Removal of Non-Steroidal Anti-Inflammatory Drugs from Drinking Water Sources by GO-SWCNT Buckypapers
Abstract
:1. Introduction
2. Results
3. Materials and Methods
3.1. Buckypaper Preparation and Characterization
3.2. NSAID Adsorption by Buckypapers
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Stancova, V.; Zikova, A.; Svobodova, Z.; Kloas, W. Effects of the non—Steroidal anti—Inflammatory drug (NSAID) naproxen on gene expression of antioxidant enzymes in zebrafish (Danio rerio). Environ. Toxicol. Pharmacol. 2015, 40, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Boxall, A.B.; Rudd, M.A.; Brooks, B.W.; Caldwell, D.J.; Choi, K.; Hickmann, S.; Innes, E.; Ostapyk, K.; Staveley, J.P.; Verslycke, T.; et al. Pharmaceuticals and personal care products in the environment: What are the big questions? Environ. Health Perspect. 2012, 120, 1221–1229. [Google Scholar] [CrossRef] [PubMed]
- Ternes, T.A. Occurrence of drugs in German sewage treatment plants and rivers. Water Res. 1998, 32, 3245–3260. [Google Scholar] [CrossRef]
- Heberer, T. Occurrence, fate and removal of pharmaceutical residues in the aquatic environment: A review of recent research data. Toxicol. Lett. 2002, 131, 5–17. [Google Scholar] [CrossRef]
- Hernando, M.D.; Mezcua, M.; Fernández-Alba, A.R.; Barceló, D. Environmental risk assessment of pharmaceutical residues in wastewater effluents, surface waters and sediments. Talanta 2006, 69, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.-C.; Chen, Y.Y.; Chiou, M.-R.; Chen, M.Y.; Fan, H.-J. Occurrence and treatment efficiency of pharmaceuticals in landfill leachates. Waste Manag. 2016, 55, 257–264. [Google Scholar] [CrossRef]
- Stackelberg, P.E.; Furlong, E.T.; Meyer, M.T.; Zaug, S.D.; Henderson, A.K.; Reissman, D.B. Persistence of pharmaceutical compounds and other organic wastewater contaminants in a conventional drinking-water treatment plant. Sci. Total Environ. 2004, 329, 99–113. [Google Scholar] [CrossRef] [Green Version]
- Okuda, T.; Kobayashi, Y.; Nagao, R.; Yamashita, N.; Tanaka, H.; Tanaka, S.; Fujii, S.; Konishi, C.; Houwa, I. Removal efficiency of 66 pharmaceuticals during wastewater treatment process in Japan. Water Sci. Technol. 2008, 57, 65–71. [Google Scholar] [CrossRef]
