Co-Fermentation of Glucose–Xylose Mixtures from Agroindustrial Residues by Ethanologenic Escherichia coli: A Study on the Lack of Carbon Catabolite Repression in Strain MS04
Abstract
:1. Introduction
2. Results and Discussion
2.1. Co-Consumption of Glucose and Xylose by E. coli MS04 in Simulated Media and Plant Hydrolysates Containing High Glucose Concentration
2.2. Co-Consumption of Glucose and Xylose by E. coli MS04 in Simulated and Plant Hydrolysates Containing High Xylose Concentration
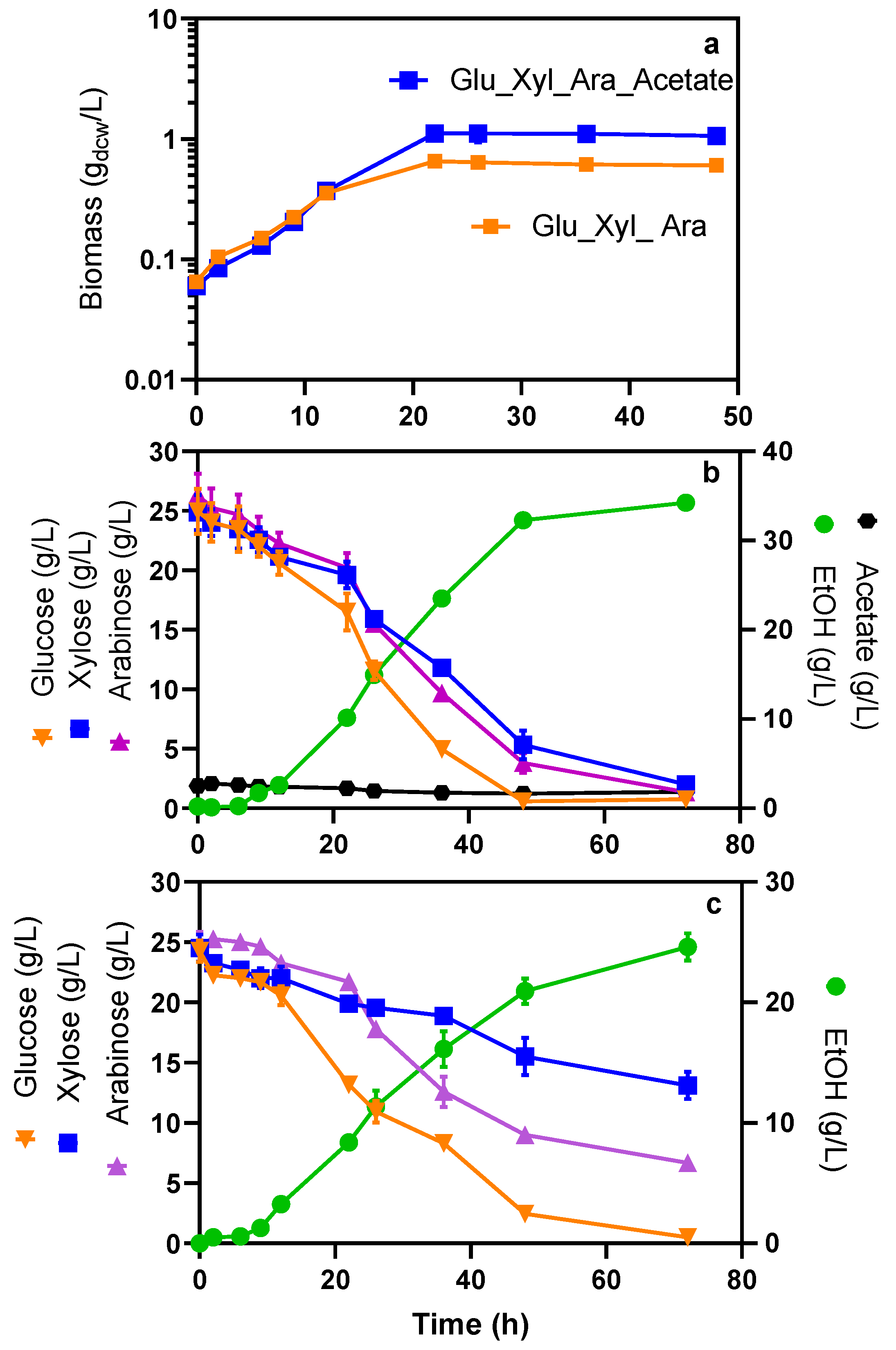
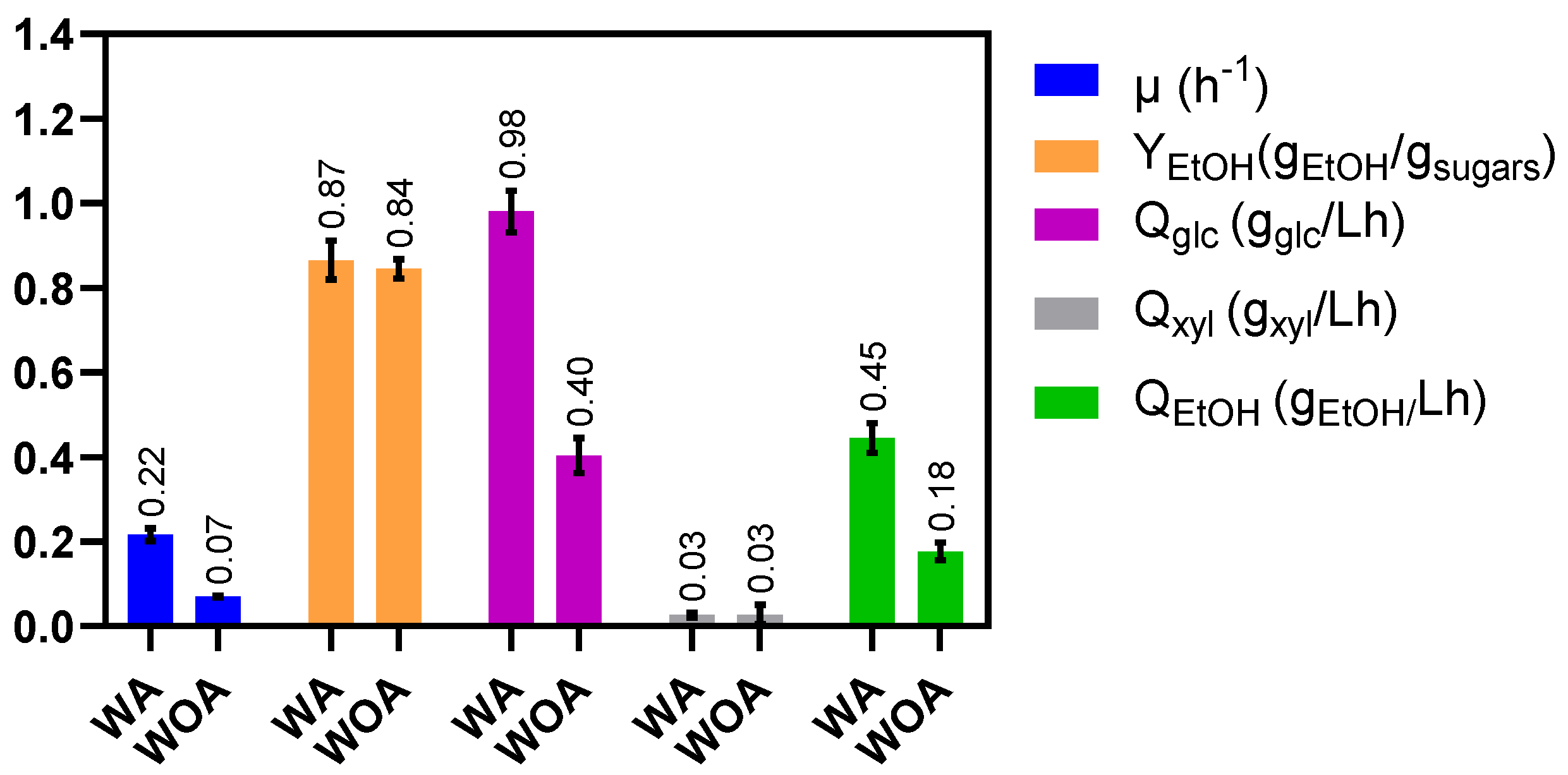
2.3. The Lack of Acetate in the Mineral Media Negatively Affects Sugar Consumption
2.4. Xylose Consumption Is Governed by the XylR Activator
3. Materials and Methods
3.1. Strains
3.1.1. Escherichia coli MS04
3.1.2. Escherichia coli MS04 ΔxylR
3.2. Culture Media
3.2.1. Laboratory-Simulated Hydrolysates
3.2.2. Lignocellulosic Hydrolysates
Teak Wood and Agave Bagasse Hydrolysates
Barley Straw and Corn Stover Hydrolysates
Spent Coffee Grounds Hydrolysates
3.3. Fermentation in Simulated and Agroindustrial Lignocellulosic Hydrolysates
3.4. Biomass, Glucose, Xylose, Arabinose, Acetate and Ethanol Determinations
3.5. Calculation of Kinetic and Stoichiometric Parameters
3.5.1. Specific Growth Rate (µ)
3.5.2. Ethanol Yield (YEtOH)
3.5.3. Volumetric Sugar Consumption Rate (QS)
3.5.4. Volumetric Productivity of Ethanol (QEtOH)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Sebayang, A.; Masjuki, H.; Ong, H.C.; Dharma, S.; Silitonga, A.; Mahlia, T.; Aditiya, H. A perspective on bioethanol production from biomass as alternative fuel for spark ignition engine. RSC Adv. 2016, 6, 14964–14992. [Google Scholar] [CrossRef]
- Balat, M.; Balat, H. Recent trends in global production and utilization of bio-ethanol fuel. Appl. Energy 2009, 86, 2273–2282. [Google Scholar] [CrossRef]
- Kumar, P.; Barrett, D.; Delwiche, M.; Stroeve, P. Methods for pretreatment of lignocellulosic biomass for efficient hydrolysis and biofuel production. Ind. Eng. Chem. Res. 2009, 48, 3713–3729. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, L.; Liu, D. Biomass recalcitrance. Part II: Fundamentals of different pre-treatments to increase the enzymatic digestibility of lignocellulose. Biofuels Bioprod. Biorefin. 2012, 6, 561–579. [Google Scholar] [CrossRef]
