“One-Pot” CuCl2-Mediated Condensation/C–S Bond Coupling Reactions to Synthesize Dibenzothiazepines by Bi-Functional-Reagent N, N′-Dimethylethane-1,2-Diamine
Abstract
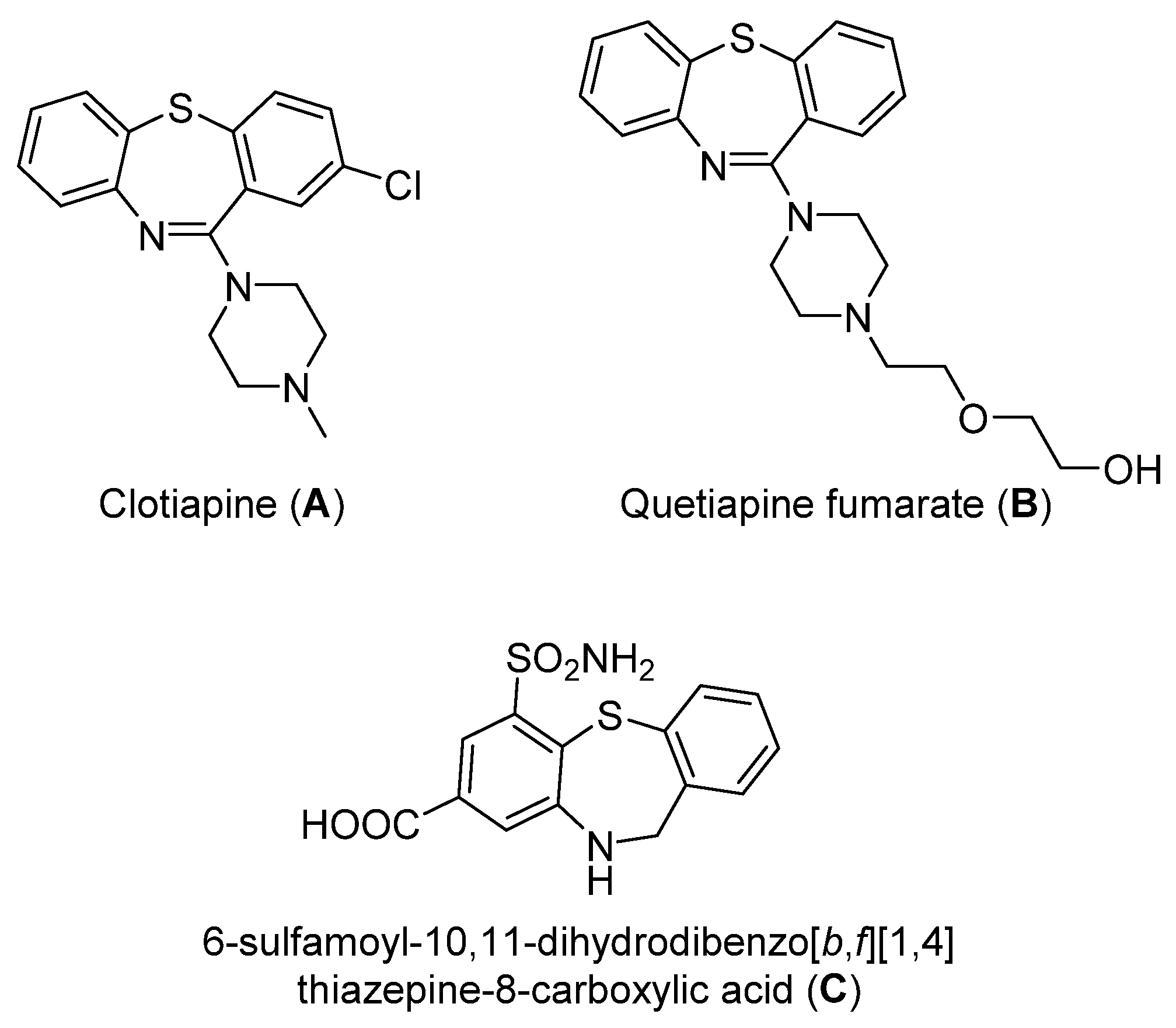
:1. Introduction
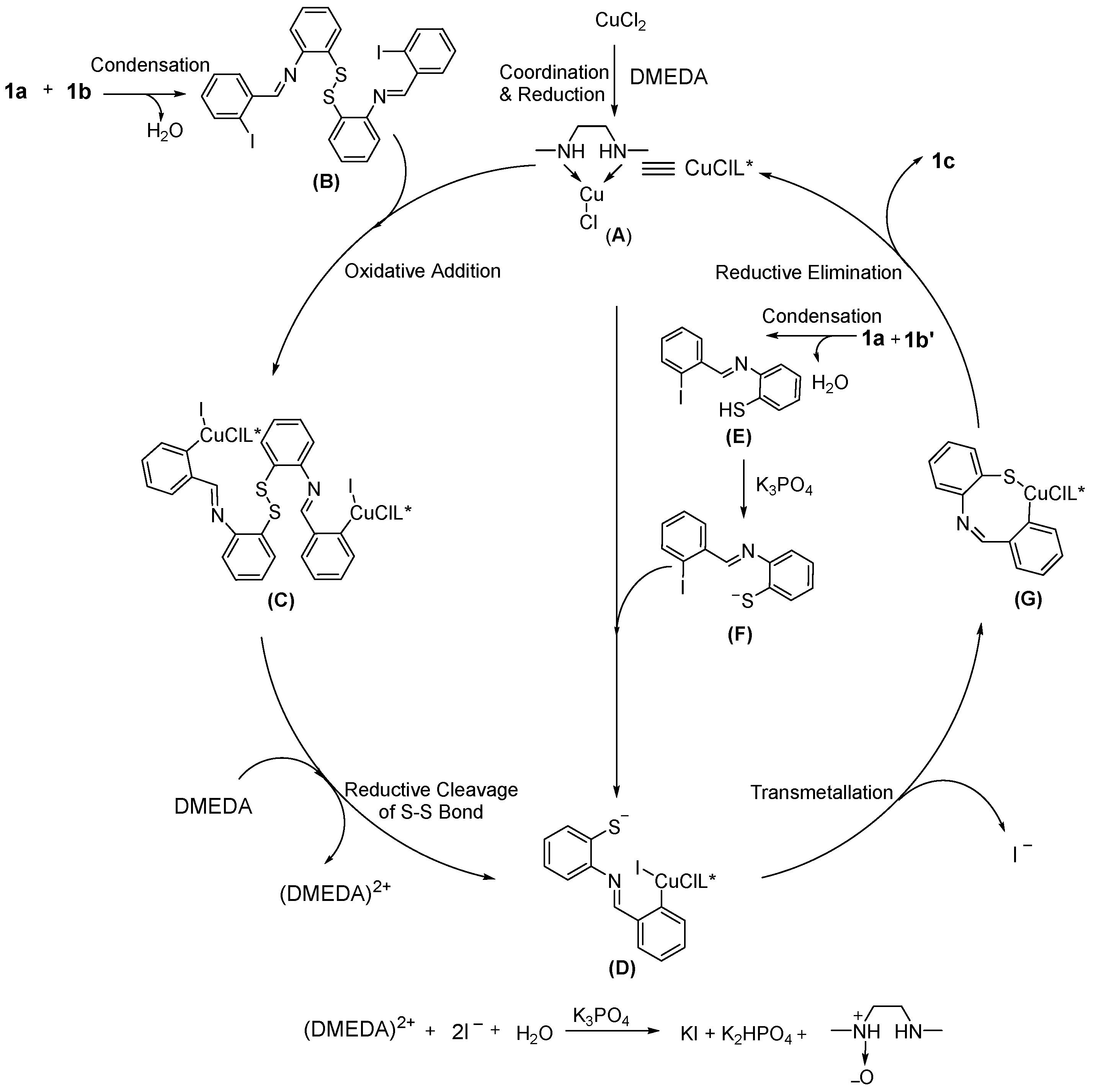
2. Results
3. Experimental Section
3.1. General
3.2. General Procedure for the Synthesis of Dibenzo[b,f][1,4]thiazepines (1c–12c) Catalyzed by CuCl2 in DMEDA
3.3. General Procedure for the Synthesis of 11-Methyldibenzo[b,f][1,4]thiazepines (1e–5e) Catalyzed by CuCl2 in DMEDA
3.4. Characterization Data
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Saha, D.; Jain, G.; Sharma, A. Benzothiazepines: Chemistry of a privileged scaffold. RSC Adv. 2015, 5, 70619–70639. [Google Scholar] [CrossRef]
- Geyer, H.M., 3rd; Watzman, N.; Buckley, J.P. Effects of a tranquilizer and two antidepressants on learned and unlearned behaviors. J. Pharm. Sci. 1970, 59, 964–968. [Google Scholar] [CrossRef] [PubMed]
- Kawakita, S.; Kinoshita, M.; Ishikawa, H.; Kagoshima, T.; Katori, R.; Ishikawa, K.; Hirota, Y. Efficacy and safety of clentiazem in patients with essential hypertension: Results of an early pilot test. Clin. Cardiol. 1991, 14, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Morton, G.C.; Salvino, J.M.; Labaudiniere, R.F.; Herpin, T.F. Novel solid-phase synthesis of 1,5-benzothiazepine-4-one derivatives. Tetrahedron Lett. 2000, 41, 3029–3033. [Google Scholar] [CrossRef]
- Geller, V.; Gorzaltsan, I.; Shleifer, T.; Belmaker, R.H.; Bersudsky, Y. Clotiapine compared with chlorpromazine in chronic schizophrenia. Schizophr. Res. 2005, 80, 343–347. [Google Scholar] [CrossRef]
- Agarwal, S.; HariKumar, S.L.; Negi, P.; Upadhyay, N.; Garg, R. Quetiapine Fumarate Loaded Nanostructured Lipid Carrier for Enhancing Oral Bioavailability: Design, Development and Pharmacokinetic Assessment. Curr. Drug Deliv. 2021, 18, 184–198. [Google Scholar] [CrossRef]
