Hierarchical Ultrathin Layered GO-ZnO@CeO2 Nanohybrids for Highly Efficient Methylene Blue Dye Degradation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Morphological Characterization
2.2. Structural Characterizations
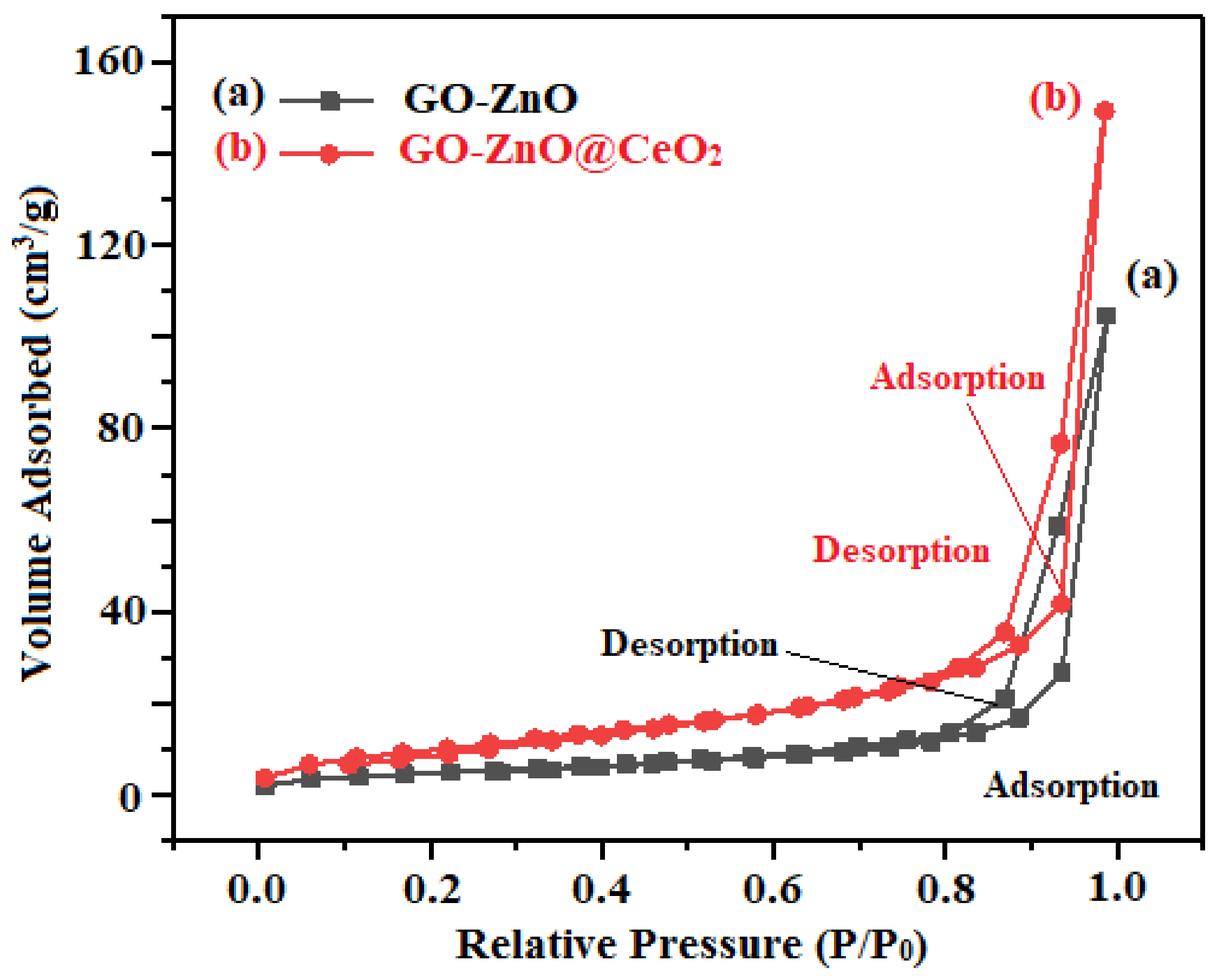
2.3. Optical and Surface Area Characterization
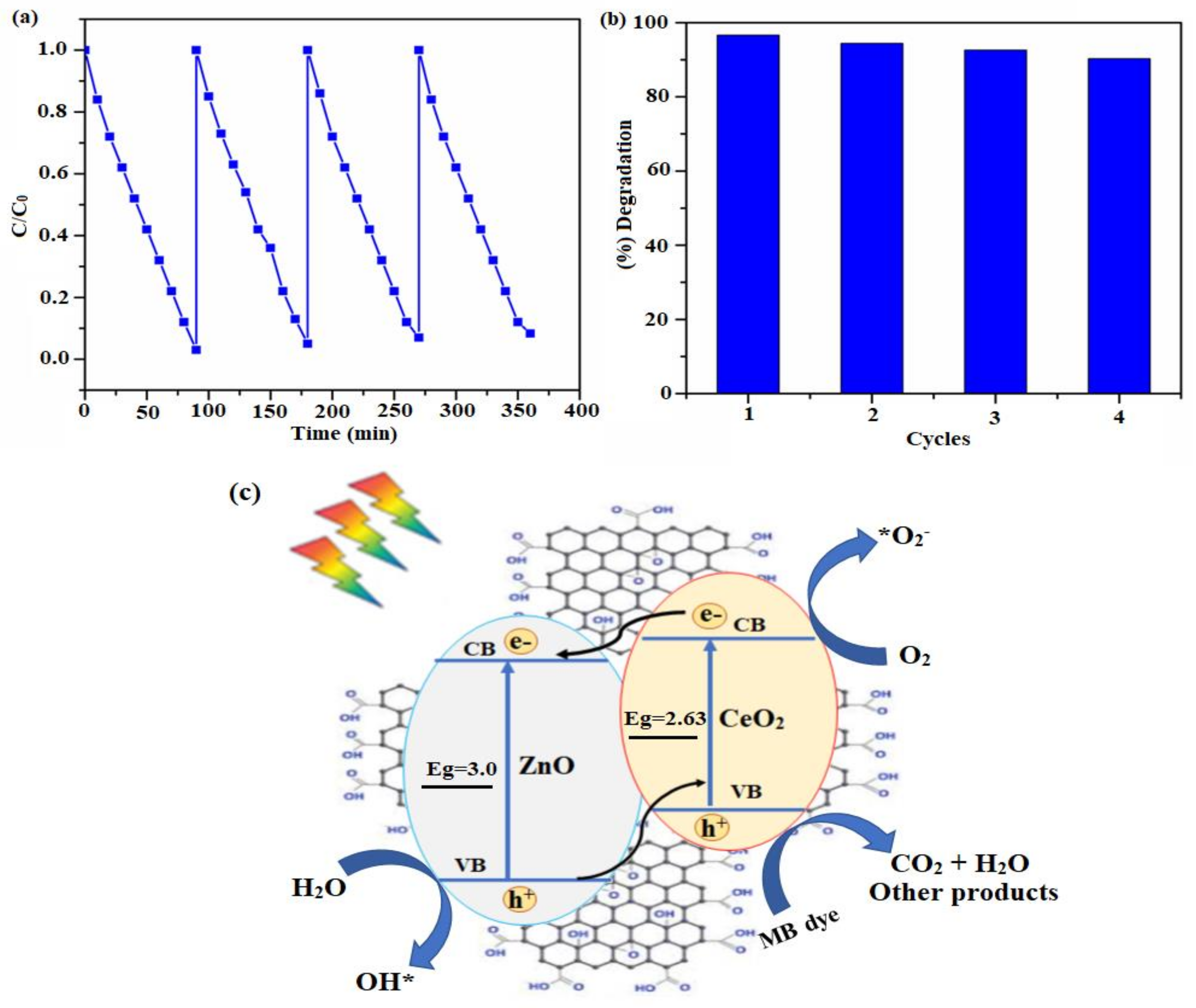
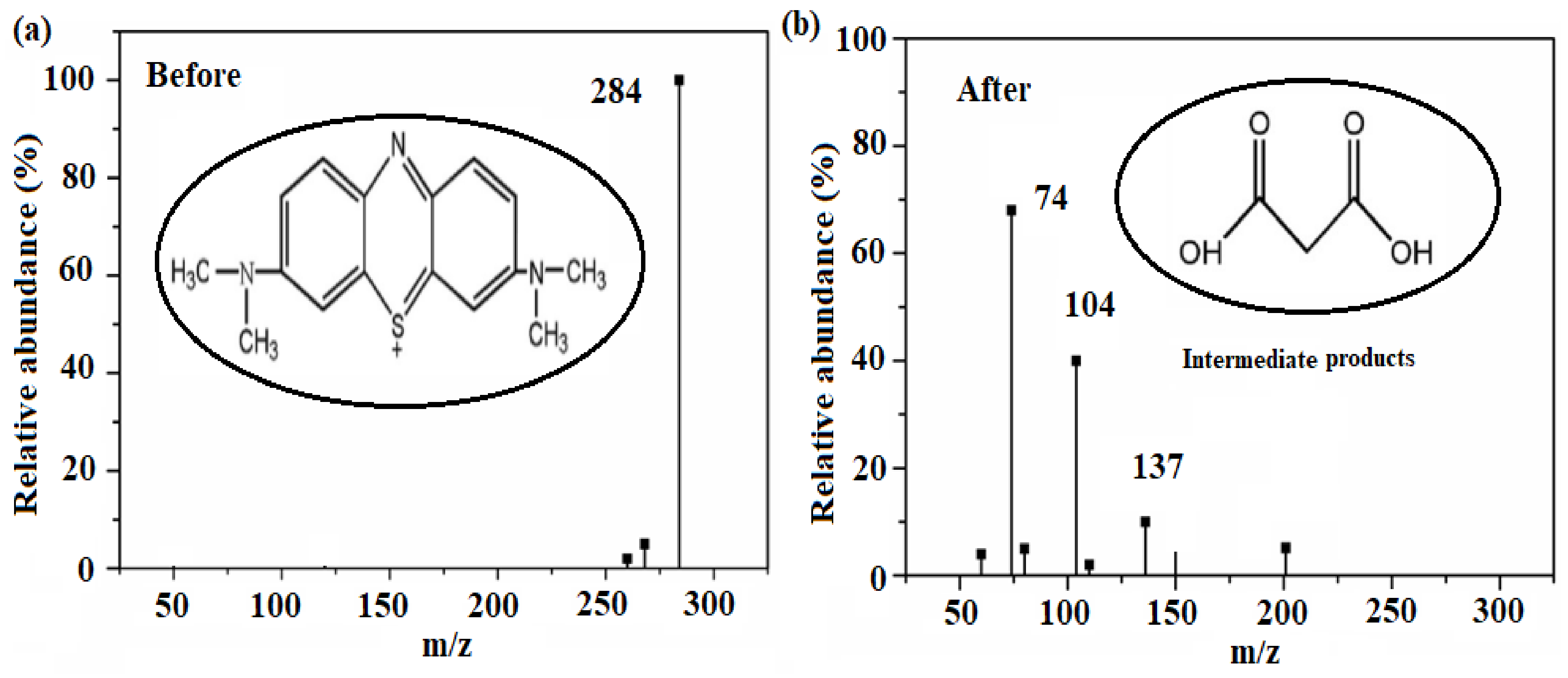
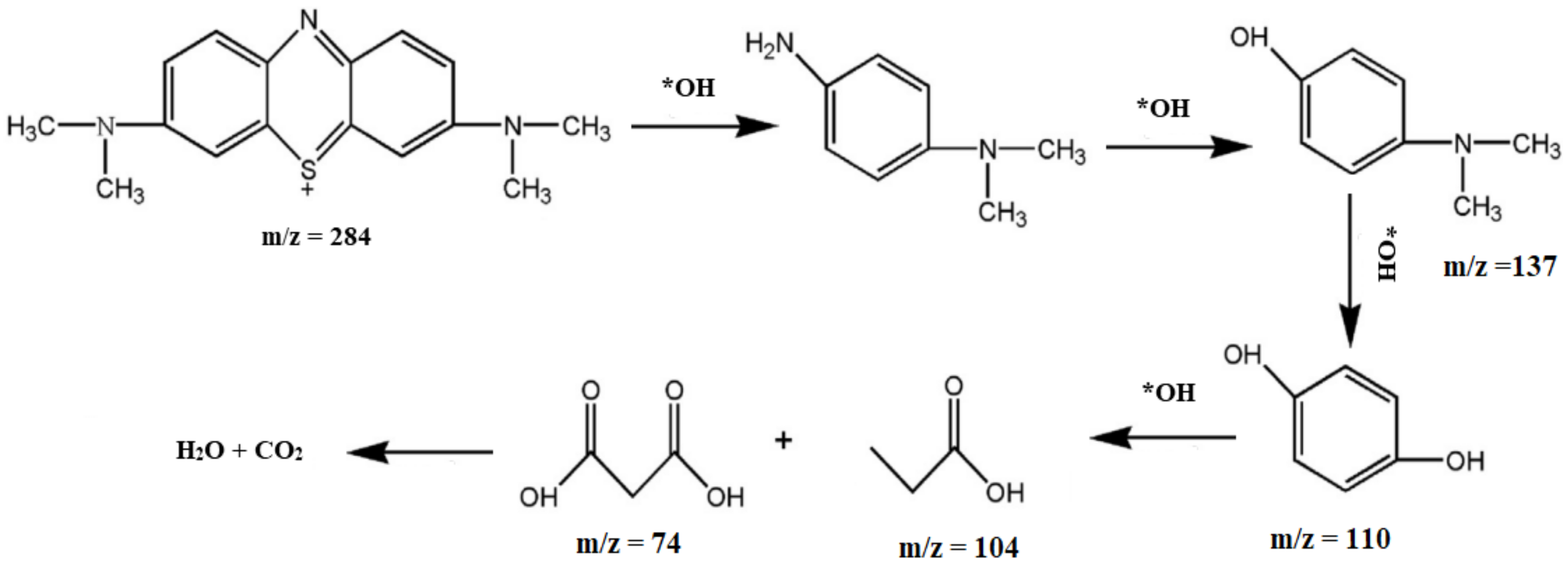
2.4. Photocatalytic Dye Degradation
3. Experimental Details
3.1. Materials
3.2. Preparation of Graphene Oxide (GO) Nanosheets
3.3. Synthesis of Ultrathin Layered GO-ZnO@CeO2 Nanohybrids
3.4. Characterizations
3.5. Photo-Catalytic Experiments
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- An, X.; Shang, Z.; Bai, Y.; Liu, H.; Qu, J. Synergetic Photocatalytic Pure Water Splitting and Self-Supplied Oxygen Activation by 2D WO3/TiO2 Heterostructures. ACS Sustain. Chem. Eng. 2019, 7, 19902–19909. [Google Scholar] [CrossRef]
- Shao, Y.; Du, J.; Li, H.; Zhao, Y.; Xu, C. Ni0.37Co0.63S2-reduced graphene oxide nanocomposites for highly efficient electrocatalytic oxygen evolution and photocatalytic pollutant degradation. J. Solid State Electrochem. 2017, 21, 183–192. [Google Scholar] [CrossRef]
- Fernandes, A.; Gągol, M.; Makoś, P.; Khan, J.A.; Boczkaj, G. Integrated photocatalytic advanced oxidation system (TiO2/UV/O3/H2O2) for degradation of volatile organic compounds. Sep. Purif. Technol. 2019, 224, 1–14. [Google Scholar] [CrossRef]
- Ahmed, L.M.; Saaed, S.I.; Marhoon, A.A. Effect of Oxidation Agents on Photo-Decolorization of Vitamin B12 in the Presence of ZnO/UV-A System. Indones. J. Chem. 2018, 18, 272–278. [Google Scholar] [CrossRef]
