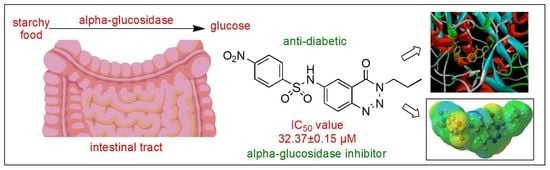
Experimental and Computational Analysis of Newly Synthesized Benzotriazinone Sulfonamides as Alpha-Glucosidase Inhibitors
Abstract
:1. Introduction
2. Results and Discussion
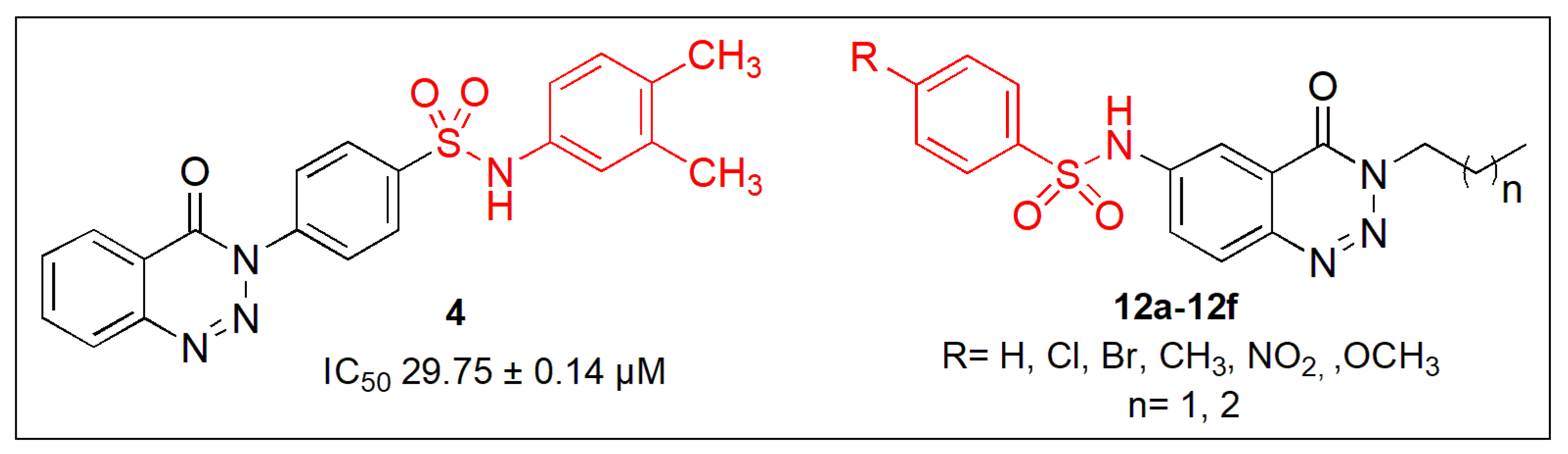
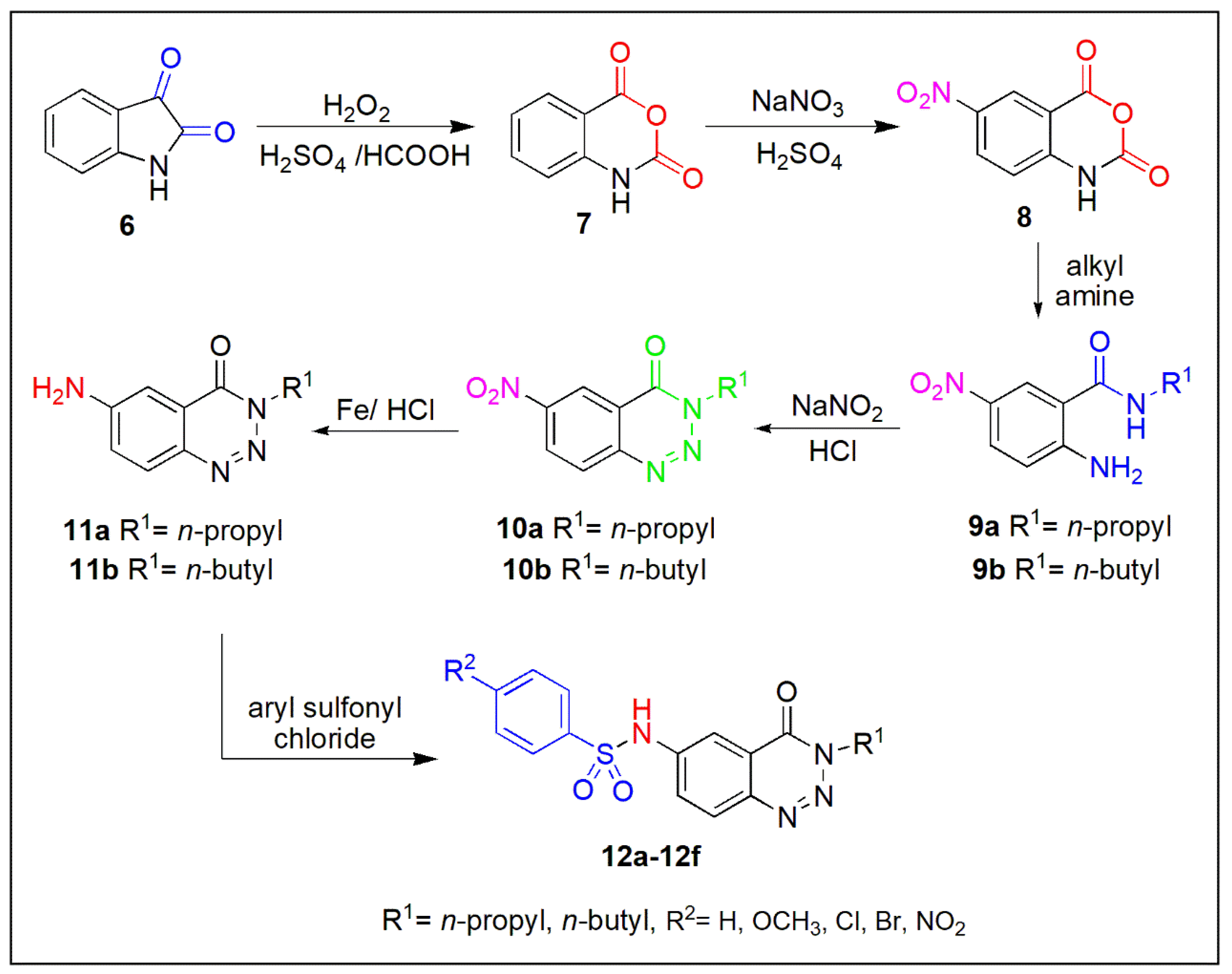
2.1. Synthetic Chemistry
2.2. Spectroscopic Characterization
2.3. Alpha-Glucosidase Inhibition Studies
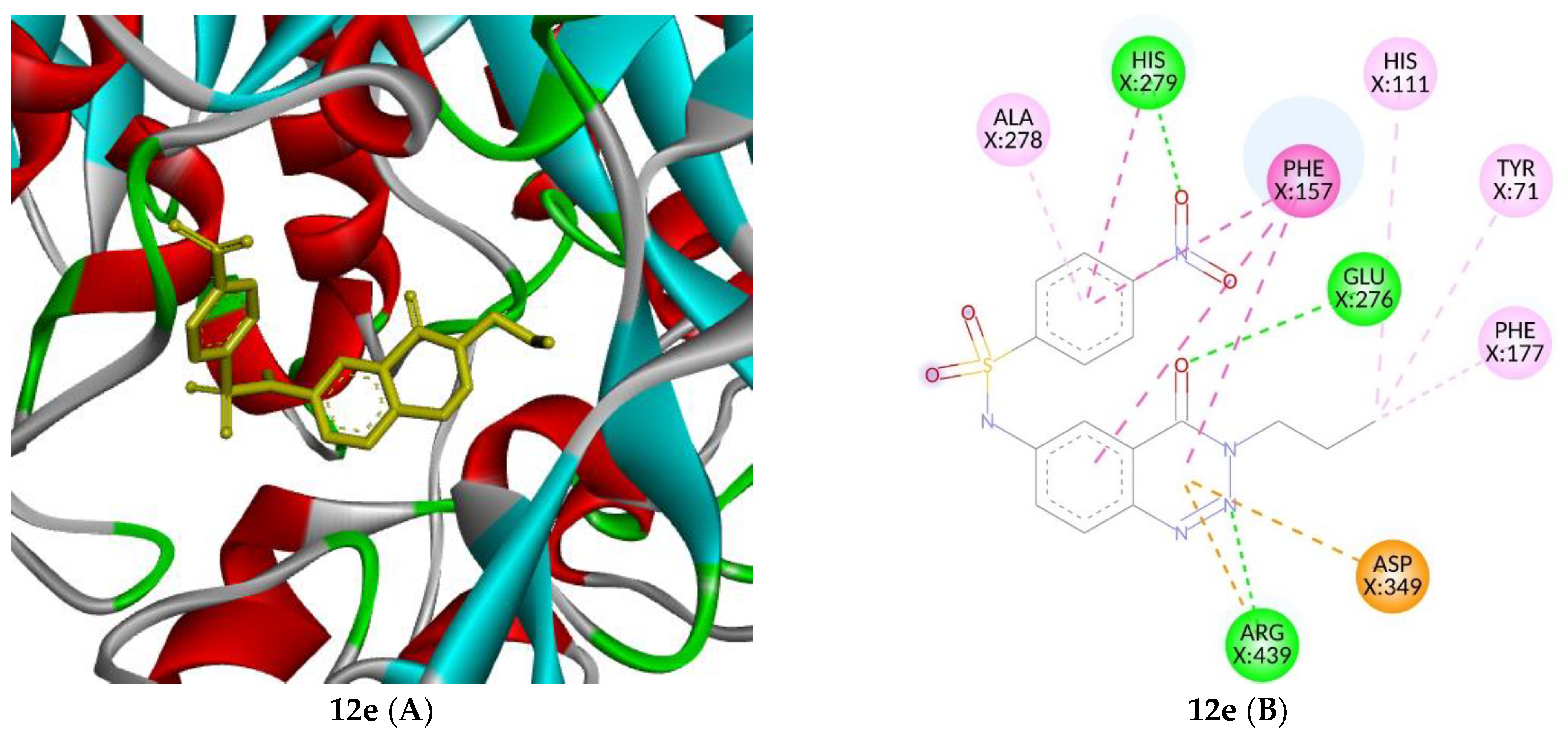
2.4. Molecular Docking
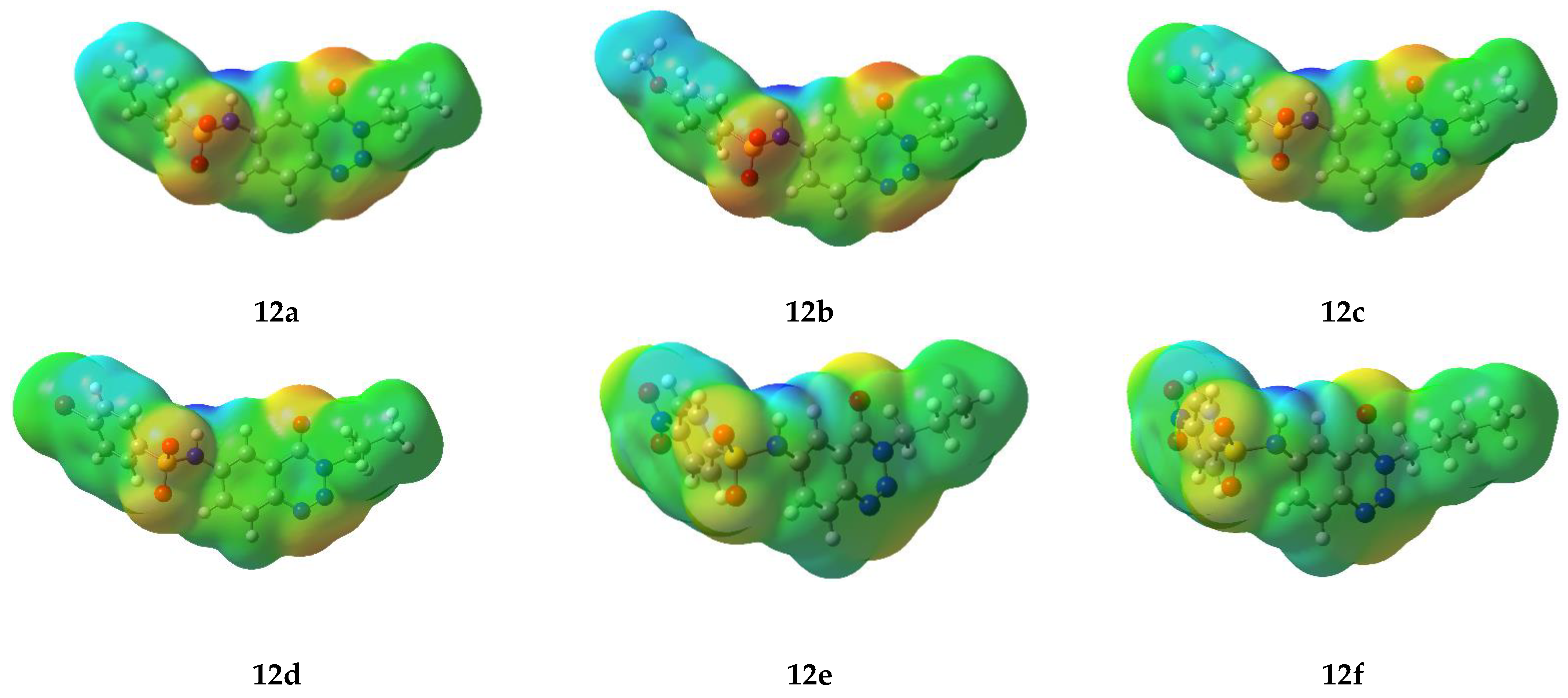
2.5. Computational Study
2.5.1. Frontier Molecular Orbital Analysis
2.5.2. Molecular Electrostatic Potential
3. Experiment Work
3.1. General
3.1.1. Synthesis of Isatoic Anhydride (7)
3.1.2. 6-Nitroisatoic Anhydride (8)
3.1.3. 2-Amino-5-nitro-N-alkylbenzamides (9a–9b)
3.1.4. General Procedure for the Synthesis of 6-Nitro-3-alkylbenzo[1,2,3]triazin-4(3H)-ones (10a–10b)
3.1.5. General Procedure for the Synthesis of 6-Amino-3-alkylbenzo[1,2,3]triazin-4(3H)-one (11a–11b)
3.1.6. General Procedure for the Synthesis of N-(4-Oxo-3-propyl/butyl-3,4-dihydrobenzo[1,2,3]triazin-6-yl)benzenesulfonamides (12a-12f)
3.2. Procedure of Alpha-Glucosidase Inhibition Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saddique, F.; Aslam, S.; Ahmad, M.; Ashfaq, U.; Muddassar, M.; Sultan, S.; Taj, S.; Hussain, M.; Lee, D.S.; Zaki, M. Synthesis and α-Glucosidase Inhibition Activity of 2-[3-(Benzoyl/4-bromobenzoyl)-4-hydroxy-1,1-dioxido-2H-benzo[e][1,2]thiazin-2-yl]-N-arylacetamides: An In Silico and Biochemical Approach. Molecules 2021, 26, 3043. [Google Scholar] [CrossRef]
- Zhang, X.; Zheng, Y.-Y.; Hu, C.-M.; Wu, X.-Z.; Lin, J.; Xiong, Z.; Zhang, K.; Xu, X.-T. Synthesis and biological evaluation of coumarin derivatives containing oxime ester as α-glucosidase inhibitors. Arab. J. Chem. 2022, 15, 104072. [Google Scholar] [CrossRef]
- Dean, L.; McEntyre, J. Introduction to diabetes. In The Genetic Landscape of Diabetes; National Center for Biotechnology Information: Bethesda, MD, USA, 2004. [Google Scholar]
- Kasturi, S.P.; Surarapu, S.; Uppalanchi, S.; Dwivedi, S.; Yogeeswari, P.; Sigalapalli, D.K.; Bathini, N.B.; Ethiraj, K.S.; Anireddy, J.S. Synthesis, molecular modeling and evaluation of α-glucosidase inhibition activity of 3,4-dihydroxy piperidines. Eur. J. Med. Chem. 2018, 150, 39–52. [Google Scholar] [CrossRef]