- Hopenwasser, J.; Mozayani, A.; Danielson, T.J.; Harbin, J.; Narula, H.S.; Posey, D.H.; Shrode, P.W.; Wilson, S.K.; Li, R.; Sanchez, L.A. Postmortem distribution of the novel antipsychotic drug quetiapine. J. Anal. Toxicol. 2004, 28, 264–268. [Google Scholar] [CrossRef] [Green Version]
- Nyavanandi, D.; Kallakunta, V.R.; Sarabu, S.; Butreddy, A.; Narala, S.; Bandari, S.; Repka, M.A. Impact of hydrophilic binders on stability of lipid-based sustained release matrices of quetiapine fumarate by the continuous twin screw melt granulation technique. Adv. Powder Technol. 2021, 32, 2591–2604. [Google Scholar] [CrossRef]
- Guo, R.N.; Gao, K.; Ye, Z.S.; Shi, L.; Li, Y.Q.; Zhou, Y.G. Iridium-catalyzed asymmetric hydrogenation of dibenzo[b,f][1,4]thiazepines. Pure. Appl. Chem. 2013, 85, 843–849. [Google Scholar] [CrossRef]
- Lin, Y.C.; Li, N.C.; Cherng, Y.J. Microwave-Assisted Synthesis of Substituted Dibenzo[b,f][1,4]thiazepines, Dibenzo[b,f][1,4]oxazepines, Benzothiazoles, and Benzimidazoles. J. Heterocyclic. Chem. 2014, 51, 808–814. [Google Scholar] [CrossRef]
- Saha, D.; Wadhwa, P.; Sharma, A. A sequential synthetic strategy towards unexplored dibenzo[b,f][1,4]thiazepine carboxamides: Copper catalysed C-S cyclisation followed by Ugi type 3CC cascade. RSC Adv. 2015, 5, 33067–33076. [Google Scholar] [CrossRef]
- De Munck, L.; Sukowski, V.; Vila, C.; Munoz, M.C.; Pedro, J.R. Catalytic enantioselective aza-Reformatsky reaction with seven-membered cyclic imines dibenzo[b,f][1,4]oxazepines. Org. Chem. Front. 2017, 4, 1624–1628. [Google Scholar] [CrossRef] [Green Version]
- Saha, D.; Kaur, T.; Sharma, A. Facile Construction of Imidazo-benzothia-/oxazepines by a Quick and Efficient van Leusen Protocol. Asian J. Org. Chem. 2017, 6, 527–533. [Google Scholar] [CrossRef]
- Shimotori, Y.; Hoshi, M.; Murata, M.; Ogawa, N.; Miyakoshi, T.; Kanamoto, T. Synthesis of dibenzothiazepine analogues by one-pot S-arylation and intramolecular cyclization of diaryl sulfides and evaluation of antibacterial properties. Heterocycl. Commun. 2018, 24, 219–230. [Google Scholar] [CrossRef]
