Desorption of Ammonia Adsorbed on Prussian Blue Analogs by Washing with Saturated Ammonium Hydrogen Carbonate Solution
Abstract
1. Introduction
2. Results and Discussion
2.1. The Amount of NH3 Adsorbed and Desorbed
2.2. Charcterization
2.3. The Crystal Structure and Chemical State during the Process
2.4. The Desorption/Adsorption Ratio
3. Materials and Methods
3.1. Materials
3.2. Synthesis of PBAs
3.3. The Experimantal Scheme of the NH3 Adsorption and Desorption
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Krupa, S. Effects of Atmospheric Ammonia (NH3) on Terrestrial Vegetation: A Review. Environ. Pollut. 2003, 124, 179–221. [Google Scholar] [CrossRef] [PubMed]
- Paulot, F.; Jacob, D.J. Hidden Cost of U.S. Agricultural Exports: Particulate Matter from Ammonia Emissions. Environ. Sci. Technol. 2014, 48, 903–908. [Google Scholar] [CrossRef] [PubMed]
- National Mineterals Information Center, U.S. Geological Survey NITROGEN (FIXED)—AMMONIA. Miner. Commod. Summ. 2022. Available online: https://pubs.usgs.gov/periodicals/mcs2022/mcs2022-nitrogen.pdf (accessed on 13 November 2022).
- Foley, J.A.; Ramankutty, N.; Brauman, K.A.; Cassidy, E.S.; Gerber, J.S.; Johnston, M.; Mueller, N.D.; O’Connell, C.; Ray, D.K.; West, P.C.; et al. Solutions for a Cultivated Planet. Nature 2011, 478, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Tista, M.; Gager, M.; Haider, S.; Pucher, I.; Ullrich, B. European Union Emission Inventory Report 1990–2015; European Environment Agency: Copenhagen, Denmark, 2017.
- The Nutrient Reduction Technology Cost Task Force A Stakeholder Group of the Chesapeake Bay Program. Nutrient Reduction Technology Cost Estimations for Point Sources in the Chesapeake Bay Watershed; The Nutrient Reduction Technology Cost Task Force: Baltimore, MD, USA, 2002. [Google Scholar]
- Fowler, D.; Coyle, M.; Skiba, U.; Sutton, M.A.; Cape, J.N.; Reis, S.; Sheppard, L.J.; Jenkins, A.; Grizzetti, B.; Galloway, J.N.; et al. The Global Nitrogen Cycle in the Twenty-First Century. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20130164. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Qiu, J.; Wang, Z.; Chen, Y.; Li, Z.; Wang, H.; Zhao, Y.; Wang, J. A Record Ammonia Adsorption by Calcium Chloride Confined in Covalent Organic Frameworks. Chem. Commun. 2022, 58, 1151–1154. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Faheem, M.; Wang, L.; Meng, Q.; Sha, H.; Yang, N.; Yuan, Y.; Zhu, G. Surface Pore Engineering of Covalent Organic Frameworks for Ammonia Capture through Synergistic Multivariate and Open Metal Site Approaches. ACS Cent. Sci. 2018, 4, 748–754. [Google Scholar] [CrossRef]
- Wonkim, D.; Wonkang, D.; Injung Kang, M.; Lee, J.U.-H.; Ong, J.; Choe, H.; Chae, Y.; Doo, S.; Choi, H.; Yun, C.; et al. Ammonia Capture Hot Paper High Ammonia UptakeofaMetal-Organic Framework Adsorbenti n AW Ide Pressure Range. Angew. Chem. Int. Ed. 2020, 59, 22531–22536. [Google Scholar] [CrossRef]
- Rieth, A.J.; Dincă, M. Controlled Gas Uptake in Metal–Organic Frameworks with Record Ammonia Sorption. J. Am. Chem. Soc. 2018, 140, 3461–3466. [Google Scholar] [CrossRef]
- Barin, G.; Peterson, G.W.; Crocellà, V.; Xu, J.; Colwell, K.A.; Nandy, A.; Reimer, J.A.; Bordiga, S.; Long, J.R. Highly Effective Ammonia Removal in a Series of Brønsted Acidic Porous Polymers: Investigation of Chemical and Structural Variations. Chem. Sci. 2017, 8, 4399–4409. [Google Scholar] [CrossRef]
- Takahashi, A.; Tanaka, H.; Parajuli, D.; Nakamura, T.; Minami, K.; Sugiyama, Y.; Hakuta, Y.; Ohkoshi, S.; Kawamoto, T. Historical Pigment Exhibiting Ammonia Gas Capture beyond Standard Adsorbents with Adsorption Sites of Two Kinds. J. Am. Chem. Soc. 2016, 138, 6376–6379. [Google Scholar] [CrossRef] [PubMed]
- Van Humbeck, J.F.; McDonald, T.M.; Jing, X.; Wiers, B.M.; Zhu, G.; Long, J.R. Ammonia Capture in Porous Organic Polymers Densely Functionalized with Brønsted Acid Groups. J. Am. Chem. Soc. 2014, 136, 2432–2440. [Google Scholar] [CrossRef] [PubMed]