- Ibrar, A.; Zaib, S.; Khan, I.; Shafique, Z.; Saeed, A.; Iqbal, J. New prospects for the development of selective inhibitors of α-glucosidase based on coumarin-iminothiazolidinone hybrids: Synthesis, in-vitro biological screening and molecular docking analysis. J. Taiwan Inst. Chem. Eng. 2017, 81, 119–133. [Google Scholar] [CrossRef]
- Temneanu, O.; Trandafir, L.; Purcarea, M.R. Type 2 diabetes mellitus in children and adolescents: A relatively new clinical problem within pediatric practice. J. Med. Life 2016, 9, 235–239. [Google Scholar] [PubMed]
- Hu, F.B.; Manson, J.E.; Stampfer, M.J.; Colditz, G.; Liu, S.; Solomon, C.G.; Willett, W.C. Diet, Lifestyle, and the Risk of Type 2 Diabetes Mellitus in Women. N. Engl. J. Med. 2001, 345, 790–797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogurtsova, K.; Guariguata, L.; Barengo, N.C.; Ruiz, P.L.-D.; Sacre, J.W.; Karuranga, S.; Sun, H.; Boyko, E.J.; Magliano, D.J. IDF diabetes Atlas: Global estimates of undiagnosed diabetes in adults for 2021. Diabetes Res. Clin. Pract. 2022, 183, 109118. [Google Scholar] [CrossRef]
- Abdullah, M.A.; Lee, Y.-R.; Mastuki, S.N.; Leong, S.W.; Ibrahim, W.N.W.; Latif, M.A.M.; Ramli, A.N.M.; Aluwi, M.F.F.M.; Faudzi, S.M.M.; Kim, C.-H. Development of diarylpentadienone analogues as alpha-glucosidase inhibitor: Synthesis, in vitro biological and in vivo toxicity evaluations, and molecular docking analysis. Bioorg. Chem. 2020, 104, 104277. [Google Scholar] [CrossRef] [PubMed]
- Fallah, Z.; Tajbakhsh, M.; Alikhani, M.; Larijani, B.; Faramarzi, M.A.; Hamedifar, H.; Mohammadi-Khanaposhtani, M.; Mahdavi, M. A review on synthesis, mechanism of action, and structure-activity relationships of 1,2,3-triazole-based α-glucosidase inhibitors as promising anti-diabetic agents. J. Mol. Struct. 2022, 1255, 132469. [Google Scholar] [CrossRef]
- Kazmi, M.; Zaib, S.; Amjad, S.T.; Khan, I.; Ibrar, A.; Saeed, A.; Iqbal, J. Exploration of aroyl/heteroaroyl iminothiazolines featuring 2,4,5-trichlorophenyl moiety as a new class of potent, selective, and in vitro efficacious glucosidase inhibitors. Bioorg. Chem. 2017, 74, 134–144. [Google Scholar] [CrossRef]