- Balakrishna, B.; Bauza, A.; Frontera, A.; Vidal-Ferran, A. Asymmetric Hydrogenation of Seven-Membered C=N-containing Heterocycles and Rationalization of the Enantioselectivity. Chem. Eur. J. 2016, 22, 10607–10613. [Google Scholar] [CrossRef] [PubMed]
- Fujii, T.; Hao, W.; Yoshimura, T. New method for the preparation of dibenzo[b,f][1,4]thiazepines. Heteroatom. Chem. 2004, 15, 246–250. [Google Scholar] [CrossRef]
- Creed, T.; Leardini, R.; McNab, H.; Nanni, D.; Nicolson, I.S.; Reed, D. Gas-phase cyclisation reactions of 1-(2-arylthiophenyl)alkaniminyl and 2-(aryliminomethyl)thiophenoxyl radicals. J. Chem. Soc. Perkin Trans. 1 2001, 9, 1079–1085. [Google Scholar] [CrossRef]
- Evano, G.; Blanchard, N.; Toumi, M. Copper-mediated coupling reactions and their applications in natural products and designed biomolecules synthesis. Chem. Rev. 2008, 108, 3054–3131. [Google Scholar] [CrossRef]
- Okamoto, N.; Sakurai, K.; Ishikura, M.; Takeda, K.; Yanada, R. One-pot concise syntheses of benzimidazo[2,1-a]isoquinolines by a microwave-accelerated tandem process. Tetrahedron Lett. 2009, 50, 4167–4169. [Google Scholar] [CrossRef]
- Luo, Y.; Xu, J. Annulation of Diaryl(aryl)phosphenes and Cyclic Imines to Access Benzo-δ-phospholactams. Org. Lett. 2020, 22, 7780–7785. [Google Scholar] [CrossRef]
- Monnier, F.; Taillefer, M. Catalytic C-C, C-N, and C-O Ullmann-type coupling reactions: Copper makes a difference. Angew. Chem. Int. Ed. 2008, 47, 3096–3099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, S.H.; Kim, J.Y.; Kwak, J.; Chang, S. Recent advances in the transition metal-catalyzed twofold oxidative C-H bond activation strategy for C-C and C-N bond formation. Chem. Soc. Rev. 2011, 40, 5068–5083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Surry, D.S.; Buchwald, S.L. Diamine ligands in copper-catalyzed reactions. Chem. Sci. 2010, 1, 13–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monnier, F.; Taillefer, M. Catalytic C-C, C-N, and C-O Ullmann-Type Coupling Reactions. Angew. Chem. Int. Ed. 2009, 48, 6954–6971. [Google Scholar] [CrossRef]