- Srinivas, K.; Chen, Y.; Wang, B.; Yu, B.; Wang, X.; Hu, Y.; Lu, Y.; Li, W.; Zhang, W.; Yang, D. Metal–Organic Framework-Derived NiS/Fe3O4 Heterostructure-Decorated Carbon Nanotubes as Highly Efficient and Durable Electrocatalysts for Oxygen Evolution Reaction. ACS Appl. Mater. Interfaces 2020, 12, 31552–31563. [Google Scholar] [CrossRef]
- Cai, Y.Z.; Cao, W.Q.; Zhang, Y.L.; He, P.; Shu, J.C.; Cao, M.S. Tailoring rGO-NiFe2O4 hybrids to tune transport of electrons and ions for supercapacitor electrodes. J. Alloys Compd. 2019, 811, 152011. [Google Scholar] [CrossRef]
- Karpuraranjith, M.; Chen, Y.; Ramadoss, M.; Wang, B.; Yang, H.; Rajaboopathi, S.; Yang, D. Magnetically recyclable magnetic biochar graphitic carbon nitride nanoarchitectures for highly efficient charge separation and stable photocatalytic activity under visible-light irradiation. J. Mol. Liq. 2021, 326, 115315. [Google Scholar] [CrossRef]
- Xu, Y.; Huang, S.; Xie, M.; Li, Y.; Xu, H.; Huang, L.; Zhang, Q.; Li, H. Magnetically separable Fe2O3/g-C3N4 catalyst with enhanced photocatalytic activity. RSC Adv. 2015, 5, 95727–95735. [Google Scholar] [CrossRef]
- Ni, X.; Chen, C.; Wang, Q.; Li, Z. One-step hydrothermal synthesis of SnO2-MoS2 composite heterostructure for improved visible light photocatalytic performance. Chem. Phys. 2019, 525, 110398. [Google Scholar] [CrossRef]
- Wang, G.; Wang, L. Hydrothermal synthesis of hierarchical flower-like α-CNTs/SnO2 architectures with enhanced photocatalytic activity. Fuller. Nanotub. Carbon Nanostruct. 2019, 27, 10–13. [Google Scholar] [CrossRef]
