Experimental Study of Halloysite Nanofluids in Pool Boiling Heat Transfer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
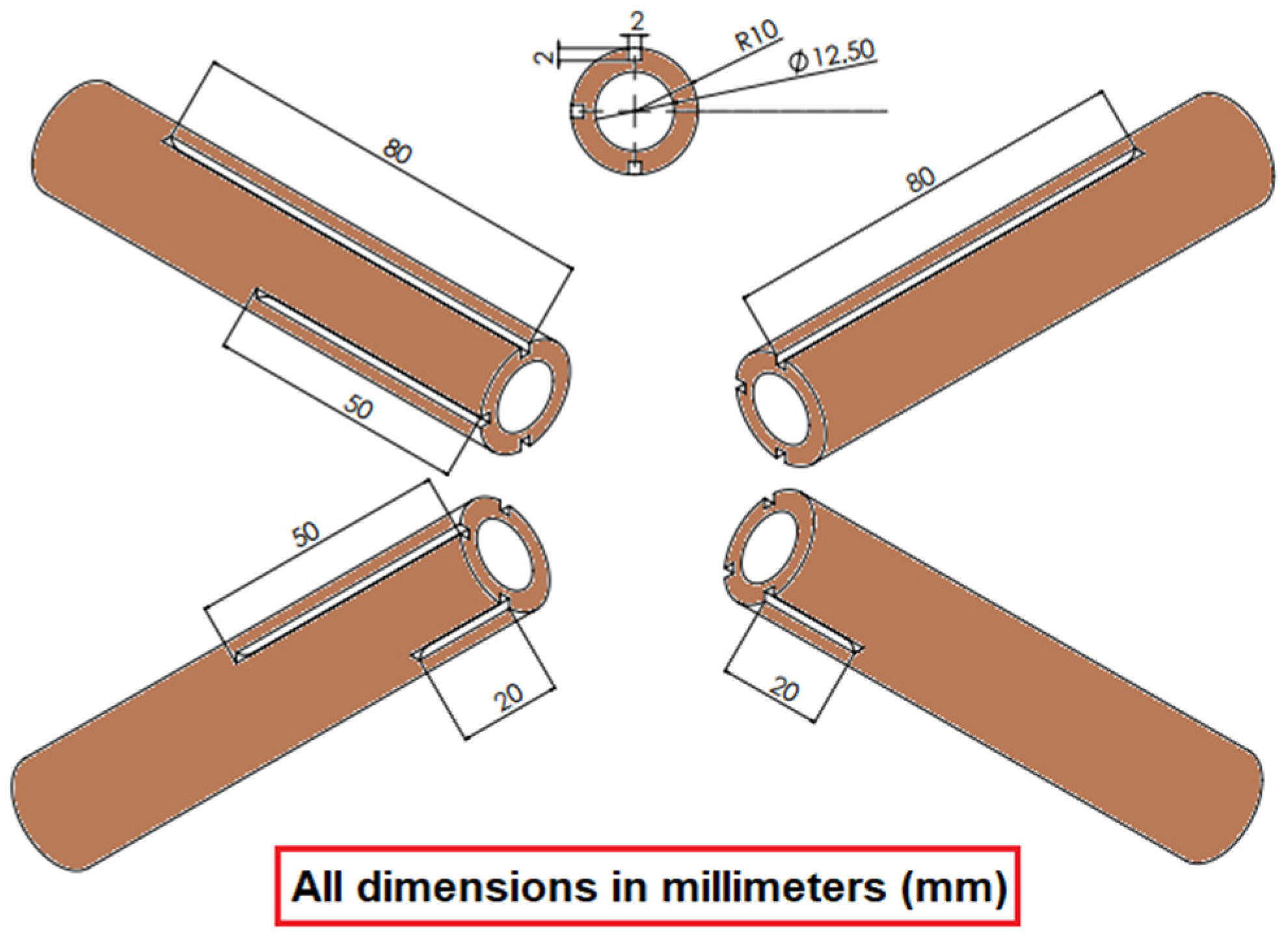
