High-Efficiency Extraction of Pantoea alhagi Exopolysaccharides Driven by pH-Related Changes in the Envelope Structure
Abstract
:1. Introduction
2. Results
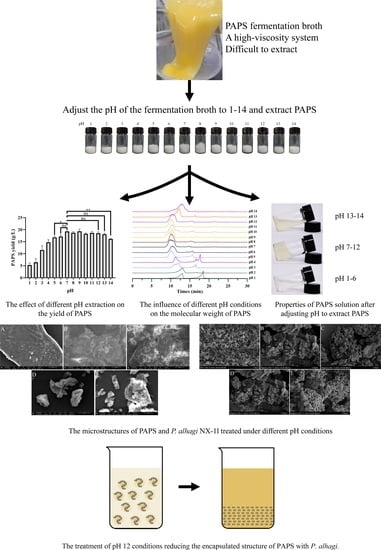
2.1. Effects of Different pH on the Extraction Process and PAPS Yield
2.2. Effects of Different pH on the Molecular Weight and Monosaccharide Composition of Crude PAPS
2.3. Effects of Different pH on the FT-IR Analysis of Crude PAPS
2.4. Effects of Different pH on the Rheological Properties of Crude PAPS
2.4.1. Apparent Viscosity and Flow Curves
2.4.2. Storage (G’) and Loss (G”) Moduli
2.5. Effects of PAPS on Water-Stable Soil Macro-Aggregates and Water Evaporation
2.6. Comparison of Microstructures of PAPS and P. alhagi NX-11 Treated under Different pH Conditions
2.7. Fatty Acid Composition of Cell Membranes Treated at Different pH
3. Materials and Methods
3.1. Materials and Chemicals
3.2. Fermentation in Shake flask
3.3. The PAPS Extraction Process under pH Conditions
3.4. Gel Permeation Chromatography
3.5. Monosaccharide Composition Analysis
3.6. Fourier-Transform Infrared Analysis
3.7. Rheological and Mechanical Properties
3.8. Determination of Soil’s Water-Stable Aggregates
3.9. Determination of Soil’s Water Evaporation Rate
3.10. Electron Microscopic Studies
3.11. Extraction and Detection of Cell-Membrane Fatty Acids
3.12. Statistical Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Castillo Pedraza, M.C.; de Oliveira Fratucelli, E.D.; Ribeiro, S.M.; Florez Salamanca, E.J.; da Silva Colin, J.; Klein, M.I. Modulation of Lipoteichoic Acids and Exopolysaccharides Prevents Streptococcus mutans Biofilm Accumulation. Molecules 2020, 25, 2232. [Google Scholar] [CrossRef] [PubMed]
- Nesic, A.; Cabrera-Barjas, G.; Dimitrijevic-Brankovic, S.; Davidovic, S.; Radovanovic, N.; Delattre, C. Prospect of Polysaccharide-Based Materials as Advanced Food Packaging. Molecules 2019, 25, 135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yilmaz, T.; Simsek, O. Potential Health Benefits of Ropy Exopolysaccharides Produced by Lactobacillus plantarum. Molecules 2020, 25, 3293. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, Z.; Zhou, X.; Hu, J.; Xue, J.; Liu, X.; Zhang, J.; Liu, P.; Tong, S. Simultaneous Use of Stimulatory Agents to Enhance the Production and Hypoglycaemic Activity of Polysaccharides from Inonotus obliquus by Submerged Fermentation. Molecules 2019, 24, 4400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barcelos, M.C.S.; Vespermann, K.A.C.; Pelissari, F.M.; Molina, G. Current status of biotechnological production and applications of microbial exopolysaccharides. Crit. Rev. Food Sci. Nutr. 2020, 60, 1475–1495. [Google Scholar] [CrossRef]
- Abdullah, N.R.; Sharif, F.; Azizan, N.H.; Hafidz, I.F.M.; Supramani, S.; Usuldin, S.R.A.; Ahmad, R.; Wan-Mohtar, W. Pellet diameter of Ganoderma lucidum in a repeated-batch fermentation for the trio total production of biomass-exopolysaccharide-endopolysaccharide and its anti-oral cancer beta-glucan response. AIMS Microbiol. 2020, 6, 379–400. [Google Scholar] [CrossRef]
