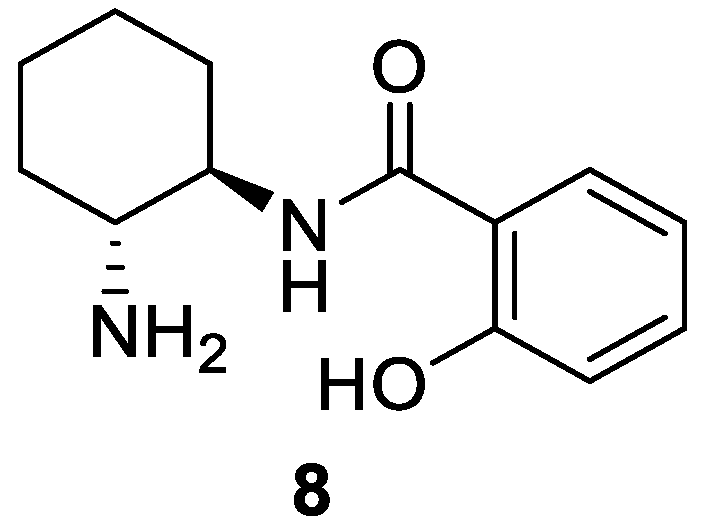
Asymmetric Conjugate Addition of Ketones to Maleimides Organocatalyzed by a Chiral Primary Amine-Salicylamide
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. Enantioselective Michael Addition of Ketones to Maleimides: General Procedure
3.3. Scaled-Up Enantioselective Michael Addition Reaction of Cyclohexanone and N-Phenylmaleimide
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Barker, D.; Lin, D.H.S.; Carland, J.E.; Chu, C.P.Y.; Chebib, M.; Brimble, M.A.; Savage, G.P.; McLeod, M.D. Methyllycaconitine analogues have mixed antagonist effects at nicotinic acetylcholine receptors. Bioorg. Med. Chem. 2005, 13, 4565–4575. [Google Scholar] [CrossRef] [PubMed]
- Uddin, J.; Ueda, K.; Siwu, E.R.O.; Kita, M.; Uemura, D. Cytotoxic labdane alkaloids from an ascidian Lissoclinum sp.: Isolation, structure elucidation, and structure-activity relationship. Bioorg. Med. Chem. 2006, 14, 6954–6961. [Google Scholar] [CrossRef] [PubMed]
- Isaka, M.; Rugseree, N.; Maithip, P.; Kongsaeree, P.; Prabpai, S.; Thebtaranonth, Y. Hirsutellones A-E, antimycobacterial alkaloids from the insect pathogenic fungus Hirsutella nivea body centered cubic 2594. Tetrahedron 2005, 61, 5577–5583. [Google Scholar] [CrossRef]
- Freiberg, C.; Brunner, N.A.; Schiffer, G.; Lampe, T.; Pohlmann, J.; Brands, M.; Raabe, M.; Haebich, D.; Ziegelbauer, K. Identification and characterization of the first class of potent bacterial Acetyl-CoA carboxylase inhibitors with antibacterial activity. J. Biol. Chem. 2004, 279, 26066–26073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ando, Y.; Fuse, E.; Figg, W.D. Thalidomide metabolism by the CYP2C subfamily. Clin. Cancer Res. 2002, 8, 1964–1973. [Google Scholar] [PubMed]
- Curtin, M.L.; Garland, R.B.; Heyman, H.R.; Frey, R.R.; Michaelides, M.R.; Li, J.; Pease, L.J.; Glaser, K.B.; Marcotte, P.A.; Davidsen, S.K. Succinimide hydroxamic acids as potent inhibitors of histone deacetylase (HDAC). Bioorg. Med. Chem. Lett. 2002, 12, 2919–2923. [Google Scholar] [CrossRef]
- Ahmed, S. Molecular modelling study of pyrrolidine-2,5-dione based aromatase inhibitors and other known inhibitors. Drug Des. Discov. 1996, 14, 77–89. [Google Scholar] [PubMed]
- Ballini, R.; Bosica, G.; Cioci, G.; Fiorini, D.; Petrini, M. Conjugate addition of nitroalkanes to N-substituted maleimides. Synthesis of 3-alkylsuccinimides and pyrrolidines. Tetrahedron 2003, 59, 3603–3608. [Google Scholar] [CrossRef]