- Saidi, M.; Ho, P.H.; Yadav, P.; Salles, F.; Charnay, C.; Girard, L.; Boukli-hacene, L.; Trens, P. Carbon Dioxide and Ethanol Vapour. A Comparative Study. Molecules 2021, 26, 7620. [Google Scholar] [CrossRef]
- Ge, J.; Ohata, Y.; Ohnishi, T.; Moteki, T.; Ogura, M. Highly Dispersed Co/Zn-Doped Zeolitic Imidazolate Framework-Derived Carbon Nanoparticles with High NO Adsorption Capacity at Low Operating Temperature. Ind. Eng. Chem. Res. 2022, 61, 3601–3609. [Google Scholar] [CrossRef]
- Barpaga, D.; Zheng, J.; Mcgrail, B.P.; Motkuri, R.K. Manipulating Pore Topology and Functionality to Promote Fluorocarbon-Based Adsorption Cooling. Acc. Chem. Res. 2021, 55, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Kraft, A. On the Discovery and History of Prussian Blue. Bull. Hist. Chem 2008, 33, 61–67. [Google Scholar]
- Loos-Neskovic, C.; Dierkes, M.H.; Jackwerth, E.; Fedoroff, M.; Garnier, E. Fixation of Palladium on Insoluble Simple or Complex Cyano Compounds. Hydrometallurgy 1993, 32, 345–363. [Google Scholar] [CrossRef]
- Manakasettharn, S.; Takahashi, A.; Kawamoto, T.; Noda, K.; Sugiyama, Y.; Nakamura, T. Differences in NH3 Gas Adsorption Behaviors of Metal-Hexacyanoferrate Nanoparticles (M [FeII(CN)6]·zH2O: M = In3+, Fe3+, and Mn2+). J. Solid State Chem. 2019, 270, 112–117. [Google Scholar] [CrossRef]
- Parajuli, D.; Kitajima, A.; Takahashi, A.; Tanaka, H.; Ogawa, H.; Hakuta, Y.; Yoshino, K.; Funahashi, T.; Yamaguchi, M.; Osada, M.; et al. Application of Prussian Blue Nanoparticles for the Radioactive Cs Decontamination in Fukushima Region. J. Environ. Radioact. 2016, 151, 233–237. [Google Scholar] [CrossRef]
- Takahashi, A.; Minami, K.; Noda, K.; Sakurai, K.; Kawamoto, T. Trace Ammonia Removal from Air by Selective Adsorbents Reusable with Water. ACS Appl. Mater. Interfaces 2020, 12, 15115–15119. [Google Scholar] [CrossRef]
- Usuda, H.; Sakurai, K.; Takahashi, A.; Kawamoto, T.; Minami, K. Ammonium Salt Production in NH3-CO2-H2O System Using a Highly Selective Adsorbent, Copper Hexacyanoferrate. Environ. Pollut. 2021, 288, 117763. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, A.; Minami, K.; Noda, K.; Sakurai, K.; Kawamoto, T. Harvesting a Solid Fertilizer Directly from Fetid Air. ACS Sustain. Chem. Eng. 2021, 9, 16865–16869. [Google Scholar] [CrossRef]
- Minami, K.; Takahashi, A.; Sakurai, K.; Mikasa, H.; Takasaki, M.; Doshu, N.; Aoyama, K.; Nakamura, T.; Iwai, R.; Kawamoto, T. Apparatus for Ammonia Removal in Livestock Farms Based on Copper Hexacyanoferrate Granules. Biosyst. Eng. 2022, 216, 98–107. [Google Scholar] [CrossRef]
- Sutter, D.; Gazzani, M.; Mazzotti, M. Formation of Solids in Ammonia-Based CO2 Capture Processes—Identification of Criticalities through Thermodynamic Analysis of the CO2–NH3–H2O System. Chem. Eng. Sci. 2015, 133, 170–180. [Google Scholar] [CrossRef]
- Sutter, D.; Mazzotti, M. Solubility and Growth Kinetics of Ammonium Bicarbonate in Aqueous Solution. Cryst. Growth Des. 2017, 17, 3048–3054. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds: Part B: Applications in Coordination, Organometallic, and Bioinorganic Chemistry; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2008; ISBN 9780471744931. [Google Scholar]
- Gates-Rector, S.; Blanton, T. The Powder Diffraction File: A Quality Materials Characterization Database. Powder Diffr. 2019, 34, 352–360. [Google Scholar] [CrossRef]
- Irving, H.; Williams, R.J.P. Order of Stability of Metal Complexes. Nature 1948, 162, 746–747. [Google Scholar] [CrossRef]
- Yatsimirskii, K.B.; Vasil’ev, V.P. Instability Constants of Complex Compounds; Springer: Berlin/Heidelberg, Germany, 1995; ISBN 978-1-4684-8406-9. [Google Scholar]
- Jannik Bjerrum Metal Ammine Formation in Aqueous Solution, Theory of the Reversible Step Reactions. Trans. Faraday Soc. 1941, 47, 297–302.
