Ag Nanoparticles Decorated CuO@RF Core-Shell Nanowires for High-Performance Surface-Enhanced Raman Spectroscopy Application
Abstract
:1. Introduction
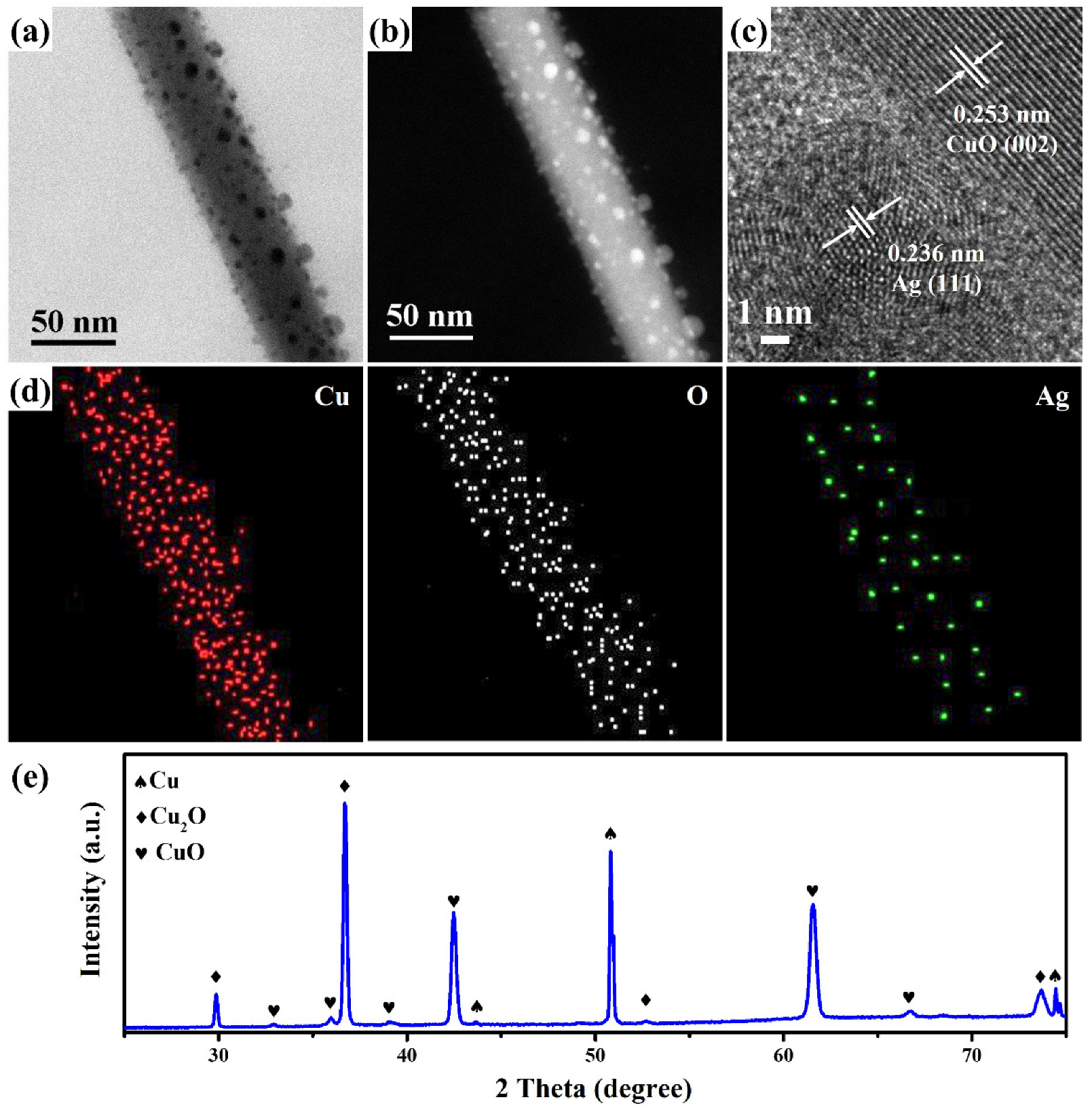
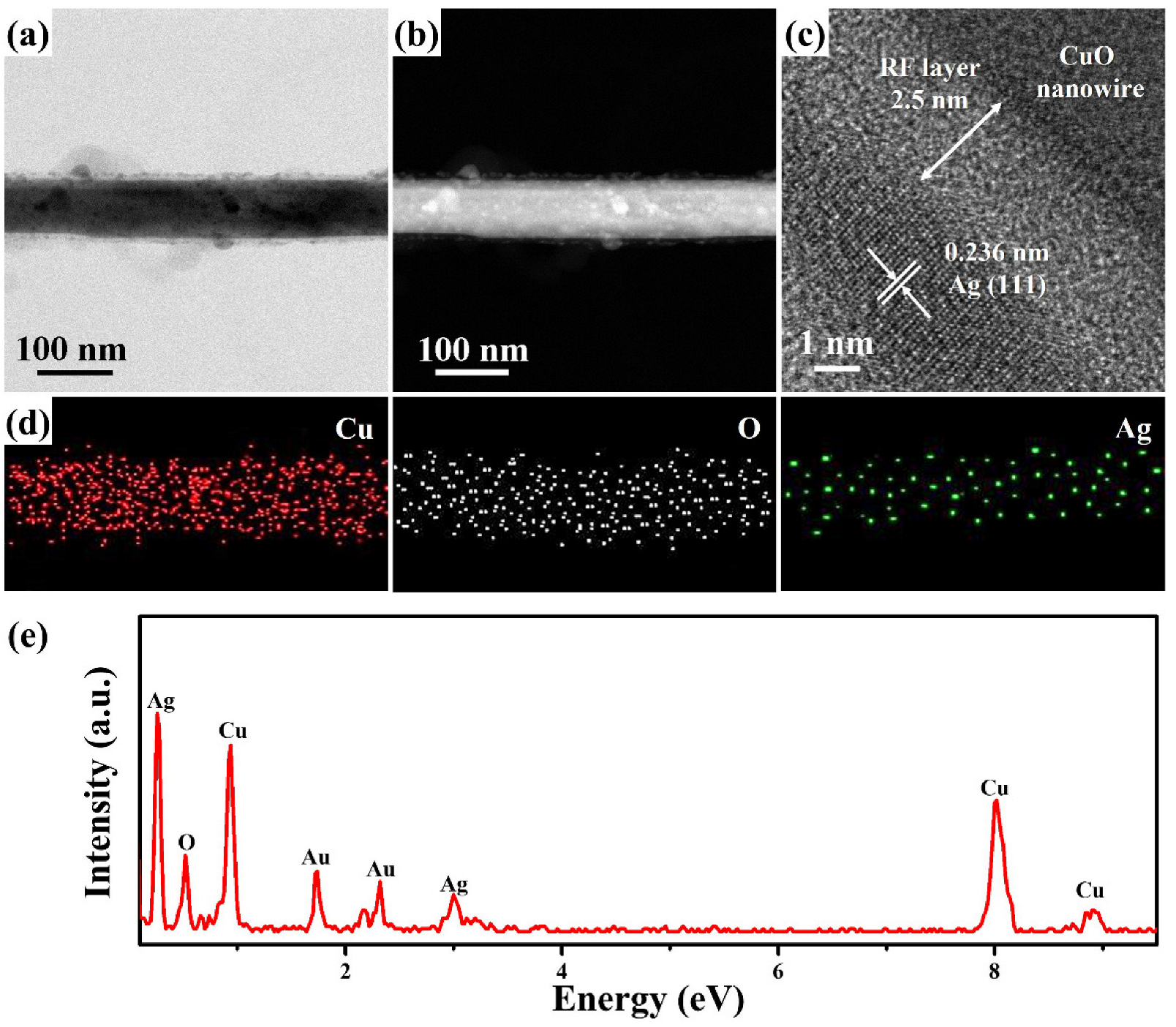
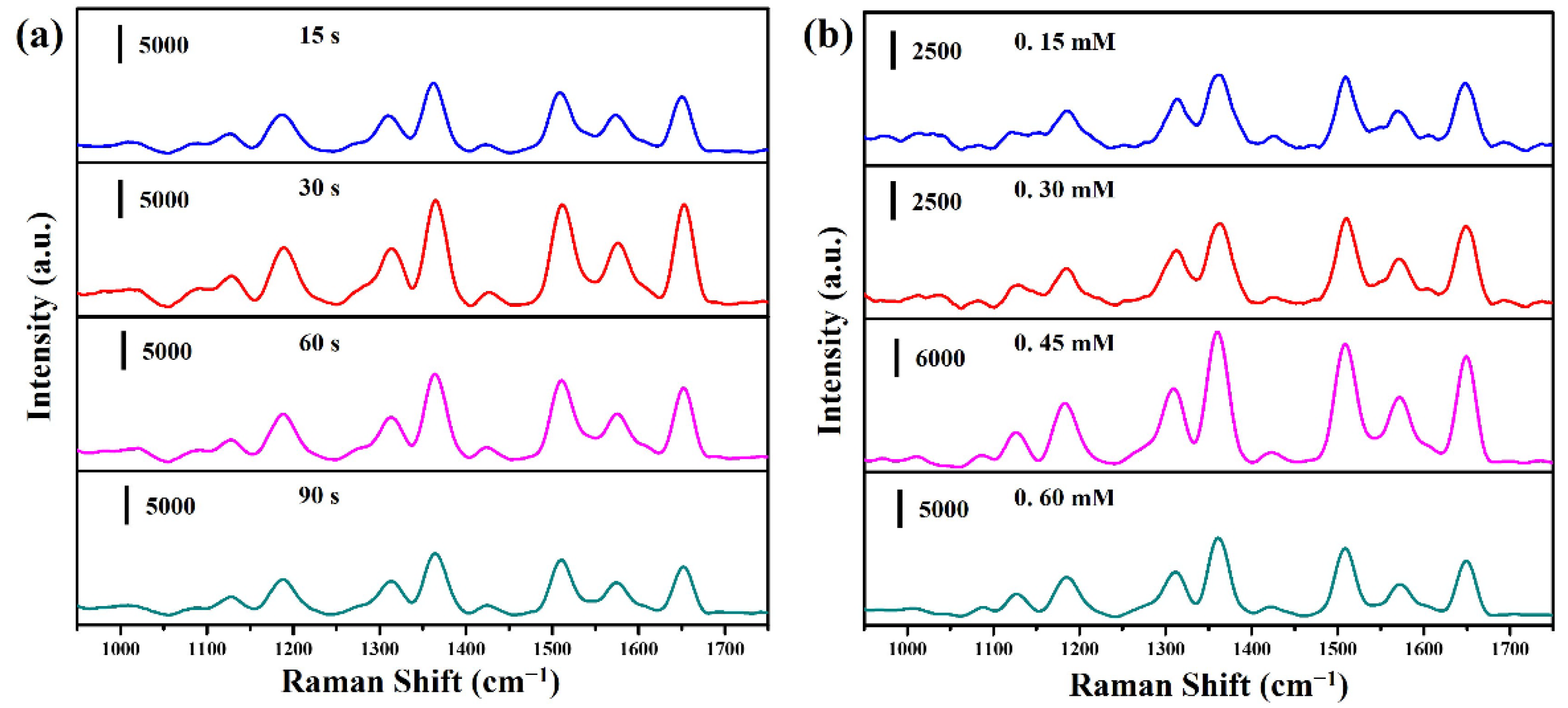
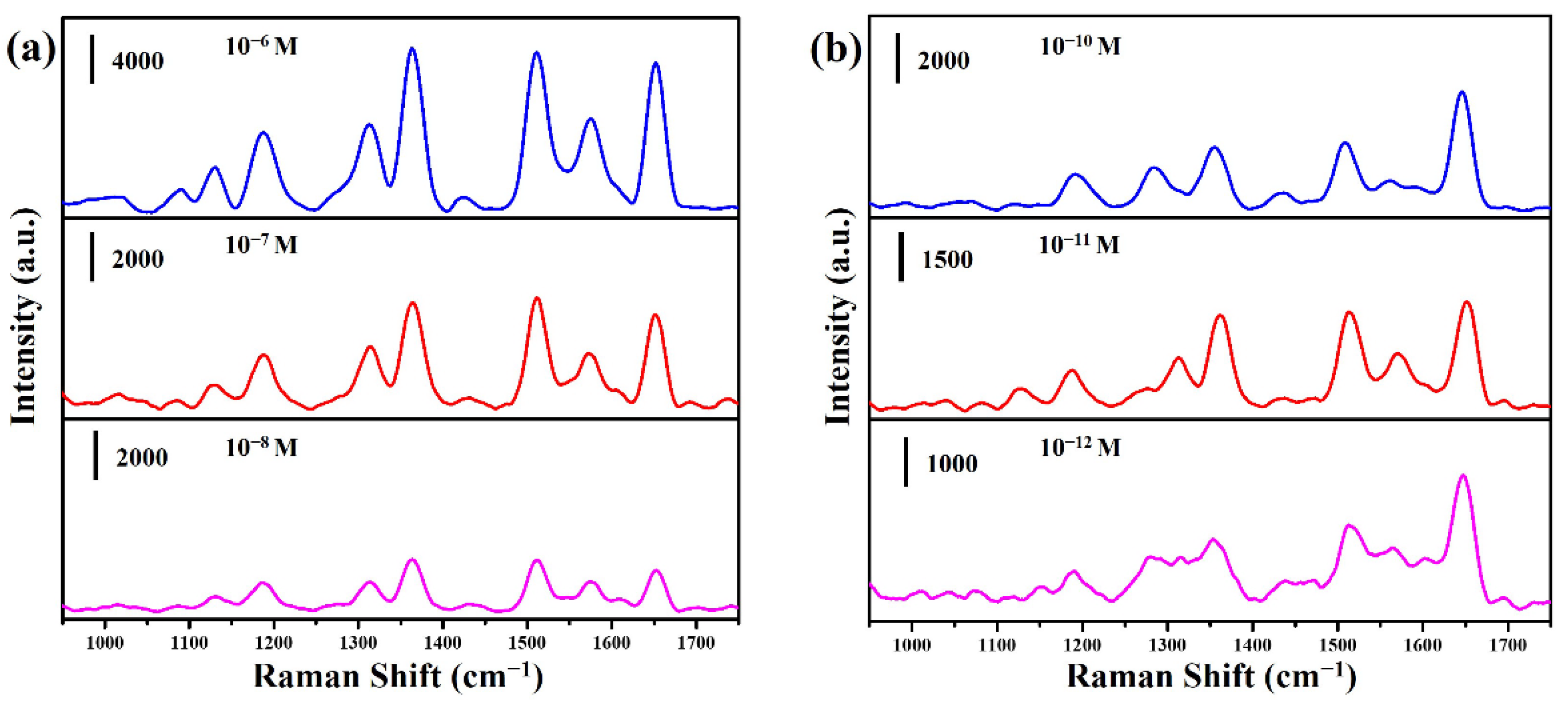
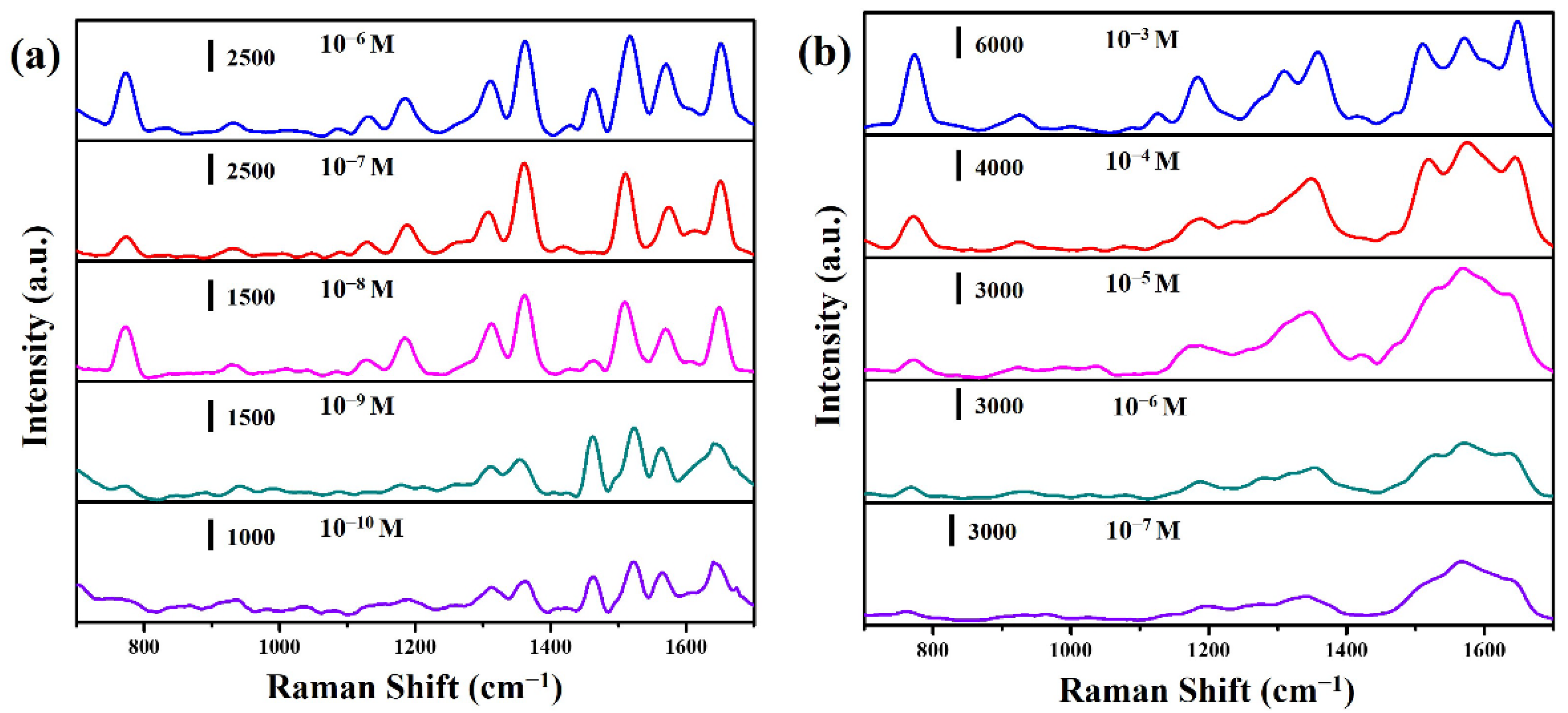
2. Results and Discussion
3. Materials and Methods
3.1. Materials
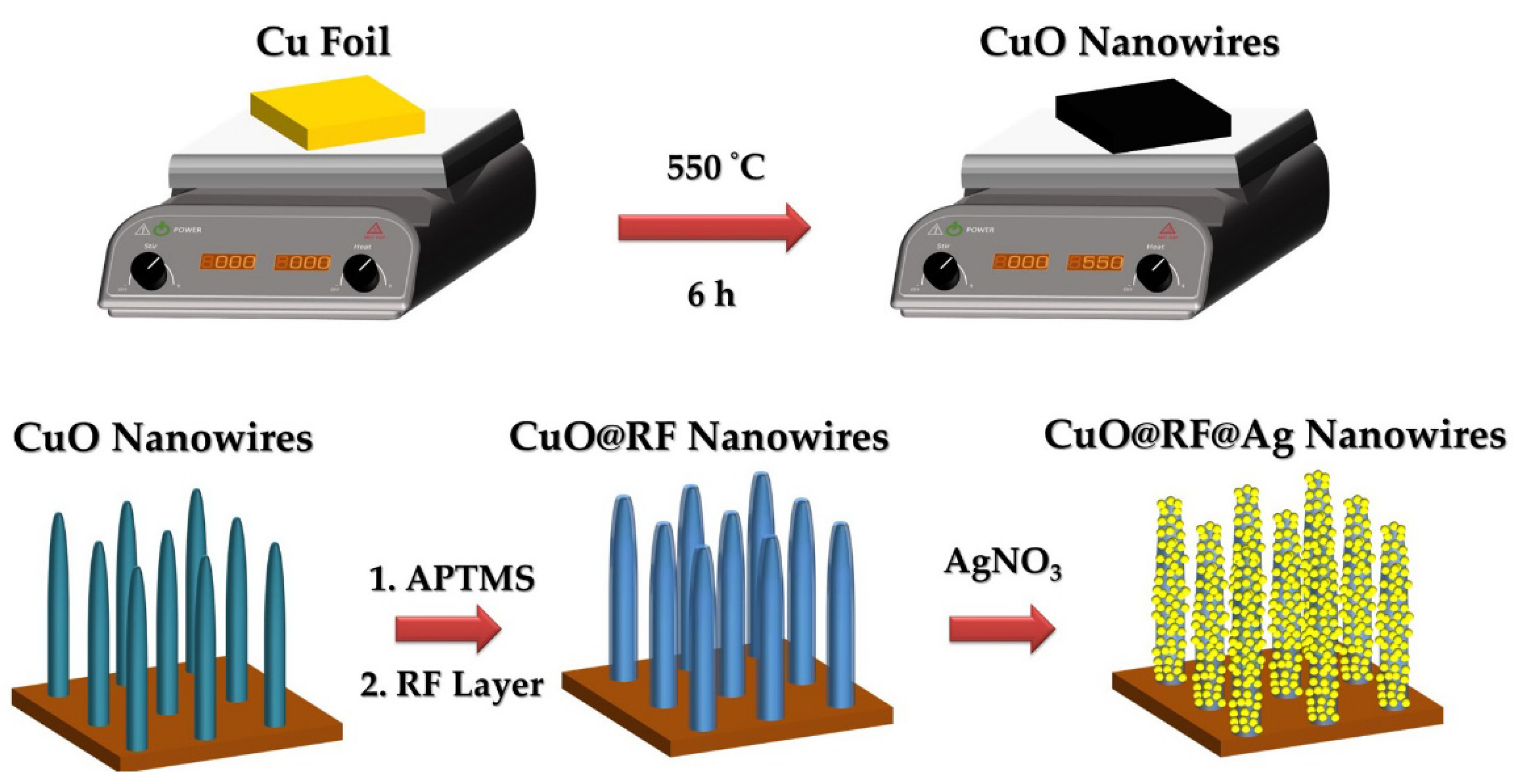
3.2. Fabrication of CuO Nanowires
3.3. Fabrication of CuO@Ag Core-Shell Nanowires
3.4. Fabrication of CuO@RF@Ag Core-Shell Nanowires
3.5. Material Characterization
3.6. SERS Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vaclavik, L.; Rosmus, J.; Popping, B.; Hajslova, J. Rapid determination of melamine and cyanuric acid in milk powder using direct analysis in real time-time-of-flight mass spectrometry. J. Chromatogr. A 2010, 1217, 4204–4211. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Lin, M.; Li, H. Potential of SERS for rapid detection of melamine and cyanuric acid extracted from milk. Sens. Instrum. Food Qual. Saf. 2010, 4, 13–19. [Google Scholar] [CrossRef]
- Sharma, B.; Frontiera, R.R.; Henry, A.-I.; Ringe, E.; Van Duyne, R.P. SERS: Materials, applications, and the future. Mater. Today 2012, 15, 16–25. [Google Scholar] [CrossRef]
- Camden, J.P.; Dieringer, J.A.; Zhao, J.; Van Duyne, R.P. Controlled plasmonic nanostructures for surface-enhanced spectroscopy and sensing. Acc. Chem. Res. 2008, 41, 1653–1661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stewart, M.E.; Anderton, C.R.; Thompson, L.B.; Maria, J.; Gray, S.K.; Rogers, J.A.; Nuzzo, R.G. Nanostructured plasmonic sensors. Chem. Rev. 2008, 108, 494–521. [Google Scholar] [CrossRef]