- Vo-Hoang, Y.; Gasse, C.; Vidal, M.; Garbay, C.; Galons, H. Efficient synthesis of N-benzyl-3-aminopyrrolidine-2,5-dione and N-benzyl-3-aminopyrrolidin-2-one. Tetrahedron Lett. 2004, 45, 3603–3605. [Google Scholar] [CrossRef]
- Nöth, J.; Frankowski, K.J.; Neuenswander, B.; Aubé, J.; Reiser, O. Efficient synthesis of γ-lactams by a tandem reductive amination/lactamization sequence. J. Comb. Chem. 2008, 10, 456–459. [Google Scholar] [CrossRef]
- Fenster, E.; Hill, D.; Reiser, O.; Aube, J. Automated three-component synthesis of a library of γ-lactams. Beilstein J. Org. Chem. 2012, 8, 1804–1813. [Google Scholar] [CrossRef] [Green Version]
- Chauhan, P.; Kaur, J.; Chimni, S.S. Asymmetric organocatalytic addition reactions of maleimides: A promising approach towards the synthesis of chiral succinimide derivatives. Chem. Asian J. 2013, 8, 328–346. [Google Scholar] [CrossRef]
- Zhao, G.-L.; Xu, Y.; Sundén, H.; Eriksson, L.; Sayah, M.; Cordova, A. Organocatalytic enantioselective conjugate addition of aldehydes to maleimides. Chem. Commun. 2007, 734–735. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.-F.; Peng, L.; Wang, L.-l.; Wang, L.-X.; Xu, X.-Y. Chiral primary amine thiourea promoted highly enantioselective Michael reactions of isobutylaldehyde with maleimides. Tetrahedron 2010, 66, 8928–8932. [Google Scholar] [CrossRef]
- Xue, F.; Liu, L.; Zhang, S.; Duan, W.; Wang, W. A simple primary amine thiourea catalyzed highly enantioselective conjugate addition of α,α-disubstituted aldehydes to maleimides. Chem. Eur. J. 2010, 16, 7979–7982. [Google Scholar] [CrossRef]
- Yu, F.; Jin, Z.; Huang, H.; Ye, T.; Liang, X.; Ye, J. A highly efficient asymmetric Michael addition of α,α-disubstituted aldehydes to maleimides catalyzed by primary amine thiourea salt. Org. Biomol. Chem. 2010, 8, 4767–4774. [Google Scholar] [CrossRef]
- Ma, Z.-w.; Liu, Y.-x.; Li, P.-l.; Ren, H.; Zhu, Y.; Tao, J.-c. A highly efficient large-scale asymmetric Michael addition of isobutyraldehyde to maleimides promoted by a novel multifunctional thiourea. Tetrahedron Asymmetry 2011, 22, 1740–1748. [Google Scholar] [CrossRef]
- Miura, T.; Masuda, A.; Ina, M.; Nakashima, K.; Nishida, S.; Tada, N.; Itoh, A. Asymmetric Michael reactions of α,α-disubstituted aldehydes with maleimides using a primary amine thiourea organocatalyst. Tetrahedron Asymmetry 2011, 22, 1605–1609. [Google Scholar] [CrossRef]