- Gulkowska, A.; Leung, H.W.; So, M.K.; Taniyasu, S.; Yamashita, N.; Yeunq, L.W.Y.; Richardson, B.J.; Lei, A.P.; Giesy, J.P.; Lam, P.K.S. Removal of antibiotics from wastewater by sewage treatment facilities in Hong Kong and Shenzhen (China). Water Res. 2008, 42, 395–403. [Google Scholar] [CrossRef]
- Manzo, V.; Honda, L.; Navarro, O.; Ascar, L.; Richter, P. Microextraction of nonsteroidal anti-inflammatory drugs from waste water samples by rotating-disk sorptive extraction. Talanta 2014, 128, 486–492. [Google Scholar] [CrossRef]
- Al-Rifai, J.; Gabelish, C.; Schäfer, A. Occurrence of pharmaceutically active and nonsteroidal estrogenic compounds in three different wastewater recycling schemes in Australia. Chemosphere 2007, 69, 803–815. [Google Scholar] [CrossRef] [PubMed]
- Kummerer, K. Drugs in the environment: Emission of drugs, diagnostic aids and disinfectants into wastewater by hospitals in relation to other sources—A review. Chemosphere 2001, 45, 957–969. [Google Scholar] [CrossRef]
- Kosma, C.I.; Lambropoulou, D.A.; Albanis, T.A. Investigation of PPCPs in wastewater treatment plants in Greece: Occurrence, removal and environmental risk assessment. Sci. Total Environ. 2014, 466–467, 421–438. [Google Scholar] [CrossRef] [PubMed]
- Lonappan, L.; Brar, S.K.; Das, R.K.; Verma, M.; Surampalli, R.Y. Diclofenac and its transformation products: Environmental occurrence and toxicity—A review. Environ. Int. 2016, 96, 127–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leng, X.-Y.; Liu, C.-N.; Wang, S.-C.; Peng, H.-D.; Wang, D.-G.; Pan, H.-F. Comparison of the efficacy of nonsteroidal anti-inflammatory drugs and opioids in the treatment of acute renal colic: A systematic review and meta-analysis. Front. Pharmacol. 2022, 12, 728908. [Google Scholar] [CrossRef] [PubMed]
- Izadi, P.; Izadi, P.; Salem, R.; Papry, S.A.; Magdouli, S.; Pulicharla, R.; Brar, S.K. Non-steroidal anti-inflammatory drugs in the environment: Where were we and how far we have come? Environ. Pollut. 2020, 267, 115370. [Google Scholar] [CrossRef]
- Mailler, R.; Gasperi, J.; Chebbo, G.; Rocher, V. Priority and emerging pollutants in sewage sludge and fate during sludge treatment. Waste Manag. 2014, 34, 1217–1226. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Zhang, P.; Ji, Y.; Tian, J.; Du, Z. Photocatalytic degradation of four non-steroidal anti-inflammatory drugs in water under visible light by P25-TiO2/tetraethyl orthosilicate film and determination via ultra-performance liquid chromatography electrospray tandem mass spectrometry. Chem. Eng. J. 2015, 262, 1108–1115. [Google Scholar] [CrossRef]
