Fabrication of Chitosan-Based Network Polysaccharide Nanogels
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Synthesis of GlcA-Glcn-GlcCOONa
3.3. Preparation of WSCS
3.4. Preparation of Nanogels
3.5. Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Schuerch, C. Polysaccharides. In Encyclopedia of Polymer Science and Engineering, 2nd ed.; Mark, H.F., Bilkales, N., Overberger, C.G., Eds.; John Wiley & Sons: New York, NY, USA, 1986; Volume 13, pp. 87–162. [Google Scholar]
- Song, E.H.; Shang, J.; Ratner, D.M. Polysaccharides. In Polymer Science: A Comprehensive Reference; Matyjaszewski, K., Möller, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 137–155. [Google Scholar]
- Lin, N.; Huang, J.; Dufresne, A. Preparation, properties and applications of polysaccharide nanocrystals in advanced functional nanomaterials: A review. Nanoscale 2012, 4, 3274–3294. [Google Scholar] [CrossRef] [PubMed]
- Akhlaghi, S.P.; Peng, B.; Yao, Z.; Tam, K.C. Sustainable nanomaterials derived from polysaccharides and amphiphilic compounds. Soft Matter 2013, 9, 7905–7918. [Google Scholar] [CrossRef]
- Wen, Y.; Oh, J.K. Recent strategies to develop polysaccharide-based nanomaterials for biomedical applications. Macromol. Rapid Commun. 2014, 35, 1819–1832. [Google Scholar] [CrossRef] [PubMed]
- Kanaoujiya, R.; Saroj, S.K.; Srivastava, S.; Chaudhary, M.K. Renewable Polysaccharide and Biomedical Application of Nanomaterials. J. Nanomater. 2022, 2022, 1050211. [Google Scholar] [CrossRef]
- Papagiannopoulos, A.; Sotiropoulos, K. Current advances of polysaccharide-based nanogels and microgels in food and biomedical sciences. Polymers 2022, 14, 813. [Google Scholar] [CrossRef]