- Ma, D.W.; Cai, Q.A. Copper/Amino Acid Catalyzed Cross-Couplings of Aryl and Vinyl Halides with Nucleophiles. Accounts. Chem. Res. 2008, 41, 1450–1460. [Google Scholar] [CrossRef]
- Dai, W.P.; Shi, H.Y.; Zhao, X.H.; Cao, S. Sterically Controlled Cu-Catalyzed or Transition-Metal-Free Cross-Coupling of gem-Difluoroalkenes with Tertiary, Secondary, and Primary Alkyl Grignard Reagents. Org. Lett. 2016, 18, 4284–4287. [Google Scholar] [CrossRef]
- Ke, J.; Tang, Y.L.; Yi, H.; Li, Y.L.; Cheng, Y.D.; Liu, C.; Lei, A.W. Copper-Catalyzed Radical/Radical C-sp3-H/P-H Cross-Coupling: Alpha-Phosphorylation of Aryl Ketone O-Acetyloximes. Angew. Chem. Int. Ed. 2015, 54, 6604–6607. [Google Scholar] [CrossRef]
- Shen, G.D.; Yang, B.C.; Huang, X.Q.; Hou, Y.X.; Gao, H.; Cui, J.C.; Cui, C.S.; Zhang, T.X. Copper- and Palladium-Catalyzed Cross-Coupling Reactions for the Synthesis of N-Fused Benzo[4,5]imidazo[2,1-b]thiazole Derivatives via Substituted trans-1,2-Diiodoalkenes, 1H-Benzo[d]imidazole-2-thiols, and Halobenzenes. J. Org. Chem. 2017, 82, 3798–3805. [Google Scholar] [CrossRef]
- Shen, G.D.; Lu, Q.C.; Wang, Z.Y.; Sun, W.W.; Zhang, Y.L.; Huang, X.Q.; Sun, M.M.; Wang, Z.M. Environmentally Friendly and Recyclable CuCl2-Mediated C-S Bond Coupling Strategy Using DMEDA as Ligand, Base, and Solvent. Synthesis 2022, 54, 184–198. [Google Scholar] [CrossRef]
- Narasimhan, N.S.; Chandrachood, P.S. Synthetic Application of Lithiation Reactions; X. Synthesis of Dibenzo[b,f][1,4]oxazepine, Dibenzo(b,f][1,4]thiazepine, and 5H-Dibenzo[b,e][1,4]-diazepine. Synthesis 1979, 8, 589–590. [Google Scholar] [CrossRef]