- Liu, F.; Dong, S.; Zhang, Z.; Li, X.; Dai, X.; Xin, Y.; Wang, X.; Liu, K.; Yuan, Z.; Zheng, Z. Synthesis of a well-dispersed CaFe2O4/g-C3N4/CNT composite towards the degradation of toxic water pollutants under visible light. RSC Adv. 2019, 9, 25750–25761. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.; Luo, Z.; Liu, J.; Li, P. Photocatalytic degradation of methylene blue in aqueous solution by using ZnO-SnO2 nanocomposites. Mater. Sci. Semicond. Process. 2018, 87, 24–31. [Google Scholar] [CrossRef]
- Khurshid, F.M.; Jeyavelan, M.; Nagarajan, S. Photocatalytic dye degradation by graphene oxide doped transition metal catalysts. Syn. Met. 2021, 278, 11683. [Google Scholar] [CrossRef]
- Naciri, Y.; Chennah, A.; Jaramillo-Páez, C.; Navío, J.A.; Bakiz, B.; Taoufyq, A.; Ezahri, M.; Villain, S.; Guinneton, F.; Benlhachemi, A. Preparation, characterization and photocatalytic degradation of Rhodamine B dye over a novel Zn3(PO4)2/BiPO4 catalyst. J. Environ. Chem. Eng. 2019, 7, 103075. [Google Scholar] [CrossRef]
- Zhu, J.; Zhou, Y.; Wu, W.; Deng, Y.; Xiang, Y. A Novel Rose-Like CuS/Bi 2 WO 6 Composite for Rhodamine B Degradation. ChemistrySelect 2019, 4, 11853–11861. [Google Scholar] [CrossRef]
- Wang, D.; Xu, Y.; Sun, F.; Zhang, Q.; Wang, P.; Wang, X. Enhanced photocatalytic activity of TiO 2 under sunlight by MoS 2 nanodots modification. Appl. Surf. Sci. 2016, 377, 221–227. [Google Scholar] [CrossRef]
- Liu, X.; Xing, Z.; Zhang, Y.; Li, Z.; Wu, X.; Tan, S.; Yu, X.; Zhu, Q.; Zhou, W. Fabrication of 3D flower-like black N-TiO2-x@MoS2 for unprecedented-high visible-light-driven photocatalytic performance. Appl. Catal. B Environ. 2017, 201, 119–127. [Google Scholar] [CrossRef]
- Saravanan, R.; Karthikeyan, N.; Govindan, S.; Narayanan, V.; Stephen, A. Photocatalytic Degradation of Organic Dyes Using ZnO/CeO2 Nanocomposite Material under Visible Light. Adv. Mater. Res. 2012, 584, 381–385. [Google Scholar] [CrossRef]