- Mauri, E.; Giannitelli, S.M.; Trombetta, M.; Rainer, A. Synthesis of nanogels: Current trends and future outlook. Gels 2021, 7, 36. [Google Scholar] [CrossRef]
- Maggi, F.; Ciccarelli, S.; Diociaiuti, M.; Casciardi, S.; Masci, G. Chitosan nanogels by template chemical cross-linking in polyion complex micelle nanoreactors. Biomacromolecules 2011, 12, 3499–3507. [Google Scholar] [CrossRef]
- Algharib, S.A.; Dawood, A.; Zhou, K.; Chen, D.; Li, C.; Meng, K.; Maa, M.K.; Ahmed, S.; Huang, L.; Xie, S. Designing, structural determination and biological effects of rifaximin loaded chitosan- carboxymethyl chitosan nanogel. Carbohydr. Polym. 2020, 248, 116782. [Google Scholar] [CrossRef]
- Kumar, M.N.V.R.; Muzzarelli, R.A.A.; Muzzarelli, C.; Sashiwa, H.; Domb, A.J. Chitosan Chemistry and Pharmaceutical Perspectives. Chem. Rev. 2004, 104, 6017–6084. [Google Scholar] [CrossRef]
- Arteche Pujana, M.; Pérez-Álvarez, L.; Cesteros Iturbe, L.C.; Katime, I. Biodegradable chitosan nanogels crosslinked with genipin. Carbohydr. Polym. 2013, 94, 836–842. [Google Scholar] [CrossRef]
- Pujana, M.A.; Pérez-Álvarez, L.; Iturbe, L.C.C.; Katime, I. Water soluble folate-chitosan nanogels crosslinked by genipin. Carbohydr. Polym. 2014, 101, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Liao, W.; Wang, W.; Zhou, J.; Tan, W.; Xiang, W.; Zhang, J.; Guo, L.; Chen, T.; Ma, D.; et al. Genipin-crosslinked carboxymethyl chitosan nanogel for lung-targeted delivery of isoniazid and rifampin. Carbohydr. Polym. 2018, 197, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, C.; Kim, T.H.; Lee, E.S.; Shin, B.S.; Chi, S.-C.; Park, E.