- Rizzo, L. Bioassays as a tool for evaluating advanced oxidation processes in water and wastewater treatment. Water Res. 2011, 45, 4311–4340. [Google Scholar] [CrossRef]
- Vergili, I. Application of nanofiltration for the removal of carbamazepine, diclofenac and ibuprofen from drinking water sources. J. Environ. Manag. 2013, 127, 177–187. [Google Scholar] [CrossRef]
- Benitez, F.J.; Acero, J.L.; Real, F.J.; Roldán, G.; Rodriguez, E. Ultrafiltration and nanofiltration membranes applied to the removal of the pharmaceuticals amoxicillin, naproxen, metoprolol and phenacetin from water. J. Chem. Technol. Biotechnol. 2011, 86, 858–866. [Google Scholar] [CrossRef]
- Patel, S.; Mondal, S.; Majumder, S.K.; Das, P.; Ghosh, P. Treatment of a pharmaceutical industrial effluent by a hybrid process of advanced oxidation and adsorption. ACS Omega 2020, 5, 32305–32317. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Peldszus, S.; Huck, P.M. Adsorption characteristics of selected pharmaceuticals and an endocrine disrupting compound-naproxen, carbamazepine and nonylphenol-on activated carbon. Water Res. 2008, 42, 2873–2882. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.J. Adsorption of non-steroidal anti-inflammatory drugs from aqueous solution using activated carbons: Review. J. Environ. Manag. 2017, 190, 274–282. [Google Scholar] [CrossRef]
- Vona, A.; di Martino, F.; Garcia-Ivars, J.; Pico, Y.; Mendoza-Roca, J.-A.; Iborra-Clar, M.-I. Comparison of different removal techniques for selected pharmaceuticals. J. Water Process Eng. 2015, 5, 48–57. [Google Scholar] [CrossRef]
- Domínguez, J.R.; González, T.; Palo, P.; Cuerda-Correa, E.M. Removal of common pharmaceuticals present in surface waters by Amberlite XAD-7 acrylic-ester-resin: Influence of pH and presence of other drugs. Desalination 2011, 269, 231–238. [Google Scholar] [CrossRef]
- Ternes, T.A.; Meisenheimer, M.; McDowell, D.; Sacher, F.; Brauch, H.J.; Haist-Gulde, B.; Preuss, G.; Wilme, U.; Zulei-Seibert, N. Removal of pharmaceuticals during drinking water treatment. Environ. Sci. Technol. 2002, 36, 3855–3863. [Google Scholar] [CrossRef]