- Miura, T.; Nishida, S.; Masuda, A.; Tada, N.; Itoh, A. Asymmetric Michael additions of aldehydes to maleimides using a recyclable fluorous thiourea organocatalyst. Tetrahedron Lett. 2011, 52, 4158–4160. [Google Scholar] [CrossRef]
- Avila, A.; Chinchilla, R.; Nájera, C. Enantioselective Michael addition of α,α-disubstituted aldehydes to maleimides organocatalyzed by chiral primary amine-guanidines. Tetrahedron Asymmetry 2012, 23, 1625–1627. [Google Scholar] [CrossRef]
- Nugent, T.C.; Sadiq, A.; Bibi, A.; Heine, T.; Zeonjuk, L.L.; Vankova, N.; Bassil, B.S. Noncovalent bifunctional organocatalysts: Powerful tools for contiguous quaternary-tertiary stereogenic carbon formation, scope, and origin of enantioselectivity. Chem. Eur. J. 2012, 18, 4088–4098. [Google Scholar] [CrossRef]
- Avila, A.; Chinchilla, R.; Gómez-Bengoa, E.; Nájera, C. Enantioselective Michael addition of aldehydes to maleimides organocatalyzed by chiral 1,2-diamines: An experimental and theoretical study. Tetrahedron Asymmetry 2013, 24, 1531–1535. [Google Scholar] [CrossRef]
- Kozma, V.; Szőllősi, G. Enantioselective Michael addition of aldehydes to maleimides catalyzed by surface-adsorbed natural amino acids. Catal. Sci. Technol. 2022, 12, 4709–4726. [Google Scholar] [CrossRef]
- Avila, A.; Chinchilla, R.; Gómez-Bengoa, E.; Nájera, C. Enantioselective synthesis of succinimides by Michael addition of aldehydes to maleimides organocatalyzed by chiral primary amine-guanidines. Eur. J. Org. Chem. 2013, 2013, 5085–5092. [Google Scholar] [CrossRef]
- Durmaz, M.; Sirit, A. Calixarene-based chiral primary amine thiourea promoted highly enantioselective asymmetric Michael reactions of α,α-disubstituted aldehydes with maleimides. Tetrahedron Asymmetry 2013, 24, 1443–1448. [Google Scholar] [CrossRef]
- Kokotos, C.G. An asymmetric Michael addition of α,α-disubstituted aldehydes to maleimides leading to a one-pot enantioselective synthesis of lactones catalyzed by amino acids. Org. Lett. 2013, 15, 2406–2409. [Google Scholar] [CrossRef]
- Qiao, Y.; Headley, A.D. A simple and highly effective water-compatible organocatalytic system for asymmetric direct Michael reactions of linear aldehydes to maleimides. Green Chem. 2013, 15, 2690–2694. [Google Scholar] [CrossRef]