- Freitas, F.; Alves, V.D.; Reis, M.A.M. Advances in bacterial exopolysaccharides: From production to biotechnological applications. Trends Biotechnol. 2011, 29, 388–398. [Google Scholar] [CrossRef]
- Welman, A.D.; Maddox, I.S. Exopolysaccharides from lactic acid bacteria: Perspectives and challenges. Trends Biotechnol. 2003, 21, 269–274. [Google Scholar] [CrossRef]
- Sun, L.; Lei, P.; Wang, Q.; Ma, J.; Zhan, Y.; Jiang, K.; Xu, Z.; Xu, H. The Endophyte Pantoea alhagi NX-11 Alleviates Salt Stress Damage to Rice Seedlings by Secreting Exopolysaccharides. Front. Microbiol. 2020, 10, 3112. [Google Scholar] [CrossRef]
- Sun, L.; Yang, Y.; Lei, P.; Li, S.; Xu, H.; Wang, R.; Qiu, Y.; Zhang, W. Structure characterization, antioxidant and emulsifying capacities of exopolysaccharide derived from Pantoea alhagi NX-11. Carbohydr. Polym. 2021, 261, 117872. [Google Scholar] [CrossRef]
- Sun, L.; Yang, Y.; Wang, R.; Li, S.; Qiu, Y.; Lei, P.; Gao, J.; Xu, H.; Zhang, F.; Lv, Y. Effects of exopolysaccharide derived from Pantoea alhagi NX-11 on drought resistance of rice and its efficient fermentation preparation. Int. J. Biol. Macromol. 2020, 162, 946–955. [Google Scholar] [CrossRef] [PubMed]
- Castillo, N.A.; Valdez, A.L.; Farina, J.I. Microbial production of scleroglucan and downstream processing. Front. Microbiol. 2015, 6, 1106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vinarta, S.C.; Yossen, M.M.; Vega, J.R.; Figueroa, L.I.C.; Farina, J.I. Scleroglucan compatibility with thickeners, alcohols and polyalcohols and downstream processing implications. Carbohydr. Polym. 2013, 92, 1107–1115. [Google Scholar] [CrossRef] [PubMed]
- Ayyash, M.; Abu-Jdayil, B.; Itsaranuwat, P.; Almazrouei, N.; Galiwango, E.; Esposito, G.; Hunashal, Y.; Hamed, F.; Najjar, Z. Exopolysaccharide produced by the potential probiotic Lactococcus garvieae C47: Structural characteristics, rheological properties, bioactivities and impact on fermented camel milk. Food Chem. 2020, 333, 127418. [Google Scholar] [CrossRef] [PubMed]
- Farina, J.I.; Sineriz, F.; Molina, O.E.; Perotti, N.I. Isolation and physicochemical characterization of soluble scleroglucan from Sclerotium rolfsii. Rheological properties, molecular weight and conformational characteristics. Carbohydr. Polym. 2001, 44, 41–50. [Google Scholar] [CrossRef]
- Zeng, H.; Zhang, Y.; Lin, S.; Jian, Y.; Miao, S.; Zheng, B. Ultrasonic-microwave synergistic extraction (UMSE) and molecular weight distribution of polysaccharides from Fortunella margarita (Lour.) Swingle. Sep. Purif. Technol. 2015, 144, 97–106. [Google Scholar] [CrossRef]
- Zhou, R.; Cui, M.; Wang, Y.; Zhang, M.; Li, F.; Liu, K. Isolation, structure identification and anti-inflammatory activity of a polysaccharide from Phragmites rhizoma. Int. J. Biol. Macromol. 2020, 161, 810–817. [Google Scholar] [CrossRef]