- Moya, R.; Tenorio, C.; Oporto, G. Short rotation wood crops in Latin American: A review on status and potential uses as biofuel. Energies 2019, 12, 705. [Google Scholar] [CrossRef] [Green Version]
- Caspeta, L.; Caro-Bermúdez, M.A.; Ponce-Noyola, T.; Martinez, A. Enzymatic hydrolysis at high-solids loadings for the conversion of agave bagasse to fuel ethanol. Appl. Energy 2014, 113, 277–286. [Google Scholar] [CrossRef]
- Vargas-Tah, A.; Moss-Acosta, C.L.; Trujillo-Martinez, B.; Tiessen, A.; Lozoya-Gloria, E.; Orencio-Trejo, M.; Gosset, G.; Martinez, A. Non-severe thermochemical hydrolysis of stover from white corn and sequential enzymatic saccharification and fermentation to ethanol. Bioresour. Technol. 2015, 198, 611–618. [Google Scholar] [CrossRef]
- Guhl, A. Coffee production intensification and landscape change in Colombia, 1970–2002. In Land-Change Science in the Tropics: Changing Agricultural Landscapes; Millington, A., Jepson, W., Eds.; Springer: Boston, MA, USA, 2008; pp. 93–116. [Google Scholar]
- Karmee, S.K. A spent coffee grounds based biorefinery for the production of biofuels, biopolymers, antioxidants and biocomposites. J. Waste Manag. 2018, 72, 240–254. [Google Scholar] [CrossRef]
- Zhou, M.X. Barley Production and Consumption. In Genetics and Improvement of Barley Malt Quality; Zhang, G., Li, C., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 1–17. [Google Scholar]
- Zaldivar, J.; Nielsen, J.; Olsson, L. Fuel ethanol production from lignocellulose: A challenge for metabolic engineering and process integration. Appl. Microbiol. Biotechnol. 2001, 56, 17–34. [Google Scholar] [CrossRef]
- Nichols, N.; Dien, B.; Bothast, R. Use of catabolite repression mutants for fermentation of sugar mixtures to ethanol. Appl. Microbiol. Biotechnol. 2001, 56, 120–125. [Google Scholar] [CrossRef]
- Postma, P.; Lengeler, J.; Jacobson, G. Phosphoenolpyruvate: Carbohydrate phosphotransferase systems of bacteria. Microbiol. Rev. 1993, 57, 543–594. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Montalvo, V.; Martínez, A.; Hernández-Chavez, G.; Bolivar, F.; Valle, F.; Gosset, G. Expression of galP and glk in an Escherichia coli PTS mutant restores glucose transport and increases glycolytic flux to fermentation products. Biotechnol. Bioeng. 2003, 83, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-H.; So, J.-H.; Shin, J.-H.; Rhee, I.-K. Role of xylR gene on the construction of xylose-inducible expression vectors using xylF promoter of Escherichia coli. J. Korean Soc. Appl. Biol. Chem. 2010, 53, 790–797. [Google Scholar] [CrossRef]
