A Novel Utilization of Water Extract of Suaeda Salsa in the Pd/C Catalyzed Suzuki–Miyaura Coupling Reaction
Abstract
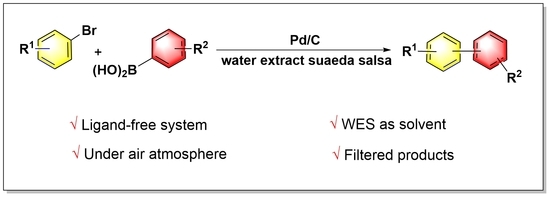
:1. Introduction
2. Results and Discussion
3. Experimental Section
3.1. General Remarks
3.2. The Procedure for Preparing Water Extract of Suaeda Salsa
3.3. General Procedure for the Synthesis of Biaryl and Heteroaryl Compounds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Sheldon, R.A.; Brady, D. Green chemistry, biocatalysis, and the chemical industry of the future. ChemSusChem 2022, 15, e202102628. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, J.B.; Anastas, P.T.; Erythropel, H.C.; Leitner, W. Designing for a green chemistry future. Science 2020, 367, 397. [Google Scholar] [CrossRef] [PubMed]
- Lasso, J.D.; Castillo-Pazos, D.J.; Li, C.-J. Green chemistry meets medicinal chemistry: A perspective on modern metal-free late-stage functionalization reactions. Chem. Soc. Rev. 2021, 50, 10955–10982. [Google Scholar] [PubMed]
- Sabatini, M.T.; Boulton, L.T.; Sneddon, H.F.; Sheppard, T.D. A green chemistry perspective on catalytic amide bond formation. Nat. Catal. 2019, 2, 10–17. [Google Scholar] [CrossRef]
- Hooshmand, S.E.; Heidari, B.; Sedghi, R.; Varma, R.S. Recent advances in the Suzuki–Miyaura cross-coupling reaction using efficient catalysts in eco-friendly media. Green Chem. 2019, 21, 381–405. [Google Scholar] [CrossRef]
- Simon, M.-O.; Li, C.-J. Green chemistry oriented organic synthesis in water. Chem. Soc. Rev. 2012, 41, 1415–1427. [Google Scholar] [CrossRef]
- Mohebat, R.; Yazdani-Elah-Abadi, A. Caffeine catalyzed green synthesis of novel benzo[a][1,3]oxazino[6,5-c]phenazines via a one-pot multi-component sequential protocol in a basic ionic liquid. Chin. Chem. Lett. 2017, 28, 1340–1344. [Google Scholar] [CrossRef]
- Fernandes, R.R.; Lasri, J.; da Silva, M.F.C.G.; Palavra, A.M.F.; da Silva, J.A.L.; da Silva, J.J.R.F.; Pombeiro, A.J.L. Oxadiazoline and Ketoimine Palladium(II) complexes as highly efficient catalysts for Suzuki–Miyaura cross-coupling reactions in supercritical carbon dioxide. Adv. Synth. Catal. 2011, 353, 1153–1160. [Google Scholar] [CrossRef]
- Peng, L.; Hu, Z.; Lu, Q.; Tang, Z.; Jiao, Y.; Xu, X. DESs: Green solvents for transition metal catalyzed organic reactions. Chin. Chem. Lett. 2019, 30, 2151–2156. [Google Scholar] [CrossRef]
- Lakshmidevi, J.; Appa, R.M.; Naidu, B.R.; Prasad, S.S.; Sarma, L.S.; Venkateswarlu, K. WEPA: A bio-derived medium for added base, π-acid and ligand free Ullmann coupling of aryl halides using Pd(OAc)2. Chem. Commun. 2018, 54, 12333–12336. [Google Scholar] [CrossRef]
- Saikia, B.; Borah, P. A new avenue to the Dakin reaction in H2O2–WERSA. RSC Adv. 2015, 5, 105583–105586. [Google Scholar] [CrossRef]
- Surneni, N.; Barua, N.C.; Saikia, B. Application of natural feedstock extract: The Henry reaction. Tetrahedron Lett. 2016, 57, 2814–2817. [Google Scholar] [CrossRef]