- Sandoval-González, A.; Robles, I.; Pineda-Arellano, C.A.; Martínez-Sánchez, C. Removal of anti-inflammatory drugs using activated carbon from agro-industrial origin: Current advances in kinetics, isotherms, and thermodynamic studies. J. Iran. Chem. Soc. 2022, 19, 4017–4033. [Google Scholar] [CrossRef]
- Costa, R.L.T.; do Nascimento, R.A.; de Araújo, R.C.S.; Vieira, M.G.A.; da Silva, M.G.C.; de Carvalho, S.M.L.; de Faria, L.J.G. Removal of non-steroidal anti-inflammatory drugs (NSAIDs) from water with activated carbons synthetized from waste murumuru (Astrocaryum murumuru Mart.): Characterization and adsorption studies. J. Mol. Liq. 2021, 343, 116980. [Google Scholar] [CrossRef]
- Phasuphan, W.; Praphairaksit, N.; Imyim, A. Removal of ibuprofen, diclofenac, and naproxen from water using chitosan-modified waste tire crumb rubber. J. Mol. Liq. 2019, 294, 111554. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Cui, J.; Li, S.; Yuan, M.; Wang, T.; Hu, Q.; Hou, X. Fabrication and characterization of metal organic frameworks/polyvinyl alcohol cryogel and their application in extraction of non-steroidal anti-inflammatory drugs in water samples. Anal. Chim. Acta 2018, 1022, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Soares, S.F.; Fernandes, T.; Sacramento, M.; Trindade, T.; Daniel-da-Silva, A.L. Magnetic quaternary chitosan hybrid nanoparticles for the efficient uptake of diclofenac from water. Carbohydr. Polym. 2019, 203, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Dong, Y.; Yang, Z.; Yang, W.; Wu, J.; Dong, C. Adsorption of pharmaceuticals on chitosan-based magnetic composite particles with core-brush topology. Chem. Eng. J. 2016, 304, 325–334. [Google Scholar] [CrossRef]
- Hanbali, G.; Jodeh, S.; Hamed, O.; Bol, R.; Khalaf, B.; Qdemat, A.; Samhan, S. Enhanced ibuprofen adsorption and desorption on synthesized functionalized magnetic multiwall carbon nanotubes from aqueous solution. Materials 2020, 13, 3329. [Google Scholar] [CrossRef]
- Mazen, N. An overview of carbon-based materials for the removal of pharmaceutical active compounds. In Carbon-Based Material for Environmental Protection and Remediation; Bartoli, M., Frediani, M., Rosi, L., Eds.; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Mohammadi Nodeh, M.K.; Radfard, M.; Zardari, L.A.; Rashidi Nodeh, H. Enhanced removal of naproxen from wastewater using silica magnetic nanoparticles decorated onto graphene oxide; parametric and equilibrium study. Sep. Sci. Technol. 2018, 53, 2476–2485. [Google Scholar] [CrossRef]