- Karrouchi, K.; Fettach, S.; Tamer, Ö.; Avci, D.; Başoğlu, A.; Atalay, Y.; Ayaz, Z.; Radi, S.; Ghabbour, H.A.; Mabkhot, Y.N.; et al. Synthesis, crystal structure, spectroscopic characterization, α-glucosidase inhibition and computational studies of (E)-5-methyl-N′-(pyridin-2-ylmethylene)-1H-pyrazole-3-carbohydrazide. J. Mol. Struct. 2022, 1248, 131506. [Google Scholar] [CrossRef]
- Kazmi, M.; Zaib, S.; Ibrar, A.; Amjad, S.T.; Shafique, Z.; Mehsud, S.; Saeed, A.; Iqbal, J.; Khan, I. A new entry into the portfolio of α-glucosidase inhibitors as potent therapeutics for type 2 diabetes: Design, bioevaluation and one-pot multi-component synthesis of diamine-bridged coumarinyl oxadiazole conjugates. Bioorg. Chem. 2018, 77, 190–202. [Google Scholar] [CrossRef] [PubMed]
- Natori, Y.; Imahori, T.; Murakami, K.; Yoshimura, Y.; Nakagawa, S.; Kato, A.; Adachi, I.; Takahata, H. The synthesis and biological evaluation of 1-C-alkyl-l-arabinoiminofuranoses, a novel class of α-glucosidase inhibitors. Bioorg. Med. Chem. Lett. 2011, 21, 738–741. [Google Scholar] [CrossRef] [PubMed]
- Khalid, Z.; Shafqat, S.S.; Ahmad, H.A.; Rehman, H.M.; Munawar, M.A.; Ahmad, M.; Asiri, A.M.; Ashraf, M. Synthesis of 1,2,3-benzotriazin-4(3H)-one derivatives as α-glucosidase inhibitor and their in-silico study. Med. Chem. Res. 2022, 31, 819–831. [Google Scholar] [CrossRef]
- Kim, H.-R.; Antonisamy, P.; Kim, Y.-S.; Lee, G.; Ham, H.-D.; Kwon, K.-B. Inhibitory effect of Amomum villosum water extracts on α-glucosidase activity. Physiol. Mol. Plant Pathol. 2022, 117, 101779. [Google Scholar] [CrossRef]
- Khan, I.; Khan, A.; Halim, S.A.; Khan, M.; Zaib, S.; Al-Yahyaei, B.E.M.; Al-Harrasi, A.; Ibrar, A. Utilization of the common functional groups in bioactive molecules: Exploring dual inhibitory potential and computational analysis of keto esters against α-glucosidase and carbonic anhydrase-II enzymes. Int. J. Biol. Macromol. 2021, 167, 233–244. [Google Scholar] [CrossRef]
- Karle, P.P.; Dhawale, S.C.; Mandade, R.J.; Navghare, V.V. Screening of Manilkara zapota (L) P. Royen stem bark ethanolic extract for in vitro α-glucosidase inhibition, preliminary antidiabetic effects, and improvement of diabetes and its complications in alloxan-induced diabetes in Wistar rats. Bull. Natl. Res. Cent. 2022, 46, 110. [Google Scholar] [CrossRef]