- Jílek, J.O.; Pelz, K.; Pavlíčková, D.; Protiva, M. Neurotrope und psychotrope Substanzen IV. Über einige neue Derivate des Dibenzo(b,f)(1,4)thiazepins. Collect. Czechoslov. Chem. Commun. 1965, 30, 1676–1683. [Google Scholar] [CrossRef]


| Entry | Catalyst | DMEDA (mL) | Base | Yield (%) b |
|---|---|---|---|---|
| 1 | CuCl2 | 0.50 | Cs2CO3 | 73 |
| 2 | CuCl2 | 0.25 | Cs2CO3 | 73 |
| 3 | CuCl2 | 0.15 | Cs2CO3 | 46 |
| 4 | CuCl2 | 0.25 | / | 22 |
| 5 | CuCl2 | 0.25 | K3PO4 | 82 |
| 6 | CuCl2 | 0.25 | K2CO3 | 62 |
| 7 | CuCl2 | 0.25 | K3PO4 | 82 c |
| 8 | CuCl2 | 0.25 | K3PO4 | 73 d |
| 9 | Cu(OAc)2 | 0.25 | K3PO4 | 66 |
| 10 | CuSO4·5H2O | 0.25 | K3PO4 | 69 |
| 11 | CuI | 0.25 | K3PO4 | 72 |
| 12 | CuCl2 | 0.25 | K3PO4 | 69 e |
| 13 | CuCl2 | 1.25 | K3PO4 | 82 f |
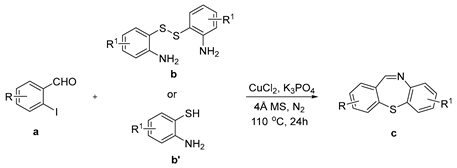
| Entry | a | b/b′ | Product | Yield (%) b |
|---|---|---|---|---|
| 1 | 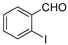 1a 1a | 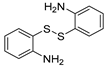 1b 1b | 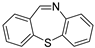 1c 1c | 82 (53 c, 35 d) |
| 2 | 1a | 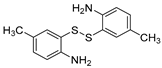 2b 2b |  2c 2c | 80 |
| 3 | 1a | 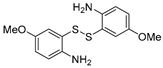 3b 3b | 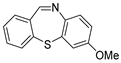 3c 3c | 86 |
| 4 | 1a | 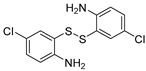 4b 4b | 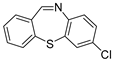 4c 4c | 89 |
| 5 | 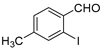 2a 2a | 1b | 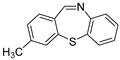 5c 5c | 84 |
| 6 |  3a 3a | 1b | 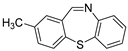 6c 6c | 73 |
| 7 | 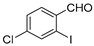 4a 4a | 1b |  7c 7c | 86 |
| 8 | 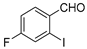 5a 5a | 1b | 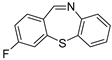 8c 8c | 78 |
| 9 |  6a 6a | 1b | 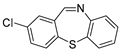 9c 9c | 83 |
| 10 | 2a | 3b | 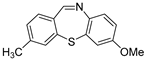 10c 10c | 74 |
| 11 | 6a | 2b | 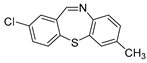 11c 11c | 84 |
| 12 | 6a | 3b |  12c 12c | 75 |
| 13 | 1a | 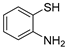 1b′ 1b′ | 1c | 81 |