- Konwar, M.; Ali, A.A.; Sarma, D. A green protocol for peptide bond formation in WEB. Tetrahedron Lett. 2016, 57, 2283–2285. [Google Scholar] [CrossRef]
- Basumatary, S.; Nath, B.; Kalita, P. Application of agro-waste derived materials as heterogeneous base catalysts for biodiesel synthesis. J. Renew. Sustain. Energy 2018, 10, 043105. [Google Scholar] [CrossRef]
- Beletskaya, I.P.; Alonso, F.; Tyurin, V. The Suzuki-Miyaura reaction after the Nobel prize. Coord. Chem. Rev. 2019, 385, 137–173. [Google Scholar] [CrossRef]
- Liu, C.; Li, X. Oxygen-promoted Suzuki–Miyaura reaction for efficient construction of biaryls. Chem. Rec. 2016, 16, 84–97. [Google Scholar] [CrossRef] [PubMed]
- Biffis, A.; Centomo, P.; Del Zotto, A.; Zecca, M. Pd Metal catalysts for cross-couplings and related reactions in the 21st Century: A Critical Review. Chem. Rev. 2018, 118, 2249–2295. [Google Scholar] [CrossRef]
- Liu, C.; Liu, C.; Li, X.-M.; Gao, Z.-M.; Jin, Z.-L. Oxygen-promoted Pd/C-catalyzed Suzuki–Miyaura reaction of potassium aryltrifluoroborates. Chin. Chem. Lett. 2016, 27, 631–634. [Google Scholar] [CrossRef]
- Mao, Z.; Gu, H.; Lin, X. Recent advances of Pd/C-catalyzed reactions. Catalysts 2021, 11, 1078. [Google Scholar] [CrossRef]
- Boruah, P.R.; Ali, A.A.; Saikia, B.; Sarma, D. A novel green protocol for ligand free Suzuki–Miyaura cross-coupling reactions in WEB at room temperature. Green Chem. 2015, 17, 1442–1445. [Google Scholar] [CrossRef]
- Boruah, P.R.; Ali, A.A.; Chetia, M.; Saikia, B.; Sarma, D. Pd(OAc)2 in WERSA: A novel green catalytic system for Suzuki–Miyaura cross-coupling reactions at room temperature. Chem. Commun. 2015, 51, 11489–11492. [Google Scholar] [CrossRef] [PubMed]
- Konwar, M.; Boruah, P.R.; Saikia, P.J.; Khupse, N.D.; Sarma, D. ESP-promoted Suzuki-Miyaura cross-coupling and peptide bond formation reactions in water at room temperature. ChemistrySelect 2017, 2, 4983–4987. [Google Scholar] [CrossRef]
- Sarmah, M.; Dewan, A.; Mondal, M.; Thakur, A.J.; Bora, U. Analysis of the water extract of waste papaya bark ash and its implications as an in situ base in the ligand-free recyclable Suzuki–Miyaura coupling reaction. RSC Adv. 2016, 6, 28981–28985. [Google Scholar] [CrossRef]
- Sarmah, M.; Dewan, A.; Thakur, A.J.; Bora, U. Extraction of Base from Eichhornia crassipes and Its Implication in Palladium-Catalyzed Suzuki Cross-Coupling Reaction. ChemistrySelect 2017, 2, 7091–7095. [Google Scholar] [CrossRef]
- Lennox, A.J.J.; Lloyd-Jones, G.C. Transmetalation in the Suzuki–Miyaura coupling: The fork in the trail. Angew. Chem., Int. Ed. 2013, 52, 7362–7370. [Google Scholar] [CrossRef] [PubMed]