- Sievert, C.; Nieves, L.M.; Panyon, L.A.; Loeffler, T.; Morris, C.; Cartwright, R.A.; Wang, X. Experimental evolution reveals an effective avenue to release catabolite repression via mutations in XylR. Proc. Natl. Acad. Sci. USA 2017, 114, 7349–7354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, S.; Park, C. Organization and regulation of the D-xylose operons in Escherichia coli K-12: XylR acts as a transcriptional activator. J. Bacteriol. 1997, 179, 7025–7032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jönsson, L.; Palmqvist, E.; Nilvebrant, N.-O.; Hahn-Hägerdal, B. Detoxification of wood hydrolysates with laccase and peroxidase from the white-rot fungus Trametes versicolor. Appl. Microbiol. Biotechnol. 1998, 49, 691–697. [Google Scholar] [CrossRef]
- Sierra-Ibarra, E.; Alcaraz-Cienfuegos, J.; Vargas-Tah, A.; Rosas-Aburto, A.; Valdivia-López, Á.; Hernández-Luna, M.G.; Vivaldo-Lima, E.; Martinez, A. Ethanol production by Escherichia coli from detoxified lignocellulosic teak wood hydrolysates with high concentration of phenolic compounds. J. Ind. Microbiol. Biotechnol. 2021, 49, kuab077. [Google Scholar] [CrossRef]
- Rios-González, L.J.; Morales-Martínez, T.K.; Rodríguez-Flores, M.F.; Rodríguez-De la Garza, J.A.; Castillo-Quiroz, D.; Castro-Montoya, A.J.; Martinez, A. Autohydrolysis pretreatment assessment in ethanol production from agave bagasse. Bioresour. Technol. 2017, 242, 184–190. [Google Scholar] [CrossRef]
- Saucedo-Luna, J.; Castro-Montoya, A.J.; Martinez-Pacheco, M.M.; Sosa-Aguirre, C.R.; Campos-Garcia, J. Efficient chemical and enzymatic saccharification of the lignocellulosic residue from Agave tequilana bagasse to produce ethanol by Pichia caribbica. J. Ind. Microbiol. Biotechnol. 2011, 38, 725–732. [Google Scholar] [CrossRef]
- Barbosa, M.F.; Beck, M.J.; Fein, J.E.; Potts, D.; Ingram, L.O. Efficient fermentation of Pinus sp. acid hydrolysates by an ethanologenic strain of Escherichia coli. Appl. Environ. Microbiol. 1992, 58, 1382–1384. [Google Scholar] [CrossRef]
