Processing of Biomass Prior to Hydrogen Fermentation and Post-Fermentative Broth Management
Abstract
:1. Introduction
2. State of the Art on the Biomass Recalcitrance
2.1. Biomass Recalcitrance and the Pre-Treatment of Raw Materials
2.2. Issues Related to the Availability and Construction of Biomass Surface
2.2.1. Overcoming Cellulose Crystallinity
2.2.2. Degree of Polymerization
2.3. Inhibitory Compounds Generation during Pretreatment
2.3.1. Inhibitors Generated during Acidic Pretreatment
2.3.2. Inhibitors Generated during Alkaline Pretreatment
2.3.3. Inhibitors Generated during Oxidative Pretreatment
2.4. Summary of Pre-Treatment Methods Advantages and Disadvantages
3. Bioconversion of Hydrolysates
3.1. Hydrogen Generation
| Substrate | Amount | Organism | Reactor type | pH | Temperature (°C) | HRT | Hydrogen Productivity | Hydrogen Yield | COD Removal (%) | % H2 | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Glucose | 10 g/L | Clostridiaceae Flexibacteraceae * | Membrane Continuous | 5.5 | 35 | 3.3 | 640 mL H2/(L·h) | 4 mol H2/mol glucose | - | 60 | [99] |
| Sucrose | 10 g/L | E. cloacae IIT-BT 08 * | Batch | 6 | 36 | - | 660 mL H2/(L·h) | 6 mol H2/mol sucrose | - | 92 | [100] |
| Glucose | 1% | E. cloacae * | Batch | 6 | 36 | - | 447 mL H2/(L·h) | 2.2 mol H2/mol glucose | - | - | [100] |
| D-Xylose | 10 g/L | E. cloacae IIT-BT 08 * | Batch | 6 | 36 | - | 348 mL H2/(L·h) | 0.95 mol H2/mol xylose | - | - | [100] |
| L-Arabinose | 10 g/L | E. cloacae IIT-BT 08 * | Batch | 6 | 36 | - | 360 mL H2/(L·h) | 1.5 mol H2/mol arabinose | - | - | [100] |
| Glucose | 10 g/L | Mixed culture from compost | Batch | 5.5 | 60 | - | 147 mL H2/(L·h) | 2.1 mol H2/mol glucose | - | - | [101] |
| Glucose | 20 g COD/L | Clostridia sp. * | CSTR Continuous | 6 | 28–32 | 6 | 7.42 mmol H2/(gVSS·h) | 1.42 mol H2/mol glucose | - | 43 | [102] |
| Glucose | 7 g/L | Mixed culture | CSTR Continuous | 5.5 | 36 | 6 | - | 2.1 molH2/mol glucose | - | 64 | [103] |
| Glucose | 4.85 g COD/L | Mixed culture | UASB Continuous | 7.2 | 70 | 26.7 | 11.15 mmol H2/d | 2.46 mol H2/mol hexose | - | 55 | [104] |
| Sucrose | 20 g COD/L | Mixed culture | Immobilized bed Continuous | 6.7 | 35 | 1 | 1.32 L H2/(L·h) | - | - | 34 | [105] |
| Sucrose | 1 g COD/L | Mixed culture | Batch | 6 | 26 | - | - | 1.8 mol H2/mol sucrose | - | - | [106] |
| Sucrose | 20 g COD/L | Mixed culture | CSTR Continuous | 6.7 | 35 | 8 | 0.105 mol H2/h | 3.47mol H2/mol sucrose | - | 42 | [107] |
| Sucrose | 25 g/L | Mixed culture | Fermenter Batch | 5.5 | 35 | - | 1504 mL H2/h | 2 mol H2/mol glucose | - | - | [108] |
| Lactose | 29 mmol/L | C.termolacticum * | CSTR Continuous | 7 | 58 | 35.7 | 2.58 mmol H2/(L·h) | 1.5 mol H2/mol hexose | - | 55 | [109] |
| Glucose | 5.5 g/L | Enterobacter aerogenes * | Bioreactors | 42 | 48 | 25.44 mL/g biomass 283.45 _ 1.87 mL/YTRS | - | - | - | [26] | |
| Paulownia | - | photosynthetic consortium HAU-M1 * | - | 7 | 30 | 26–38 | 338.41 mL | 67.11 mL/g | 62 | - | [110] |
| Xylose | 20 g/L | Lactobacillus and Sporolactobacillus spp., Clostridium sp * | Dynamic membrane module bioreactor (DMBR) | 7.5 | 37 | 3–12 | 30.26 L H2/L-d | 1.40 mol H2/mol xylose | - | - | [111] |
| Glucose | 10 g/L | Caldicellulosiruptor * | Batch | - | 70 | 20 | 10.55 mmol/L/h | 4 mol H2/mol glucose | - | - | [112] |
| Glucose and Xylose | 50 % mol/mol | Rhodopseudomonas palustris * | Bioreactor | 7 | 30 | - | 30.6 mL h−1 L−1 | 1.63 (mol H2/mol carbon) | - | - | [113] |
| Glucose, xylose | 41.17 g/L | Rhodospirillum rubrum * | Batch | 4.5 | 60 | - | 819 mL H2/L medium m/7 d | - | 82 | - | [114] |
| Rice husk | 5 g dw | Clostridium termitidis ATCC-21846 * and Clostridium intestinale ATCC-BAA 1027 | Batch | 7.5 | 37 | - | 0.023 mL H2 g−1 dw rice husk h | 5.9 mL g−1 dw Rice husk | - | 29.26 mL | [115] |
| Corncob |
10 g | HAU-M1 * photosynthetic bacteria | Batch | 7 | 50 | 48 | - | 27.34 mL/g TS | - | 80.94 | [116] |
| Duckweed and corn straw | 5:1 | photosynthetic strain HAU-M1 | Batch | 8 | 30 | 18.57 | - | 85.6 mL/g TS | - | [117] |
3.2. Genetic Modifications of Microorganisms
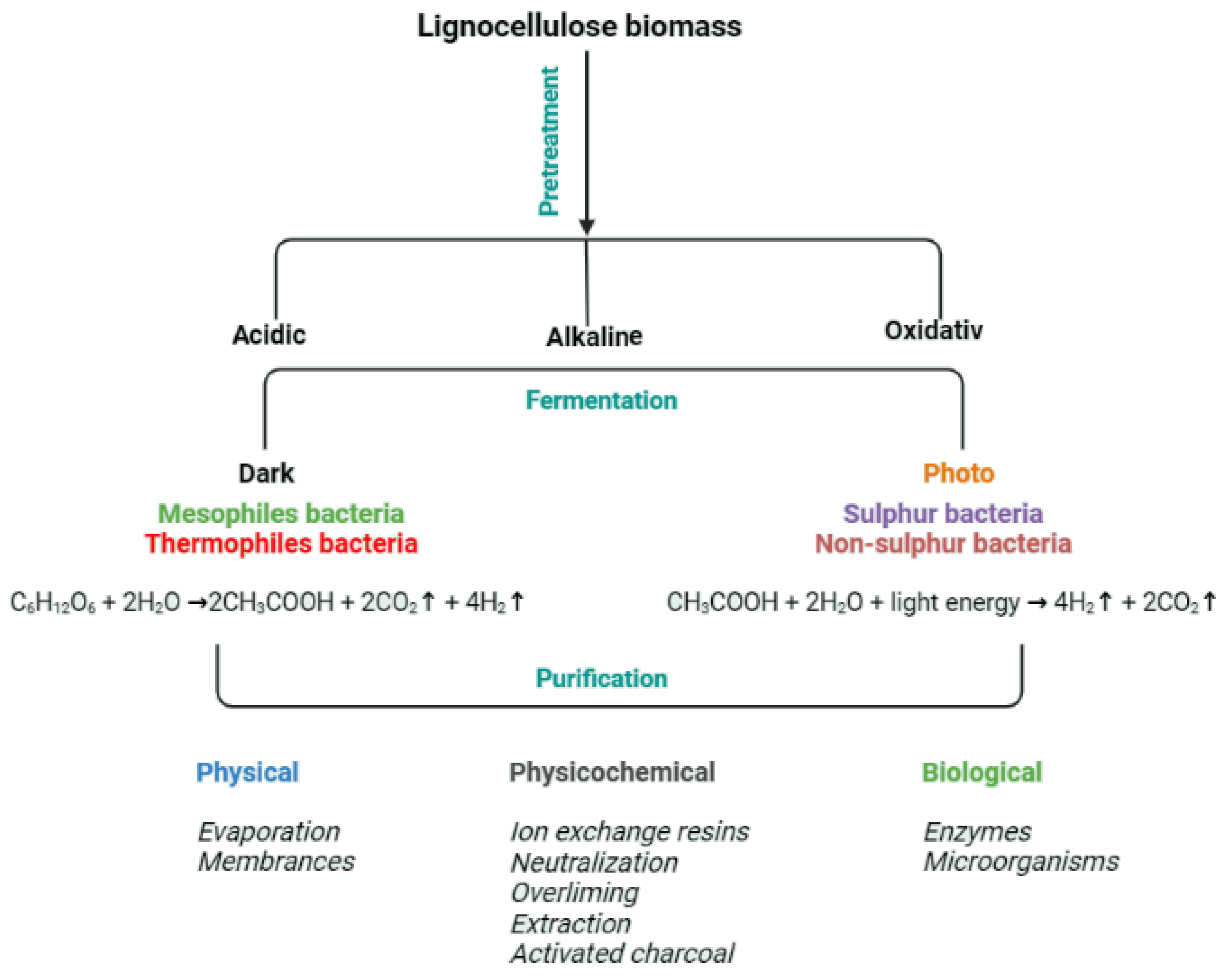
4. Post-Fermentative Broth Detoxification and Management Methods
4.1. Physical Methods
4.1.1. Evaporation
4.1.2. Membranes
4.2. Physicochemical
4.2.1. Ion Exchange Resins
4.2.2. Neutralization
4.2.3. Overliming
4.2.4. Activated Charcoal
4.2.5. Extraction
4.3. Biological Methods
4.4. Perspectives of Broth Detoxification and Management Methods
5. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Peng, L.; Fu, D.; Chu, H.; Wang, Z.; Qi, H. Biofuel production from microalgae: A review. Environ. Chem. Lett. 2020, 18, 285–297. [Google Scholar] [CrossRef]
- Sharma, R.B.; Parey, A. Modelling of acoustic emission generated due to pitting on spur gear. Eng. Fail. Anal. 2018, 86, 1–20. [Google Scholar] [CrossRef]
- Lay, C.; Dharmaraja, J.; Shobana, S.; Arvindnarayan, S.; Priya, R.K.; Jeyakumar, R.B.; Saratale, R.G.; Park, Y.; Kumar, V.; Kumar, G. Lignocellulose biohydrogen towards net zero emission: A review on recent developments. Bioresour. Technol. 2022, 364, 128084. [Google Scholar] [CrossRef] [PubMed]
- Jayakody, L.N.; Ferdouse, J.; Hayashi, N.; Kitagaki, H. Identification and detoxification of glycolaldehyde, an unattended bioethanol fermentation inhibitor. Crit. Rev. Biotechnol. 2017, 37, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Soltanian, S.; Aghbashlo, M.; Almasi, F.; Hosseinzadeh-Bandbafha, H.; Nizami, A.-S.; Ok, Y.S.; Lam, S.S.; Tabatabaei, M. A critical review of the effects of pretreatment methods on the exergetic aspects of lignocellulosic biofuels. Energy Convers. Manag. 2020, 212, 112792. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Jagtap, S.S.; Bedekar, A.A.; Bhatia, R.K.; Rajendran, K.; Pugazhendhi, A.; Rao, C.V.; Atabani, A.; Kumar, G.; Yang, Y.-H. Renewable biohydrogen production from lignocellulosic biomass using fermentation and integration of systems with other energy generation technologies. Sci. Total Environ. 2021, 765, 144429. [Google Scholar] [CrossRef]