- West, P.C.; Gerber, J.S.; Engstrom, P.M.; Mueller, N.D.; Brauman, K.A.; Carlson, K.M.; Cassidy, E.S.; Johnston, M.; MacDonald, G.K.; Ray, D.K.; et al. Leverage Points for Improving Global Food Security and the Environment. Science 2014, 345, 325–328. [Google Scholar] [CrossRef]
- Monk, C.B. Electrolytes in solutions of amino acids part 1v.-dissociation constants of metal complexes of glycine, alanine and glycyl-glycine from ph titrations. Trans. Faraday Soc. 1951, 47, 297–302. [Google Scholar] [CrossRef]
- Basolo, F.; Chen, Y.T. Steric Effects and the Stability of Complex Compounds. III. The Chelating Tendencies of N-Alkylglycines and N-Dialkylglycines with Copper(II) and Nickel(II) Ions. J. Am. Chem. Soc. 1954, 76, 953–955. [Google Scholar] [CrossRef]
- Mellor, D.P.; Maley, L. Stability Constants of Internal Complexes. Nature 1947, 159, 370. [Google Scholar] [CrossRef]

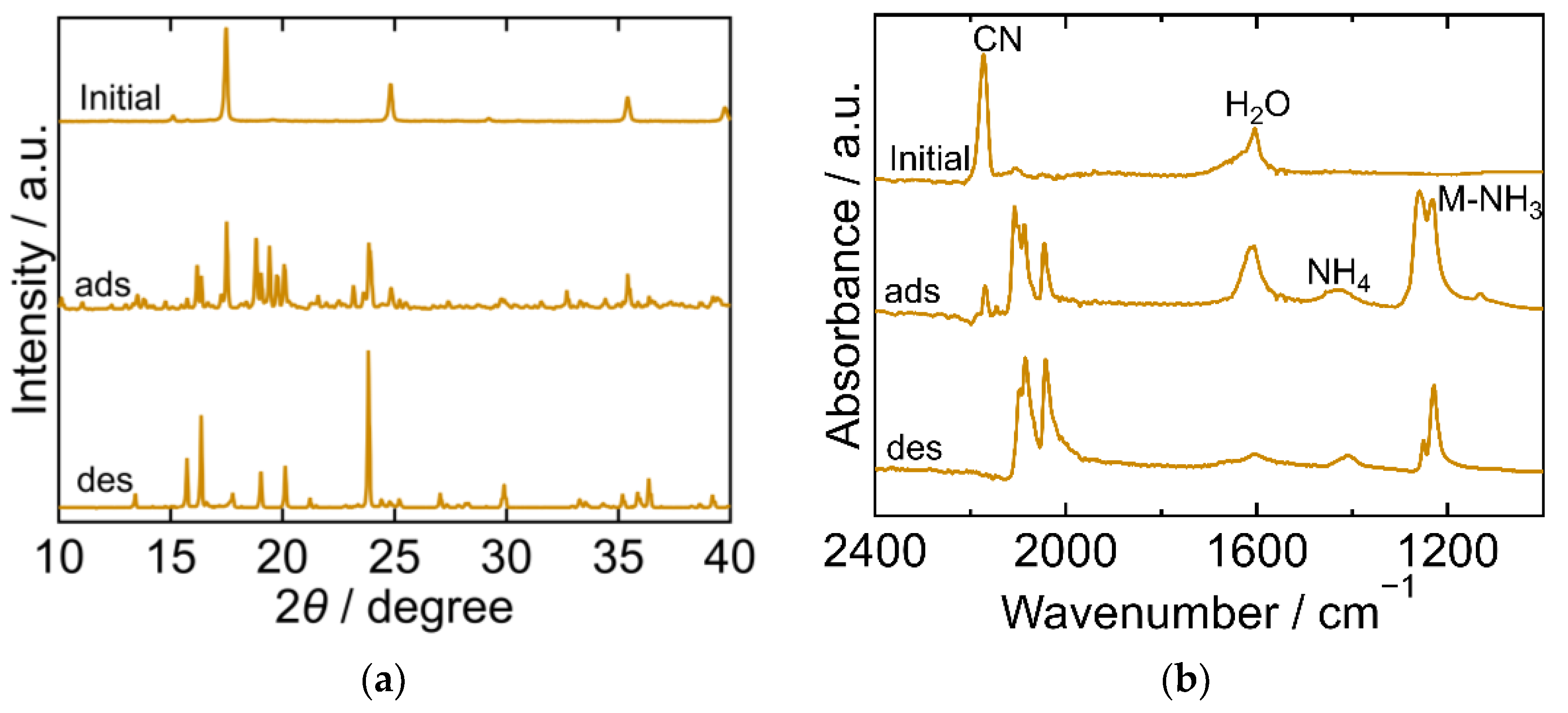
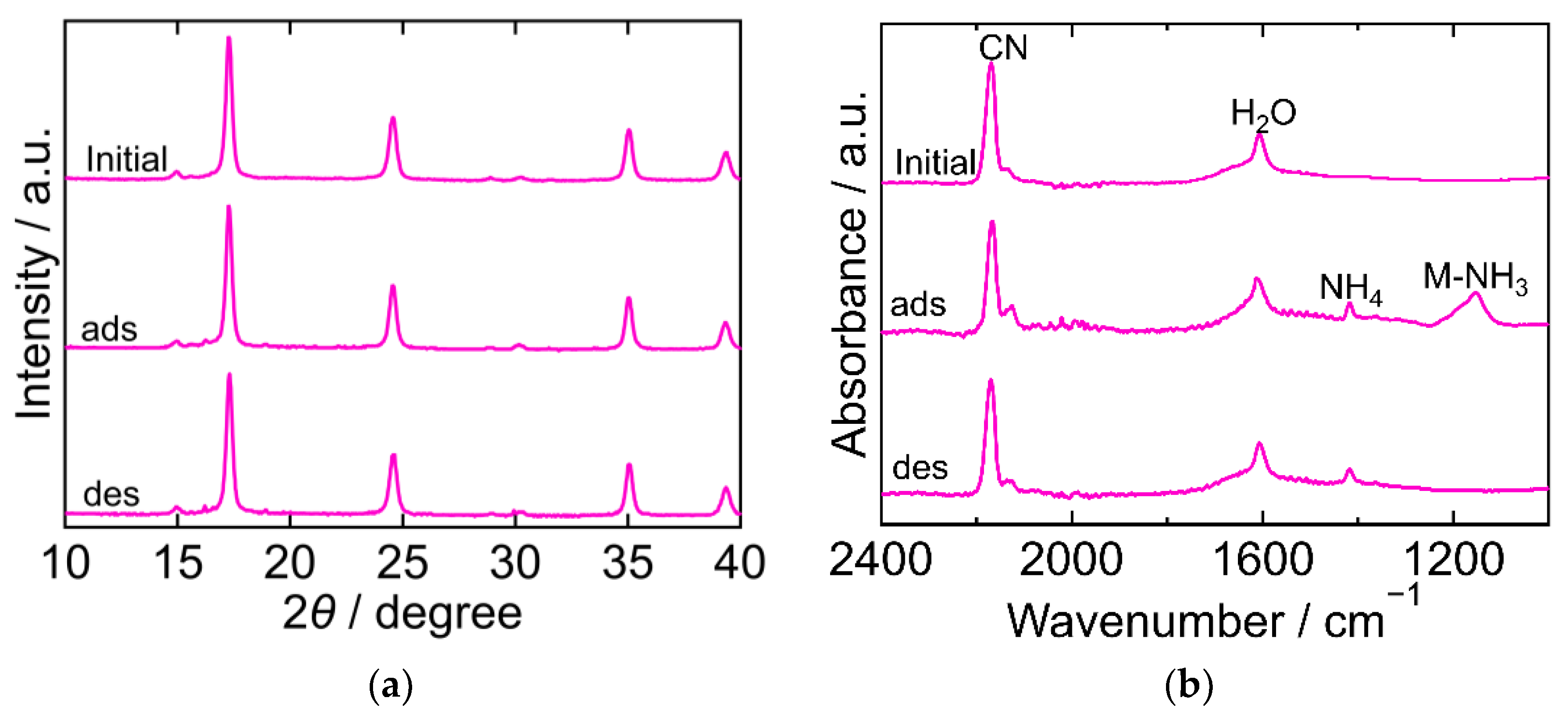


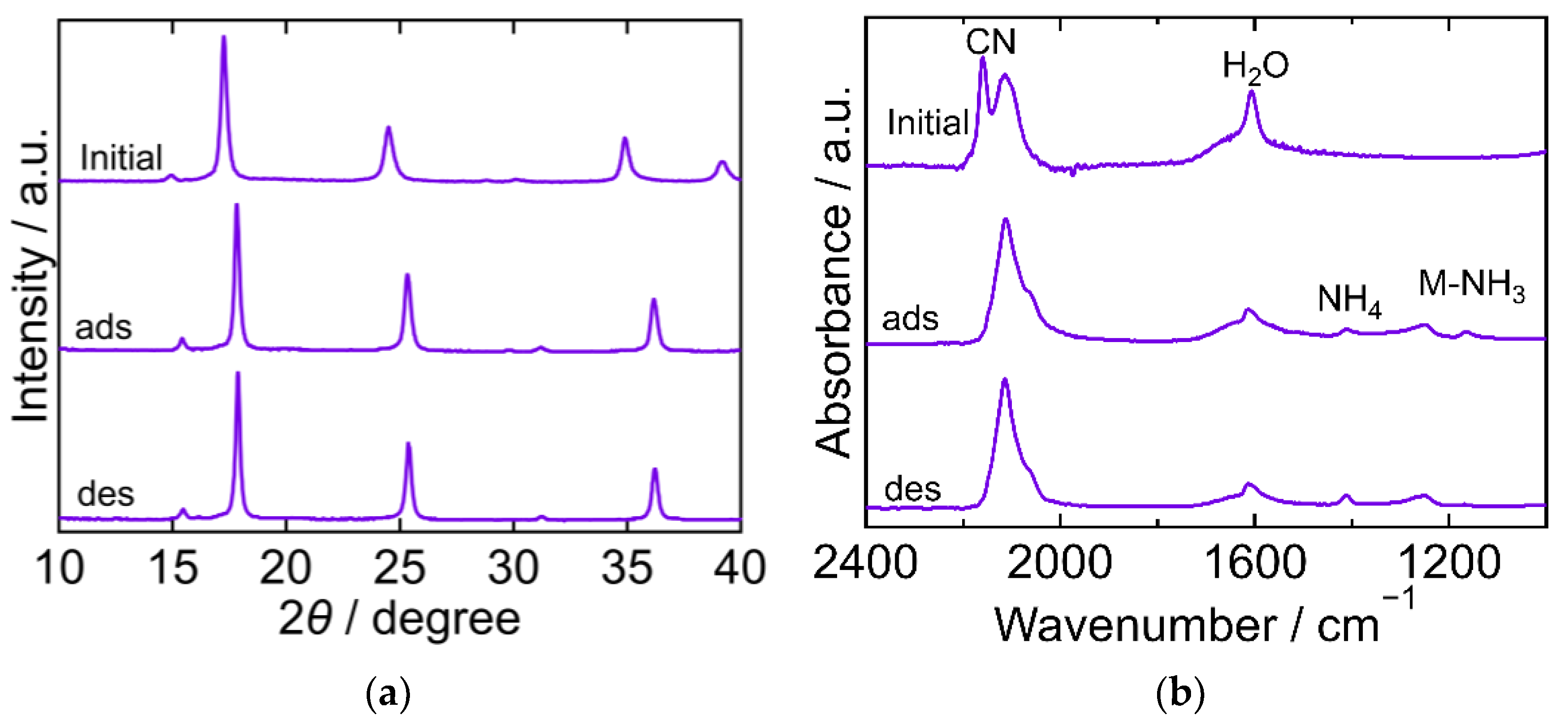
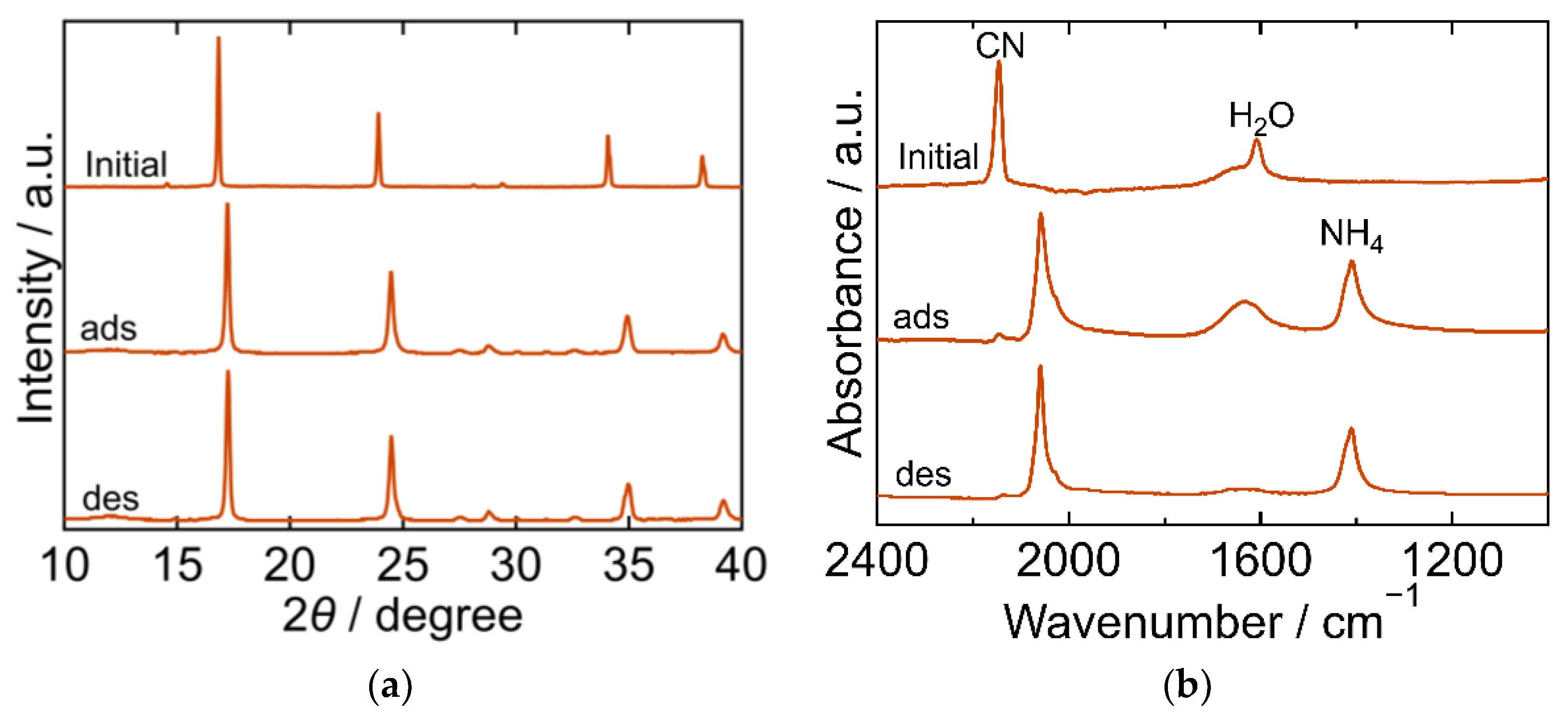
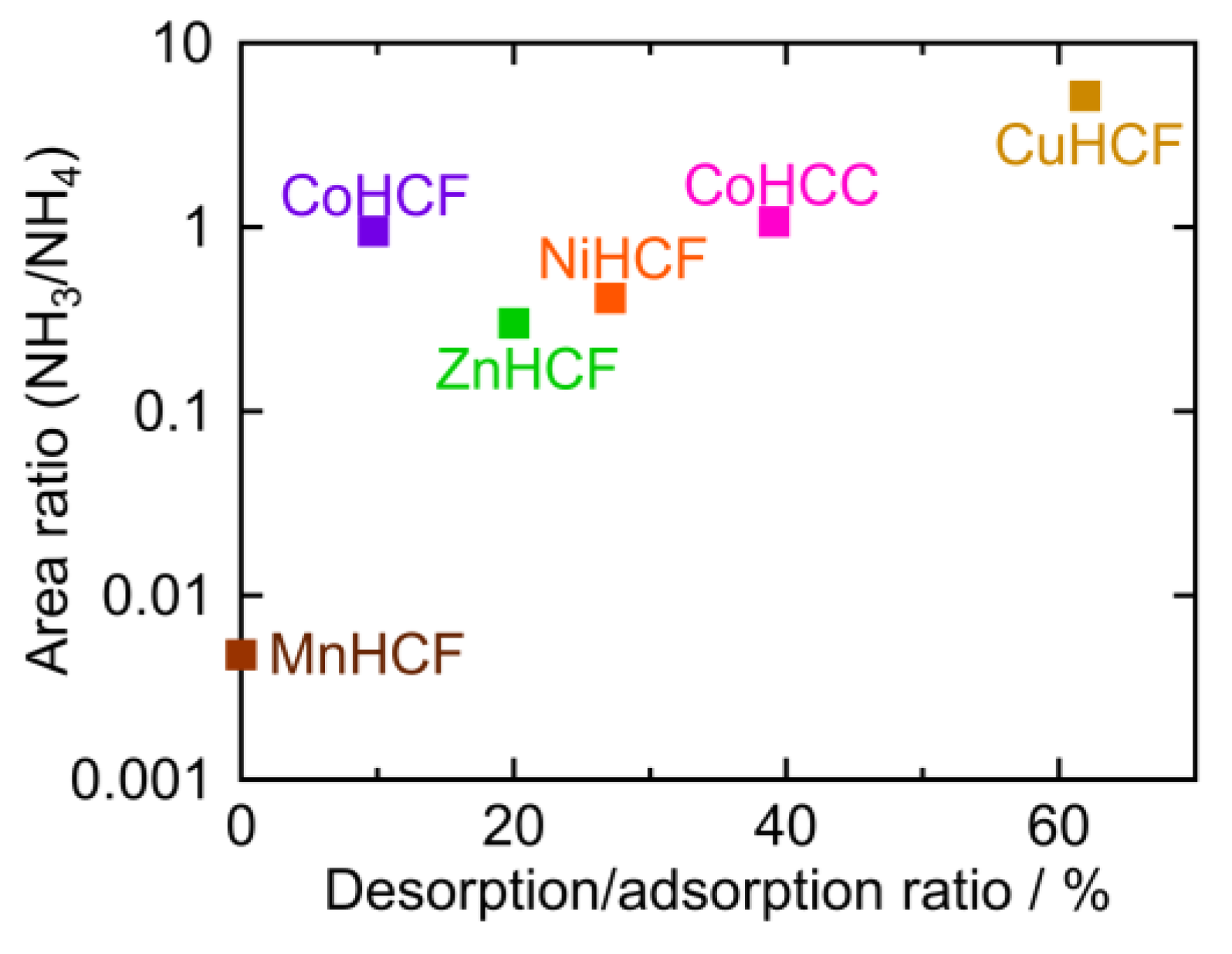
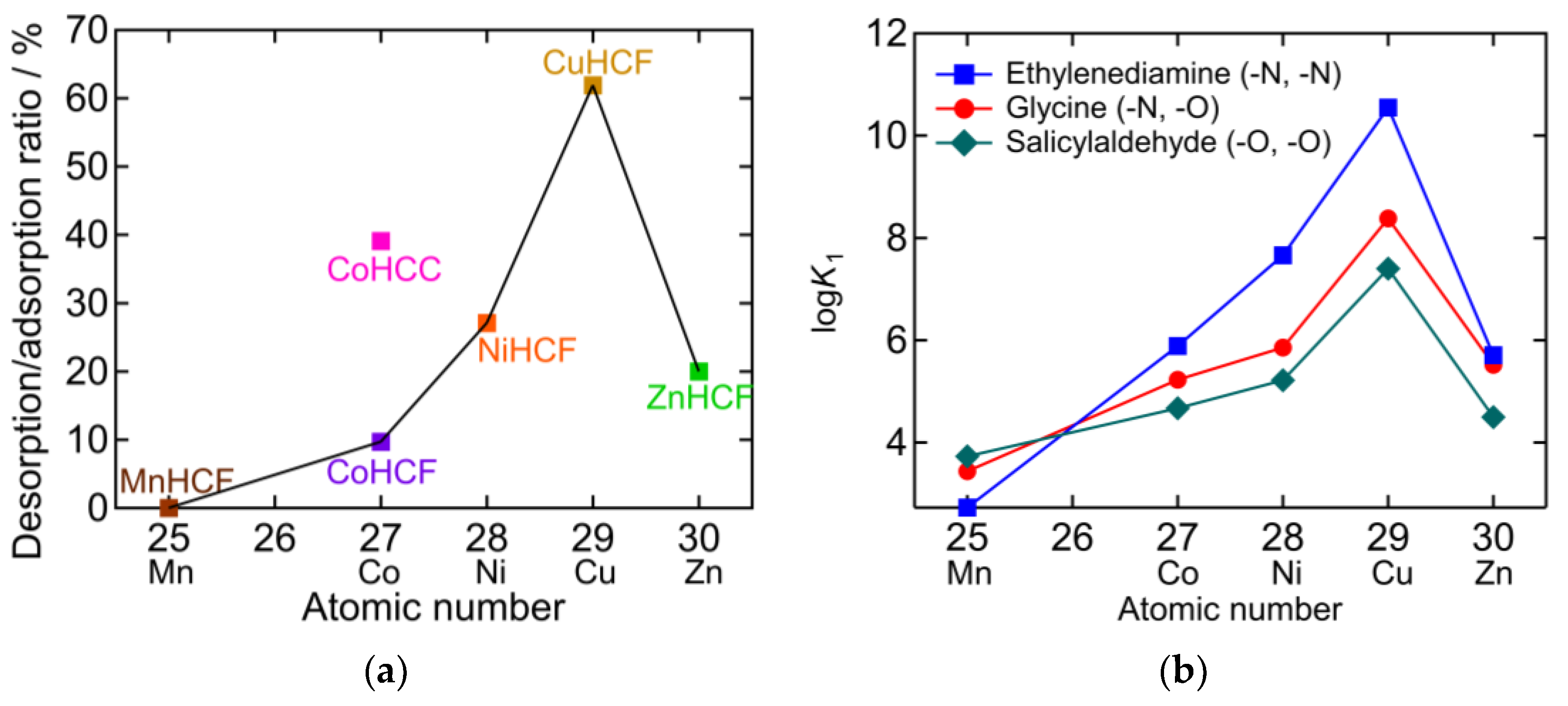

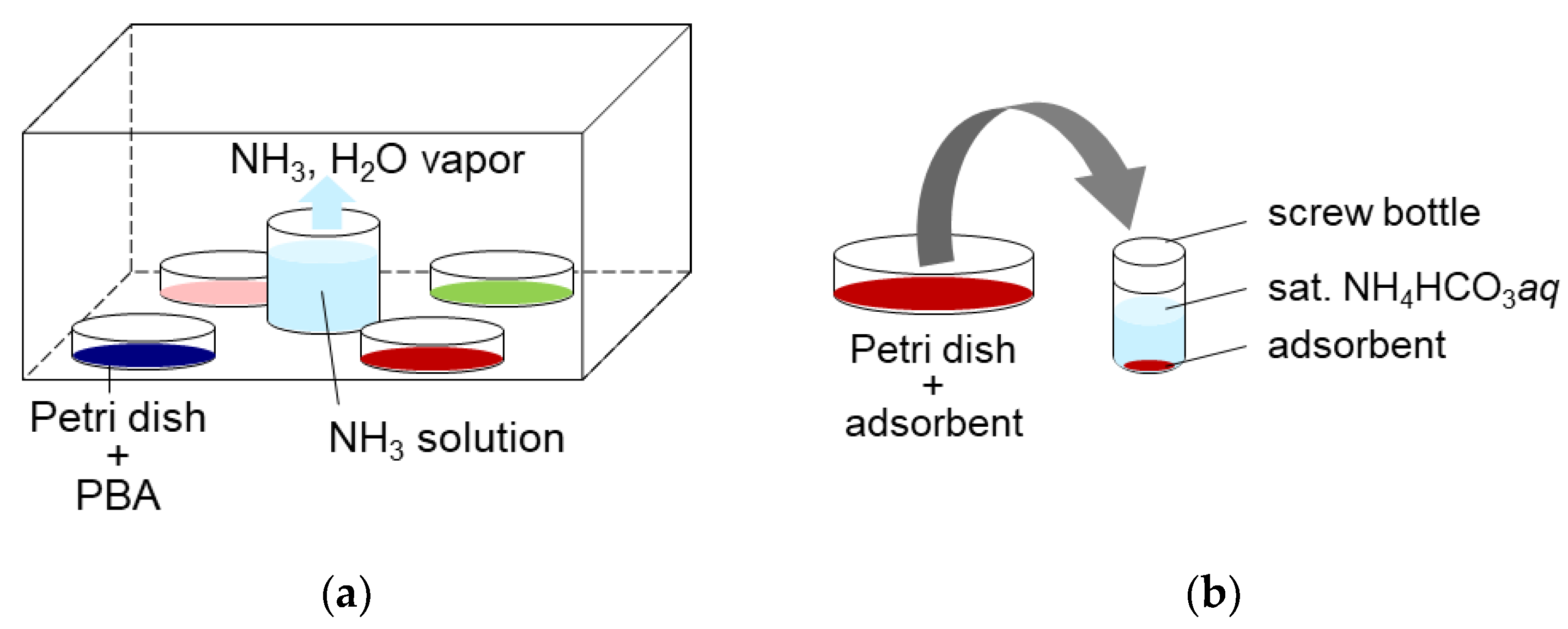
| PBA | Adsorption Amount mmol/g | Desorption Amount mmol/g | Desorption Ratio % |