- Pérez-Pimienta, J.A.; Vargas-Tah, A.; López-Ortega, K.M.; Medina-López, Y.N.; Mendoza-Pérez, J.A.; Avila, S.; Singh, S.; Simmons, B.A.; Loaces, I.; Martinez, A. Sequential enzymatic saccharification and fermentation of ionic liquid and organosolv pretreated agave bagasse for ethanol production. Bioresour. Technol. 2017, 225, 191–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ballesteros, L.F.; Teixeira, J.A.; Mussatto, S.I. Chemical, functional, and structural properties of spent coffee grounds and coffee silverskin. Food Bioprocess Technol. 2014, 7, 3493–3503. [Google Scholar] [CrossRef] [Green Version]
- Atabani, A.E.; Al-Muhtaseb, A.a.H.; Kumar, G.; Saratale, G.D.; Aslam, M.; Khan, H.A.; Said, Z.; Mahmoud, E. Valorization of spent coffee grounds into biofuels and value-added products: Pathway towards integrated bio-refinery. Fuel 2019, 254, 115640. [Google Scholar] [CrossRef]
- Battista, F.; Barampouti, E.M.; Mai, S.; Bolzonella, D.; Malamis, D.; Moustakas, K.; Loizidou, M. Added-value molecules recovery and biofuels production from spent coffee grounds. Renew. Sustain. Energy Rev. 2020, 131, 110007. [Google Scholar] [CrossRef]
- Negrete, A.; Ng, W.-I.; Shiloach, J. Glucose uptake regulation in E. coli by the small RNA SgrS: Comparative analysis of E. coli K-12 (JM109 and MG1655) and E. coli B (BL21). Microb. Cell Factories 2010, 9, 75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neubauer, P.; Häggström, L.; Enfors, S.O. Influence of substrate oscillations on acetate formation and growth yield in Escherichia coli glucose limited fed-batch cultivations. Biotechnol. Bioeng. 1995, 47, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Saha, B.C.; Qureshi, N.; Kennedy, G.J.; Cotta, M.A. Enhancement of xylose utilization from corn stover by a recombinant Escherichia coli strain for ethanol production. Bioresour. Technol. 2015, 190, 182–188. [Google Scholar] [CrossRef]
- Saha, B.C.; Cotta, M.A. Comparison of pretreatment strategies for enzymatic saccharification and fermentation of barley straw to ethanol. N. Biotechnol. 2010, 27, 10–16. [Google Scholar] [CrossRef]
- Fernández-Sandoval, M.T.; Galíndez-Mayer, J.; Moss-Acosta, C.L.; Gosset, G.; Martinez, A. Volumetric oxygen transfer coefficient as a means of improving volumetric ethanol productivity and a criterion for scaling up ethanol production with Escherichia coli. J. Chem. Technol. Biotechnol. 2017, 92, 981–989. [Google Scholar] [CrossRef]