- Lu, L.; Eychmüller, A. Ordered macroporous bimetallic nanostructures: Design, characterization, and applications. Acc. Chem. Res. 2008, 41, 244–253. [Google Scholar] [CrossRef]
- Langer, J.; Jimenez de Aberasturi, D.; Aizpurua, J.; Alvarez-Puebla, R.A.; Auguié, B.; Baumberg, J.J.; Bazan, G.C.; Bell, S.E.J.; Boisen, A.; Brolo, A.G.; et al. Present and future of surface-enhanced raman scattering. ACS Nano 2020, 14, 28–117. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Huang, Y.; Li, X.; Zhang, Y.; Chen, Q.; Ye, Z.; Alqarni, Z.; Bell, S.E.J.; Xu, Y. Towards practical and sustainable SERS: A review of recent developments in the construction of multifunctional enhancing substrates. J. Mater. Chem. C 2021, 9, 11517–11552. [Google Scholar] [CrossRef]
- Moldovan, R.; Vereshchagina, E.; Milenko, K.; Iacob, B.-C.; Bodoki, A.E.; Falamas, A.; Tosa, N.; Muntean, C.M.; Farcău, C.; Bodoki, E. Review on combining surface-enhanced Raman spectroscopy and electrochemistry for analytical applications. Anal. Chim. Acta 2022, 1209, 339250. [Google Scholar] [CrossRef]
- Abdelsalam, M.E.; Bartlett, P.N.; Baumberg, J.J.; Cintra, S.; Kelf, T.A.; Russell, A.E. Electrochemical SERS at a structured gold surface. Electrochem. Commun. 2005, 7, 740–744. [Google Scholar] [CrossRef]
- Panyathip, R.; Sucharitakul, S.; Phaduangdhitidhada, S.; Ngamjarurojana, A.; Kumnorkaew, P.; Choopun, S. Surface enhanced raman scattering in graphene quantum dots grown via electrochemical process. Molecules 2021, 26, 5484. [Google Scholar] [CrossRef] [PubMed]