- Ahmad, H.A.; Aslam, M.; Gul, S.; Mehmood, T.; Munawar, M.A. In vivo Anti Inflammation Studies of Novel 1, 2, 5 Oxadiazole Sulfonamide Hybrids. Pak. J. Zool 2021, 54, 1001–1500. [Google Scholar] [CrossRef]
- Gulçin, İ.; Taslimi, P. Sulfonamide inhibitors: A patent review 2013–present. Expert Opin. Ther. Pat. 2018, 28, 541–549. [Google Scholar] [CrossRef]
- Wang, G.; Chen, M.; Wang, J.; Peng, Y.; Li, L.; Xie, Z.; Deng, B.; Chen, S.; Li, W. Synthesis, biological evaluation and molecular docking studies of chromone hydrazone derivatives as α-glucosidase inhibitors. Bioorg. Med. Chem. Lett. 2017, 27, 2957–2961. [Google Scholar] [CrossRef]
- Taha, M.; Alrashedy, A.S.; Almandil, N.B.; Iqbal, N.; Anouar, E.H.; Nawaz, M.; Uddin, N.; Chigurupati, S.; Wadood, A.; Rahim, F.; et al. Synthesis of indole derivatives as diabetics II inhibitors and enzymatic kinetics study of α-glucosidase and α-amylase along with their in-silico study. Int. J. Biol. Macromol. 2021, 190, 301–318. [Google Scholar] [CrossRef] [PubMed]
- Taha, M.; Imran, S.; Salahuddin, M.; Iqbal, N.; Rahim, F.; Uddin, N.; Shehzad, A.; Farooq, R.K.; Alomari, M.; Khan, K.M. Evaluation and docking of indole sulfonamide as a potent inhibitor of α-glucosidase enzyme in streptozotocin –induced diabetic albino wistar rats. Bioorg. Chem. 2021, 110, 104808. [Google Scholar] [CrossRef] [PubMed]
- Selim, Y.A.; El-Azim, M.H.M.A.; El-Farargy, A.F. Synthesis and Anti-inflammatory Activity of Some New 1,2,3-Benzotriazine Derivatives Using 2-(4-Oxo-6-phenylbenzo[d][1,2,3]triazin-3(4H)-yl)acetohydrazide as a Starting Material. J. Heterocycl. Chem. 2018, 55, 1756–1764. [Google Scholar] [CrossRef]
- Fiorino, F.; Severino, B.; De Angelis, F.; Perissutti, E.; Frecentese, F.; Massarelli, P.; Bruni, G.; Collavoli, E.; Santagada, V.; Caliendo, G. Synthesis and In-Vitro Pharmacological Evaluation of New 5-HT1A Receptor Ligands Containing a Benzotriazinone Nucleus. Arch. Pharm. 2008, 341, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Fadda, A.A.; Abdel-Latif, E.; Fekri, A.; Mostafa, A.R. Synthesis and Docking Studies of Some 1,2,3-Benzotriazine-4-one Derivatives as Potential Anticancer Agents. J. Heterocycl. Chem. 2019, 56, 804–814. [Google Scholar] [CrossRef]