- Lv, Y.; Liu, X.; Zhou, S.; Yu, Q.; Xu, Y. Microbial saccharification—Biorefinery platform for lignocellulose. Ind. Crops Prod. 2022, 189, 115761. [Google Scholar] [CrossRef]
- Mahmoodi, P.; Karimi, K.; Taherzadeh, M.J. Efficient conversion of municipal solid waste to biofuel by simultaneous dilute-acid hydrolysis of starch and pretreatment of lignocelluloses. Energy Convers. Manag. 2018, 166, 569–578. [Google Scholar] [CrossRef]
- Zoghlami, A.; Paës, G. Lignocellulosic biomass: Understanding recalcitrance and predicting hydrolysis. Front. Chem. 2019, 7, 874. [Google Scholar] [CrossRef] [Green Version]
- Sharma, R.; Joshi, R.; Kumar, D. Present status and future prospect of genetic and metabolic engineering for biofuels production from lignocellulosic biomass. In Genetic and Metabolic Engineering for Improved Biofuel Production from Lignocellulosic Biomass; Elsevier: Amsterdam, The Netherlands, 2020; pp. 171–192. [Google Scholar]
- Vu, H.P.; Nguyen, L.N.; Vu, M.T.; Johir, M.A.H.; McLaughlan, R.; Nghiem, L.D. A comprehensive review on the framework to valorise lignocellulosic biomass as biorefinery feedstocks. Sci. Total Environ. 2020, 743, 140630. [Google Scholar] [CrossRef]
- Li, H.; Liu, Y.; Gao, X.; Li, X. Preparation and characterization of cassava starch-based adsorbents for separating of azeotropic ethanol-water in biofuels ethanol production. J. Chem. Technol. Biotechnol. 2016, 91, 977–984. [Google Scholar] [CrossRef]
- Kucharska, K.; Rybarczyk, P.; Hołowacz, I.; Łukajtis, R.; Glinka, M.; Kamiński, M. Pretreatment of lignocellulosic materials as substrates for fermentation processes. Molecules 2018, 23, 2937. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Huang, W.; Sathitsuksanoh, N.; Zhu, Z.; Zhang, Y.-H.P. Biohydrogenation from biomass sugar mediated by in vitro synthetic enzymatic pathways. Chem. Biol. 2011, 18, 372–380. [Google Scholar] [CrossRef] [Green Version]
- Łukajtis, R.; Hołowacz, I.; Kucharska, K.; Glinka, M.; Rybarczyk, P.; Przyjazny, A.; Kamiński, M. Hydrogen production from biomass using dark fermentation. Renew. Sustain. Energy Rev. 2018, 91, 665–694. [Google Scholar] [CrossRef]
- Hawkes, F.R.; Hussy, I.; Kyazze, G.; Dinsdale, R.; Hawkes, D.L. Continuous dark fermentative hydrogen production by mesophilic microflora: Principles and progress. Int. J. Hydrogen Energy 2007, 32, 172–184. [Google Scholar] [CrossRef]
- Adessi, A.; Philippis, R.D. Hydrogen production: Photofermentation. In Microbial Technologies in Advanced Biofuels Production; Springer: Berlin/Heidelberg, Germany, 2012; pp. 53–75. [Google Scholar]
- Ashokkumar, V.; Venkatkarthick, R.; Jayashree, S.; Chuetor, S.; Dharmaraj, S.; Kumar, G.; Chen, W.; Ngamcharussrivichai, C. Recent advances in lignocellulosic biomass for biofuels and value-added bioproducts—A critical review. Bioresour. Technol. 2022, 344, 126195. [Google Scholar] [CrossRef]
- Kamm, B.; Kamm, M. Biorefineries–multi product processes. White Biotechnol. 2007, 105, 175–204. [Google Scholar]
- Claassen, P.; Van Lier, J.; Lopez Contreras, A.; Van Niel, E.; Sijtsma, L.; Stams, A.; de Vries, S.S.; Weusthuis, R.A. Utilisation of biomass for the supply of energy carriers. Appl. Microbiol. Biotechnol. 1999, 52, 741–755. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, L.; Liu, D. Biomass recalcitrance. Part I: The chemical compositions and physical structures affecting the enzymatic hydrolysis of lignocellulose. Biofuels Bioprod. Biorefin. 2012, 6, 465–482. [Google Scholar] [CrossRef]
- Zheng, Y.; Pan, Z.; Zhang, R. Overview of biomass pretreatment for cellulosic ethanol production. Int. J. Agric. Biol. Eng. 2009, 2, 51–68. [Google Scholar]
- Pauly, M.; Keegstra, K. Plant cell wall polymers as precursors for biofuels. Curr. Opin. Plant Biol. 2010, 13, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Alvira, P.; Tomás-Pejó, E.; Ballesteros, M.; Negro, M. Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: A review. Bioresour. Technol. 2010, 101, 4851–4861. [Google Scholar] [CrossRef] [PubMed]
- Kucharska, K.; Hołowacz, I.; Konopacka-Łyskawa, D.; Rybarczyk, P.; Kamiński, M. Key issues in modeling and optimization of lignocellulosic biomass fermentative conversion to gaseous biofuels. Renew. Energy 2018, 129, 384–408. [Google Scholar] [CrossRef]
- Kucharska, K.; Cieśliński, H.; Rybarczyk, P.; Słupek, E.; Łukajtis, R.; Wychodnik, K.; Kamiński, M. Fermentative conversion of two-step pre-treated lignocellulosic biomass to hydrogen. Catalysts 2019, 9, 858. [Google Scholar] [CrossRef] [Green Version]
- Łukajtis, R.; Kucharska, K.; Hołowacz, I.; Rybarczyk, P.; Wychodnik, K.; Słupek, E.; Nowak, P.; Kamiński, M. Comparison and optimization of saccharification conditions of alkaline pre-treated triticale straw for acid and enzymatic hydrolysis followed by ethanol fermentation. Energies 2018, 11, 639. [Google Scholar] [CrossRef] [Green Version]
- Kucharska, K.; Słupek, E.; Cieśliński, H.; Kamiński, M. Advantageous conditions of saccharification of lignocellulosic biomass for biofuels generation via fermentation processes. Chem. Pap. 2020, 74, 1199–1209. [Google Scholar] [CrossRef] [Green Version]
- Kucharska, K.; Łukajtis, R.; Słupek, E.; Cieśliński, H.; Rybarczyk, P.; Kamiński, M. Hydrogen production from energy poplar preceded by MEA pre-treatment and enzymatic hydrolysis. Molecules 2018, 23, 3029. [Google Scholar] [CrossRef] [Green Version]
- Yu, G.; Yano, S.; Inoue, H.; Inoue, S.; Endo, T.; Sawayama, S. Pretreatment of Rice Straw by a Hot-Compressed Water Process for Enzymatic Hydrolysis. Appl. Biochem. Biotechnol. 2010, 160, 539–551. [Google Scholar] [CrossRef]
- Sharma, H.K.; Xu, C.; Qin, W. Biological pretreatment of lignocellulosic biomass for biofuels and bioproducts: An overview. Waste Biomass Valorization 2019, 10, 235–251. [Google Scholar] [CrossRef]