- Pilot, R.; Signorini, R.; Durante, C.; Orian, L.; Bhamidipati, M.; Fabris, L. A Review on Surface-Enhanced Raman Scattering. Biosensors 2019, 9, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, M.; Andrade, G.F.S.; Brolo, A.G. A review on recent advances in the applications of surface-enhanced Raman scattering in analytical chemistry. Anal. Chim. Acta 2020, 1097, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liu, Y.; Zhou, T.; Zhou, P.; Li, J.; Deng, A. Ultrasensitive and specific detection of anticancer drug 5-fluorouracil in blood samples by a surface-enhanced raman scattering (SERS)-based lateral flow immunochromatographic assay. Molecules 2022, 27, 4019. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.-H.; Chuang, K.-W.; Chang, Y.-C.; Chou, C.-M. Optimizing and improving the growth of Ag nanoparticles decorated on the silicon pyramid for surface-enhanced Raman spectroscopy. Mater. Chem. Phys. 2022, 280, 125823. [Google Scholar] [CrossRef]
- Yang, Y.; Zhong, X.-L.; Zhang, Q.; Blackstad, L.G.; Fu, Z.-W.; Li, Z.-Y.; Qin, D. The role of etching in the formation of Ag nanoplates with straight, curved and wavy edges and comparison of their SERS properties. Small 2014, 10, 1430–1437. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Mao, N.; Zhang, J. Graphene: A platform for surface-enhanced raman spectroscopy. Small 2013, 9, 1206–1224. [Google Scholar] [CrossRef]
- Chang, T.-H.; Chuang, K.-W.; Chang, Y.-C. Ag/Ga-doped ZnO/pyramidal silicon as a multifunctional surface-enhanced Raman scattering substrate. J. Alloys Compd. 2022, 893, 162288. [Google Scholar] [CrossRef]
