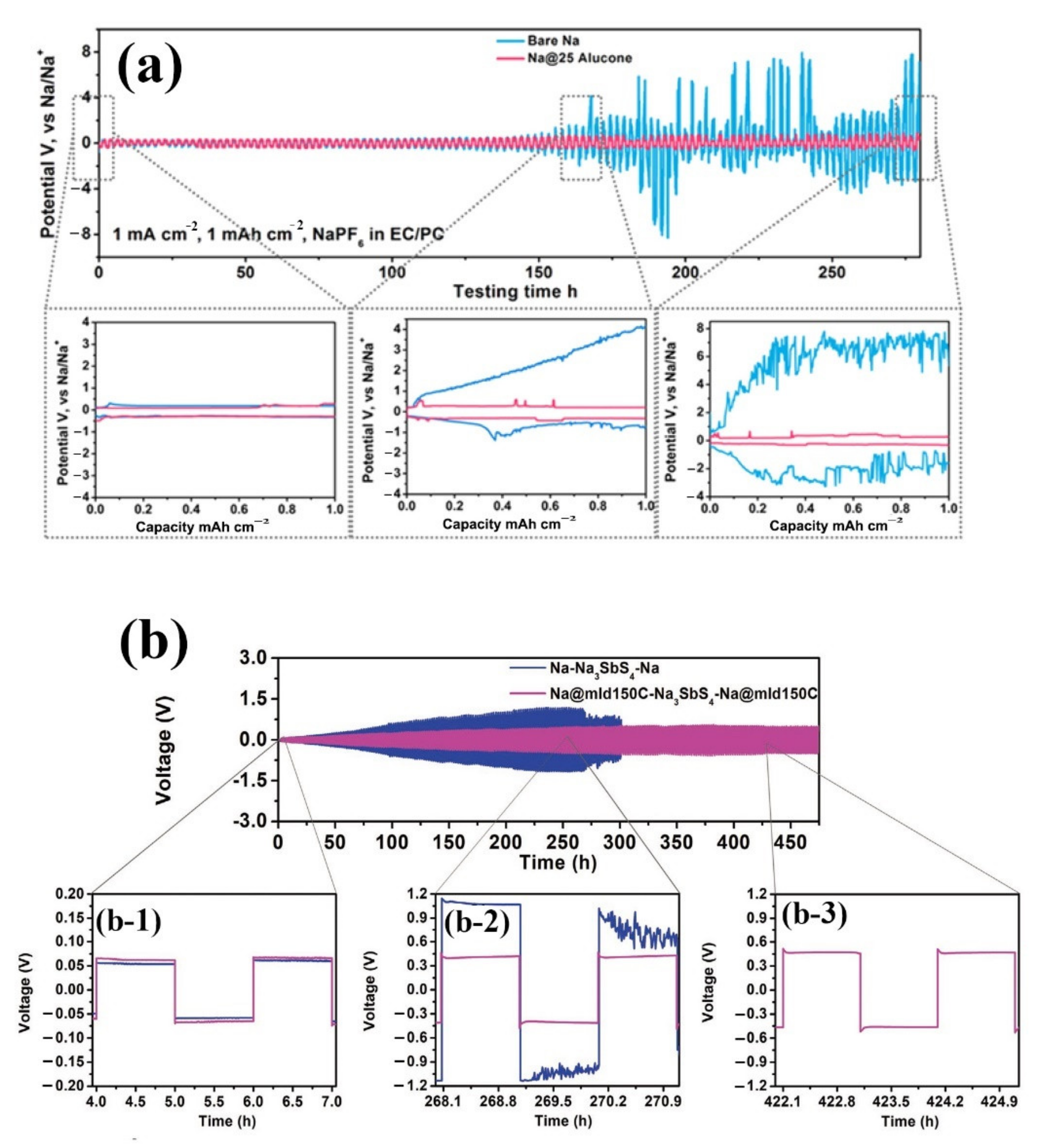
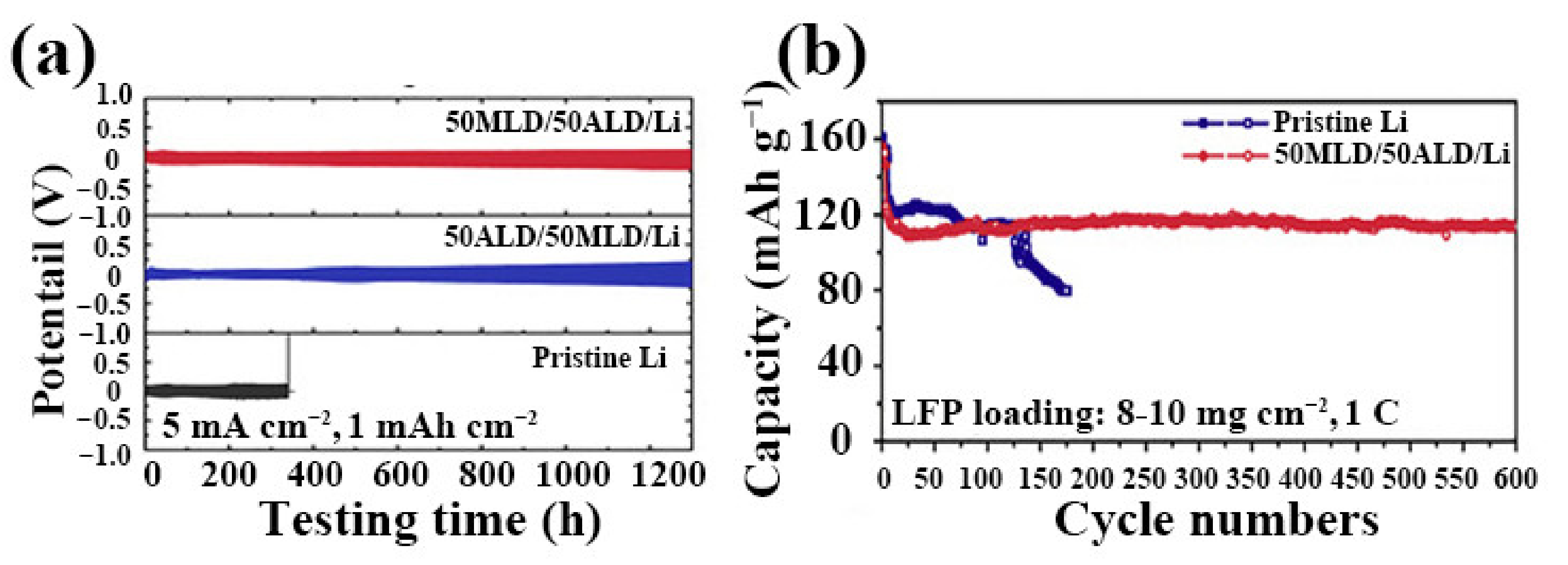
| No. | Material | ALD Process | Temp./(°C) | Thickness and ALD Cycles | Electrochemical Performance | Year | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | Al2O3 | TMA pulse/purge/plasma O2 pulse/purge 0.06 s/30 s/10 s/5 s | 150 | 14 nm and ~117 | ~90% capacity retention after 100 cycles (current density: ~1200 mAhg−1) in Li-O2 cells | 2015 | [30] |
| 2 | Al2O3 | TMA pulse/purge/H2O pulse/purge 0.1 s/30 s/10 s/30 s | 100 | ~2–3 nm and 20 | 1259 cycles (current density: 1 mA cm−2) in symmetric cells | 2015 | [85] |
| 3 | LixAlyS | LTB pulse/purge/H2S pulse/purge 5 s/5 s/5 s/5 sTDMAA pulse/purge/H2S pulse/purge 5 s/5 s/10 s/5 s | 140 80 | 50 nm and 100 | ~80% and ~90% capacity retention in 1 M LiPF6-EC/EMC and 4 M LiTFSI-DME in Li-Cu cells | 2016 | [86] |
| 4 | Al2O3 | TMA pulse/purge/H2O pulse/purge 1 s/5 s/1 s/5 s | 150 | 4 nm and 20 6 nm and 50 | CE > 95% for 50 cycles with 5, 10, and 20 µL electrolyte in Li-Cu cells, 130 cycles before cells failure (current density: 1 mA cm−2) in Li-S cells | 2017 | [87] |
| 5 | ZrO2 | H2O pulse/purge/TDMAZ pulse/purge 0.06 s/10 s/1.5 s/10 s | 145 | ~190 nm and 100 | Maintaining ~150 mAh g−1 (current density: 1.25 mA cm−2) in Li-LTO with SSEs | 2018 | [88] |
| 6 | LiF | LTB pulse/purge/HF pulse/purge 5 s/5 s/1 s/5 s | 135 | 8 nm and ~91 | Stable Coulombic efficiency as high as 99.5% for over 170 cycles (current density: 1 mA cm−2) in Li-Cu cells | 2018 | [89] |
| 7 | AlF3 | TDMAA pulse/purge/HF pulse/purge 8 s/15 s/15 ms/45 s | 150 | ~3 nm and 50 | Maintaining ~600 mAh g−1 at 85 charge/discharge cycles Li-Cu cells | 2020 | [90] |