- Wang, T.; Dong, Z.; Zhou, D.; Sun, K.; Zhao, Y.; Wang, B.; Chen, Y. Structure and immunostimulating activity of a galactofuranose-rich polysaccharide from the bamboo parasite medicinal fungus Shiraia bambusicola. J. Ethnopharmacol. 2020, 257, 112833. [Google Scholar] [CrossRef]
- Zou, Y.-F.; Zhang, Y.-Y.; Paulsen, B.S.; Fu, Y.-P.; Huang, C.; Feng, B.; Li, L.-X.; Chen, X.-F.; Jia, R.-Y.; Song, X.; et al. Prospects of Codonopsis pilosula polysaccharides: Structural features and bioactivities diversity. Trends Food Sci. Technol. 2020, 103, 1–11. [Google Scholar] [CrossRef]
- Harding, N.E.; Patel, Y.N.; Coleman, R.J. Targeted Gene Deletions for Polysaccharide Slime Formers. U.S. Patent 8759071 B2, 24 June 2014. [Google Scholar]
- Wang, Y.; Hu, L.; Huang, H.; Wang, H.; Zhang, T.; Chen, J.; Du, G.; Kang, Z. Eliminating the capsule-like layer to promote glucose uptake for hyaluronan production by engineered Corynebacterium glutamicum. Nat. Commun. 2020, 11, 3120. [Google Scholar] [CrossRef]
- Zhao, M.; Zhang, H.; Xu, X.; Li, S.; Xu, H. A strategy for the synthesis of low-molecular-weight welan gum by eliminating capsule form of Sphingomonas strains. Int. J. Biol. Macromol. 2021, 178, 11–18. [Google Scholar] [CrossRef] [PubMed]
- de Moura, F.A.; Macagnan, F.T.; da Silva, L.P. Oligosaccharide production by hydrolysis of polysaccharides: A review. Int. J. Food Sci. Tech. 2015, 50, 275–281. [Google Scholar] [CrossRef]
- de Oliveira, J.M.; Amaral, S.A.; Veiga Burkert, C.A. Rheological, textural and emulsifying properties of an exopolysaccharide produced by Mesorhizobium loti grown on a crude glycerol-based medium. Int. J. Biol. Macromol. 2018, 120, 2180–2187. [Google Scholar] [CrossRef] [PubMed]
- Calvo, C.; Martinez-Checa, F.; Mota, A.; Bejar, V.; Quesada, E. Effect of cations, pH and sulfate content on the viscosity and emulsifying activity of the Halomonas eurihalina exopolysaccharide. J. Ind. Microbiol. Biotechnol. 1998, 20, 205–209. [Google Scholar] [CrossRef]
- Wang, K.; Li, M.; Wang, Y.; Liu, Z.; Ni, Y. Effects of extraction methods on the structural characteristics and functional properties of dietary fiber extracted from kiwifruit (Actinidia deliciosa). Food Hydrocoll. 2021, 110, 106162. [Google Scholar] [CrossRef]
- Chen, S.; Shang, H.; Yang, J.; Li, R.; Wu, H. Effects of different extraction techniques on physicochemical properties and activities of polysaccharides from comfrey (Symphytum officinale L.) root. Ind. Crops Prod. 2018, 121, 18–25. [Google Scholar] [CrossRef]
- Ahmad Usuldin, S.R.; Mahmud, N.; Ilham, Z.; Khairul Ikram, N.K.; Ahmad, R.; Wan-Mohtar, W.A.A.Q.I. In-depth spectral characterization of antioxidative (1,3)-β-D-glucan from the mycelium of an identified tiger milk mushroom Lignosus rhinocerus strain ABI in a stirred-tank bioreactor. Biocatal. Agric. Biotechnol. 2020, 23, 101455. [Google Scholar] [CrossRef]