- Chang, T.-H.; Chang, Y.-C.; Wu, S.-H. Ag nanoparticles decorated ZnO: Al nanoneedles as a high-performance surface-enhanced Raman scattering substrate. J. Alloys Compd. 2020, 843, 156044. [Google Scholar] [CrossRef]
- Liao, W.; Liu, K.; Chen, Y.; Hu, J.; Gan, Y. Au–Ag bimetallic nanoparticles decorated silicon nanowires with fixed and dynamic hot spots for ultrasensitive 3D SERS sensing. J. Alloys Compd. 2021, 868, 159136. [Google Scholar] [CrossRef]
- Sun, Q.; Zhang, Q.Y.; Zhou, N.; Zhang, L.Y.; Hu, Q.; Ma, C.Y. FDTD simulation of Ag-decorated ZnO nanorods for optimization of 3D SERS substrates. Appl. Surf. Sci. 2021, 565, 150524. [Google Scholar] [CrossRef]
- Filippin, N.; Castillo-Seoane, J.; López-Santos, M.C.; Rojas, C.T.; Ostrikov, K.; Barranco, A.; Sánchez-Valencia, J.R.; Borrás, A. Plasma-enabled amorphous TiO2 nanotubes as hydrophobic support for molecular sensing by SERS. ACS Appl. Mater. Interfaces 2020, 12, 50721–50733. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.K.; Pagal, S.; Prashanth, K.; Chandra, G.K.; Umapathy, S. Ag/ZnO/Au 3D hybrid structured reusable SERS substrate as highly sensitive platform for DNA detection. Sens. Actuators B Chem. 2019, 279, 157–169. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, J.; Wu, H.; Qin, S.; Pan, J.; Li, C. Hierarchically rough CuO/Ag composite film with controlled morphology as recyclable SERS-active substrate. Appl. Surf. Sci. 2022, 598, 153746. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, M.; Sun, X.; Li, X.; Li, J.