- Yang, W.; Jiang, K.-Z.; Lu, X.; Yang, H.-M.; Li, L.; Lu, Y.; Xu, L.-W. Molecular assembly of an achiral phosphine and a chiral primary amine: A highly efficient supramolecular catalyst for the enantioselective Michael reaction of aldehydes with maleimides. Chem. Asian J. 2013, 8, 1182–1190. [Google Scholar] [CrossRef]
- Flores-Ferrándiz, J.; Chinchilla, R. Solvent-dependent enantioswitching in the Michael addition of α,α-disubstituted aldehydes to maleimides organocatalyzed by mono-N-BOC-protected cyclohexa-1,2-diamines. Tetrahedron Asymmetry 2014, 25, 1091–1094. [Google Scholar] [CrossRef] [Green Version]
- Song, Z.T.; Zhang, T.; Du, H.L.; Ma, Z.W.; Zhang, C.H.; Tao, J.C. Highly enantioselective Michael addition promoted by a new diterpene-derived bifunctional thiourea catalyst: A doubly stereocontrolled approach to chiral succinimide derivatives. Chirality 2014, 26, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Flores-Ferrándiz, J.; Fiser, B.; Gómez-Bengoa, E.; Chinchilla, R. Solvent-induced reversal of enantioselectivity in the synthesis of succinimides by the addition of aldehydes to maleimides catalysed by carbamate-monoprotected 1,2-diamines. Eur. J. Org. Chem. 2015, 2015, 1218–1225. [Google Scholar] [CrossRef]
- Avila-Ortiz, C.G.; Díaz-Corona, L.; Jiménez-González, E.; Juaristi, E. Asymmetric Michael addition organocatalyzed by α,β-dipeptides under solvent-free reaction conditions. Molecules 2017, 22, 1328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colin, O.; Boufroura, H.; Thomassigny, C.; Perato, S.; Gaucher, A.; Marrot, J.; Prim, D. Modular Urea-Based Catalytic Platforms Bearing Flexible Pyridylmethylamine and Rigid Pyridyl-Imidazolidine Fragments. Eur. J. Org. Chem. 2017, 2017, 746–752. [Google Scholar] [CrossRef]
- Flores-Ferrándiz, J.; Chinchilla, R. Organocatalytic enantioselective conjugate addition of aldehydes to maleimides in deep eutectic solvents. Tetrahedron Asymmetry 2017, 28, 302–306. [Google Scholar] [CrossRef] [Green Version]
- Kochetkov, S.V.; Kucherenko, A.S.; Zlotin, S.G. Asymmetric Michael addition of aldehydes to maleimides in primary amine-based aqueous ionic liquid-supported recyclable catalytic system. Mendeleev Commun. 2017, 27, 473–475. [Google Scholar] [CrossRef]
- Ma, Z.-W.; Liu, X.-F.; Liu, J.-T.; Liu, Z.-J.; Tao, J.-C. Highly enantioselective Michael addition of α,α-disubstituted aldehydes to maleimides catalyzed by new primary amine-squaramide bifunctional organocatalysts. Tetrahedron Lett. 2017, 58, 4487–4490. [Google Scholar] [CrossRef]