- Fiorino, F.; Caliendo, G.; Perissutti, E.; Severino, B.; Frecentese, F.; Preziosi, B.; Izzo, A.A.; Capasso, R.; Santagada, V. Synthesis by Microwave Irradiation and Antidiarrhoeal Activity of Benzotriazinone and Saccharine Derivatives. Arch. Pharm. 2005, 338, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, E.S.; Macedo, A.B.; Resop, R.S.; Howard, J.N.; Nell, R.; Sarabia, I.; Newman, D.; Ren, Y.; Jones, R.B.; Planelles, V.; et al. Structure-Activity Relationship Analysis of Benzotriazine Analogues as HIV-1 Latency-Reversing Agents. Antimicrob. Agents Chemother. 2020, 64, e00888-20. [Google Scholar] [CrossRef] [PubMed]
- Caliendo, G.; Fiorino, F.; Grieco, P.; Perissutti, E.; Santagada, V.; Meli, R.; Raso, G.M.; Zanesco, A.; De Nucci, G. Preparation and local anaesthetic activity of benzotriazinone and benzoyltriazole derivatives. Eur. J. Med. Chem. 1999, 34, 1043–1051. [Google Scholar] [CrossRef]
- Yan, Y.-C.; Wu, W.; Huang, G.-Y.; Yang, W.-C.; Chen, Q.; Qu, R.-Y.; Lin, H.-Y.; Yang, G.-F. Pharmacophore-Oriented Discovery of Novel 1,2,3-Benzotriazine-4-one Derivatives as Potent 4-Hydroxyphenylpyruvate Dioxygenase Inhibitors. J. Agric. Food Chem. 2022, 70, 6644–6657. [Google Scholar] [CrossRef]
- Khalid, Z.; Ahmad, H.A.; Munawar, M.A.; Khan, M.-U.; Gul, S. 1,2,3-Benzotriazin-4(3H)-ones: Synthesis, Reactions and Applications. Heterocycles 2017, 94, 3–54. [Google Scholar] [CrossRef]
- Reddy, G.S.; Snehalatha, A.V.; Edwin, R.K.; Hossain, K.A.; Giliyaru, V.B.; Hariharapura, R.C.; Shenoy, G.G.; Misra, P.; Pal, M. Synthesis of 3-indolylmethyl substituted (pyrazolo/benzo)triazinone derivatives under Pd/Cu-catalysis: Identification of potent inhibitors of chorismate mutase (CM). Bioorg. Chem. 2019, 91, 103155. [Google Scholar] [CrossRef] [PubMed]
- El Rayes, S.M.; Ali, I.A.I.; Fathalla, W.; Mahmoud, M.A.A. Synthesis and Biological Activities of Some New Benzotriazinone Derivatives Based on Molecular Docking; Promising HepG2 Liver Carcinoma Inhibitors. ACS Omega 2020, 5, 6781–6791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moghimi, S.; Goli-Garmroodi, F.; Pilali, H.; Mahdavi, M.; Firoozpour, L.; Nadri, H.; Moradi, A.; Asadipour, A.; Shafiee, A.; Foroumadi, A. Synthesis and anti-acetylcholinesterase activity of benzotriazinone-triazole systems. J. Chem. Sci. 2016, 128, 1445–1449. [Google Scholar] [CrossRef] [Green Version]