- Agbor, V.B.; Cicek, N.; Sparling, R.; Berlin, A.; Levin, D.B. Biomass pretreatment: Fundamentals toward application. Biotechnol. Adv. 2011, 29, 675–685. [Google Scholar] [CrossRef]
- Yoo, C.G.; Yang, Y.; Pu, Y.; Meng, X.; Muchero, W.; Yee, K.L.; Thompson, O.A.; Rodriguez, M.; Bali, G.; Engle, N.L.; et al. Insights of biomass recalcitrance in natural Populus trichocarpa variants for biomass conversion. Green Chem. 2017, 19, 5467–5478. [Google Scholar] [CrossRef]
- Lu, H.; Wang, X.; Zang, M.; Zhou, J.; Wang, J.; Guo, W. Degradation pathways and kinetics of anthraquinone compounds along with nitrate removal by a newly isolated Rhodococcus pyridinivorans GF3 under aerobic conditions. Bioresour. Technol. 2019, 285, 121336. [Google Scholar] [CrossRef]
- Chandel, A.K.; Garlapati, V.K.; Singh, A.K.; Antunes, F.A.F.; da Silva, S.S. The path forward for lignocellulose biorefineries: Bottlenecks, solutions, and perspective on commercialization. Bioresour. Technol. 2018, 264, 370–381. [Google Scholar] [CrossRef]
- Mota, T.R.; Oliveira, D.; Marchiosi, R.; Ferrarese-Filho, O.; Santos, W. Plant cell wall composition and enzymatic deconstruction. AIMS Bioeng. 2018, 5, 63–77. [Google Scholar] [CrossRef]
- Kruyeniski, J.; Ferreira, P.J.; Carvalho, M.d.G.V.S.; Vallejos, M.E.; Felissia, F.E.; Area, M.C. Physical and chemical characteristics of pretreated slash pine sawdust influence its enzymatic hydrolysis. Ind. Crops Prod. 2019, 130, 528–536. [Google Scholar] [CrossRef] [Green Version]
- Ragauskas, A.J.; Beckham, G.T.; Biddy, M.J.; Chandra, R.; Chen, F.; Davis, M.F.; Davison, B.H.; Dixon, R.A.; Gilna, P.; Keller, M.; et al. Lignin valorization: Improving lignin processing in the biorefinery. Science 2014, 344, 1246843. [Google Scholar] [CrossRef]
- Whitham, J.M.; Moon, J.-W.; Rodriguez, M.; Engle, N.L.; Klingeman, D.M.; Rydzak, T.; Abel, M.M.; Tschaplinski, T.J.; Guss, A.M.; Brown, S.D. Clostridium thermocellum LL1210 pH homeostasis mechanisms informed by transcriptomics and metabolomics. Biotechnol. Biofuels 2018, 11, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.; Wang, G.; Pan, X.; Gleisner, R. Specific surface to evaluate the efficiencies of milling and pretreatment of wood for enzymatic saccharification. Chem. Eng. Sci. 2009, 64, 474–485. [Google Scholar] [CrossRef]
- Yeh, A.-I.; Huang, Y.-C.; Chen, S.H. Effect of particle size on the rate of enzymatic hydrolysis of cellulose. Carbohydr. Polym. 2010, 79, 192–199. [Google Scholar] [CrossRef]
- Wen, Z.; Liao, W.; Chen, S. Hydrolysis of animal manure lignocellulosics for reducing sugar production. Bioresour. Technol. 2004, 91, 31–39. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Dale, B.; Hespell, R.; Bothast, R. Enzymatic hydrolysis of high-moisture corn fiber pretreated by AFEX and recovery and recycling of the enzyme complex. Appl. Biochem. Biotechnol. 1997, 67, 113–126. [Google Scholar] [CrossRef]
- Cosgrove, D.J. Growth of the plant cell wall. Nat. Rev. Mol. Cell Biol. 2005, 6, 850–861. [Google Scholar] [CrossRef] [PubMed]
- Grous, W.R.; Converse, A.O.; Grethlein, H.E. Effect of steam explosion pretreatment on pore size and enzymatic hydrolysis of poplar. Enzym. Microb. Technol. 1986, 8, 274–280. [Google Scholar] [CrossRef]
- Shevchenko, S.; Chang, K.; Robinson, J.; Saddler, J. Optimization of monosaccharide recovery by post-hydrolysis of the water-soluble hemicellulose component after steam explosion of softwood chips. Bioresour. Technol. 2000, 72, 207–211. [Google Scholar] [CrossRef]
- Zhao, X.; Peng, F.; Cheng, K.; Liu, D. Enhancement of the enzymatic digestibility of sugarcane bagasse by alkali–peracetic acid pretreatment. Enzym. Microb. Technol. 2009, 44, 17–23. [Google Scholar] [CrossRef]
- Zhao, X.; Song, Y.; Liu, D. Enzymatic hydrolysis and simultaneous saccharification and fermentation of alkali/peracetic acid-pretreated sugarcane bagasse for ethanol and 2, 3-butanediol production. Enzym. Microb. Technol. 2011, 49, 413–419. [Google Scholar] [CrossRef]
- Ximenes, E.; Kim, Y.; Mosier, N.; Dien, B.; Ladisch, M. Deactivation of cellulases by phenols. Enzym. Microb. Technol. 2011, 48, 54–60. [Google Scholar] [CrossRef]
- Nakagame, S.; Chandra, R.P.; Saddler, J.N. The influence of lignin on the enzymatic hydrolysis of pretreated biomass substrates. Sustain. Prod. Fuels Chem. Fibers For. Biomass 2011, 1067, 145–167. [Google Scholar]
- Wang, Q.; He, Z.; Zhu, Z.; Zhang, Y.H.; Ni, Y.; Luo, X.; Zhu, J. Evaluations of cellulose accessibilities of lignocelluloses by solute exclusion and protein adsorption techniques. Biotechnol. Bioeng. 2012, 109, 381–389. [Google Scholar] [CrossRef]
- Luo, X.; Zhu, J. Effects of drying-induced fiber hornification on enzymatic saccharification of lignocelluloses. Enzym. Microb. Technol. 2011, 48, 92–99. [Google Scholar] [CrossRef]
- Huang, R.; Su, R.; Qi, W.; He, Z. Understanding the key factors for enzymatic conversion of pretreated lignocellulose by partial least square analysis. Biotechnol. Prog. 2010, 26, 384–392. [Google Scholar] [CrossRef]
- Luo, X.; Zhu, J.; Gleisner, R.; Zhan, H. Effects of wet-pressing-induced fiber hornification on enzymatic saccharification of lignocelluloses. Cellulose 2011, 18, 1055–1062. [Google Scholar] [CrossRef]
- Bian, J.; Peng, F.; Peng, X.-P.; Xiao, X.; Peng, P.; Xu, F.; Sun, R.-C. Effect of [Emim] Ac pretreatment on the structure and enzymatic hydrolysis of sugarcane bagasse cellulose. Carbohydr. Polym. 2014, 100, 211–217. [Google Scholar] [CrossRef]
- Morais, A.R.C.; Pinto, J.V.; Nunes, D.; Roseiro, L.B.; Oliveira, M.C.; Fortunato, E.; Bogel-Łukasik, R. Imidazole: Prospect Solvent for Lignocellulosic Biomass Fractionation and Delignification. ACS Sustain. Chem. Eng. 2016, 4, 1643–1652. [Google Scholar] [CrossRef]
- Thygesen, A.; Oddershede, J.; Lilholt, H.; Thomsen, A.B.; Ståhl, K. On the determination of crystallinity and cellulose content in plant fibres. Cellulose 2005, 12, 563–576. [Google Scholar] [CrossRef]