- Chi, Y.; Li, Y.; Zhang, G.; Gao, Y.; Ye, H.; Gao, J.; Wang, P. Effect of extraction techniques on properties of polysaccharides from Enteromorpha prolifera and their applicability in iron chelation. Carbohydr. Polym. 2018, 181, 616–623. [Google Scholar] [CrossRef]
- Bai, Y.; Shi, Y.-C.; Wetzel, D.L. Fourier Transform Infrared (FT-IR) Microspectroscopic Census of Single Starch Granules for Octenyl Succinate Ester Modification. J. Agric. Food. Chem. 2009, 57, 6443–6448. [Google Scholar] [CrossRef]
- Lohumi, S.; Lee, S.; Lee, W.-H.; Kim, M.S.; Mo, C.; Bae, H.; Cho, B.-K. Detection of Starch Adulteration in Onion Powder by FT-NIR and FT-IR Spectroscopy. J. Agric. Food. Chem. 2014, 62, 9246–9251. [Google Scholar] [CrossRef]
- Li, C.; Fu, X.; Huang, Q.; Luo, F.; You, L. Ultrasonic extraction and structural identification of polysaccharides from Prunella vulgaris and its antioxidant and antiproliferative activities. Eur. Food Res. Technol. 2015, 240, 49–60. [Google Scholar] [CrossRef]
- Zeng, F.; Chen, W.; He, P.; Zhan, Q.; Wang, Q.; Wu, H.; Zhang, M. Structural characterization of polysaccharides with potential antioxidant and immunomodulatory activities from Chinese water chestnut peels. Carbohydr. Polym. 2020, 246, 116551. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Guo, L.; Yan, A.; Feng, L.; Wan, Y. Fractionation, structure and conformation characterization of polysaccharides from Anoectochilus roxburghii. Carbohydr. Polym. 2020, 231, 115688. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.-H.; Liu, Y.; Wu, X.-L.; Liu, L.-Z.; Fu, W.; Song, D.-D. Isolation, purification, characterization and immunostimulatory activity of polysaccharides derived from American ginseng. Carbohydr. Polym. 2017, 156, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Chachlioutaki, K.; Tzimtzimis, E.K.; Tzetzis, D.; Chang, M.-W.; Ahmad, Z.; Karavasili, C.; Fatouros, D.G. Electrospun Orodispersible Films of Isoniazid for Pediatric Tuberculosis Treatment. Pharmaceutics 2020, 12, 470. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, S.; Shen, M.; Jiang, L.; Ren, Y.; Luo, Y.; Wen, H.; Xie, J. Physicochemical, rheological and thermal properties of Mesona chinensis polysaccharides obtained by sodium carbonate assisted and cellulase assisted extraction. Int. J. Biol. Macromol. 2019, 126, 30–36. [Google Scholar] [CrossRef]
- Garcia-Diaz, S.; Hernandez-Jaimes, C.; Escalona-Buendia, H.B.; Bello-Perez, L.A.; Vernon-Carter, E.J.; Alvarez-Ramirez, J. Effects of CaCO3 treatment on the morphology, crystallinity, rheology and hydrolysis of gelatinized maize starch dispersions. Food Chem. 2016, 207, 139–147. [Google Scholar] [CrossRef]
- Renou, F.; Petibon, O.; Malhiac, C.; Grisel, M. Effect of xanthan structure on its interaction with locust bean gum: Toward prediction of rheological properties. Food Hydrocoll. 2013, 32, 331–340. [Google Scholar] [CrossRef]
- Ji, Y.-H.; Liao, A.-M.; Huang, J.-H.; Thakur, K.; Li, X.-L.; Hu, F.; Wei, Z.-J. The rheological properties and emulsifying behavior of polysaccharides sequentially extracted from Amana edulis. Int. J. Biol. Macromol. 2019, 137, 160–168. [Google Scholar] [CrossRef]