- Clark, R.H.; Wagner, E.C. Isatoic Anhydride. I. Reactions with Primary and Secondary Amines and with Some Amides1. J. Org. Chem. 1944, 9, 55–67. [Google Scholar] [CrossRef]
- Quadbeck-Seeger, H.-J.; Tonne, P. Production of Unsubstituted or Substituted Isatoic Anhydride. U.S. Patent 3,984,406, 5 October 1976. [Google Scholar]
- Kanışkan, N.; Kökten, Ş.; Çelik, I. A new protocol for the synthesis of primary, secondary and tertiary anthranilamides utilizing N-(2-aminoarylacyl)benzotriazoles. Arkivoc 2012, 8, 198–213. [Google Scholar] [CrossRef] [Green Version]
- Ege, G.; Arnold, P.; Beisiegel, E.; Lehrer, I.; Suschitzky, H.; Price, D. Ringspaltung cyclischer Azoverbindungen, VII. 6-Fluor- sowie 6-Nitro-3-phenyl-3,4-dihydro-1,2,3-benzotriazin-4-on und deren Photolyse; nucleophile Substitution zur Erprobung der Fluor-Markierungsmethode von Suschitzky. Eur. J. Org. Chem. 1976, 1976, 946–968. [Google Scholar] [CrossRef]
- Bashir, N.; Gilchrist, T.L. Formation of fused azetinones by photolysis and pyrolysis of triazinones. N-aminonaphth[2,3-b]azet-2(1H)-one and N-1-adamantylbenzazet-2-(1H)-one. J. Chem. Soc. Perkin Trans. 1 1973, 868–872. [Google Scholar] [CrossRef]
- Ganesan, M.; Raja, K.K.; Murugesan, S.; Kumar, B.K.; Rajagopal, G.; Thirunavukkarasu, S. Synthesis, biological evaluation, molecular docking, molecular dynamics and DFT studies of quinoline-fluoroproline amide hybrids. J. Mol. Struct. 2020, 1217, 128360. [Google Scholar] [CrossRef]
- Aslam, S.; Ali, H.S.; Ahmad, M.; Mansha, A.; Ali, N.; Khan, S.; Naqvi, S.A.R.; Khalid, Z.; Asim, S.; Parvez, M.; et al. A combined experimental and theoretical study of alkyl 2-(3-benzoyl-4-hydroxy-1,1-dioxido-2H-benzo[e][1,2]thiazin-2-yl)acetates: Synthesis, X-ray crystallography and DFT. J. Mol. Struct. 2022, 1258, 132671. [Google Scholar] [CrossRef]
- Karrouchi, K.; Sert, Y.; Ansar, M.; Radi, S.; El Bali, B.; Imad, R.; Alam, A.; Irshad, R.; Wajid, S.; Altaf, M. Synthesis, α-Glucosidase Inhibition, Anticancer, DFT and Molecular Docking Investigations of Pyrazole Hydrazone Derivatives. Polycycl. Aromat. Compd. 2022, 1–20. [Google Scholar] [CrossRef]