- Sarko, A.; Muggli, R. Packing analysis of carbohydrates and polysaccharides. III. Valonia cellulose and cellulose II. Macromolecules 1974, 7, 486–494. [Google Scholar] [CrossRef]
- Nishiyama, Y.; Langan, P.; Chanzy, H. Crystal structure and hydrogen-bonding system in cellulose Iβ from synchrotron X-ray and neutron fiber diffraction. J. Am. Chem. Soc. 2002, 124, 9074–9082. [Google Scholar] [CrossRef]
- Felby, C.; Klinke, H.; Olsen, H.; Thomsen, A. Ethanol from Wheat Straw Cellulose by Wet Oxidation Pretreatment and Simultaneous Saccharifleation and Fermentation; ACS Publications: Columbus, OH, USA, 2003. [Google Scholar]
- Varga, E.; Réczey, K.; Zacchi, G. (Eds.) Optimization of steam pretreatment of corn stover to enhance enzymatic digestibility. In Proceedings of the Twenty-Fifth Symposium on Biotechnology for Fuels and Chemicals, Breckenridge, CO, USA, 4–7 May 2003; Springer: Berlin/Heidelberg, Germany, 2004. [Google Scholar]
- Pihlajaniemi, V.; Sipponen, M.H.; Liimatainen, H.; Sirviö, J.A.; Nyyssölä, A.; Laakso, S. Weighing the factors behind enzymatic hydrolyzability of pretreated lignocellulose. Green Chem. 2016, 18, 1295–1305. [Google Scholar] [CrossRef]
- Liu, Z.-H.; Qin, L.; Li, B.-Z.; Yuan, Y.-J. Physical and Chemical Characterizations of Corn Stover from Leading Pretreatment Methods and Effects on Enzymatic Hydrolysis. ACS Sustain. Chem. Eng. 2015, 3, 140–146. [Google Scholar] [CrossRef]
- Chang, V.S.; Holtzapple, M.T. (Eds.) Fundamental factors affecting biomass enzymatic reactivity. In Twenty-First Symposium on Biotechnology for Fuels and Chemicals; Springer: Berlin/Heidelberg, Germany, 2000. [Google Scholar]
- Mansfield, S.D.; Mooney, C.; Saddler, J.N. Substrate and enzyme characteristics that limit cellulose hydrolysis. Biotechnol. Prog. 1999, 15, 804–816. [Google Scholar] [CrossRef]
- Ioelovich, M.; Morag, E. Effect of cellulose structure on enzymatic hydrolysis. BioResources 2011, 6, 2818–2835. [Google Scholar] [CrossRef]
- Aldaeus, F.; Larsson, K.; Srndovic, J.S.; Kubat, M.; Karlström, K.; Peciulyte, A.; Olsson, L.; Larsson, P.T. The supramolecular structure of cellulose-rich wood pulps can be a determinative factor for enzymatic hydrolysability. Cellulose 2015, 22, 3991–4002. [Google Scholar] [CrossRef]
- Auxenfans, T.; Crônier, D.; Chabbert, B.; Paës, G. Understanding the structural and chemical changes of plant biomass following steam explosion pretreatment. Biotechnol. Biofuels 2017, 10, 36. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Pu, Y.; Yoo, C.G.; Li, M.; Bali, G.; Park, D.Y.; Gjersing, E.; Davis, M.F.; Muchero, W.; Tuskan, G.A.; et al. An in-depth understanding of biomass recalcitrance using natural poplar variants as the feedstock. ChemSusChem 2017, 10, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, J.; Huang, G.; Yang, Z.; Han, L. Understanding the synergistic effect and the main factors influencing the enzymatic hydrolyzability of corn stover at low enzyme loading by hydrothermal and/or ultrafine grinding pretreatment. Bioresour. Technol. 2018, 264, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Yu, Y.; Wu, H. Differences in water-soluble intermediates from slow pyrolysis of amorphous and crystalline cellulose. Energy Fuels 2013, 27, 1371–1380. [Google Scholar] [CrossRef]
- Ahvenainen, P.; Kontro, I.; Svedström, K. Comparison of sample crystallinity determination methods by X-ray diffraction for challenging cellulose I materials. Cellulose 2016, 23, 1073–1086. [Google Scholar] [CrossRef] [Green Version]
- Sathitsuksanoh, N.; Zhu, Z.; Wi, S.; Percival Zhang, Y.H. Cellulose solvent-based biomass pretreatment breaks highly ordered hydrogen bonds in cellulose fibers of switchgrass. Biotechnol. Bioeng. 2011, 108, 521–529. [Google Scholar] [CrossRef]
- Yao, L.; Yoo, C.G.; Meng, X.; Li, M.; Pu, Y.; Ragauskas, A.J.; Yang, H. A structured understanding of cellobiohydrolase I binding to poplar lignin fractions after dilute acid pretreatment. Biotechnol. Biofuels 2018, 11, 96. [Google Scholar] [CrossRef]
- Sánchez, C. Lignocellulosic residues: Biodegradation and bioconversion by fungi. Biotechnol. Adv. 2009, 27, 185–194. [Google Scholar] [CrossRef]
- Gupta, R.; Lee, Y. Mechanism of cellulase reaction on pure cellulosic substrates. Biotechnol. Bioeng. 2009, 102, 1570–1581. [Google Scholar] [CrossRef]
- Nazhad, M.; Ramos, L.; Paszner, L.; Saddler, J. Structural constraints affecting the initial enzymatic hydrolysis of recycled paper. Enzym. Microb. Technol. 1995, 17, 68–74. [Google Scholar] [CrossRef]
- Maki, M.; Leung, K.T.; Qin, W. The prospects of cellulase-producing bacteria for the bioconversion of lignocellulosic biomass. Int. J. Biol. Sci. 2009, 5, 500. [Google Scholar] [CrossRef]
- Jönsson, L.J.; Martín, C. Pretreatment of lignocellulose: Formation of inhibitory by-products and strategies for minimizing their effects. Bioresour. Technol. 2016, 199, 103–112. [Google Scholar] [CrossRef] [Green Version]
- Galbe, M.; Zacchi, G. A review of the production of ethanol from softwood. Appl. Microbiol. Biotechnol. 2002, 59, 618–628. [Google Scholar] [CrossRef]
- Sanchez, O.J.; Cardona, C.A. Trends in biotechnological production of fuel ethanol from different feedstocks. Bioresour. Technol. 2008, 99, 5270–5295. [Google Scholar] [CrossRef]
- Tomas-Pejo, E.; Oliva, J.; Ballesteros, M. Realistic approach for full-scale bioethanol production from lignocellulose: A review. J. Sci. Ind. Res. 2008, 67, 874–884. [Google Scholar]
- Talebnia, F.; Karakashev, D.; Angelidaki, I. Production of bioethanol from wheat straw: An overview on pretreatment, hydrolysis and fermentation. Bioresour. Technol. 2010, 101, 4744–4753. [Google Scholar] [CrossRef]
- Taherzadeh, M.J.; Karimi, K. Acid-based hydrolysis processes for ethanol from lignocellulosic materials: A review. BioResources 2007, 2, 472–499. [Google Scholar]