- De Simone, N.A.; Meninno, S.; Talotta, C.; Gaeta, C.; Neri, P.; Lattanzi, A. Solvent-free enantioselective Michael reactions catalyzed by a calixarene-based primary amine thiourea. J. Org. Chem. 2018, 83, 10318–10325. [Google Scholar] [CrossRef] [PubMed]
- Schiza, A.; Spiliopoulou, N.; Shahu, A.; Kokotos, C.G. Combining organocatalysis with photoorganocatalysis: Photocatalytic hydroacylation of asymmetric organocatalytic Michael addition products. New J. Chem. 2018, 42, 18844–18849. [Google Scholar] [CrossRef]
- Szollosi, G.; Kozma, V. Design of heterogeneous organocatalyst for the asymmetric Michael addition of aldehydes to maleimides. ChemCatChem 2018, 10, 4362–4368. [Google Scholar] [CrossRef]
- Torregrosa-Chinillach, A.; Moragues, A.; Pérez-Furundarena, H.; Chinchilla, R.; Gómez-Bengoa, E.; Guillena, G. Enantioselective Michael addition of aldehydes to maleimides organocatalyzed by a chiral primary amine-salicylamide. Molecules 2018, 23, 3299. [Google Scholar] [CrossRef]
- Landeros, J.M.; Suchy, L.; Avila-Ortiz, C.G.; Maulide, N.; Juaristi, E. Dendrimeric α,β-dipeptidic conjugates as organocatalysts in the asymmetric Michael addition reaction of isobutyraldehyde to N-phenylmaleimides. Monatsh. Chem. 2019, 150, 777–788. [Google Scholar] [CrossRef]
- Du, Z.-H.; Qin, W.-J.; Tao, B.-X.; Yuan, M.; Da, C.-S. N-Primary-amine tetrapeptide-catalyzed highly asymmetric Michael addition of aliphatic aldehydes to maleimides. Org. Biomol. Chem. 2020, 18, 6899–6904. [Google Scholar] [CrossRef] [PubMed]
- Kozma, V.; Fueloep, F.; Szollosi, G. 1,2-Diamine-derived (thio)phosphoramide organocatalysts in asymmetric Michael additions. Adv. Synth. Catal. 2020, 362, 2444–2458. [Google Scholar] [CrossRef]
- Sadiq, A.; Nugent, T.C. Catalytic Access to Succinimide Products Containing Stereogenic Quaternary Carbons. ChemistrySelect 2020, 5, 11934–11938. [Google Scholar] [CrossRef]
- Torregrosa-Chinillach, A.; Sanchez-Laó, A.; Santagostino, E.; Chinchilla, R. Organocatalytic asymmetric conjugate addition of aldehydes to maleimides and nitroalkenes in deep eutectic solvents. Molecules 2019, 24, 4058. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Zhang, M.-M.; Zhang, S.; Xu, Z.-A.; Li, H.; Yu, X.-H.; Wang, W. Chiral pyrrolidine sulfonamide catalyzed enantioselective Michael addition of cyclohexanones to maleimides. Synlett 2011, 2011, 473–476. [Google Scholar] [CrossRef]