|---|---|---|---|
| CuHCF | 13.2 | 8.19 | 61.9 |
| CoHCC | 4.87 | 1.90 | 39.1 |
| NiHCF | 5.48 | 1.49 | 27.1 |
| ZnHCF | 2.66 | 0.53 | 20.0 |
| CoHCF | 1.47 | 0.14 | 9.7 |
| MnHCF | 4.57 | −0.63 | −13.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Usuda, H.; Mishima, Y.; Kawamoto, T.; Minami, K. Desorption of Ammonia Adsorbed on Prussian Blue Analogs by Washing with Saturated Ammonium Hydrogen Carbonate Solution. Molecules 2022, 27, 8840. https://doi.org/10.3390/molecules27248840
Usuda H, Mishima Y, Kawamoto T, Minami K. Desorption of Ammonia Adsorbed on Prussian Blue Analogs by Washing with Saturated Ammonium Hydrogen Carbonate Solution. Molecules. 2022; 27(24):8840. https://doi.org/10.3390/molecules27248840
Chicago/Turabian StyleUsuda, Hatsuho, Yoshie Mishima, Tohru Kawamoto, and Kimitaka Minami. 2022. "Desorption of Ammonia Adsorbed on Prussian Blue Analogs by Washing with Saturated Ammonium Hydrogen Carbonate Solution" Molecules 27, no. 24: 8840. https://doi.org/10.3390/molecules27248840
APA StyleUsuda, H., Mishima, Y., Kawamoto, T., & Minami, K. (2022). Desorption of Ammonia Adsorbed on Prussian Blue Analogs by Washing with Saturated Ammonium Hydrogen Carbonate Solution. Molecules, 27(24), 8840. https://doi.org/10.3390/molecules27248840