- Alyar, S.; Şen, T.; Özmen, Ü.Ö.; Alyar, H.; Adem, S.; Şen, C. Synthesis, spectroscopic characterizations, enzyme inhibition, molecular docking study and DFT calculations of new Schiff bases of sulfa drugs. J. Mol. Struct. 2019, 1185, 416–424. [Google Scholar] [CrossRef]
- Kharul, R.K.; Prajapati, P.N.; Thorave, A.A.; Shah, H.A.; Dhar, A.; Joshi, D.A.; Jain, M.R.; Patel, P.R.; Pancholi, S.S. Effective Synthesis of 1,5-Disubstituted 2,1-Benzisothiazol-3(1H)-ones. Synth. Commun. 2011, 41, 3265–3279. [Google Scholar] [CrossRef]



| Sr. No. | Codes | Structures | % Inhibition at 0.5 mM | IC50 (μM) |
|---|---|---|---|---|
| 1. | 11a | 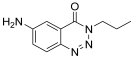 | 26.12 ± 0.13 | ND |
| 2. | 11b | 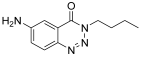 | 27.12 ± 0.13 | ND |
| 3. | 12a | 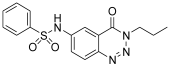 | 51.25 ± 0.16 | ˂500 |
| 4. | 12b | 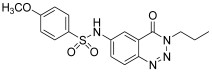 | 91.73 ± 0.17 | 39.21 ± 0.12 |
| 5. | 12c | 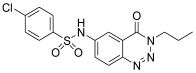 | 78.12 ± 0.16 | 53.76 ± 0.14 |
| 6. | 12d | 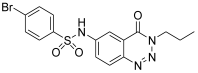 | 79.42 ± 0.15 | 55.21 ± 0.12 |
| 7. | 12e | 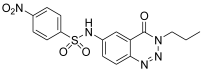 | 96.27 ± 0.19 | 32.37 ± 0.15 |
| 8. | 12f | 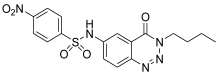 | 93.27 ± 0.14 | 37.75 ± 0.11 |
| 9. | Standard | acarbose | 92.23 ± 0.16 | 37.38 ± 0.12 |
| Compounds | EHOMO | ELUMO | Egap | I | A | η | S | μ | Χ | ω | D |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 12a | −6.62 | −2.06 | 4.55 | 6.62 | 2.06 | 2.28 | 0.22 | 4.34 | 4.34 | 4.14 | 5.54 |
| 12b | −6.52 | −1.98 | 4.54 | 6.52 | 1.98 | 2.27 | 0.22 | 4.25 | 4.25 | 3.98 | 7.07 |
| 12c | −6.73 | −2.17 | 4.56 | 6.73 | 2.17 | 2.28 | 0.22 | 4.45 | 4.45 | 4.34 | 3.67 |
| 12d | −6.70 | −2.15 | 4.55 | 6.70 | 2.15 | 2.27 | 0.22 | 4.43 | 4.43 | 4.31 | 3.89 |
| 12e | −7.02 | −3.33 | 3.70 | 7.02 | 3.33 | 1.85 | 0.27 | 5.18 | 5.18 | 7.25 | 2.30 |
| 12f | −7.01 | −3.33 | 3.68 | 7.01 | 3.33 | 1.84 | 0.27 | 5.17 | 5.17 | 7.26 | 2.35 |
| Entry | Compound | Molecular Formula | Molecular Weight | Physical Appearance | % Yield | m.p. (℃) | Solubility |
|---|---|---|---|---|---|---|---|