- Yu, F.; Sun, X.; Jin, Z.; Wen, S.; Liang, X.; Ye, J. Enantioselective Michael addition of ketones to maleimides catalyzed by bifunctional monosulfonyl DPEN salt. Chem. Commun. 2010, 46, 4589–4591. [Google Scholar] [CrossRef]
- Muramulla, S.; Ma, J.-A.; Zhao, J.C.-G. Michael addition of ketones and aldehydes to maleimides catalyzed by modularly designed organocatalysts. Adv. Synth. Catal. 2013, 355, 1260–1264. [Google Scholar] [CrossRef]
- Nakashima, K.; Kawada, M.; Hirashima, S.-I.; Kato, M.; Koseki, Y.; Miura, T. Asymmetric conjugate addition of ketones to maleimides using diaminomethyleneindenedione organocatalyst. Synlett 2015, 26, 1248–1252. [Google Scholar] [CrossRef] [Green Version]
- Nakashima, K.; Kawada, M.; Hirashima, S.-I.; Kosugi, A.; Kato, M.; Yoshida, A.; Koseki, Y.; Miura, T. Stereoselective conjugate addition of carbonyl compounds to maleimides using a diaminomethyleneindenedione organocatalyst. Tetrahedron Asymmetry 2016, 27, 888–895. [Google Scholar] [CrossRef]
- Jan, M.S.; Ahmad, S.; Hussain, F.; Ahmad, A.; Mahmood, F.; Rashid, U.; Abid, O.-U.-R.; Ullah, F.; Ayaz, M.; Sadiq, A. Design, synthesis, in-vitro, in-vivo and in-silico studies of pyrrolidine-2,5-dione derivatives as multitarget anti-inflammatory agents. Eur. J. Med. Chem. 2020, 186, 111863. [Google Scholar] [CrossRef] [PubMed]


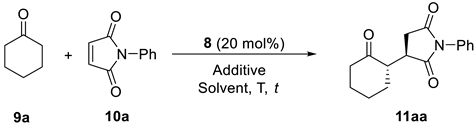 | |||||||
|---|---|---|---|---|---|---|---|
| Entry | Additive (mol%) a | Solvent a | T (°C) | t (d) | Conv. (%) b | drc | ee (%) d |
| 1 | - | Toluene | 25 | 3 | 93 | 76/24 | 94 (55) |
| 2 | - | Xylene | 25 | 3 | 76 | 79/21 | 89 (49) |
| 3 | - | Hexane | 25 | 3 | 91 | 63/37 | 31 (8) |
| 4 | - | CH2Cl2 | 25 | 3 | 100 | 80/20 | 99 (40) |
| 5 | - | CHCl3 | 25 | 3 | 100 | 80/20 | 92 (50) |
| 6 | - | DCE | 25 | 3 | 100 | 78/22 | 97 (30) |
| 7 | - | Et2O | 25 | 3 | 55 | 72/28 | 78 (25) |
| 8 | - | THF | 25 | 3 | 63 | 67/33 | 38 (32) |
| 9 | - | MeCN | 25 | 3 | 69 | 71/29 | 41 (5) |
| 10 | - | MeOH | 25 | 3 | n.r. | n.d. | n.d. |
| 11 | - | iPrOH | 25 | 3 | 49 | 71/29 | 50 (11) |
| 12 | - | H2O | 25 | 3 | 100 | 67/33 | 95 (6) |
| 13 | - | CH2Cl2 | 25 | 5 | 100 | 80/20 | 99 (41) |
| 14 | PhCO2H (10) | CH2Cl2 | 25 | 3 | 100 | 77/23 | 51 (40) |
| 15 | 4-O2NC6H4CO2H (10) | CH2Cl2 | 25 | 3 | 100 | 77/23 | 47 (40) |