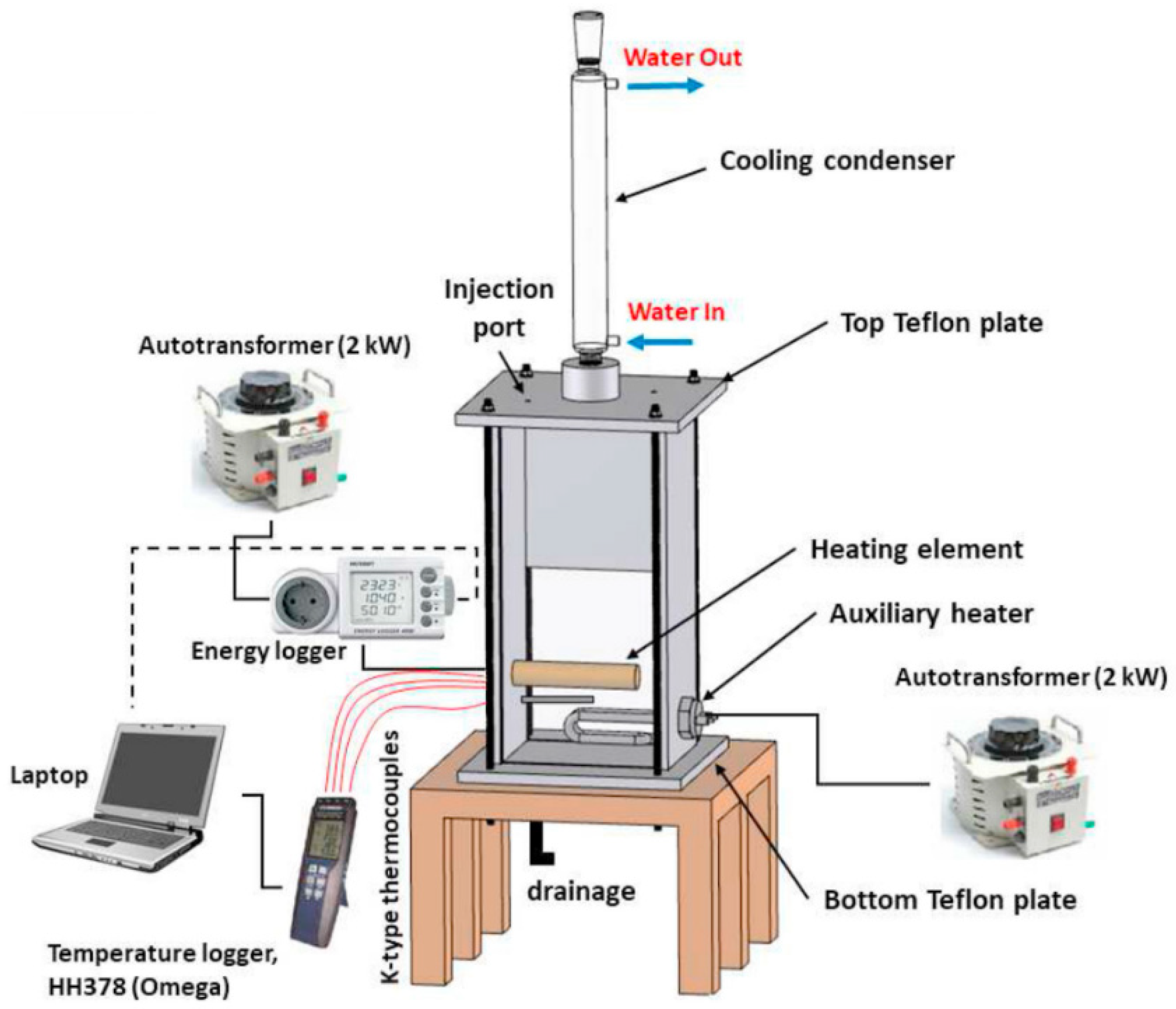
2.2. Pool Boiling Experimental Test