- Doyle, J.P.; Lyons, G.; Morris, E.R. New proposals on “hyperentanglement” of galactomannans: Solution viscosity of fenugreek gum under neutral and alkaline conditions. Food Hydrocoll. 2009, 23, 1501–1510. [Google Scholar] [CrossRef]
- Yang, L.; Du, K. A comprehensive review on the natural, forced, and mixed convection of non-Newtonian fluids (nanofluids) inside different cavities. J. Therm. Anal. Calorim. 2020, 140, 2033–2054. [Google Scholar] [CrossRef]
- Ognyanov, M.; Remoroza, C.; Schols, H.A.; Georgiev, Y.N.; Petkova, N.T.; Krystyjan, M. Structural, rheological and functional properties of galactose-rich pectic polysaccharide fraction from leek. Carbohydr. Polym. 2020, 229, 115549. [Google Scholar] [CrossRef] [PubMed]
- Crawford, R.J.; Edler, K.J.; Lindhoud, S.; Scott, J.L.; Unali, G. Formation of shear thinning gels from partially oxidised cellulose nanofibrils. Green Chem. 2012, 14, 300–303. [Google Scholar] [CrossRef]
- Yoo, D.; Kim, C.; Yoo, B. Steady and dynamic shear rheology of rice starch-galactomannan mixtures. Starch-Starke 2005, 57, 310–318. [Google Scholar] [CrossRef]
- Zhang, L.; Shi, W.; Wang, J.; Jin, L.; Hu, G.; Zheng, Q.; Xie, H.; Chen, P. Unique gelation and rheological properties of the cellulose/CO2-based reversible ionic liquid/DMSO solutions. Carbohydr. Polym. 2019, 222, 115024. [Google Scholar] [CrossRef]
- Blankinship, J.C.; Fonte, S.J.; Six, J.; Schimel, J.P. Plant versus microbial controls on soil aggregate stability in a seasonally dry ecosystem. Geoderma 2016, 272, 39–50. [Google Scholar] [CrossRef] [Green Version]
- Sun, T.; Chen, Q.; Chen, Y.; Cruse, R.M.; Li, X.F.; Song, C.Y.; Kravchenko, Y.S.; Zhang, X.Y. A novel soil wetting technique for measuring wet stable aggregates. Soil Tillage Res. 2014, 141, 19–24. [Google Scholar] [CrossRef]
- Alami, Y.; Achouak, W.; Marol, C.; Heulin, T. Rhizosphere soil aggregation and plant growth promotion of sunflowers by an exopolysaccharide-producing Rhizobium sp. strain isolated from sunflower roots. Appl. Environ. Microbiol. 2000, 66, 3393–3398. [Google Scholar] [CrossRef] [Green Version]
- Kohler, J.; Roldan, A.; Campoy, M.; Caravaca, F. Unraveling the role of hyphal networks from arbuscular mycorrhizal fungi in aggregate stabilization of semiarid soils with different textures and carbonate contents. Plant Soil 2017, 410, 273–281. [Google Scholar] [CrossRef]
- Leifheit, E.F.; Veresoglou, S.D.; Lehmann, A.; Morris, E.K.; Rillig, M.C. Multiple factors influence the role of arbuscular mycorrhizal fungi in soil aggregation-a meta-analysis. Plant Soil 2014, 374, 523–537. [Google Scholar] [CrossRef]