| 8 | Li3PO4 | LTB pulse/purge/TMP/purge/vacuum 3 s/15 s/2 s/15 s/10 s | 160–180 | 10 nm and 150 | Reaching 1000 h without the change of the overpotential (current density: 1 mA cm−2) in symmetric cells | 2021 | [91] |
| 9 | TiO2 | TTIP pulse/purge/H2O pulse/purge/vacuum 3 s/15 s/2 s/15 s/10 s | 150 | 5 nm and 50 | Stable cycling over 800 cycles (current density: 1 mA cm−2), stable cycling over 500 h (current density: 3 and 10 mA cm−2 | 2021 | [92] |
| 10 | LiAlOx | TMA pulse/purge/H2O pulse/purge 0.1 s/30 s/0.1 s/50 s | 120 | ~50 nm and 200 | The annealed LiAlOx-coated Li extended cycling lifespan twice as much as that of the bare Li in symmetric cells | 2022 | [93] |
| Material | ALD Process | Temp./(°C) | Thickness and ALD Cycles | Electrochemical Performance | Year | Ref. |
|---|---|---|---|---|---|---|
| Al2O3 | TMA pulse/purge/plasma O2 pulse/purge 0.06 s/80 s/20 s/40 s | 75 | 2.8 nm and 25 | Highest stable cycling over 450 h (current density: 0.25 mA cm−2) in symmetric cells | 2016 | [94] |
| Al2O3 | TMA pulse/purge/H2O pulse/purge (Not given) | 85 | 1.4 nm and 103.5 nm and 257 nm and 50 | Highest stable cycling over 500 h (current density: 3 mA cm−2) for of Na@25Al2O3 in symmetric cells | 2017 | [95] |
| NaAlOx | TMA pulse/purge/H2O pulse/purge 0.1 s/30 s/0.1 s/50 s | 65 | ~50 nm and 200 | ~40 mV overpotential beyond 1000 h at 1 and 3 mA cm−2 | 2022 | [96] |