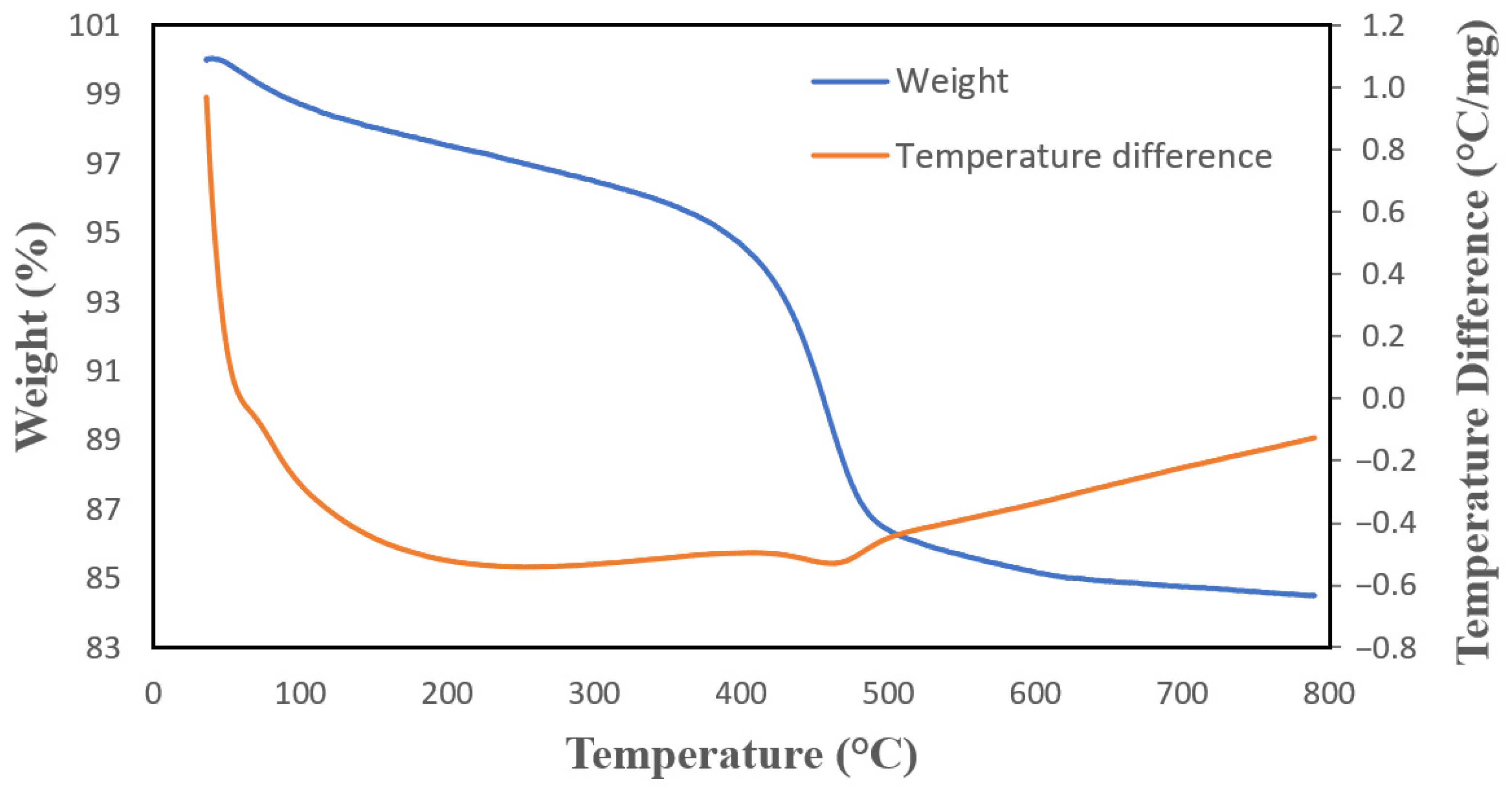
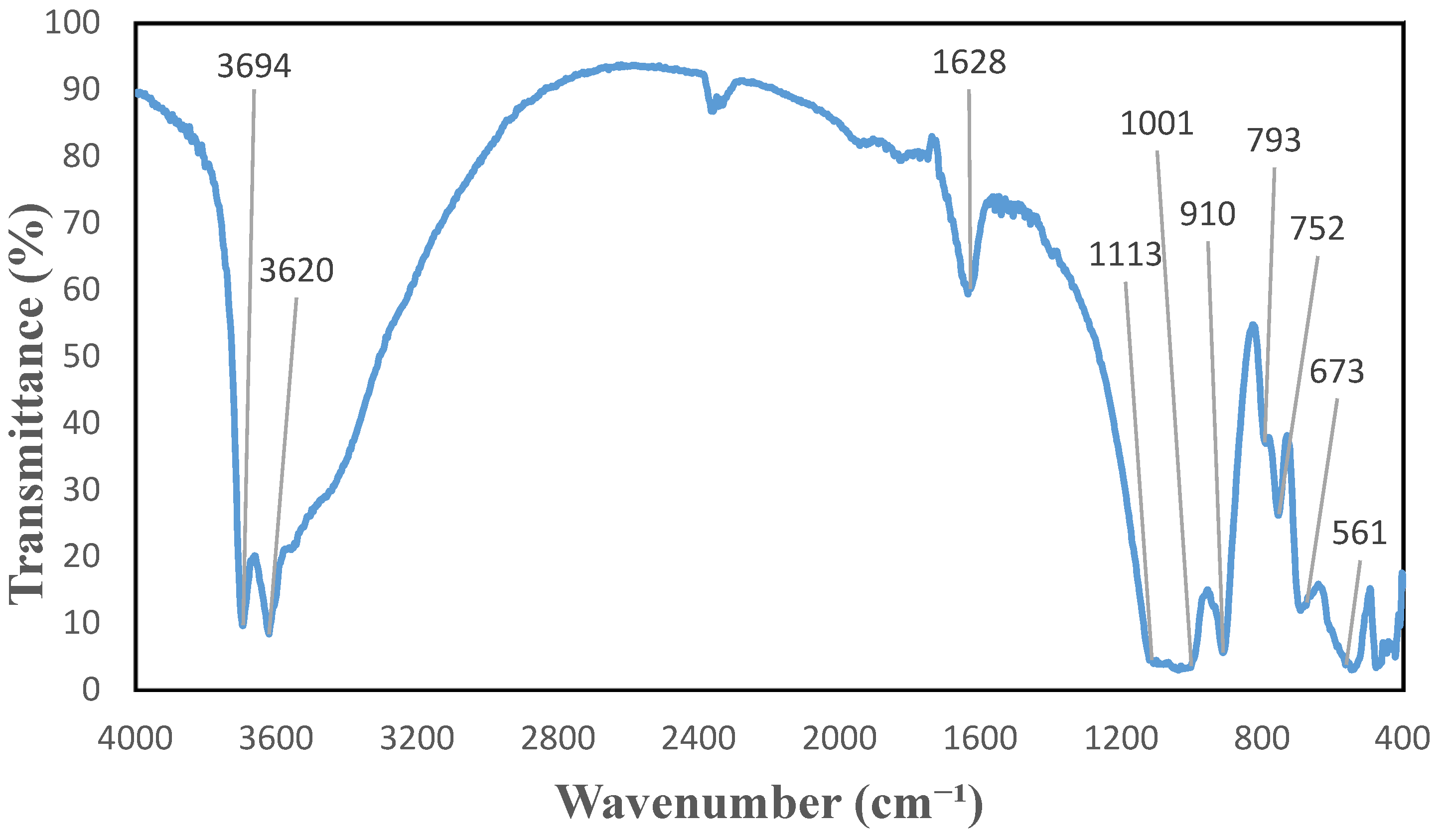