-S.; Lee, K.C.; Youn, Y.S. Self-assembled glycol chitosan nanogels containing palmityl-acylated exendin-4 peptide as a long-acting anti-diabetic inhalation system. J. Control. Release 2012, 161, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Pereira, P.; Morgado, D.; Crepet, A.; David, L.; Gama, F.M. Glycol chitosan-based nanogel as a potential targetable carrier for siRNA. Macromol. Biosci. 2013, 13, 1369–1378. [Google Scholar] [CrossRef] [Green Version]
- Pereira, P.; Correia, A.; Gama, F.M. In vivo imaging of glycol chitosan-based nanogel biodistribution. Macromol. Biosci. 2016, 16, 432–440. [Google Scholar] [CrossRef] [Green Version]
- Kadokawa, J.; Chigita, H.; Yamamoto, K. Chemoenzymatic synthesis of carboxylate-terminated maltooligosaccharides and their use for cross-linking of chitin. Int. J. Biol. Macromol. 2020, 159, 510–516. [Google Scholar] [CrossRef]
- Umegatani, Y.; Izawa, H.; Nawaji, M.; Yamamoto, K.; Kubo, A.; Yanase, M.; Takaha, T.; Kadokawa, J. Enzymatic α-glucuronylation of maltooligosaccharides using α-glucuronic acid 1-phosphate as glycosyl donor catalyzed by a thermostable phosphorylase from Aquifex aeolicus vf5. Carbohydr. Res. 2012, 350, 81–85. [Google Scholar] [CrossRef]
- Kobayashi, K.; Kamiya, S.; Enomoto, N. Amylose-carrying styrene macromonomer and its homo- and copolymers: Synthesis via enzyme-catalyzed polymerization and complex formation with iodine. Macromolecules 1996, 29, 8670–8676. [Google Scholar] [CrossRef]
- Kadokawa, J. Glucan phosphorylase-catalyzed enzymatic synthesis of unnatural oligosaccharides and polysaccharides using nonnative substrates. Polym. J. 2022, 54, 413–426. [Google Scholar] [CrossRef]
- Kurita, K. Chitin and chitosan: Functional biopolymers from marine crustaceans. Marine Biotechnol. 2006, 8, 203–226. [Google Scholar] [CrossRef]