- Khan, F.S.A.; Mubarak, N.M.; Tan, Y.H.; Khalid, M.; Karri, R.R.; Walvekar, R.; Abdullah, E.C.; Nizamuddin, S.; Mazari, S.A. A comprehensive review on magnetic carbon nanotubes and carbon nanotube-based buckypaper for removal of heavy metals and dyes. J. Hazard. Mater. 2021, 413, 125375. [Google Scholar] [CrossRef] [PubMed]
- Alshahrani, A.; Alharbi, A.; Alnasser, S.; Almihdar, M.; Alsuhybani, M.; AlOtaibi, B. Enhanced heavy metals removal by a novel carbon nanotubes buckypaper membrane containing a mixture of two biopolymers: Chitosan and i-carrageenan. Separ. Purif. Technol. 2021, 276, 119300. [Google Scholar] [CrossRef]
- De Filpo, G.; Pantuso, E.; Mashin, A.I.; Baratta, M.; Nicoletta, F.P. WO3/buckypaper membranes for advanced oxidation processes. Membranes 2020, 10, 157. [Google Scholar] [CrossRef]
- Rashid, M.H.-O.; Ralph, S.F. Carbon nanotube membranes: Synthesis, properties, and future filtration applications. Nanomaterials 2017, 7, 99. [Google Scholar] [CrossRef]
- Patole, S.P.; Arif, M.F.; Susantyoko, R.A.; Almheiri, S.; Kumar, S. A wet-filtration-zipping approach for fabricating highly electroconductive and auxetic graphene/carbon nanotube hybrid buckypaper. Sci. Rep. 2018, 8, 12188. [Google Scholar] [CrossRef] [Green Version]
- Xia, Q.; Zhang, Z.; Liu, Y.; Leng, J. Buckypaper and its composites for aeronautic applications. Compos. Part B Eng. 2020, 199, 108231. [Google Scholar] [CrossRef]
- Aslam, M.M.-A.; Kuo, H.-W.; Den, W.; Usman, M.; Sultan, M.; Ashraf, H. Functionalized carbon nanotubes (CNTs) for water and wastewater treatment: Preparation to application. Sustainability 2021, 13, 5717. [Google Scholar] [CrossRef]
- Shawky, H.A.; El-Aassar, A.H.M.; Abo-Zeid, D.E. Chitosan/carbon nanotube composite beads: Preparation, characterization, and cost evaluation for mercury removal from wastewater of some industrial cities in Egypt. J. Appl. Polym. Sci. 2012, 125, E93–E101. [Google Scholar] [CrossRef]
- Baratta, M.; Mastropietro, T.F.; Bruno, R.; Tursi, A.; Negro, C.; Ferrando-Soria, J.; Mashin, A.I.; Nezhdanov, A.; Nicoletta, F.P.; De Filpo, G.; et al. Multivariate metal–organic framework/single–walled carbon nanotube buckypaper for selective lead decontamination. ACS Appl. Nano Mater. 2022, 5, 5223–5233. [Google Scholar] [CrossRef]