2.3. HNTs Properties
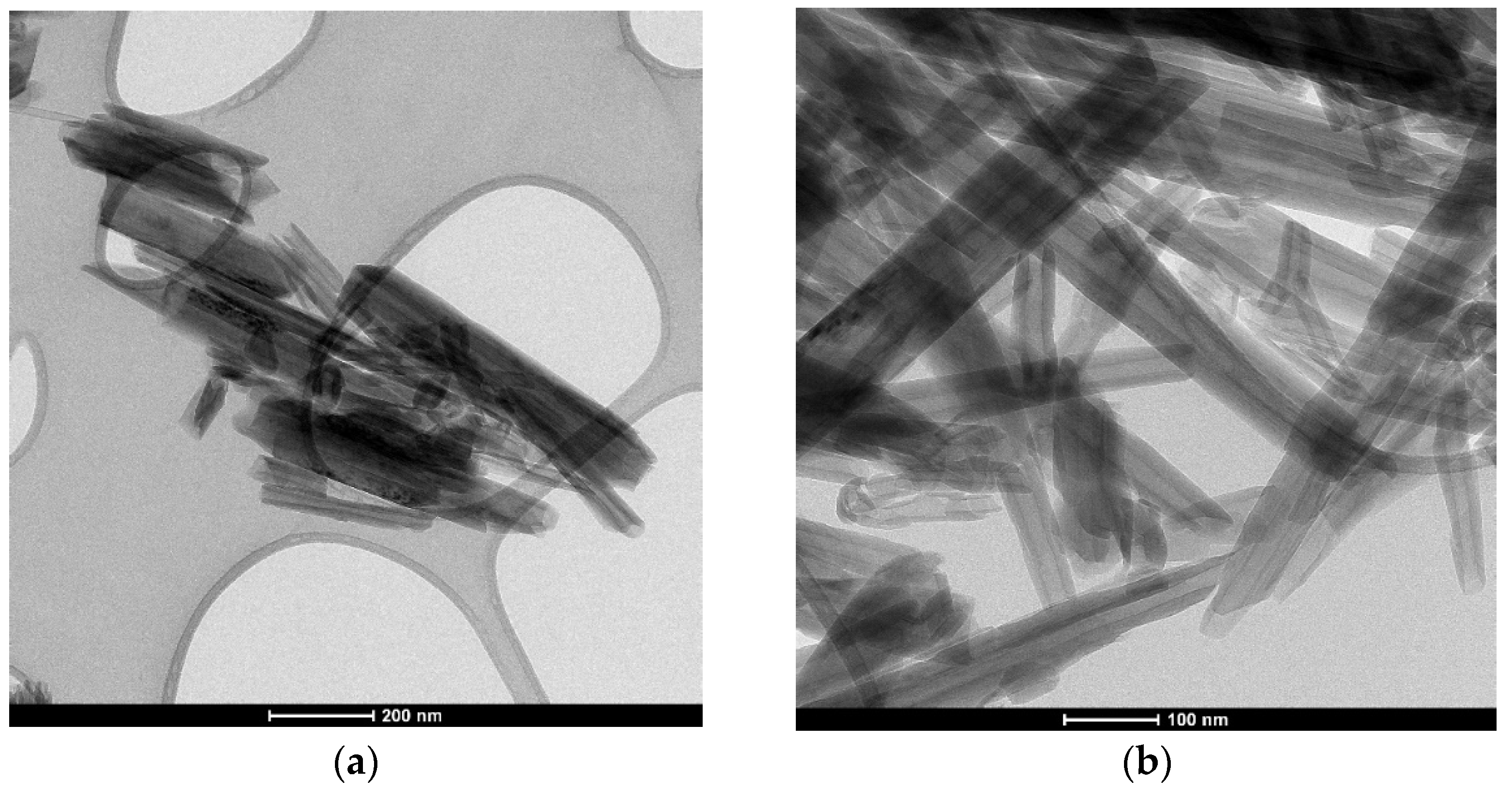
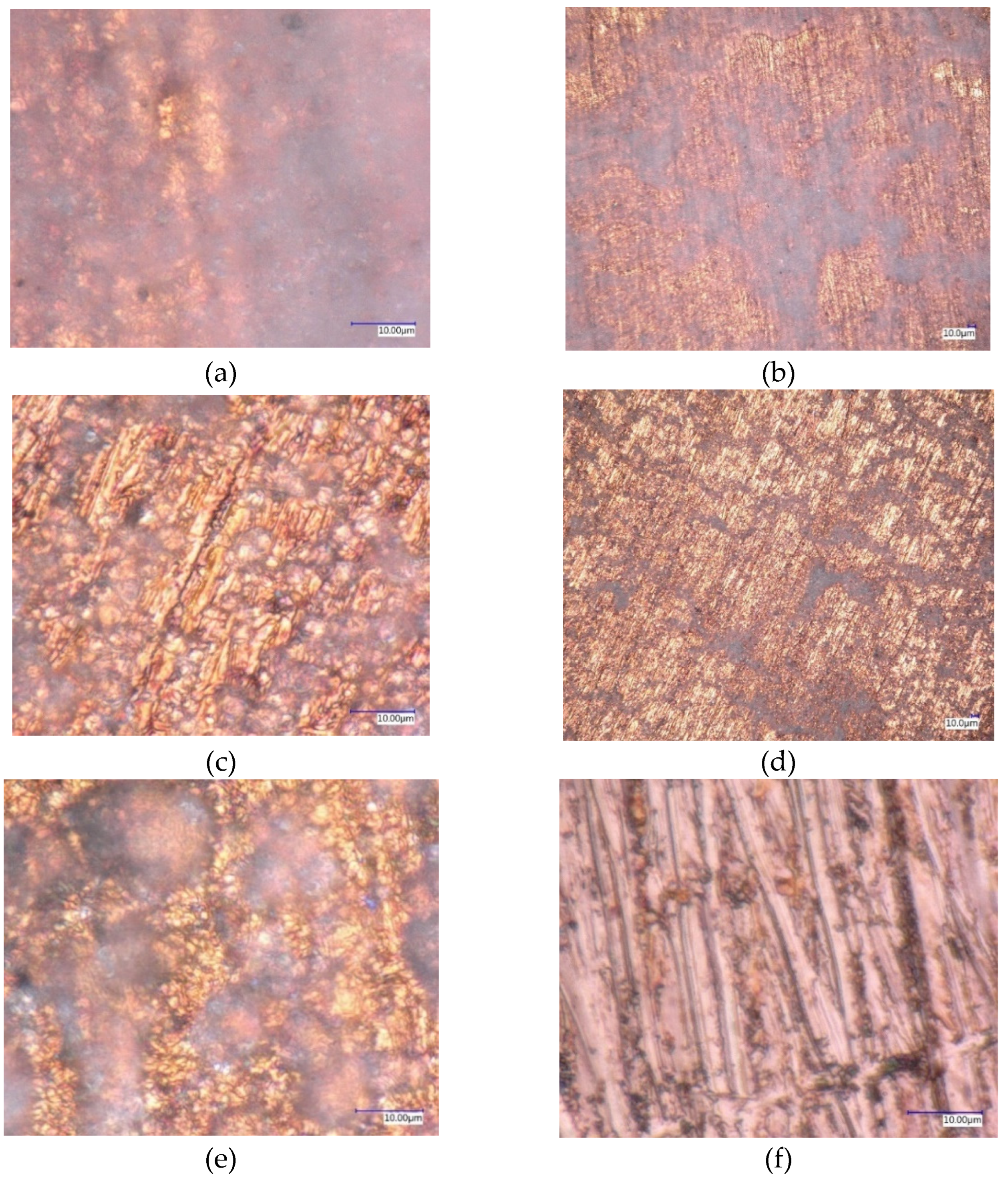