| 14 | 1a | 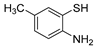 2b′ 2b′ | 2c | 80 |
| 15 | 1a | 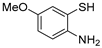 3b′ 3b′ | 3c | 86 |
| 16 | 2a | 1b′ | 5c | 78 |
| 17 | 3a | 1b′ | 6c | 83 |
| 18 | 4a | 1b′ | 7c | 88 |
| 19 | 5a | 1b′ | 8c | 89 |
| 20 | 6a | 1b′ | 9c | 80 |
| 21 | 6a | 2b′ | 11c | 78 |
| 22 | 6a | 3b′ | 12c | 80 |

| Entry | d | b/b′ | Product | Yield (%) b |
|---|---|---|---|---|
| 1 | 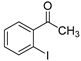 1d 1d | 1b | 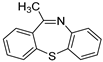 1e 1e | 75 |
| 2 | 1d | 2b | 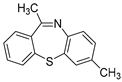 2e 2e | 74 |
| 3 | 1d | 3b |  3e 3e | 69 |
| 4 | 1d |  4b 4b | 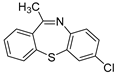 4e 4e | 61 |
| 5 | 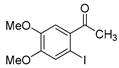 2d 2d | 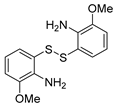 5b 5b | 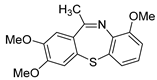 5e 5e | 77 |
| 6 | 1d | 1b′ | 1e | 95 |
| 7 | 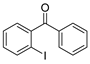 3d 3d | 1b | 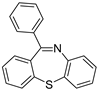 6e 6e |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Lu, Q.; Li, Z.; Fang, C.; Liu, R.; Yang, B.; Shen, G. “One-Pot” CuCl2-Mediated Condensation/C–S Bond Coupling Reactions to Synthesize Dibenzothiazepines by Bi-Functional-Reagent N, N′-Dimethylethane-1,2-Diamine. Molecules 2022, 27, 7392. https://doi.org/10.3390/molecules27217392
Wang D, Lu Q, Li Z, Fang C, Liu R, Yang B, Shen G. “One-Pot” CuCl2-Mediated Condensation/C–S Bond Coupling Reactions to Synthesize Dibenzothiazepines by Bi-Functional-Reagent N, N′-Dimethylethane-1,2-Diamine. Molecules. 2022; 27(21):7392. https://doi.org/10.3390/molecules27217392
Chicago/Turabian StyleWang, Dehe, Qichao Lu, Zhanjun Li, Chen Fang, Ran Liu, Bingchuan Yang, and Guodong Shen. 2022. "“One-Pot” CuCl2-Mediated Condensation/C–S Bond Coupling Reactions to Synthesize Dibenzothiazepines by Bi-Functional-Reagent N, N′-Dimethylethane-1,2-Diamine" Molecules 27, no. 21: 7392. https://doi.org/10.3390/molecules27217392