- Tien, H.N.; Luan, V.H.; Hoa, L.T.; Khoa, N.T.; Hahn, S.H.; Chung, J.S.; Shin, E.W.; Hur, S.H. One-pot synthesis of a reduced graphene oxide–zinc oxide sphere composite and its use as a visible light photocatalyst. Chem. Eng. J. 2013, 229, 126–133. [Google Scholar] [CrossRef]
- Gao, P.; Ng, K.; Sun, D.D. Sulfonated graphene oxide-ZnO-Ag photocatalyst for fast photodegradation and disinfection under visible light. J. Hazard. Mater. 2013, 262, 826–835. [Google Scholar] [CrossRef]
- Ramkumar, J.; Chen, Y.; Manigandan, R.; Karpuraranjith, M.; Wang, B.; Li, W.; Zhang, X. A three-dimensional porous CoSnS@CNT nanoarchitecture as a highly efficient bifunctional catalyst for boosted OER performance and photocatalytic degradation. Nanoscale 2020, 12, 3879–3887. [Google Scholar]
- Karpuraranjith, M.; Chen, Y.; Wang, X.; Yu, B.; Rajaboopathi, S.; Yang, D. Hexagonal SnSe nanoplate supported SnO2-CNTs nanoarchitecture for enhanced photocatalytic degradation under visible light driven. Appl. Surf. Sci. 2020, 507, 145026. [Google Scholar] [CrossRef]
- Atchudan, R.; Edison, T.N.J.I.; Perumal, S.; Karthikeyan, D.; Lee, Y.R. Facile synthesis of zinc oxide nanoparticles decorated graphene oxide composite via simple solvothermal route and their photocatalytic activity on methylene blue degradation. J. Photochem. Photobiol. B Biol. 2016, 162, 500–510. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Tang, Q.; Fan, H.; Liu, Z.; Hu, A.; Zhang, S.; Deng, W.; Chen, X. Dual-Confined and Hierarchical-Porous Graphene/C/SiO2 Hollow Microspheres through Spray Drying Approach for Lithium-Sulfur Batteries. Electrochim. Acta 2017, 255, 179–186. [Google Scholar] [CrossRef]