| No. | Material | MLD Process | Temp./(°C) | Thickness and MLD Cycles | Electrochemical Performance | Year | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | AlEG | TMA pulse/purge/EG pulse/purge 1 s/5 s/1 s/15 s | 150 | ~6 nm and 60 | Higher capacity after 140 cycles (1 mA cm−2) in Li-S full cell | 2018 | [113] |
| 2 | AlEG | TMA pulse/purge/EG pulse/purge 0.01 s/40 s/0.01 s/70 s | 120 | Ether: 5 nm and 10 Carbonate: 25 nm and 50 | Stable overpotential beyond 70 h (5 mA cm−2) in symmetric cells with ether electrolyte | 2018 | [114] |
| 3 | AlEG | TMA pulse/purge/EG pulse/purge 0.01 s/40 s/0.01 s/70 s | 85 | ~12 nm and 50 | Overpotential growth after 150 h (0.1 mA cm−2) with Li10SnP2S12 in symmetric cells with SSEs | 2018 | [115] |
| 4 | PU | ED pulse/purge/PDIC pulse/purge 0.1 s/30 s/1 s/30 s | 65 | ~4 nm and 10 | 240 cycles at ~400 mV overpotential (3 mA cm−2) in symmetric cells | 2018 | [116] |
| 5 | ZrEG | ZTB pulse/purge/EG pulse/purge 0.25 s/45 s/0.25 s/45 s | 130 | 5 nm and 25 | Highly air stable and stable overpotential for 120 h (3 mA cm−2) in symmetric cells | 2019 | [117] |
| 6 | LiGL | LTB pulse/purge/GL pulse/purge 3 s/60 s/2 s/60 s | 150 | 162 nm and 60 | Stable overpotential beyond 5500 cycles (1 mA cm−2). Stable overpotential beyond 13,500 cycles (5 mA cm−2) in symmetric cells with ether electrolyte | 2021 | [57] |
| Material | MLD Process | Temp./(°C) | Thickness and MLD Cycles | Electrochemical Performance | Year | Ref. |
|---|---|---|---|---|---|---|
| AlEG | TMA pulse/purge/EG pulse/purge 0.01 s/40 s/0.01 s/70 s | 85 | ~20 nm and 25 | Stable overpotential beyond 300 h (1 mA cm−2) and stable overpotential beyond 120 h (3 mA cm−2) in symmetric cells with carbonate electrolyte | 2017 | [112] |