- Qin, C.Q.; Du, Y.M.; Xiao, L. Effect of hydrogen peroxide treatment on the molecular weight and structure of chitosan. Polym. Degrad. Stab. 2002, 76, 211–218. [Google Scholar] [CrossRef]
- Du, Y.; Zhao, Y.; Dai, S.; Yang, B. Preparation of water-soluble chitosan from shrimp shell and its antibacterial activity. Innov. Food Sci. Emerg. Technol. 2009, 10, 103–107. [Google Scholar] [CrossRef]
- Jin, Q.; Yu, H.; Wang, X.; Li, K.; Li, P. Effect of the molecular weight of water-soluble chitosan on its fat-/cholesterol-binding capacities and inhibitory activities to pancreatic lipase. PeerJ 2017, 5, e3279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yaghobi, N. Controlling chitosan’s molecular weight via multistage deacetylation. Biotechnol. Bioprocess Eng. 2012, 17, 812–817. [Google Scholar] [CrossRef]
- Einbu, A.; Vårum, K.M. Characterization of chitin and its hydrolysis to GlcNAc and GlcN. Biomacromolecules 2008, 9, 1870–1875. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Yamamoto, K.; Kadokawa, J. Facile nanofibrillation of chitin derivatives by gas bubbling and ultrasonic treatments in water. Carbohydr. Res. 2014, 398, 25–30. [Google Scholar] [CrossRef]
- Teng, Y.; Jin, H.; Nan, D.; Li, M.; Fan, C.; Liu, Y.; Lv, P.; Cui, W.; Sun, Y.; Hao, H.; et al. In vivo evaluation of urokinase-loaded hollow nanogels for sonothrombolysis on suture embolization-induced acute ischemic stroke rat model. Bioact. Mater. 2018, 3, 102–109. [Google Scholar] [CrossRef]
- Bhuiyan, S.H.; Rus’d, A.A.; Kitaoka, M.; Hayashi, K. Characterization of a hyperthermostable glycogen phosphorylase from Aquifex aeolicus expressed in Escherichia coli. J. Mol. Catal. B Enzym. 2003, 22, 173–180. [Google Scholar] [CrossRef]