- Rashid, M.I.; Mujawar, L.H.; Shahzad, T.; Almeelbi, T.; Ismail, I.M.I.; Oves, M. Bacteria and fungi can contribute to nutrients bioavailability and aggregate formation in degraded soils. Microbiol. Res. 2016, 183, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Wangli, S.-G.; He, L.; Sheng, X. Effect of Exopolysaccharide-Producing Bacteria on Water-Stable Macro-Aggregate Formation in Soil. Geomicrobiol. J. 2020, 37, 738–745. [Google Scholar] [CrossRef]
- Lin, D.; Ma, W.; Jin, Z.; Wang, Y.; Huang, Q.; Cai, P. Interactions of EPS with soil minerals: A combination study by ITC and CLSM. Colloids Surf. B-Biointerfaces 2016, 138, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.; Hasnain, S.; Berge, O.; Mahmood, T. Inoculating wheat seedlings with exopolysaccharide-producing bacteria restricts sodium uptake and stimulates plant growth under salt stress. Biol. Fertil. Soils 2004, 40, 157–162. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Hashem, A.; Abd Allah, E.F. Bacillus: A Biological Tool for Crop Improvement through Bio-Molecular Changes in Adverse Environments. Front. Physiol. 2017, 8, 667. [Google Scholar] [CrossRef] [Green Version]
- Hanafiah, Z.M.; Mohtar, W.H.M.W.; Abu Hasan, H.; Jensen, H.S.; Klaus, A.; Wan-Mohtar, W.A.Q.I. Performance of wild-Serbian Ganoderma lucidum mycelium in treating synthetic sewage loading using batch bioreactor. Sci. Rep. 2019, 9, 16109. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Zhang, X.; Yan, X.H.; Zhang, J.L.; Wang, L.Y.; Xue, H.; Jiang, G.C.; Ma, X.T.; Liu, X.J. Characterization, hypolipidemic and antioxidant activities of degraded polysaccharides from Ganoderma lucidum. Int. J. Biol. Macromol. 2019, 135, 706–716. [Google Scholar] [CrossRef]
- McElhaney, R.N.; de Gier, J.; van der Neut-Kok, E.C. The effect of alterations in fatty acid composition and cholesterol content on the nonelectrolyte permeability of Acholeplasma laidlawii B cells and derived liposomes. Biochim. Biophys. Acta 1973, 298, 500–512. [Google Scholar] [CrossRef]
- Kelly, S.J.; Muszynski, A.; Kawaharada, Y.; Hubber, A.M.; Sullivan, J.T.; Sandal, N.; Carlson, R.W.; Stougaard, J.; Ronson, C.W. Conditional Requirement for Exopolysaccharide in the Mesorhizobium-Lotus Symbiosis. Mol. Plant-Microbe Interact. 2013, 26, 319–329. [Google Scholar] [CrossRef] [Green Version]
- Honda, S.; Akao, E.; Suzuki, S.; Okuda, M.; Kakehi, K.; Nakamura, J. High-Performance Liquid-Chromatography of Reducing Carbohydrates as Strongly Ultraviolet-Absorbing and Electrochemically Sensitive 1-Phenyl-3-Methyl-5-Pyrazolone Derivatives. Anal. Biochem. 1989, 180, 351–357. [Google Scholar] [CrossRef]
- Yang, R.; Wang, X.; Liu, S.; Zhang, W.; Wang, P.; Liu, X.; Ren, Y.; Tan, X.; Chi, B. Bioinspired poly (gamma-glutamic acid) hydrogels for enhanced chondrogenesis of bone marrow-derived mesenchymal stem cells. Int. J. Biol. Macromol. 2020, 142, 332–344. [Google Scholar] [CrossRef] [PubMed]
- Amellal, N.; Burtin, G.; Bartoli, F.; Heulin, T. Colonization of wheat roots by an exopolysaccharide-producing Pantoea agglomerans strain and its effect on rhizosphere soil aggregation. Appl. Environ. Microbiol. 1998, 64, 3740–3747. [Google Scholar] [CrossRef] [PubMed]