- Wang, B.; Chen, Y.; Wang, X.; Ramkumar, J.; Zhang, X.; Yu, B.; Yang, D.; Karpuraranjith, M.; Zhang, W. rGO wrapped trimetallic sulfide nanowires as an efficient bifunctional catalyst for electrocatalytic oxygen evolution and photocatalytic organic degradation. J. Mater. Chem. A 2020, 8, 13558–13571. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, N.; Xu, Y.-J. Synthesis of graphene–ZnO nanorod nanocomposites with improved photoactivity and anti-photocorrosion. CrystEngComm 2013, 15, 3022–3030. [Google Scholar] [CrossRef]
- Bai, X.; Wang, L.; Zong, R.; Lv, Y.; Sun, Y.; Zhu, Y. Performance Enhancement of ZnO Photocatalyst via Synergic Effect of Surface Oxygen Defect and Graphene Hybridization. Langmuir 2013, 29, 3097–3105. [Google Scholar] [CrossRef]
- Liu, I.T.; Hon, M.H.; Teoh, L.G. The preparation, characterization and photocatalytic activity of radical-shaped CeO2/ZnO microstructures. Ceram. Int. 2014, 40, 4019–4024. [Google Scholar] [CrossRef]
- Lamba, R.; Umar, A.; Mehta, S.K.; Kansal, S.K. CeO2ZnO hexagonal nanodisks: Efficient material for the degradation of direct blue 15 dye and its simulated dye bath effluent under solar light. J. Alloys Compd. 2015, 620, 67–73. [Google Scholar] [CrossRef]
- Choi, J.; Reddy, D.A.; Islam, M.J.; Ma, R.; Kim, T.K. Self-assembly of CeO2 nanostructures/reduced graphene oxide composite aerogels for efficient photocatalytic degradation of organic pollutants in water. J. Alloys Compd. 2016, 688, 527–536. [Google Scholar] [CrossRef]
- Raja, V.R.; Karthika, A.; Kirubahar, S.L.; Suganthi, A.; Rajarajan, M. Sonochemical synthesis of novel ZnFe2O4/CeO2 heterojunction with highly enhanced visible light photocatalytic activity. Solid State Ion. 2019, 332, 55–62. [Google Scholar] [CrossRef]