- Amatore, C.; Le Duc, G.; Jutand, A. Mechanism of Palladium-catalyzed Suzuki–Miyaura reactions: Multiple and antagonistic roles of anionic “Bases” and their countercations. Chem.–Eur. J. 2013, 19, 10082–10093. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, X.; Gao, Z.; Wang, X.; Jin, Z. In situ-generated nano-palladium-catalyzed ligand-free Suzuki–Miyaura reaction of potassium aryltrifluoroborates at room temperature. Tetrahedron 2015, 71, 3954–3959. [Google Scholar] [CrossRef]






| Entry | Pd Catalyst | Loading (mol%) | Temperature (°C) | Yield (%) |
|---|---|---|---|---|
| 1 | Pd/C | 1 | 25 | trace |
| 2 | Pd/C | 1 | 50 | 17 |
| 3 | Pd/C | 1 | 80 | 71 |
| 4 | Pd/C | 1 | 100 | 94 |
| 5 | Pd/C | 0.5 | 100 | 95 |
| 6 | Pd/C | 0.2 | 100 | 94 |
| 7 | Pd/C | 0.1 | 100 | 48 |
| 8 | Pd/Al2O3 | 0.2 | 100 | 16 |
| 9 | Pd/CaCO3 | 0.2 | 100 | 91 |
| 10 | Pd/BaSO4 | 0.2 | 100 | 92 |

| Entry | R1 | R2 | Time (h) | Number | Yield (%) |
|---|---|---|---|---|---|
| 1 | 4-CN | H | 1 | 3e | 92 a 94 b |
| 2 | 4-NO2 | H | 1 | 3f | 89 a |
| 3 | 4-CHO | H | 2 | 3g | 91 a |
| 4 | 4-COCH3 | H | 2 | 3d | 94 a |
| 5 | 4-OH | H | 1 | 3h | 87 a |
| 6 | 4-COOH | H | 8 | 3i | 36 a |
| 7 | 4-OCH3 | H | 1 | 3j | 67 b |
| 8 | 4-CH3 | H | 2 | 3k | 13 b |
| 9 | 3-CN | H | 1 | 3l | 90 b |
| 10 | 3-NO2 | H | 2 | 3m | 82 b |
| 11 | 3-OCH3 | H | 2 | 3n | 63 b |
| 12 | 2-CN | H | 1 | 3o | 88 b |
| 13 | 2-OCH3 | H | 2 | 3p | 72 b |
| 14 | 4-CN | 4-F | 1 | 3q | 88 a |
| 15 | 4-CN | 4-OCH3 | 1 | 3r | 90 a |
| 16 | 4-CN | 3,4-(OCH3)2 | 2 | 3s | 60 b |
| 17 | 4-CN | 4-CH3 | 2 | 3b | 88 b |
| 18 | 4-CHO | 4-CH3 | 1 | 3t | 92 a |
| 19 | 4-CHO | 4-OCH3 | 1 | 3u | 90 a |
| 20 | 4-CHO | 4-F | 2 | 3a | 82 b |
| 21 | 4-OCH3 | 4-CH3 | 4 | 3v | 71 b |
| 22 | 4-OCH3 | 4-OCH3 | 4 | 3w | 68 b |
| 23 | 4-OCH3 | 3,4-(OCH3)2 | 4 | 3c | 85 b |
| 24 | 4-F | 4-OCH3 | 2 | 3x | 72 b |
| 25 | 4-Cl | 4-OCH3 | 2 | 3y | 70 b |
| 26 | 2-OCH3 | 2-CH3 | 2 | 3z | 30 b |
| 27 | 2-CN | 2-CH3 | 2 | 3aa | 36 b |
| 28 | 4-CN | H | 8 | 3e | trace c |
| 29 | 4-CN | H | 4 | 3e | 49 d |
| 30 | 4-CN | H | 4 | 3e | trace e |

| Entry | Time (h) | Yield (%) |
|---|---|---|
| 1 | 1.0 | 95 |
| 2 | 1.0 | 90 |
| 3 | 1.0 | 83 |
| 4 | 2.0 | 71 |
| 5 | 8.0 | 52 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, C.; Zhang, H.; Chen, Z.; Gao, J.; Yang, M.; Yuan, Z.; Li, X. A Novel Utilization of Water Extract of Suaeda Salsa in the Pd/C Catalyzed Suzuki–Miyaura Coupling Reaction. Molecules 2022, 27, 6623. https://doi.org/10.3390/molecules27196623
Ren C, Zhang H, Chen Z, Gao J, Yang M, Yuan Z, Li X. A Novel Utilization of Water Extract of Suaeda Salsa in the Pd/C Catalyzed Suzuki–Miyaura Coupling Reaction. Molecules. 2022; 27(19):6623. https://doi.org/10.3390/molecules27196623
Chicago/Turabian StyleRen, Changyue, Hang Zhang, Zhengjun Chen, Jie Gao, Mingyan Yang, Zeli Yuan, and Xinmin Li. 2022. "A Novel Utilization of Water Extract of Suaeda Salsa in the Pd/C Catalyzed Suzuki–Miyaura Coupling Reaction" Molecules 27, no. 19: 6623. https://doi.org/10.3390/molecules27196623