| 1. | 7 | C8H5NO3 | 163.13 | Light yellow powder | 85% | 230–232 | Ethanol, DMSO |
| 2. | 8 | C8H4N2O5 | 208.13 | Yellow powder | 62% | 219–221 | Ethanol, DMSO |
| 3. | 9a | C10H13N3O3 | 223.23 | Mustard powder | 60% | 195–197 | Ethanol, DMSO |
| 4. | 9b | C11H15N3O3 | 237.26 | Mustard powder | 58% | 197–199 | Ethanol, DMSO |
| 5. | 10a | C10H10N4O3 | 234.21 | Light yellow powder | 92% | 215–217 | Ethanol, DMSO |
| 6. | 10b | C11H12N4O3 | 248.24 | White powder | 91% | 217–218 | Ethanol, DMSO |
| 7. | 11a | C10H12N4O | 204.23 | Transparent crystals | 80% | 185–186 | Methanol, DMSO |
| 8. | 11b | C11H14N4O | 218.26 | Transparent crystals | 72% | 191–192 | Methanol, DMSO |
| 9. | 12a | C16H16N4O3S | 344.39 | Brown powder | 82% | 171–173 | Ethanol, DMSO |
| 10. | 12b | C17H18N4O4S | 374.41 | Brown powder | 75% | 175–177 | Ethanol, DMSO |
| 11. | 12c | C16H15ClN4O3S | 378.83 | Brown powder | 68% | 169–171 | Ethanol, DMSO |
| 12. | 12d | C16H15BrN4O3S | 423.28 | Brown powder | 78% | 171–179 | Ethanol, DMSO |
| 13. | 12e | C16H15N5O5S | 389.39 | Brown powder | 71% | 167–169 | Ethanol, DMSO |
| 14. | 12f | C17H17N5O5S | 403.41 | Brown powder | 62% | 179–181 | Ethanol, DMSO |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalid, Z.; Alnuwaiser, M.A.; Ahmad, H.A.; Shafqat, S.S.; Munawar, M.A.; Kamran, K.; Abbas, M.M.; Kalam, M.A.; Ewida, M.A. Experimental and Computational Analysis of Newly Synthesized Benzotriazinone Sulfonamides as Alpha-Glucosidase Inhibitors. Molecules 2022, 27, 6783. https://doi.org/10.3390/molecules27206783
Khalid Z, Alnuwaiser MA, Ahmad HA, Shafqat SS, Munawar MA, Kamran K, Abbas MM, Kalam MA, Ewida MA. Experimental and Computational Analysis of Newly Synthesized Benzotriazinone Sulfonamides as Alpha-Glucosidase Inhibitors. Molecules. 2022; 27(20):6783. https://doi.org/10.3390/molecules27206783
Chicago/Turabian StyleKhalid, Zunera, Maha Abdallah Alnuwaiser, Hafiz Adnan Ahmad, Syed Salman Shafqat, Munawar Ali Munawar, Kashif Kamran, Muhammad Mujtaba Abbas, M. A. Kalam, and Menna A. Ewida. 2022. "Experimental and Computational Analysis of Newly Synthesized Benzotriazinone Sulfonamides as Alpha-Glucosidase Inhibitors" Molecules 27, no. 20: 6783. https://doi.org/10.3390/molecules27206783