- Sun, Y.; Lin, H.; Wang, C.; Wu, Q.; Wang, X.; Yang, M. Morphology-controlled synthesis of TiO2/MoS2 nanocomposites with enhanced visible-light photocatalytic activity. Inorg. Chem. Front. 2018, 5, 145. [Google Scholar] [CrossRef]
- Wang, S.; Zhu, B.C.; Liu, M.J.; Zhang, L.Y.; Yu, J.G.; Zhou, M.H. Direct Z-scheme ZnO/ CdS hierarchical photocatalyst for enhanced photocatalytic H2-production activity. Appl. Catal. B Environ. 2019, 243, 19–26. [Google Scholar] [CrossRef]
- Wolski, P.L.; Grzelak, K.; Munko, M.; Frankowski, M.; Grzyb, T.; Nowaczyk, G. Insight into photocatalytic degradation of ciprofloxacin over CeO2/ZnO nanocomposites: Unravelling the synergy between the metal oxides and analysis of reaction pathways. Appl. Surf. Sci. 2021, 563, 150338. [Google Scholar] [CrossRef]
- Jia, P.; Tan, H.; Liu, K.; Gao, W. Synthesis, characterization and photocatalytic property of novel ZnO/bone char composite. Mater. Res. Bull. 2018, 102, 45–50. [Google Scholar] [CrossRef]
- Xiao, Y.; Yu, H.; Dong, X. Ordered mesoporous CeO2/ZnO composite with photodegradation concomitant photocatalytic hydrogen production performance. J. Solid State Chem. 2019, 278, 120893. [Google Scholar] [CrossRef]
- Rauf, M.A.; Meetani, M.; Khaleel, A.; Ahmed, A. Photocatalytic degradation of Methylene Blue using a mixed catalyst and product analysis by LC/MS. Chem. Eng. J. 2010, 157, 373–378. [Google Scholar] [CrossRef]
- Fang, S.; Xin, Y.; Ge, L.; Han, C.; Qiu, P.; Wu, L. Facile synthesis of CeO2 hollow structures with controllable morphology by template-engaged etching of Cu2O and their visible light photocatalytic performance. Appl. Catal. B Environ. 2015, 179, 458–467. [Google Scholar] [CrossRef]