-G. Comprehensive understanding of the formation process on monodisperse resorcinol-formaldehyde polymer and carbon spheres and their use as substrates for surface-enhanced Raman spectroscopy. Appl. Surf. Sci. 2020, 506, 144591. [Google Scholar] [CrossRef]
- Yang, T.; Liu, J.; Zheng, Y.; Monteiro, M.J.; Qiao, S.Z. Facile fabrication of core–shell-structured Ag@Carbon and mesoporous yolk–shell-structured Ag@Carbon@Silica by an extended stöber method. Chem.—A Eur. J. 2013, 19, 6942–6945. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Xu, Y.; Chen, L.; Wang, X.; Zhang, Q. One-pot synthesis of monodisperse noble metal @ resorcinol-formaldehyde (M@RF) and M@Carbon core–shell nanostructure and their catalytic applications. Langmuir 2015, 31, 11701–11708. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Guo, J.-Y.; Chen, C.-M.; Di, H.-W.; Hsu, C.-C. Construction of CuO/In2S3/ZnO heterostructure arrays for enhanced photocatalytic efficiency. Nanoscale 2017, 9, 13235–13244. [Google Scholar] [CrossRef]
- Yuan, L.; Wang, Y.; Mema, R.; Zhou, G. Driving force and growth mechanism for spontaneous oxide nanowire formation during the thermal oxidation of metals. Acta Mater. 2011, 59, 2491–2500. [Google Scholar] [CrossRef]
- He, X.N.; Gao, Y.; Mahjouri-Samani, M.; Black, P.N.; Allen, J.; Mitchell, M.; Xiong, W.; Zhou, Y.S.; Jiang, L.; Lu, Y.F. Surface-enhanced Raman spectroscopy using gold-coated horizontally aligned carbon nanotubes. Nanotechnology 2012, 23, 205702. [Google Scholar] [CrossRef]