| 16 | Salicylic acid (10) | CH2Cl2 | 25 | 3 | 100 | 74/26 | 33 (30) |
| 17 | HDA (10) | CH2Cl2 | 25 | 3 | 100 | 76/24 | 83 (58) |
| 18 | DMAP (10) | CH2Cl2 | 25 | 3 | 100 | 69/31 | 87 (22) |
| 19 | Imidazole (10) | CH2Cl2 | 25 | 3 | 100 | 83/17 | 83 (74) |
| 20 | DBU (10) | CH2Cl2 | 25 | 3 | n.r. | n.d. | n.d |
| 21 | 2,6-Lutidine (10) | CH2Cl2 | 25 | 3 | 100 | 81/19 | 84 (6) |
| 22 | DABCO (10) | CH2Cl2 | 25 | 3 | 100 | 85/15 | 99 (9) |
| 23 | DABCO (5) | CH2Cl2 | 25 | 3 | 100 | 85/15 | 97 (9) |
| 24 | DABCO (20) | CH2Cl2 | 25 | 3 | 100 | 85/15 | 64 (16) |
| 25 | DABCO (10) | CH2Cl2 | 5 | 4 | 100 | 93/7 | 99 (19) |
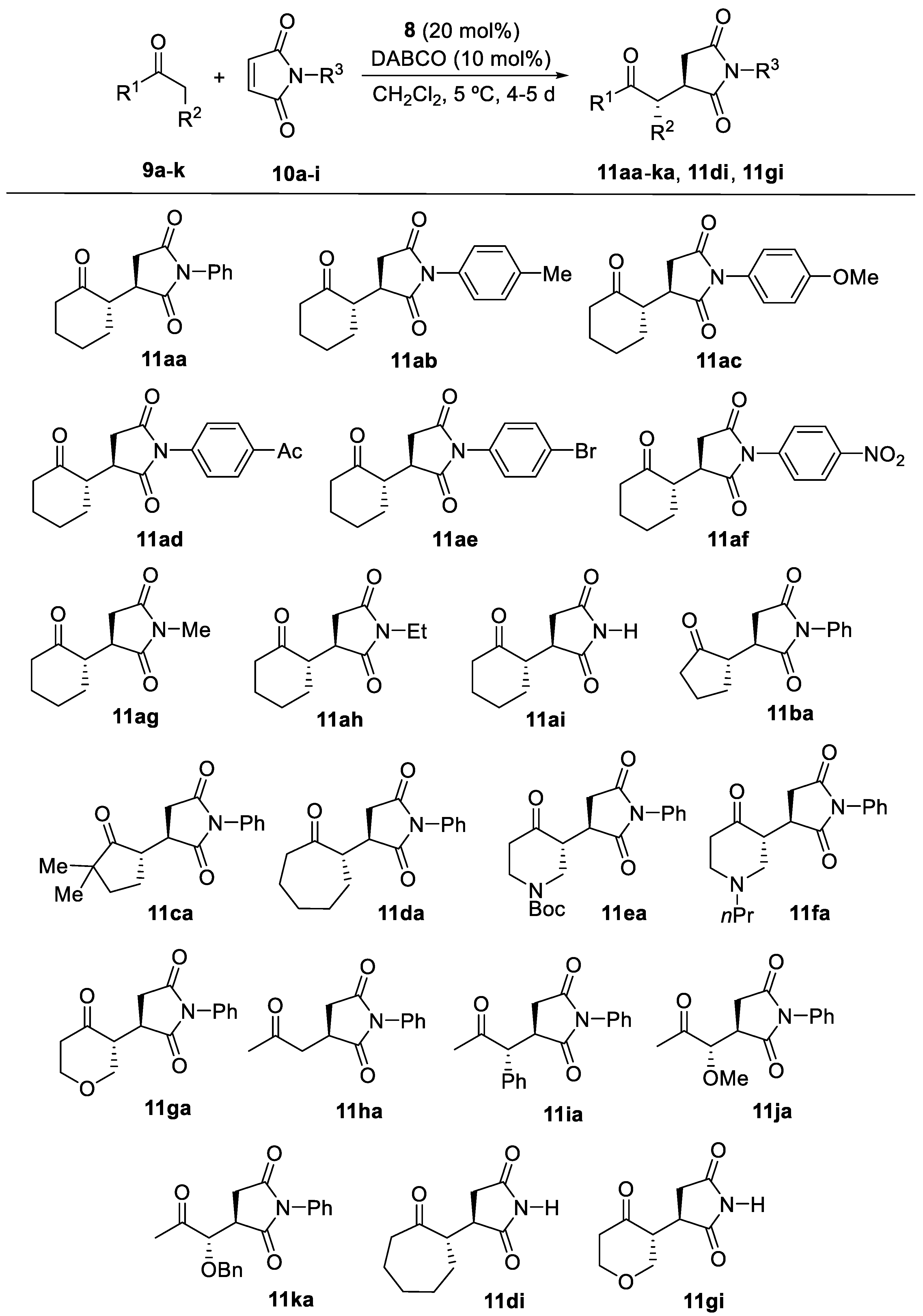
| Entry | t (d) | Product 11 | Yield (%) a | drb | ee (%) c,d |
|---|---|---|---|---|---|
| 1 | 4 | 11aa | 98 | 93/7 | 99 (19) |
| 2 | 4 | 11ab | 91 | 90/10 | 76 (53) |
| 3 | 4 | 11ac | 90 | 85/15 | 64 (53) |
| 4 | 4 | 11ad | 82 | 88/12 | 78 (46) |
| 5 | 4 | 11ae | 95 | 92/8 | 73 (18) |
| 6 | 4 | 11af | 80 | 84/16 | 62 (40) |
| 7 | 4 | 11ag | 94 | 91/9 | 76 (25) |
| 8 | 4 | 11ah | 93 | 83/17 | 77 (63) |
| 9 | 4 | 11ai | 92 | 76/24 | 99 (99) |
| 10 e,f | 5 | 11ba | 89 | 51/49 | 62 (22) |
| 11 e,f | 5 | 11ca | 65 | 64/36 | 93 (81) |
| 12 e,f | 5 | 11da | 92 | 66/34 | 85 (21) |
| 13 f | 4 | 11ea | 94 | 56/44 | 62 (54) |
| 14 f | 4 | 11fa | 91 | 65/35 | 76 (76) |
| 15 f | 4 | 11ga | 93 | 58/42 | 99 (99) |
| 16 | 4 | 11ha | 71 | - | 72 |
| 17 | 5 | 11ia | 56 | 52/48 | 96 (26) |
| 18 | 4 | 11ja | 94 | 62/38 | 36 (15) |
| 19 | 4 | 11ka | 96 | 75/25 | 75 (25) |
| 20 e,f | 4 | 11di | 85 | 60/40 | 95 (75) |
| 21 f | 4 | 11gi | 90 | 51/49 | 99 (99) |
| 22 g | 4 | 11aa | 92 | 91/9 | 97 (19) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torregrosa-Chinillach, A.; Chinchilla, R. Asymmetric Conjugate Addition of Ketones to Maleimides Organocatalyzed by a Chiral Primary Amine-Salicylamide. Molecules 2022, 27, 6668. https://doi.org/10.3390/molecules27196668
Torregrosa-Chinillach A, Chinchilla R. Asymmetric Conjugate Addition of Ketones to Maleimides Organocatalyzed by a Chiral Primary Amine-Salicylamide. Molecules. 2022; 27(19):6668. https://doi.org/10.3390/molecules27196668
Chicago/Turabian StyleTorregrosa-Chinillach, Alejandro, and Rafael Chinchilla. 2022. "Asymmetric Conjugate Addition of Ketones to Maleimides Organocatalyzed by a Chiral Primary Amine-Salicylamide" Molecules 27, no. 19: 6668. https://doi.org/10.3390/molecules27196668