- Sun, W.; Meng, S.; Zhang, S.; Zheng, X.; Ye, X.; Fu, X.; Chen, S. Insight into the Transfer Mechanisms of Photogenerated Carriers for Heterojunction Photocatalysts with the Analogous Positions of Valence Band and Conduction Band: A Case Study of ZnO/TiO2. J. Phys. Chem C. 2018, 122, 15409–15420. [Google Scholar] [CrossRef]
- Zhang, X.; Li, R.; Jia, M.; Wang, S.; Huang, Y.; Chen, C. Degradation of ciprofloxacin in aqueous bismuth oxybromide (BiOBr) suspensions under visible light irradiation: A direct hole oxidation pathway. Chem. Eng. J. 2015, 274, 290–297. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, Y.; Wang, Y.; Zhu, A.; Zhang, Y. Efficient photocatalytic reduction of aqueous Cr (VI) by Zr4+ doped and polyaniline coupled SnS2 nanoflakes. Sep. Purif. Technol. 2022, 283, 120161. [Google Scholar] [CrossRef]
- Wang, Y.; Su, Y.; Fang, W.; Zhang, Y.; Li, X.; Zhang, G.; Sun, W. SnO2/SnS2 nanocomposite anchored on nitrogen-doped RGO for improved photocatalytic reduction of aqueous Cr(VI). Powder Technol. 2020, 363, 337–348. [Google Scholar] [CrossRef]
- Gayathri, S.; Jayabal, P.M.; Kottaisamy, M.; Ramakrishnan, V. Synthesis of ZnO decorated graphene nanocomposite for enhanced photocatalytic properties. J. Appl. Phys. 2014, 115, 173504. [Google Scholar] [CrossRef]
- Ma, D.; Shi, J.W.; Sun, D. Au decorated hollow ZnO@ZnS heterostructure for enhanced photocatalytic hydrogen evolution: The insight into the roles of hollow channel and Au nanoparticles. Appl. Catal. B Environ. 2019, 244, 748–757. [Google Scholar] [CrossRef]