- Liu, C.; Yang, M.; Yu, J.; Lei, F.; Wei, Y.; Peng, Q.; Li, C.; Li, Z.; Zhang, C.; Man, B. Fast multiphase analysis: Self-separation of mixed solution by a wettability-controlled CuO@Ag SERS substrate and its applications in pollutant detection. Sens. Actuators B Chem. 2020, 307, 127663. [Google Scholar] [CrossRef]
- Ye, F.; Ju, S.; Liu, Y.; Jiang, Y.; Chen, H.; Ge, L.; Yan, C.; Yuan, A. Ag-CuO nanocomposites: Surface-enhanced raman scattering substrate and photocatalytic performance. Cryst. Res. Technol. 2019, 54, 1800257. [Google Scholar] [CrossRef]
- Tran, T.H.; Nguyen, M.H.; Nguyen, T.H.T.; Dao, V.P.T.; Nguyen, Q.H.; Sai, C.D.; Pham, N.H.; Bach, T.C.; Ngac, A.B.; Nguyen, T.T.; et al. Facile fabrication of sensitive surface enhanced Raman scattering substrate based on CuO/Ag core/shell nanowires. Appl. Surf. Sci. 2020, 509, 145325. [Google Scholar] [CrossRef]
- Wu, H.-Y.; Lin, H.-C.; Liu, Y.-H.; Chen, K.-L.; Wang, Y.-H.; Sun, Y.-S.; Hsu, J.-C. Highly sensitive, robust, and recyclable TiO2/AgNP substrate for SERS detection. Molecules 2022, 27, 6755. [Google Scholar] [CrossRef]
- Chou, C.-M.; Chang, T.-T.; Chen, C.-Y.; Chang, Y.-C. Constructing er-doped ZnO/CuS/Au core-shell nanowires with enhanced photocatalytic and SERS properties. Catalysts 2021, 11, 1347. [Google Scholar] [CrossRef]
- Skarp, K.-P.; Shams, A.; Montelin, H.; Lagerbäck, P.; Tängdén, T. Synergistic and bactericidal activities of mecillinam, amoxicillin and clavulanic acid combinations against extended-spectrum β-lactamase (ESBL)-producing Escherichia coli in 24-h time—Kill experiments. Int. J. Antimicrob. Agents 2019, 53, 74–79. [Google Scholar] [CrossRef]