- Tursi, A.; Mastropietro, T.F.; Bruno, R.; Baratta, M.; Ferrando-Soria, J.; Mashin, A.I.; Nicoletta, F.P.; Pardo, E.; De Filpo, G.; Armentano, D. Synthesis and enhanced capture properties of a new BioMOF@SWCNT-BP: Recovery of the endangered rare-earth elements from aqueous systems. ACS Adv. Mater. Interfaces 2021, 8, 2100730. [Google Scholar] [CrossRef]
- Baratta, M.; Tursi, A.; Curcio, M.; Cirillo, G.; Nicoletta, F.P.; De Filpo, G. GO-SWCNT Buckypapers as an enhanced technology for water decontamination from lead. Molecules 2022, 27, 4044. [Google Scholar] [CrossRef]
- Huang, J.; Her, S.-C.; Yang, X.; Zhi, M. Synthesis and characterization of multi-walled carbon nanotube/graphene nanoplatelet hybrid film for flexible strain sensors. Nanomaterials 2018, 8, 786. [Google Scholar] [CrossRef] [Green Version]
- Musielak, M.; Gagor, A.; Zawisza, B.; Talik, E.; Sitko, R. Graphene oxide/carbon nanotube membranes for highly efficient removal of metal ions from water. ACS Appl. Mater. Interfaces 2019, 11, 28582–28590. [Google Scholar] [CrossRef]
- Jauris, I.M.; Matos, C.F.; Saucier, C.; Lima, E.C.; Zarbin, A.J.G.; Fagan, S.B.; Machado, F.M.; Zanella, I. Adsorption of sodium diclofenac on graphene: A combined experimental and theoretical study. Phys. Chem. Chem. Phys. 2016, 18, 1526–1536. [Google Scholar] [CrossRef]
- Czech, B.; Oleszczuk, P. Sorption of diclofenac and naproxen onto MWCNT in model wastewater treated by H2O2 and/or UV. Chemosphere 2016, 149, 272–278. [Google Scholar] [CrossRef]
- Hiew, B.Y.Z.; Lee, L.Y.; Lee, X.J.; Gan, S.; Thangalazhy-Gopakumar, S.; Lim, S.S.; Pan, G.-T.; Yang, T.C.-K. Adsorptive removal of diclofenac by graphene oxide: Optimization, equilibrium, kinetic and thermodynamic studies. J. Taiwan Inst. Chem. Eng. 2019, 98, 150–162. [Google Scholar] [CrossRef]
- Seo, P.W.; Bhadra, B.N.; Ahmed, I.; Khan, N.A.; Jhung, S.H. Adsorptive removal of pharmaceuticals and personal care products from water with functionalized metalorganic frameworks: Remarkable adsorbents with hydrogen-bonding abilities. Sci. Rep. 2016, 6, 34462. [Google Scholar] [CrossRef] [Green Version]