- Mima, S.; Miya, M.; Iwamoto, R.; Yoshikawa, S. Highly deacetylated chitosan and its properties. J. Appl. Polym. Sci. 1983, 28, 1909–1917. [Google Scholar] [CrossRef]
- Heeres, A.; Van Doren, H.A.; Gotlieb, K.F.; Bleeker, I.P. Synthesis of α- and β-D-glucopyranuronate 1-phosphate and α-d-glucopyranuronate 1-fluoride: Intermediates in the synthesis of D-glucuronic acid from starch. Carbohydr. Res. 1997, 299, 221–227. [Google Scholar] [CrossRef]
- von Braunmühl, V.; Jonas, G.; Stadler, R. Enzymatic grafting of amylose from poly(dimethylsiloxanes). Macromolecules 1995, 28, 17–24. [Google Scholar] [CrossRef]

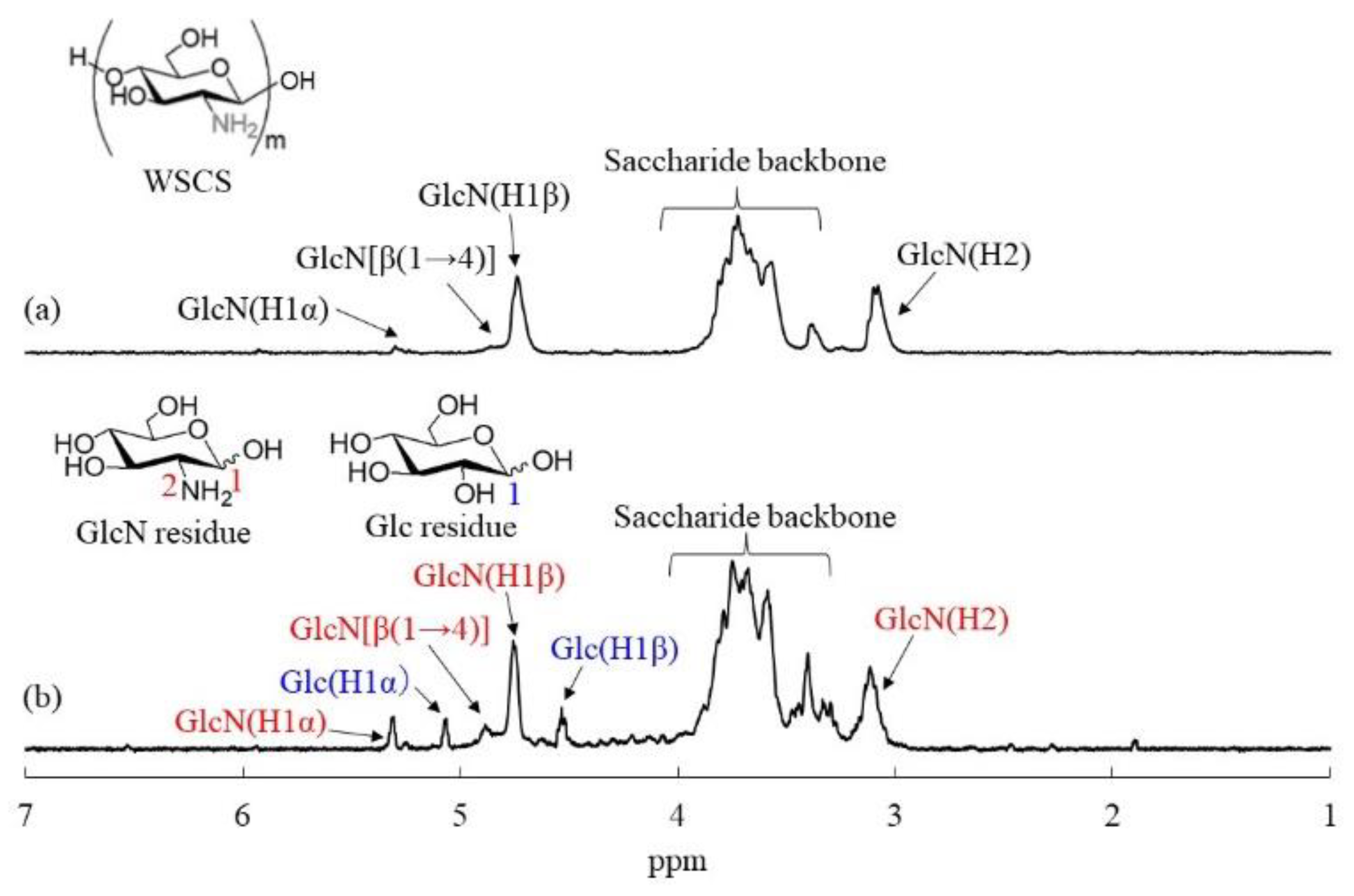
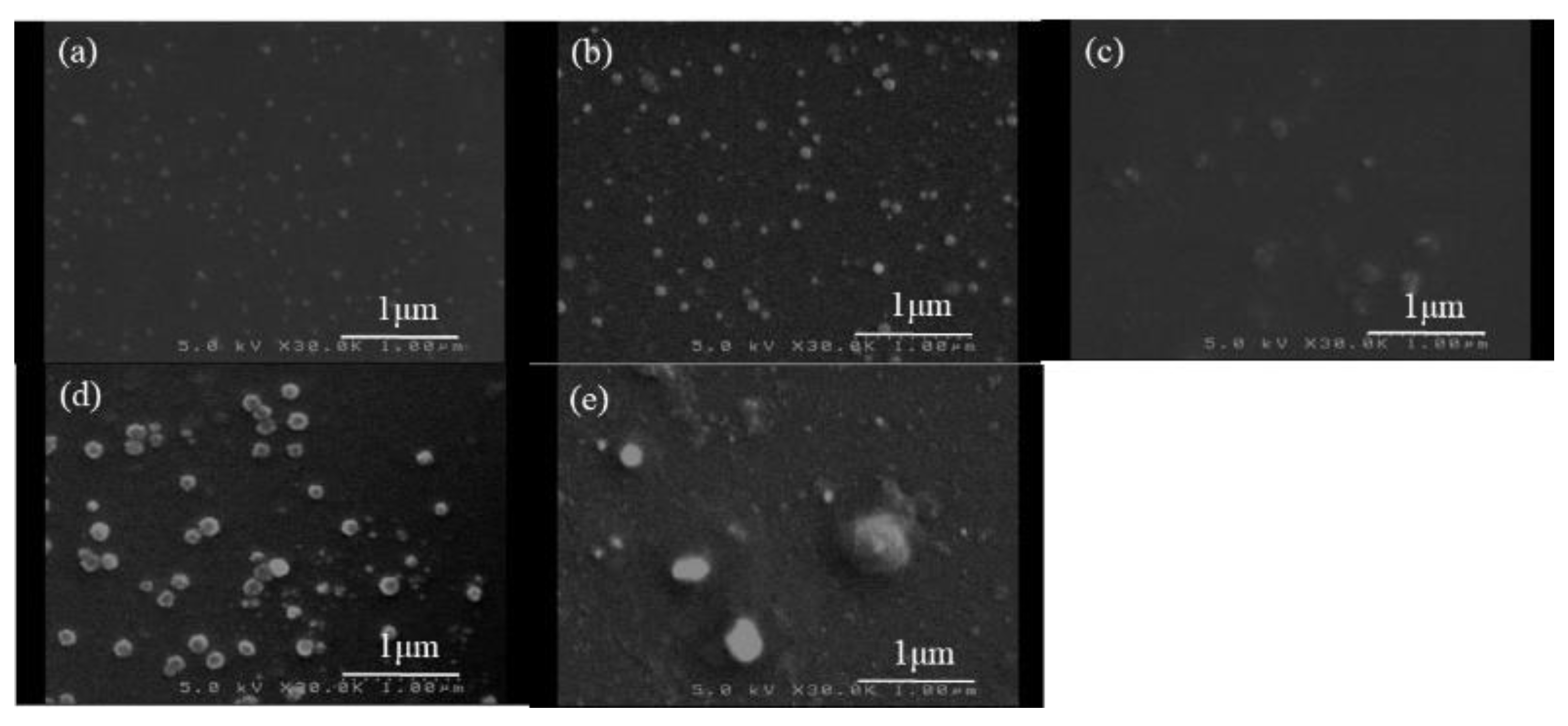
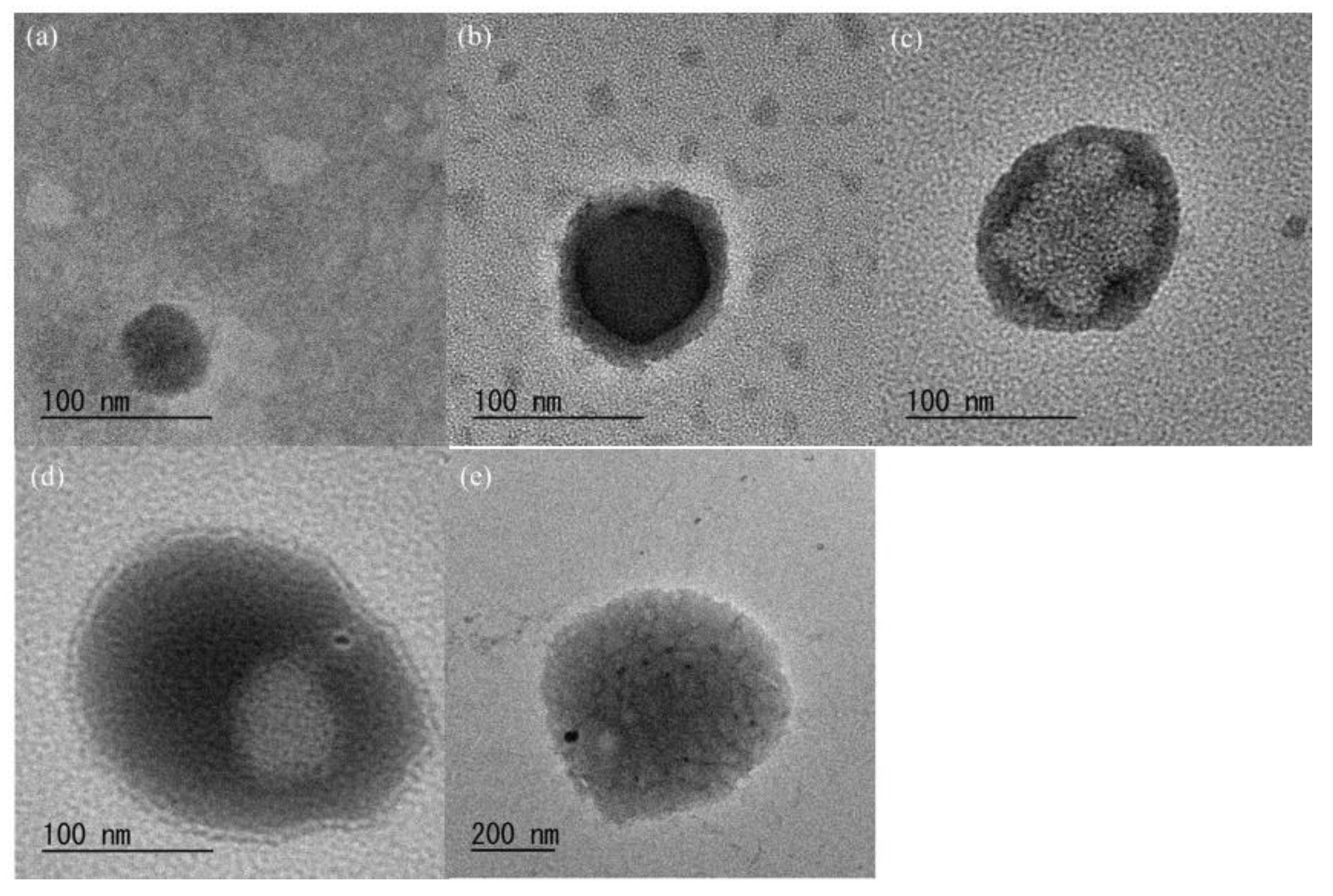
| Run | NH2:COONa | Glc/GlcN Unit Ratio (b) | Particle Size by SEM (nm) | Standard Deviation by SEM | Particle Size by DLS (nm) |
|---|---|---|---|---|---|
| 1 | 1:0.04 | 0.23 | 44 | 0.63 | 720.0 |
| 2 | 1:0.05 | 0.33 | 90 | 0.82 | 729.6 |
| 3 | 1:0.06 | 0.39 | 128 | 1.11 | 909.8 |
| 4 | 1:0.10 | 0.45 | 135 | 1.09 | 975.2 |
| 5 | 1:0.12 | 0.58 | 242 | 2.07 | 1366.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakamichi, A.; Kadokawa, J.-i. Fabrication of Chitosan-Based Network Polysaccharide Nanogels. Molecules 2022, 27, 8384. https://doi.org/10.3390/molecules27238384
Nakamichi A, Kadokawa J-i. Fabrication of Chitosan-Based Network Polysaccharide Nanogels. Molecules. 2022; 27(23):8384. https://doi.org/10.3390/molecules27238384
Chicago/Turabian StyleNakamichi, Aina, and Jun-ichi Kadokawa. 2022. "Fabrication of Chitosan-Based Network Polysaccharide Nanogels" Molecules 27, no. 23: 8384. https://doi.org/10.3390/molecules27238384