- Fernández-Sandoval, M.; Huerta-Beristain, G.; Trujillo-Martinez, B.; Bustos, P.; González, V.; Bolivar, F.; Gosset, G.; Martinez, A. Laboratory metabolic evolution improves acetate tolerance and growth on acetate of ethanologenic Escherichia coli under non-aerated conditions in glucose-mineral medium. Appl. Microbiol. Biotechnol. 2012, 96, 1291–1300. [Google Scholar] [CrossRef]
- Xia, T.; Eiteman, M.A.; Altman, E. Simultaneous utilization of glucose, xylose and arabinose in the presence of acetate by a consortium of Escherichia coli strains. Microb. Cell Factories 2012, 11, 77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fox, K.J.; Prather, K.L.J. Carbon catabolite repression relaxation in Escherichia coli: Global and sugar-specific methods for glucose and secondary sugar co-utilization. Curr. Opin. Chem. Eng. 2020, 30, 9–16. [Google Scholar] [CrossRef]
- Utrilla, J.; Licona-Cassani, C.; Marcellin, E.; Gosset, G.; Nielsen, L.K.; Martinez, A. Engineering and adaptive evolution of Escherichia coli for D-lactate fermentation reveals GatC as a xylose transporter. Metab. Eng. 2012, 14, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.M.; Kim, H.J.; Lee, S.J. Efficient anaerobic consumption of D-xylose by E. coli BL21 (DE3) via xylR adaptive mutation. BMC Microbiol. 2021, 21, 332. [Google Scholar] [CrossRef]
- Thomason, L.C.; Costantino, N.; Court, D.L. E. coli Genome Manipulation by P1 Transduction. Curr. Protoc. Mol. Biol. 2007, 79, 1171–1178. [Google Scholar] [CrossRef]
- Baba, T.; Ara, T.; Hasegawa, M.; Takai, Y.; Okumura, Y.; Baba, M.; Datsenko, K.A.; Tomita, M.; Wanner, B.L.; Mori, H. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: The Keio collection. Mol. Syst. Biol. 2006, 2, 2006. [Google Scholar] [CrossRef] [Green Version]
- Martinez, A.; Grabar, T.B.; Shanmugam, K.T.; Yomano, L.P.; York, S.W.; Ingram, L.O. Low salt medium for lactate and ethanol production by recombinant Escherichia coli B. Biotechnol. Lett. 2007, 29, 397–404. [Google Scholar] [CrossRef]
- Hernández Luna, M.G.; Vivaldo Lima, E.; Alcaraz Cienfuegos, J.; Valdivia López, M.A. Proceso de Tratamiento ácido en Fase de Gas de Materiales Lignocelulosicos. MX Publication No. WO 2018/004327 A1, 1 April 2018. [Google Scholar]


| Parameter | Control LSH | Teak Wood | Agave Bagasse |
|---|---|---|---|
| Xmax (gDCW/L) | 1.69 | ND | ND |