- Qiao, H.; Huang, Z.; Liu, S.; Tao, Y.; Zhou, H.; Li, M.; Qi, X. Novel Mixed-Dimensional Photocatalysts Based on 3D Graphene Aerogel Embedded with TiO2/MoS2 Hybrid. J. Phys. Chem. C 2019, 123, 10949–10955. [Google Scholar] [CrossRef]
- Priyadharsan, A.; Vasanthakumar, V.; Karthikeyan, S.; Raj, V.; Shanavas, S.; Anbarasan, P.M. Multi-functional properties of ternary CeO2/SnO2/rGO nanocomposites: Visible light driven photocatalyst and heavy metal removal. J. Photochem. Photobiol. A Chem. 2017, 346, 32–45. [Google Scholar] [CrossRef]





| Photocatalyst | Method | Degradation (%) | Degradation Target | Times (min) | Ref. |
|---|---|---|---|---|---|
| TiO2/MoS2/RGO | Hydrothermal | 92.4 | MB | 100 | [35] |
| CeO2/SnO2/RGO | Hydrothermal | 95.0 | MB | 90 | [36] |
| gCN@TiO2/MoS2 | Hydrothermal | 94.0 | MB | 60 | [37] |
| SnO2/MoS2/RGO | Hydrothermal | 90.0 | MB | 75 | [38] |
| GO/ZnO@CeO2 | Hydrothermal | 96.6 | MB | 90 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karpuraranjith, M.; Chen, Y.; Manigandan, R.; Srinivas, K.; Rajaboopathi, S. Hierarchical Ultrathin Layered GO-ZnO@CeO2 Nanohybrids for Highly Efficient Methylene Blue Dye Degradation. Molecules 2022, 27, 8788. https://doi.org/10.3390/molecules27248788
Karpuraranjith M, Chen Y, Manigandan R, Srinivas K, Rajaboopathi S. Hierarchical Ultrathin Layered GO-ZnO@CeO2 Nanohybrids for Highly Efficient Methylene Blue Dye Degradation. Molecules. 2022; 27(24):8788. https://doi.org/10.3390/molecules27248788
Chicago/Turabian StyleKarpuraranjith, Marimuthu, Yuanfu Chen, Ramadoss Manigandan, Katam Srinivas, and Sivamoorthy Rajaboopathi. 2022. "Hierarchical Ultrathin Layered GO-ZnO@CeO2 Nanohybrids for Highly Efficient Methylene Blue Dye Degradation" Molecules 27, no. 24: 8788. https://doi.org/10.3390/molecules27248788