- Talebnia, F.; Taherzadeh, M.J. In situ detoxification and continuous cultivation of dilute-acid hydrolyzate to ethanol by encapsulated S. cerevisiae. J. Biotechnol. 2006, 125, 377–384. [Google Scholar] [CrossRef]
- Singh, D.P.; Trivedi, R.K. Acid and Alkaline pretreatment of lignocellulosic biomass to produce ethanol as biofuel. Int. J. ChemTech Res. 2013, 5, 727–734. [Google Scholar]
- Kumar, P.; Barrett, D.M.; Delwiche, M.J.; Stroeve, P. Methods for pretreatment of lignocellulosic biomass for efficient hydrolysis and biofuel production. Ind. Eng. Chem. Res. 2009, 48, 3713–3729. [Google Scholar] [CrossRef]
- Mosier, N.; Hendrickson, R.; Ho, N.; Sedlak, M.; Ladisch, M.R. Optimization of pH controlled liquid hot water pretreatment of corn stover. Bioresour. Technol. 2005, 96, 1986–1993. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Holtzapple, M.T. Effect of structural features on enzyme digestibility of corn stover. Bioresour. Technol. 2006, 97, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Ozmihci, S.; Kargi, F. Effects of feed sugar concentration on continuous ethanol fermentation of cheese whey powder solution (CWP). Enzym. Microb. Technol. 2007, 41, 876–880. [Google Scholar] [CrossRef]
- Rabelo, S.C.; Amezquita Fonseca, N.A.; Andrade, R.R.; Maciel Filho, R.; Costa, A.C. Ethanol production from enzymatic hydrolysis of sugarcane bagasse pretreated with lime and alkaline hydrogen peroxide. Biomass Bioenergy 2011, 35, 2600–2607. [Google Scholar] [CrossRef]
- Hendriks, A.T.W.M.; Zeeman, G. Pretreatments to enhance the digestibility of lignocellulosic biomass. Bioresour. Technol. 2009, 100, 10–18. [Google Scholar] [CrossRef]
- Klinke, H.B.; Ahring, B.K.; Schmidt, A.S.; Thomsen, A.B. Characterization of degradation products from alkaline wet oxidation of wheat straw. Bioresour. Technol. 2002, 82, 15–26. [Google Scholar] [CrossRef]
- Da Costa Lopes, A.M.; João, K.G.; Morais, A.R.C.; Bogel-Łukasik, E.; Bogel-Łukasik, R. Ionic liquids as a tool for lignocellulosic biomass fractionation. Sustain. Chem. Process. 2013, 1, 3. [Google Scholar] [CrossRef] [Green Version]
- Escobar, E.L.N.; Da Silva, T.A.; Pirich, C.L.; Corazza, M.L.; Pereira Ramos, L. Supercritical fluids: A promising technique for biomass pretreatment and fractionation. Front. Bioeng. Biotechnol. 2020, 8, 252. [Google Scholar] [CrossRef] [Green Version]
- Toscan, A.; Morais, A.R.C.; Paixão, S.M.; Alves, L.; Andreaus, J.; Camassola, M.; Dillon, A.J.P.; Lukasik, R.M. High-pressure carbon dioxide/water pre-treatment of sugarcane bagasse and elephant grass: Assessment of the effect of biomass composition on process efficiency. Bioresour. Technol. 2017, 224, 639–647. [Google Scholar] [CrossRef]
- Wang, W.; Lee, D.-J. Lignocellulosic biomass pretreatment by deep eutectic solvents on lignin extraction and saccharification enhancement: A review. Bioresour. Technol. 2021, 339, 125587. [Google Scholar] [CrossRef]
- Margeot, A.; Hahn-Hagerdal, B.; Edlund, M.; Slade, R.; Monot, F. New improvements for lignocellulosic ethanol. Curr. Opin. Biotechnol. 2009, 20, 372–380. [Google Scholar] [CrossRef]
- Oh, S.E.; Iyer, P.; Bruns, M.A.; Logan, B.E. Biological hydrogen production using a membrane bioreactor. Biotechnol. Bioeng. 2004, 87, 119–127. [Google Scholar] [CrossRef]
- Kumar, N.; Das, D. Enhancement of hydrogen production by Enterobacter cloacae IIT-BT 08. Process Biochem. 2000, 35, 589–593. [Google Scholar] [CrossRef]
- Morimoto, M.; Atsuko, M.; Atif, A.; Ngan, M.; Fakhru’l-Razi, A.; Iyuke, S.; Bakir, A.M. Biological production of hydrogen from glucose by natural anaerobic microflora. Int. J. Hydrogen Energy 2004, 29, 709–713. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Chang, R.-C. Fermentative hydrogen production at ambient temperature. Int. J. Hydrogen Energy 2004, 29, 715–720. [Google Scholar] [CrossRef]
- Fang, H.H.; Liu, H. Effect of pH on hydrogen production from glucose by a mixed culture. Bioresour. Technol. 2002, 82, 87–93. [Google Scholar] [CrossRef]
- Kotsopoulos, T.A.; Zeng, R.J.; Angelidaki, I. Biohydrogen production in granular up-flow anaerobic sludge blanket (UASB) reactors with mixed cultures under hyper-thermophilic temperature (70 °C). Biotechnol. Bioeng. 2006, 94, 296–302. [Google Scholar] [CrossRef]
- Chang, J.-S.; Lee, K.-S.; Lin, P.-J. Biohydrogen production with fixed-bed bioreactors. Int. J. Hydrogen Energy 2002, 27, 1167–1174. [Google Scholar] [CrossRef]
- Logan, B.E.; Oh, S.-E.; Kim, I.S.; Van Ginkel, S. Biological hydrogen production measured in batch anaerobic respirometers. Environ. Sci. Technol. 2002, 36, 2530–2535. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Lin, C.; Chang, J. Kinetics of hydrogen production with continuous anaerobic cultures utilizing sucrose as the limiting substrate. Appl. Microbiol. Biotechnol. 2001, 57, 56–64. [Google Scholar] [PubMed]
- Mu, Y.; Yu, H.-Q.; Wang, G. Evaluation of three methods for enriching H2-producing cultures from anaerobic sludge. Enzym. Microb. Technol. 2007, 40, 947–953. [Google Scholar] [CrossRef]
- Collet, C.; Adler, N.; Schwitzguébel, J.-P.; Péringer, P. Hydrogen production by Clostridium thermolacticum during continuous fermentation of lactose. Int. J. Hydrogen Energy 2004, 29, 1479–1485. [Google Scholar] [CrossRef]
- Zhang, Q.; Jin, P.; Li, Y.; Zhang, Z.; Zhang, H.; Ru, G.; Jiang, D.; Jing, Y.; Zhang, X. Analysis of the characteristics of paulownia lignocellulose and hydrogen production potential via photo fermentation. Bioresour. Technol. 2022, 344, 126361. [Google Scholar] [CrossRef]
- Baik, J.-H.; Jung, J.-H.; Sim, Y.-B.; Park, J.-H.; Yang, J.; Kim, S.-H. High-rate biohydrogen production from xylose using a dynamic membrane bioreactor. Bioresour. Technol. 2022, 344, 126205. [Google Scholar] [CrossRef]