| Fatty Acid Species | pH 2 (%) | pH 7.0 (%) | pH 9 (%) | pH 12 (%) | pH 13 (%) |
|---|---|---|---|---|---|
| C8:0 | 0.50 ± 0.01 d | 7.53 ± 0.18 c | 5.21 ± 0.18 c | 11.89 ± 0.15 b | 32.09 ± 0.99 a |
| C10:0 | 1.39 ± 0.04 d | 2.80 ± 0.17 b | 0.85 ± 0.02 d | 1.81 ± 0.04 c | 9.56 ± 0.43 a |
| C11:0 | 0.04 ± 0.01 e | 0.84 ± 0.03 b | 0.17 ± 0.02 d | 0.37 ± 0.01 c | 2.08 ± 0.14 a |
| C12:0 | 17.71 ± 0.57 b | 8.72 ± 0.11 c | 8.26 ± 0.03 c | 11.36 ± 0.06 a | 2.65 ± 0.12 d |
| C13:0 | 0.07 ± 0.01 e | 0.48 ± 0.01 d | 0.79 ± 0.01 b | 0.67 ± 0.01 c | 0.99 ± 0.02 a |
| C14:1 | 0.52 ± 0.01 d | 0.70 ± 0.02 d | 1.46 ± 0.10 c | 2.42 ± 0.04 b | 5.35 ± 0.16 a |
| C14:0 | 3.82 ± 0.24 c | 4.44 ± 0.05 c | 4.92 ± 0.05 c | 7.14 ± 0.05 b | 14.48 ± 0.84 a |
| C15:0 | 0.30 ± 0.01 d | 1.02 ± 0.03 b | 0.52 ± 0.01 c | 0.87 ± 0.01 c | 2.32 ± 0.14 a |
| C16:1 | 8.72 ± 0.32 d | 17.6 ± 0.09 b | 16.73 ± 0.08 c | 22.25 ± 0.02 a | 2.42 ± 0.13 e |
| C16:0 | 22.62 ± 0.5 b | 25.45 ± 0.10 a | 26.69 ± 1.90 a | 0.03 ± 0.01 d | 3.92 ± 0.17 c |
| C17:1 | 0.43 ± 0.01 d | 0.65 ± 0.02 c | 0.17 ± 0.01 e | 0.80 ± 0.01 b | 2.16 ± 0.14 a |
| C17:0 | 0.38 ± 0.01 c | 0.29 ± 0.02 c | 0.42 ± 0.02 c | 0.68 ± 0.01 b | 2.41 ± 0.11 a |
| C18:3 | 0.25 ± 0.02 c | 0.79 ± 0.01 a | 0.43 ± 0.01 b | 0.75 ± 0.01 a | 0.78 ± 0.02 a |
| C18:2 | 0.18 ± 0.01 e | 0.53 ± 0.01 c | 0.41 ± 0.01 d | 1.06 ± 0.02 b | 3.01 ± 0.03 a |
| C18:1 | 22.37 ± 0.29 c | 21.25 ± 0.14 d | 25.3 ± 0.05 b | 29.43 ± 0.07 a | 0.99 ± 0.04 e |
| C18:0 | 18.86 ± 0.47 a | 2.81 ± 0.03 d | 2.83 ± 0.13 d | 4.63 ± 0.02 c | 7.18 ± 0.28 b |
| C20:4 | 0.22 ± 0.01 c | 0.59 ± 0.05 b | 0.27 ± 0.02 c | 0.63 ± 0.01 b | 2.57 ± 0.10 a |
| C20:5 | 0.11 ± 0.01 c | 0.61 ± 0.02 b | 0.27 ± 0.01 c | 0.59 ± 0.01 b | 1.33 ± 0.05 a |
| C20:3 | 0.28 ± 0.02 d | 1.49 ± 0.08 a | 0.27 ± 0.01 d | 0.80 ± 0.01 c | 1.09 ± 0.01 b |
| C20:2 | 0.38 ± 0.03 e | 0.85 ± 0.06 c | 0.64 ± 0.02 d | 0.97 ± 0.01 a | 0.90 ± 0.03 b |
| C20:1 | 0.15 ± 0.01 d | 0.22 ± 0.02 d | 0.32 ± 0.01 c | 0.53 ± 0.01 b | 0.73 ± 0.03 a |
| U/S | 0.51 ± 1.01 d | 0.83 ± 0.01 c | 0.91 ± 0.04 b | 1.53 ± 0.01 a | 0.27 ± 0.01 e |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Sun, L.; Wang, R.; Gu, Y.; Xu, H.; Lei, P. High-Efficiency Extraction of Pantoea alhagi Exopolysaccharides Driven by pH-Related Changes in the Envelope Structure. Molecules 2022, 27, 7209. https://doi.org/10.3390/molecules27217209
Ma Y, Sun L, Wang R, Gu Y, Xu H, Lei P. High-Efficiency Extraction of Pantoea alhagi Exopolysaccharides Driven by pH-Related Changes in the Envelope Structure. Molecules. 2022; 27(21):7209. https://doi.org/10.3390/molecules27217209
Chicago/Turabian StyleMa, Yuhang, Liang Sun, Rui Wang, Yian Gu, Hong Xu, and Peng Lei. 2022. "High-Efficiency Extraction of Pantoea alhagi Exopolysaccharides Driven by pH-Related Changes in the Envelope Structure" Molecules 27, no. 21: 7209. https://doi.org/10.3390/molecules27217209