- Belkadi, A.; Meliani, M.F.; Mokhtar, A.; Djelad, A.; Abdelkrim, S.; Kebir-Medjhouda, Z.A.; Bengueddach, A.; Sassi, M. Amoxicillin loaded bentonite, advanced low-cost antibacterial and environmentally friendly materials. J. Mol. Struct. 2022, 1270, 133880. [Google Scholar] [CrossRef]
- Lee, B.W.H.; Sidhu, A.S.; Francis, I.C.; Coroneo, M.T. 5-fluorouracil in primary, impending recurrent and recurrent pterygium: Systematic review of the efficacy and safety of a surgical adjuvant and intralesional antimetabolite. Ocul. Surf. 2022, 26, 128–141. [Google Scholar] [CrossRef] [PubMed]
- Farquharson, S.; Gift, A.; Shende, C.; Inscore, F.; Ordway, B.; Farquharson, C.; Murren, J. Surface-enhanced Raman spectral measurements of 5-fluorouracil in saliva. Molecules 2008, 13, 2608–2627. [Google Scholar] [CrossRef]
- Khrushchev, A.Y.; Akmaev, E.R.; Gulyaeva, A.Y.; Likhikh, T.N.; Khodkova, J.S.; Kolyachkina, S.V. Quantitative measurement of trace amoxicillin using SERS under the conditions of controlled agglomeration of silver nanoparticles. Vib. Spectrosc. 2022, 120, 103388. [Google Scholar] [CrossRef]
- Dikmen, G. Ultrasensitive detection of amoxicillin using the plasmonic silver nanocube as SERS active substrate. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 278, 121308. [Google Scholar] [CrossRef] [PubMed]
- Alwan, A.M.; Mahmood, M.J. Using laser duty cycles for modifying the performance of Au-NPs/Si nano column hot spot SERS sensors. Plasmonics 2020, 15, 1709–1717. [Google Scholar] [CrossRef]