- Byrne, E.; Björkmalm, J.; Bostick, J.P.; Sreenivas, K.; Willquist, K.; van Niel, E.W. Characterization and adaptation of Caldicellulosiruptor strains to higher sugar concentrations, targeting enhanced hydrogen production from lignocellulosic hydrolysates. Biotechnol. Biofuels 2021, 14, 199–212. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, G.; Ma, S.; Huang, H.; Ma, Y.; Li, Z. Enhancing Hydrogen Productivity of Photosynthetic Bacteria from the Formulated Carbon Source by Mixing Xylose with Glucose. Appl. Biochem. Biotechnol. 2021, 193, 3996–4017. [Google Scholar] [CrossRef]
- Kucharska, K.; Makoś-Chełstowska, P.; Słupek, E.; Gębicki, J. Management of Dark Fermentation Broth via Bio Refining and Photo Fermentation. Energies 2021, 14, 6268. [Google Scholar] [CrossRef]
- Tosuner, Z.V.; Taylan, G.; Özmıhçı, S. Effects of rice husk particle size on biohydrogen production under solid state fermentation. Int. J. Hydrogen Energy 2019, 44, 18785–18791. [Google Scholar] [CrossRef]
- Zhang, Z.; Fan, X.; Li, Y.; Jin, P.; Jiao, Y.; Ai, F.; Zhang, H.; Zhang, Q. Photo-fermentative biohydrogen production from corncob treated by microwave irradiation. Bioresour. Technol. 2021, 340, 125460. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Jiang, D.; Zhang, H.; Wang, Y.; Zhang, Z.; Lu, C.; Zhang, Q. Enhancement of the biohydrogen production performance from the mixed substrate by photo-fermentation: Effects of initial pH and inoculation volume ratio. Bioresour. Technol. 2021, 319, 124153. [Google Scholar] [CrossRef] [PubMed]
- Cha, M.; Chung, D.; Elkins, J.G.; Guss, A.M.; Westpheling, J. Metabolic engineering of Caldicellulosiruptor bescii yields increased hydrogen production from lignocellulosic biomass. Biotechnol. Biofuels 2013, 6, 85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colletti, P.F.; Goyal, Y.; Varman, A.M.; Feng, X.; Wu, B.; Tang, Y.J. Evaluating factors that influence microbial synthesis yields by linear regression with numerical and ordinal variables. Biotechnol. Bioeng. 2011, 108, 893–901. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, A.; Nishimura, T.; Kawaguchi, H.; Inui, M.; Yukawa, H. Enhanced hydrogen production from glucose using ldh- and frd-inactivated Escherichia coli strains. Appl. Microbiol. Biotechnol. 2006, 73, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Maeda, T.; Sanchez-Torres, V.; Wood, T.K. Enhanced hydrogen production from glucose by metabolically engineered Escherichia coli. Appl. Microbiol. Biotechnol. 2007, 77, 879–890. [Google Scholar] [CrossRef]
- Redwood, M.D.; Mikheenko, I.P.; Sargent, F.; Macaskie, L.E. Dissecting the roles of Escherichia coli hydrogenases in biohydrogen production. FEMS Microbiol. Lett. 2008, 278, 48–55. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, A.; Nishimura, T.; Kawaguchi, H.; Inui, M.; Yukawa, H. Enhanced hydrogen production from formic acid by formate hydrogen lyase-overexpressing Escherichia coli strains. Appl. Environ. Microbiol. 2005, 71, 6762–6768. [Google Scholar] [CrossRef]
- Chittibabu, G.; Nath, K.; Das, D. Feasibility studies on the fermentative hydrogen production by recombinant Escherichia coli BL-21. Process Biochem. 2006, 41, 682–688. [Google Scholar] [CrossRef]
- Akhtar, M.K.; Jones, P.R. Construction of a synthetic YdbK-dependent pyruvate: H2 pathway in Escherichia coli BL21 (DE3). Metab. Eng. 2009, 11, 139–147. [Google Scholar] [CrossRef]
- Morimoto, K.; Kimura, T.; Sakka, K.; Ohmiya, K. Overexpression of a hydrogenase gene in Clostridium paraputrificum to enhance hydrogen gas production. FEMS Microbiol. Lett. 2005, 246, 229–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klein, M.; Ansorge-Schumacher, M.B.; Fritsch, M.; Hartmeier, W. Influence of hydrogenase overexpression on hydrogen production of Clostridium acetobutylicum DSM 792. Enzym. Microb. Technol. 2010, 46, 384–390. [Google Scholar] [CrossRef]
- Liu, X.; Zhu, Y.; Yang, S.T. Construction and characterization of ack deleted mutant of Clostridium tyrobutyricum for enhanced butyric acid and hydrogen production. Biotechnol. Prog. 2006, 22, 1265–1275. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhu, Y.; Yang, S.-T. Butyric acid and hydrogen production by Clostridium tyrobutyricum ATCC 25755 and mutants. Enzym. Microb. Technol. 2006, 38, 521–528. [Google Scholar] [CrossRef]
- Zhao, H.; Ma, K.; Lu, Y.; Zhang, C.; Wang, L.; Xing, X.-H. Cloning and knockout of formate hydrogen lyase and H2-uptake hydrogenase genes in Enterobacter aerogenes for enhanced hydrogen production. Int. J. Hydrogen Energy 2009, 34, 186–194. [Google Scholar] [CrossRef]
- Zhao, J.-F.; Song, W.-L.; Cheng, J.; Zhang, C.-X. Heterologous expression of a hydrogenase gene in Enterobacter aerogenes to enhance hydrogen gas production. World J. Microbiol. Biotechnol. 2010, 26, 177–181. [Google Scholar] [CrossRef]
- Lu, Y.; Zhao, H.; Zhang, C.; Lai, Q.; Xing, X.-H. Perturbation of formate pathway for hydrogen production by expressions of formate hydrogen lyase and its transcriptional activator in wild Enterobacter aerogenes and its mutants. Int. J. Hydrogen Energy 2009, 34, 5072–5079. [Google Scholar] [CrossRef]
- Lu, Y.; Zhao, H.; Zhang, C.; Lai, Q.; Wu, X.; Xing, X.-H. Alteration of hydrogen metabolism of ldh-deleted Enterobacter aerogenes by overexpression of NAD (+)-dependent formate dehydrogenase. Appl. Microbiol. Biotechnol. 2010, 86, 255–262. [Google Scholar] [CrossRef]
- Anish, R.; Rao, M. Bioethanol from lignocellulosic biomass part III hydrolysis and fermentation. In Handbook of Plant-based Biofuels; CRC Press: Boca Raton, FL, USA, 2009; pp. 159–173. [Google Scholar]
- Canilha, L.; Chandel, A.K.; Suzane dos Santos Milessi, T.; Antunes, F.A.F.; Luiz da Costa Freitas, W.; das Graças Almeida Felipe, M.; da Silva, S.S. Bioconversion of sugarcane biomass into ethanol: An overview about composition, pretreatment methods, detoxification of hydrolysates, enzymatic saccharification, and ethanol fermentation. J. Biomed. Biotechnol. 2012, 2012, 989572. [Google Scholar] [CrossRef] [Green Version]
- Grzenia, D.L.; Schell, D.J.; Wickramasinghe, S.R. Membrane extraction for detoxification of biomass hydrolysates. Bioresour. Technol. 2012, 111, 248–254. [Google Scholar] [CrossRef]