| AlEG | TMA pulse/purge/EG pulse/purge 0.01 s/40 s/0.01 s/70 s | 85 | >50 nm and 150 | Overpotential ~450 mV beyond 475 h (0.1 mA cm−2) in symmetric cells with Na3SbS4 SSEs | 2020 | [118] |
| Material | MLD Process | Temp./(°C) | Thickness and Total Cycles | Electrochemical Performance | Year | Ref. |
|---|---|---|---|---|---|---|
| AlEG/Al2O3 | TMA pulse/purge/H2O pulse/purge/TMA pulse/purge/EG pulse/purge 0.1 s/20 s/0.1 s/50 s | 120 | 5.55 nm and 50 | Highest stability in MLD inner layers, stable overpotential beyond 1200 h (5 mA cm−2) in symmetric cells | 2019 | [131] |
| HPU-ZnGr | DEZ pulse/purge/ED pulse/purge/PDIC pulse/purge/ED pulse/purge 0.2 s/50 s/0.1 s/50/1 s/50 s/0.1 s/50 s | 65 | 8 nm and 10 | Stable overpotential beyond 2000 h (1 mA cm−2) in symmetric cells | 2021 | [132] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sullivan, M.; Tang, P.; Meng, X. Atomic and Molecular Layer Deposition as Surface Engineering Techniques for Emerging Alkali Metal Rechargeable Batteries. Molecules 2022, 27, 6170. https://doi.org/10.3390/molecules27196170
Sullivan M, Tang P, Meng X. Atomic and Molecular Layer Deposition as Surface Engineering Techniques for Emerging Alkali Metal Rechargeable Batteries. Molecules. 2022; 27(19):6170. https://doi.org/10.3390/molecules27196170
Chicago/Turabian StyleSullivan, Matthew, Peng Tang, and Xiangbo Meng. 2022. "Atomic and Molecular Layer Deposition as Surface Engineering Techniques for Emerging Alkali Metal Rechargeable Batteries" Molecules 27, no. 19: 6170. https://doi.org/10.3390/molecules27196170