- Lin, S.; Zhao, Y.; Yun, Y.-S. Highly effective removal of nonsteroidal anti-inflammatory pharmaceuticals from water by Zr(IV)-based metal–organic framework: Adsorption performance and mechanisms. ACS Appl. Mater. Interfaces 2018, 10, 28076–28085. [Google Scholar] [CrossRef] [PubMed]
- Bhadra, B.N.; Ahmed, I.; Kim, S.; Jhung, S.H. Adsorptive removal of ibuprofen and diclofenac from water using metal-organic framework-derived porous carbon. Chem. Eng. J. 2017, 314, 50–58. [Google Scholar] [CrossRef]
- Liu, W.; Shen, X.; Han, Y.; Liu, Z.; Dai, W.; Dutta, A.; Kumar, A.; Liu, J. Selective adsorption and removal of drug contaminants by using an extremely stable Cu(II)-based 3D metal-organic framework. Chemosphere 2019, 215, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Da̧browski, A. Adsorption-from theory to practice. Adv. Colloid Interface Sci. 2001, 93, 135–224. [Google Scholar] [CrossRef]
- Song, J.Y.; Jhung, S.H. Adsorption of pharmaceuticals and personal care products over metal-organic frameworks functionalized with hydroxyl groups: Quantitative analyses of H-bonding in adsorption. Chem. Eng. J. 2017, 322, 366–374. [Google Scholar] [CrossRef]
- White, B.; Banerjee, S.; O’Brien, S.; Turro, N.J.; Herman, I.P. Zeta-potential measurements of surfactant-wrapped individual single-walled carbon nanotubes. J. Phys. Chem. C 2007, 111, 13684–13690. [Google Scholar] [CrossRef]
- Sangster, J. LOGKOW Databank; Sangster Res. Lab.: Montreal, QC, Canada, 1994. [Google Scholar]
- Karami, A.; Sabouni, R.; Ghommem, M. Experimental investigation of competitive co-adsorption of naproxen and diclofenac from water by an aluminum-based metalorganic framework. J. Mol. Liq. 2020, 305, 112808. [Google Scholar] [CrossRef]
- Sun, W.; Li, H.; Li, H.; Li, S.; Cao, X. Adsorption mechanisms of ibuprofen and naproxen to UiO-66 and UiO-66-NH2: Batch experiment and DFT calculation. Chem. Eng. J. 2019, 360, 645–653. [Google Scholar] [CrossRef]
- Tomul, F.; Arslan, Y.; Kabak, B.; Trak, D.; Kendüzler, E.; Lima, E.C.; Tran, H.N. Peanut shells-derived biochars prepared from different carbonization processes: Comparison of characterization and mechanism of naproxen adsorption in water. Sci. Total Env. 2020, 726, 137828. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, G.; Curcio, M.; Madeo, L.F.; Iemma, F.; De Filpo, G.; Hampel, S.; Nicoletta, F.P. Carbon Nanotubes Hybrid Hydrogels for Environmental Remediation: Evaluation of Adsorption Efficiency under Electric Field. Molecules 2021, 26, 7001. [Google Scholar] [CrossRef] [PubMed]