- Chandel, A.K.; da Silva, S.S.; Singh, O.V. Detoxification of lignocellulosic hydrolysates for improved bioethanol production. Biofuel Prod.-Recent Dev. Prospect. 2011, 10, 225. [Google Scholar]
- Canilha, L.; Carvalho, W.; Giulietti, M.; Felipe, M.D.G.A.; Almeida, E.; Silva, J.B. Clarification of a wheat straw-derived medium with ion-exchange resins for xylitol crystallization. J. Chem. Technol. Biotechnol. 2008, 83, 715–721. [Google Scholar] [CrossRef]
- Gírio, F.M.; Fonseca, C.; Carvalheiro, F.; Duarte, L.C.; Marques, S.; Bogel-Łukasik, R. Hemicelluloses for fuel ethanol: A review. Bioresour. Technol. 2010, 101, 4775–4800. [Google Scholar] [CrossRef]
- Zhu, J.J.; Yong, Q.; Xu, Y.; Yu, S.Y. Comparative detoxification of vacuum evaporation/steam stripping combined with overliming on corn stover prehydrolyzate. In Proceedings of the 2009 International Conference on Energy and Environment Technology, Guilin, China, 16–18 October 2009. [Google Scholar]
- Antunes, F.; Milessi, T.; Oliveira, I.; Chandel, A.; Silva, S. Characterization of sugarcane bagasse hemicellulosic hydrolysate after detoxification with overliming and activated charcoal. In Proceedings of the 20th European Biomass Conference and Exhibition, Milan, Italy, 18–22 June 2012. [Google Scholar]
- Carvalho, W.; Canilha, L.; Silva, S.S. Semi-continuous xylose-to-xylitol bioconversion by Ca-alginate entrapped yeast cells in a stirred tank reactor. Bioprocess Biosyst. Eng. 2008, 31, 493–498. [Google Scholar] [CrossRef]
- Converti, A.; Domínguez, J.M.; Perego, P.; Da Silva, S.; Zilli, M. Wood hydrolysis and hydrolyzate detoxification for subsequent xylitol production. Chem. Eng. Technol. 2000, 23, 1013–1020. [Google Scholar] [CrossRef]
- Cantarella, M.; Cantarella, L.; Gallifuoco, A.; Spera, A.; Alfani, F. Comparison of different detoxification methods for steam-exploded poplar wood as a substrate for the bioproduction of ethanol in SHF and SSF. Process Biochem. 2004, 39, 1533–1542. [Google Scholar] [CrossRef]
- Zhang, H.-R.; Qin, X.-X.; Silva, S.S.; Sarrouh, B.F.; Cai, A.-H.; Zhou, Y.-H.; Jin, K.; Xiang, Q. Novel isolates for biological detoxification of lignocellulosic hydrolysate. Appl. Biochem. Biotechnol. 2009, 152, 199–212. [Google Scholar] [CrossRef]
- Ch, A.K.; Ch, G.; Radhika, K.; Ravinder, R.; Ravindra, P. Bioconversion of pentose sugars into ethanol: A review and future directions. Biotechnol. Mol. Biol. Rev. 2011, 6, 8–20. [Google Scholar]
- Moreno, A.D.; Ibarra, D.; Fernández, J.L.; Ballesteros, M. Different laccase detoxification strategies for ethanol production from lignocellulosic biomass by the thermotolerant yeast Kluyveromyces marxianus CECT 10875. Bioresour. Technol. 2012, 106, 101–109. [Google Scholar] [CrossRef]
- Parawira, W.; Tekere, M. Biotechnological strategies to overcome inhibitors in lignocellulose hydrolysates for ethanol production. Crit. Rev. Biotechnol. 2011, 31, 20–31. [Google Scholar] [CrossRef]
- Makoś-Chełstowska, P.; Słupek, E.; Kucharska, K.; Kramarz, A.; Gębicki, J. Efficient Extraction of Fermentation Inhibitors by Means of Green Hydrophobic Deep Eutectic Solvents. Molecules 2022, 27, 157. [Google Scholar] [CrossRef] [PubMed]

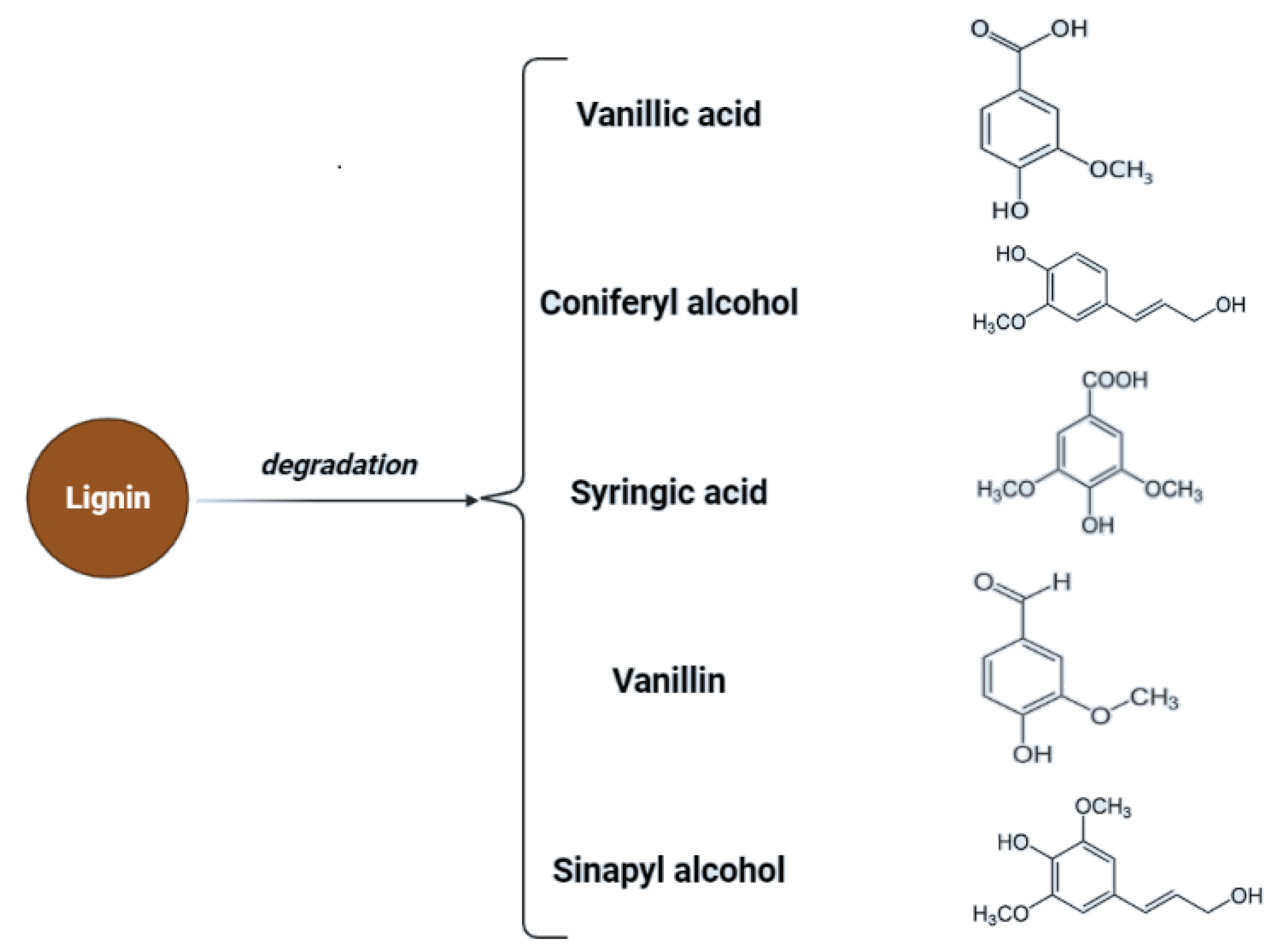
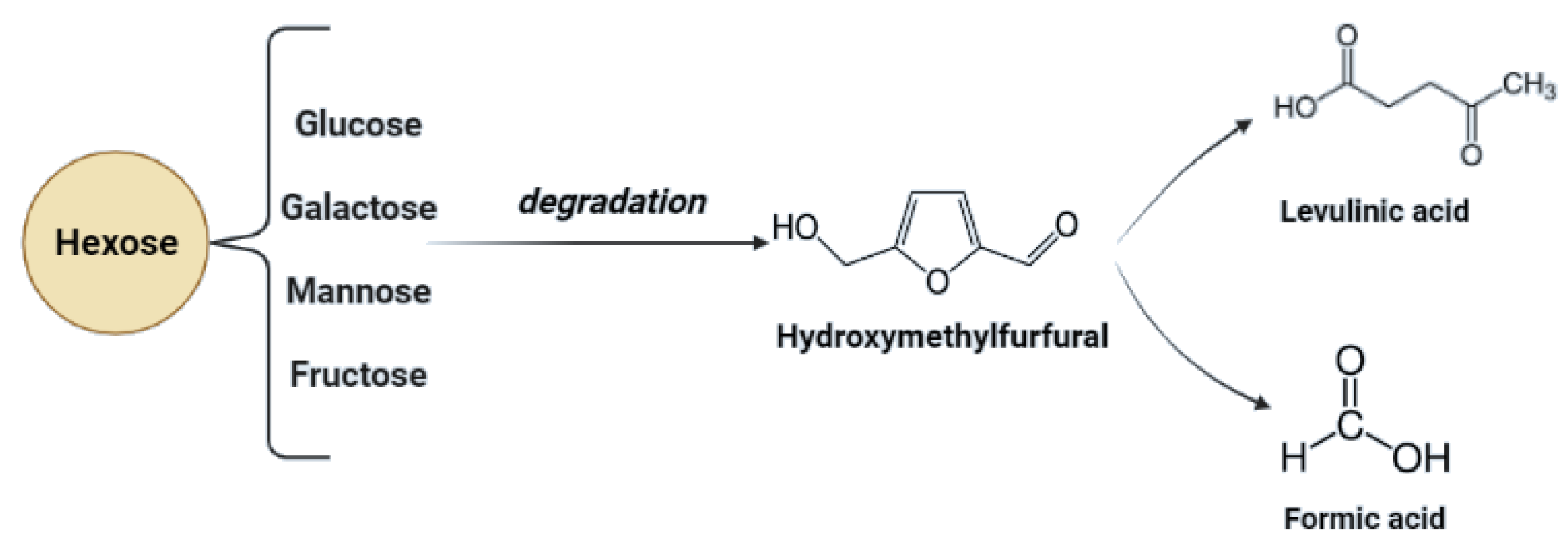
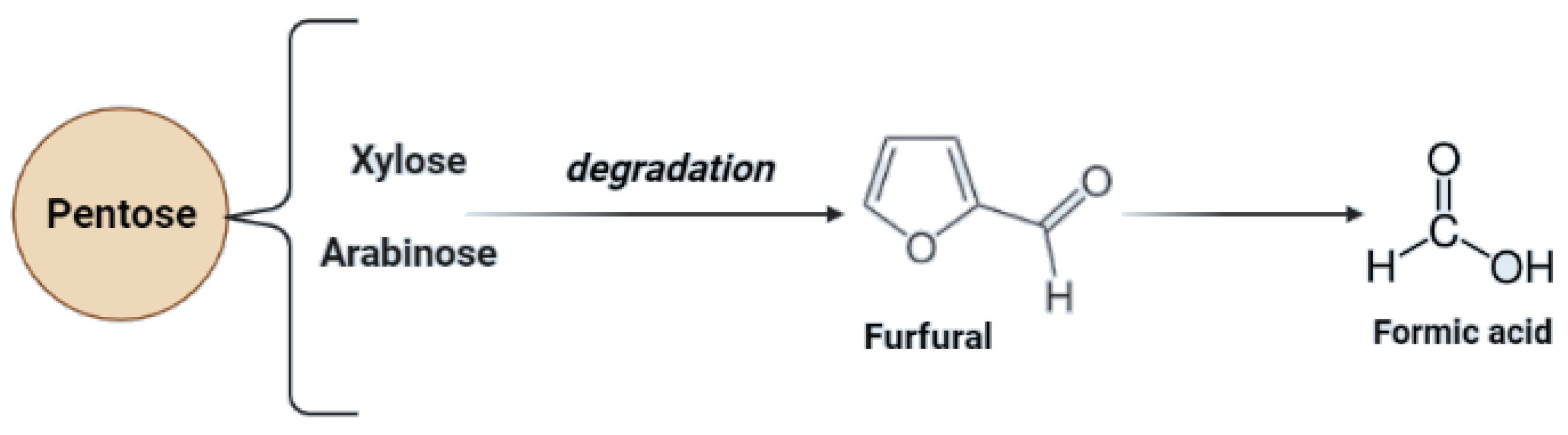
| Criterion | Starch | Lignocellulose |
|---|---|---|
| Microbial resistance | Biodegradable, excluding granular amylase-resistant α-glycans | Resistant to biodegradation due to the strong and compact structure of plant cell walls |
| Factors affecting access to monosugars | Fiber, physical form | Available surface, pore size, volume, particle size, specific surface area, and degree of polymerization |
| Chemical compounds or units | Glucose monomers linked with 1,4 and 1,6 linkages; linear polymer—amylose; branched form—amylopectin. | Lignin (amorphous heteropolymer of phenylpropanoid building units, i.e., p-coumaryl, coniferyl, and sinapyl alcohol); hemicellulose (various monosaccharide subunits to form xylans, xyloglucan, mannans, and glucomannans); cellulose (ß-D-glucopyranose units linked via ß-(1,4) glycosidic bonds, with cellobiose as the fundamental repeating unit), extractives |
| Chemical interactions between polymers | α -1,4 glycosidic bonds;α-1,6 glycosidic bonds | Hydrogen bonds (between cellulose-hemicellulose); Lignin-carbohydrate complex, i.e., the occurrence of phenyl glycosides, γ-esters, benzyl ethers, ferulate esters, coumarate esters; Hemiacetal and acetal linkages at 4-OH and 4-O positions (lignin covalently linked to hemicellulose) |
| Possible pre-treatment by-products | Monosaccharides conversion by-products and secondary transformation products, i.e., levulinic acid, HMF, furfural | Lignin derivatives, HMF, vanillin, syringole, furfural, p-coumaryl, coniferyl, sinapyl alcohol, oligopeptides, terpenoids, and levulinic acid, monosaccharides conversion by-products, i.e., levulinic acid, HMF, furfural |
| Pre-Treatment Method | Advantages | Disadvantages |
|---|---|---|
| Enzymatic hydrolysis | The precise method for saccharification, the possibility of process planning, and the selection of an enzymatic liqueur for a specific raw material | The process parameters must be carefully designed and controlled. Enzymes are expensive and not always able to recirculate, loss of activity if the local temperature is unstable, and some saccharification by-products are recognized as enzyme inhibitors |
| Biological pre-treatment | Ability to design a microbial consortium, reducing the number of pre-treatment steps. Allows the design of a precise liqueur of enzymes at lower costs, a wide range of process parameters, and the possibility to obtain wild rot species for precise raw material | Time-consuming process, the possibility of monosaccharide self-consumption if the consortium is designed inappropriately, risk of infection of the bacterial culture, difficulties in separating the products, and toxicity of the fermentation broth; work in a two-phase system is necessary |