| µ (h−1) | 0.16 | ND | ND |
| YEtOH(%) | 88 | 92 (5) | 81 (5) |
| Qglc (gglu/Lh) | 1.61 | 0.72 (0.00) | 1.59 (0.02) |
| Qxyl (gxyl/Lh) | 0.69 | 0.18 (0.02) | 0.73 (0.01) |
| QEtOH (gEtOH/Lh) | 0.92 | 0.42 (0.02) | 0.96 (0.05) |
| Parameter | Control LSH | Corn Stover | Barley Straw |
|---|---|---|---|
| Xmax (gDCW/L) | 1.61 | ND | ND |
| µ (h−1) | 0.19 | ND | ND |
| YEtOH(%) | 75 | 81 (1) | 82 (4) |
| Qglc (gglc/Lh) | 0.61 | 0.31 (0.01) | 0.35 (0.02) |
| Qxyl (gxyl/Lh) | 1.29 | 0.52 (0.02) | 0.77 (0.03) |
| QEtOH (gETOH/Lh) | 0.72 | 0.25 (0.02) | 0.36 (0.01) |
| Parameter | WA | WOA |
|---|---|---|
| µ (h−1) | 0.14 (0.00) | 0.13 (0.00) |
| Xmax (gDCW/L) | 1.12 (0.09) | 0.65 (0.06) |
| YEtOH (%) | 95 (5) | 90 (2) |
| Qglc (gglu/Lh) | 0.51 (0.04) | 0.33 (0.01) |
| Qxyl (gxyl/Lh) | 0.32(0.02) | 0.16 (0.00) |
| Qara (gara/Lh) | 0.35 (0.02) | 0.25 (0.01) |
| QEtOH (gETOH/Lh) | 0.66 (0.01) | 0.34 (0.02) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sierra-Ibarra, E.; Vargas-Tah, A.; Moss-Acosta, C.L.; Trujillo-Martínez, B.; Molina-Vázquez, E.R.; Rosas-Aburto, A.; Valdivia-López, Á.; Hernández-Luna, M.G.; Vivaldo-Lima, E.; Martínez, A. Co-Fermentation of Glucose–Xylose Mixtures from Agroindustrial Residues by Ethanologenic Escherichia coli: A Study on the Lack of Carbon Catabolite Repression in Strain MS04. Molecules 2022, 27, 8941. https://doi.org/10.3390/molecules27248941
Sierra-Ibarra E, Vargas-Tah A, Moss-Acosta CL, Trujillo-Martínez B, Molina-Vázquez ER, Rosas-Aburto A, Valdivia-López Á, Hernández-Luna MG, Vivaldo-Lima E, Martínez A. Co-Fermentation of Glucose–Xylose Mixtures from Agroindustrial Residues by Ethanologenic Escherichia coli: A Study on the Lack of Carbon Catabolite Repression in Strain MS04. Molecules. 2022; 27(24):8941. https://doi.org/10.3390/molecules27248941
Chicago/Turabian StyleSierra-Ibarra, Estefanía, Alejandra Vargas-Tah, Cessna L. Moss-Acosta, Berenice Trujillo-Martínez, Eliseo R. Molina-Vázquez, Alberto Rosas-Aburto, Ángeles Valdivia-López, Martín G. Hernández-Luna, Eduardo Vivaldo-Lima, and Alfredo Martínez. 2022. "Co-Fermentation of Glucose–Xylose Mixtures from Agroindustrial Residues by Ethanologenic Escherichia coli: A Study on the Lack of Carbon Catabolite Repression in Strain MS04" Molecules 27, no. 24: 8941. https://doi.org/10.3390/molecules27248941