- Chang, T.-H.; Chang, Y.-C.; Chen, C.-M.; Chuang, K.-W.; Chou, C.-M. A facile method to directly deposit the large-scale Ag nanoparticles on a silicon substrate for sensitive, uniform, reproducible and stable SERS substrate. J. Alloys Compd. 2019, 782, 887–892. [Google Scholar] [CrossRef]
- Zhou, G.; Li, P.; Ge, M.; Wang, J.; Chen, S.; Nie, Y.; Wang, Y.; Qin, M.; Huang, G.; Lin, D.; et al. Controlling the shrinkage of 3D hot spot droplets as a microreactor for quantitative SERS detection of anticancer drugs in serum using a handheld raman spectrometer. Anal. Chem. 2022, 94, 4831–4840. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.-H.; Chang, Y.-C.; Chen, C.-M.; Chuang, K.-W. Optimizing pyramidal silicon substrates through the electroless deposition of Ag nanoparticles for high-performance surface-enhanced Raman scattering. Thin Solid Film. 2019, 676, 108–112. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, T.-H.; Di, H.-W.; Chang, Y.-C.; Chou, C.-M. Ag Nanoparticles Decorated CuO@RF Core-Shell Nanowires for High-Performance Surface-Enhanced Raman Spectroscopy Application. Molecules 2022, 27, 8460. https://doi.org/10.3390/molecules27238460
Chang T-H, Di H-W, Chang Y-C, Chou C-M. Ag Nanoparticles Decorated CuO@RF Core-Shell Nanowires for High-Performance Surface-Enhanced Raman Spectroscopy Application. Molecules. 2022; 27(23):8460. https://doi.org/10.3390/molecules27238460
Chicago/Turabian StyleChang, Tung-Hao, Hsin-Wei Di, Yu-Cheng Chang, and Chia-Man Chou. 2022. "Ag Nanoparticles Decorated CuO@RF Core-Shell Nanowires for High-Performance Surface-Enhanced Raman Spectroscopy Application" Molecules 27, no. 23: 8460. https://doi.org/10.3390/molecules27238460