| Acidic hydrolysis | A method that is cheap, easy to control, widely used, and allows comparison of the results of the pre-treatment. The application of a wide range of acid concentrations allows for controlling the generation of inhibitory compounds | High temperatures, specific requirements of reactor materials, decomposition of parts of the main product and its transformation into inhibitors, and emission of oxides as a result of the fusion of acid particles |
| Alkaline hydrolysis | An effective method to increase the concentration of reducing sugars in the hydrolysis process. Alkaline processes use less temperature and pressure than other methods | The decomposition of sugars in this method is less than in acidic pretreatment. Possibility of fermentation inhibitors generation and secondary transformation of saccharification products |
| Oxidative hydrolysis | Dissolves amorphous cellulose and lignin, possibility to remove lignin without derivatives generation, COD lowering effect | Reagents are unstable under alkaline conditions and decompose easily in the presence of transition metals and the application of hydrogen peroxide requires special processing conditions |
| Ionic liquids | It is an effective method for dissolving the plant cell wall that does not require high temperature to dissolve the cell wall. This method is used in mild processing conditions. It also has low volatility and reusability, selective removal of lignin and hemicellulose as well as cellulose release | Failure to recycle solvents creates toxic substances in the environment and deactivates enzymes |
| Supercritical fluid CO2Water | The decomposition of sugars is low and, unlike acid methods, the amount of corrosiveness is significantly reduced. It prevents the degradation of xylose at low temperatures, recovers, and reusesNo need to dry biomass before pretreatment and reduces resistance to mass transfer. Requires a very short reaction time; therefore, the decomposition of glucose, xylose, and arabinose sugars is prevented. | Requires high pressure and temperature, non-change of lignin and hemicellulose, increasing the concentration of xylan and furan for pretreatment of corn |
| DES | High recovery of sugars during the pre-treatment process, improving the rate of enzymatic saccharification, preventing the degradation of polysaccharides, and preserving carbohydrates. Excellent performance on lignin extraction and biomass saccharification enhancement. Ability to selectively dissolve lignin and hemicellulose | The high viscosity limits their application and the pretreatments are often very complex, with the inhibition effect toward cellulase and acidic DESs destroying polysaccharides |
| Microorganism | Strain | Genetic Modification | mol H2/mol Glucose | Reference |
|---|---|---|---|---|
| Caldicellulosiruptor bescii * | - | deletion of L-lactate dehydrogenase gene (ldh) | 2.5 | [118] |
| Escherichia coli * | SR15 | modifying ΔldhA, ΔfrdBC | 1.82 | [120] |
| Escherichia coli * | - | production BW25113 hyaB hybC hycA fdoG frdC ldhA aceE | 2 | [121] |
| Escherichia coli * | MC4100, wild-type FTD89, mutant | deletion of Hyd-1 + Hyd-2; hyaB + hybC | 1.043 | [122] |
| Escherichia coli * | FTD67, mutant | deletion of Hyd-2; hybC | 1.024 | [122] |
| Escherichia coli * | W3110, wild-type SR15, mutant | deletion of ldhA + frdBC | 1.82 | [123] |
| Escherichia coli * | W3110, wild-type SR14, mutant | deletion of ldhA + frdBC overexpression of fhlA | 1.87 | [123] |
| Escherichia coli * | Bl-21 recombinant, mutant | deletion of hydA | 3.12 | [124] |
| Escherichia coli * | BL21(DE3] _iscR pYdbK pAF, mutant | deletion of iscR + MCS2 overexpression of YdbK + CpFdx + hydA + hydF + hydG + hydE | 1.46 | [125] |
| Clostridium paraputrificum * | M-21 pJIR751, mutant | overexpression of hydA | 2.4 | [126] |
| Clostridium acetobutylicum * | DSM 792 [pSOS], mutant | overexpression of thl promoter | 1.77 | [127] |
| Clostridium acetobutylicum * | DSM 792 [pSOShydACa], mutant | overexpression of hydA | 1.81 | [127] |
| Clostridium acetobutylicum * | DSM 792 (pSOShydACb), mutant | overexpression of hydA | 1.80 | [127] |
| Clostridium tyrobutyricum * | PAK-Em, mutant | deletion of ack | 2.16 | [128] |
| Clostridium tyrobutyricum * | PAK-Em, mutant | deletion of ack | 2.61 | [129] |
| Clostridium tyrobutyricum * | ATCC 25,755 PPTA-Em, mutant | deletion of pta | 1.08 | [129] |
| Enterobacter aerogenes * | IAM1183 A, mutant | deletion of hycA | 1.20 | [130] |
| Enterobacter aerogenes * | IAM1183 O, mutant | deletion of hybO | 1.27 | [130] |
| Enterobacter aerogenes * | IAM1183 AO, mutant | deletion of hycA + hybO | 1.36 | [130] |
| Enterobacter aerogenes * | ATCC 13048/hydA, mutant | overexpression of hydA | 2.31 | [131] |
| Enterobacter aerogenes * | IAM1183 Ea (pMCL-fdhF), mutant | overexpression of fdhF | 1.16 | [132] |
| Enterobacter aerogenes * | IAM1183 A (pMCL-fdhF), mutant | deletion of hycA overexpression of fdhF | 1.19 | [133] |
| Enterobacter aerogenes * | IAM1183 (pCOM 10-fdh1), mutant | deletion of ldh overexpression of Fdh1 | 1.70 | [134] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Honarmandrad, Z.; Kucharska, K.; Gębicki, J. Processing of Biomass Prior to Hydrogen Fermentation and Post-Fermentative Broth Management. Molecules 2022, 27, 7658. https://doi.org/10.3390/molecules27217658
Honarmandrad Z, Kucharska K, Gębicki J. Processing of Biomass Prior to Hydrogen Fermentation and Post-Fermentative Broth Management. Molecules. 2022; 27(21):7658. https://doi.org/10.3390/molecules27217658
Chicago/Turabian StyleHonarmandrad, Zhila, Karolina Kucharska, and Jacek Gębicki. 2022. "Processing of Biomass Prior to Hydrogen Fermentation and Post-Fermentative Broth Management" Molecules 27, no. 21: 7658. https://doi.org/10.3390/molecules27217658