- Chun, M.S.; Lee, S.Y.; Yang, S.M. Estimation of zeta potential by electrokinetic analysis of ionic fluid flows through a divergent microchannel. J. Colloid Interface Sci. 2003, 266, 120–126. [Google Scholar] [CrossRef]
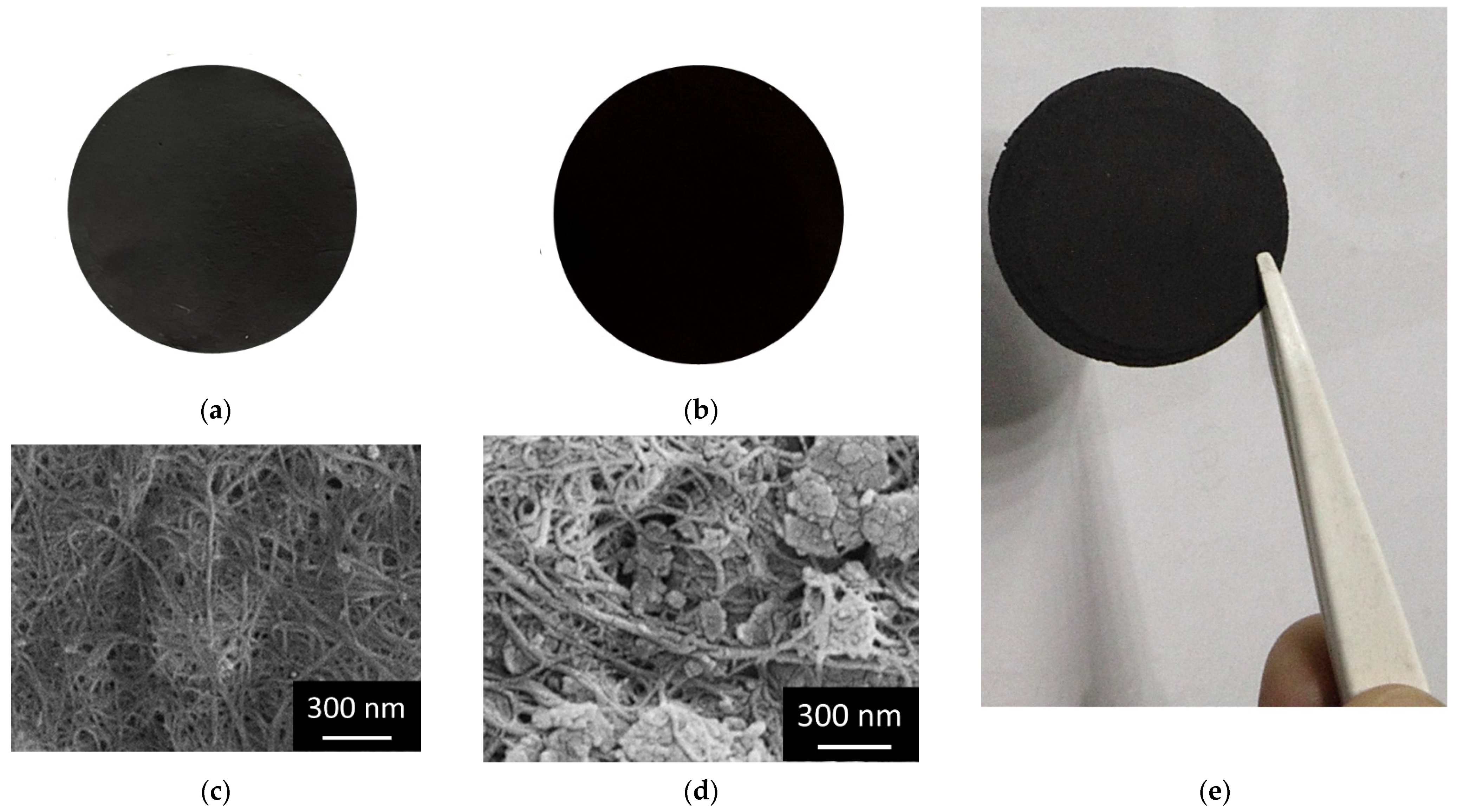


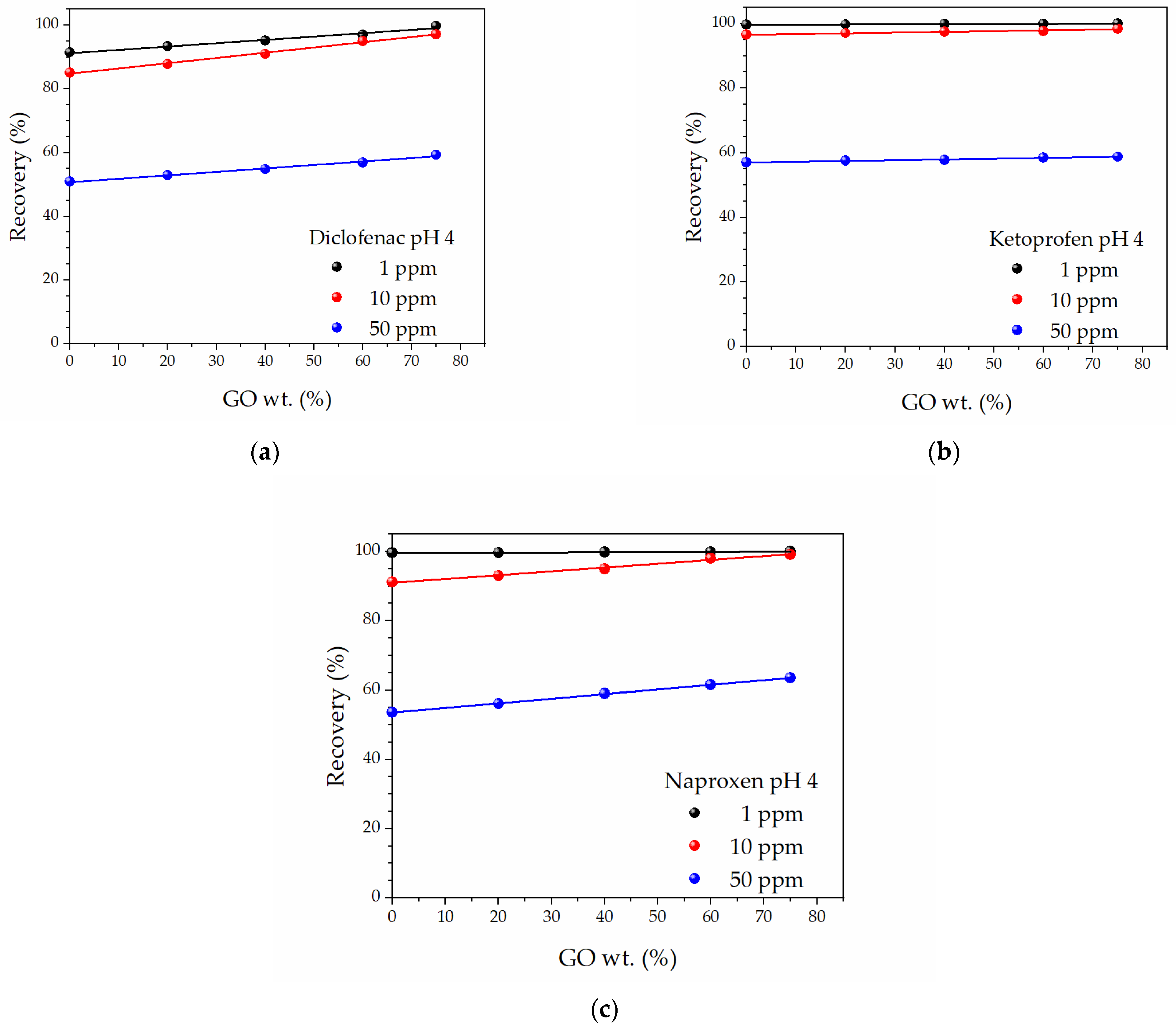
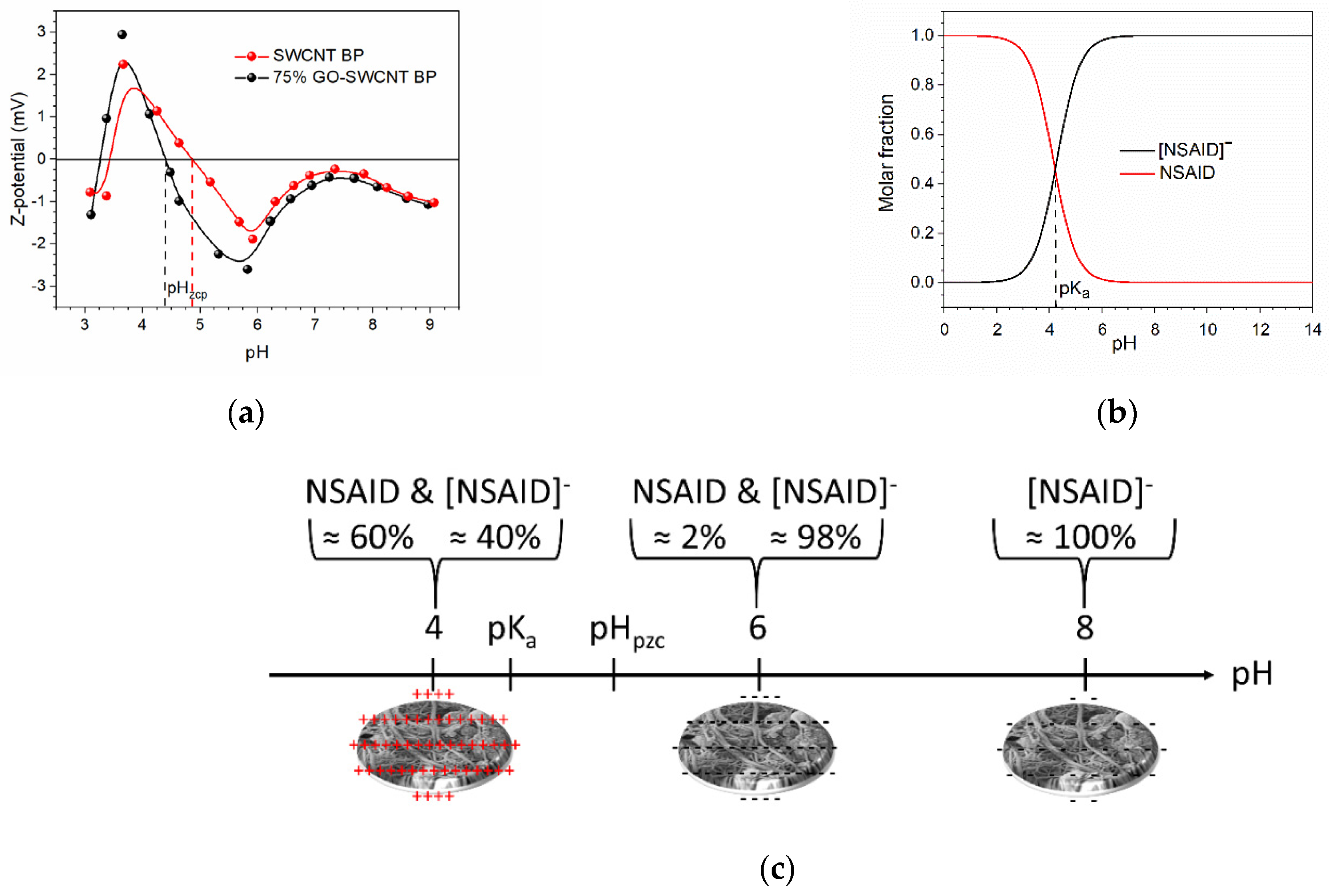
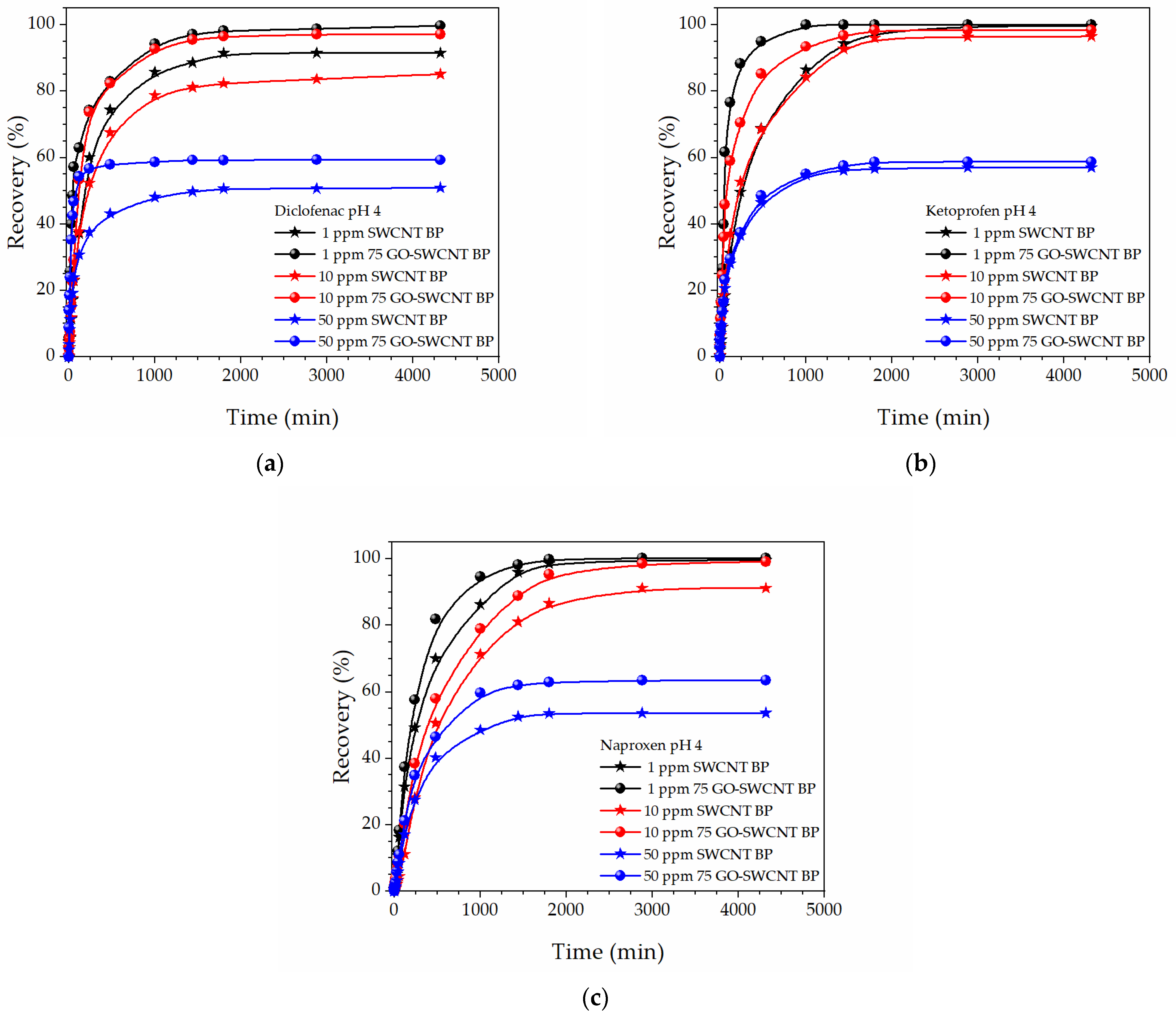
| Pseudo-First Order Kinetics | Pseudo-Second Order Kinetics | |||||
|---|---|---|---|---|---|---|
| Diclofenac | k1 × 103 (min−1) | qe (mg g−1) | R2 | k2 × 104 (g mg−1 min−1) | qe (mg g−1) | R2 |
| SWCNT BP | 10.1 ± 1.1 | 96.4 ± 2.5 | 0.9823 | 2.6 ± 0.4 | 103.0 ± 5.4 | 0.9961 |
| 75% GO-SWCNT BP | 32.5 ± 2.5 | 116.1 ± 2.0 | 0.9752 | 8.4 ± 1.8 | 120.5 ± 8.3 | 0.9914 |
| Ketoprofen | k1 × 103 (min−1) | qe (mg g−1) | R2 | k2 × 103 (g mg−1 min−1) | qe (mg g−1) | R2 |
| SWCNT BP | 6.1 ± 0.7 | 110.2 ± 3.0 | 0.9756 | 1.4 ± 0.3 | 118.7 ± 8.3 | 0.9939 |
| 75% GO-SWCNT BP | 6.4 ± 0.7 | 112.8 ± 3.1 | 0.9777 | 1.5 ± 0.3 | 121.8 ± 8.8 | 0.9934 |
| Naproxen | k1 × 103 (min−1) | qe (mg g−1) | R2 | k2 × 103 (g mg−1 min−1) | qe (mg g−1) | R2 |
| SWCNT BP | 2.9 ± 0.1 | 106.6 ± 0.8 | 0.9943 | 5.3 ± 1.1 | 121.1 ± 9.5 | 0.9980 |
| 75% GO-SWCNT BP | 3.2 ± 0.1 | 125.9 ± 0.8 | 0.9960 | 5.7 ± 1.4 | 142.2 ± 9.4 | 0.9988 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baratta, M.; Tursi, A.; Curcio, M.; Cirillo, G.; Nezhdanov, A.V.; Mashin, A.I.; Nicoletta, F.P.; De Filpo, G. Removal of Non-Steroidal Anti-Inflammatory Drugs from Drinking Water Sources by GO-SWCNT Buckypapers. Molecules 2022, 27, 7674. https://doi.org/10.3390/molecules27227674
Baratta M, Tursi A, Curcio M, Cirillo G, Nezhdanov AV, Mashin AI, Nicoletta FP, De Filpo G. Removal of Non-Steroidal Anti-Inflammatory Drugs from Drinking Water Sources by GO-SWCNT Buckypapers. Molecules. 2022; 27(22):7674. https://doi.org/10.3390/molecules27227674
Chicago/Turabian StyleBaratta, Mariafrancesca, Antonio Tursi, Manuela Curcio, Giuseppe Cirillo, Aleksey Vladimirovich Nezhdanov, Alexandr Ivanovic Mashin, Fiore Pasquale Nicoletta, and Giovanni De Filpo. 2022. "Removal of Non-Steroidal Anti-Inflammatory Drugs from Drinking Water Sources by GO-SWCNT Buckypapers" Molecules 27, no. 22: 7674. https://doi.org/10.3390/molecules27227674