2.4. Digital Optical Microscopy (DOM)
3. Results and Discussion
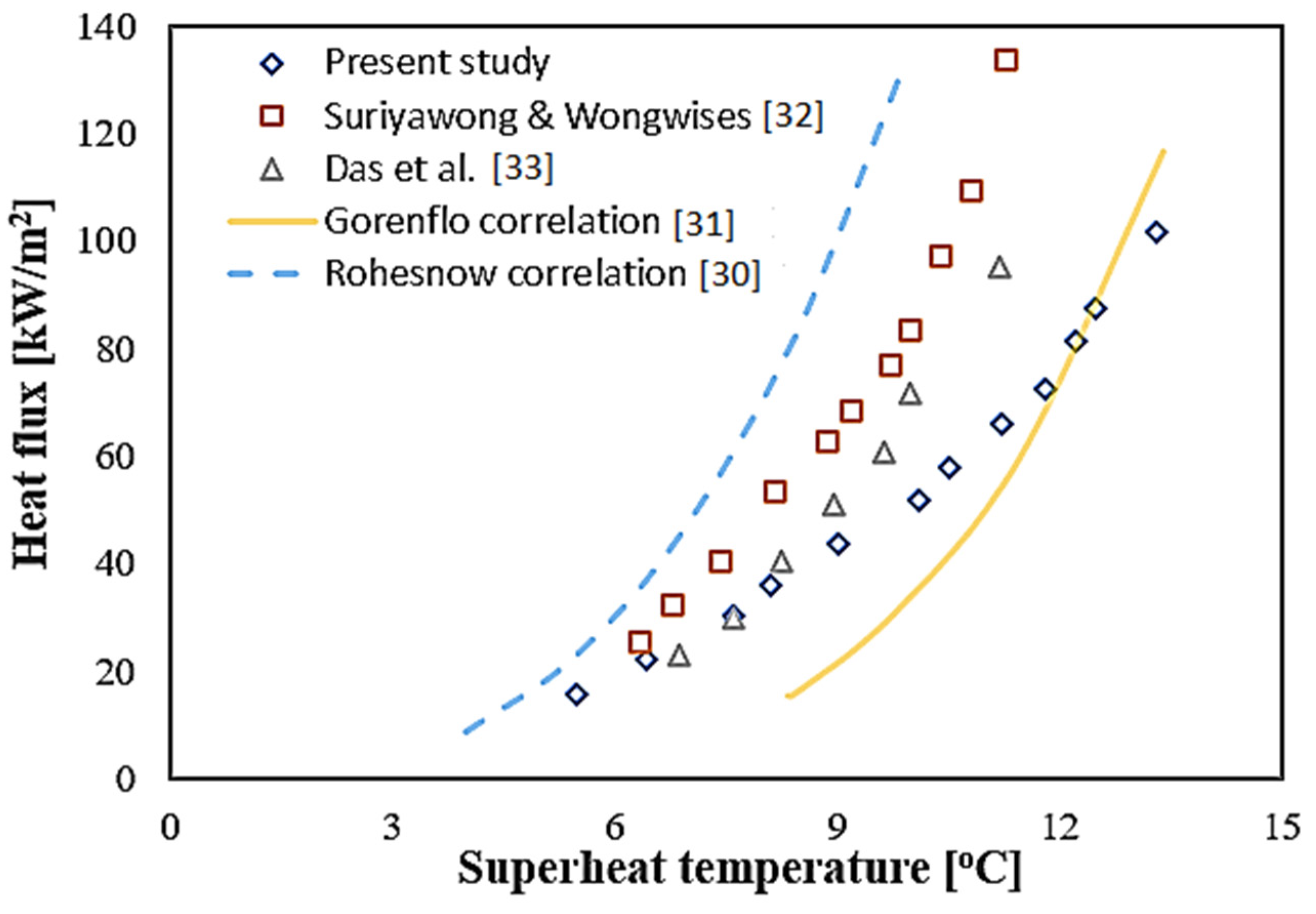
3.1. Validation of Pool Boiling Chamber
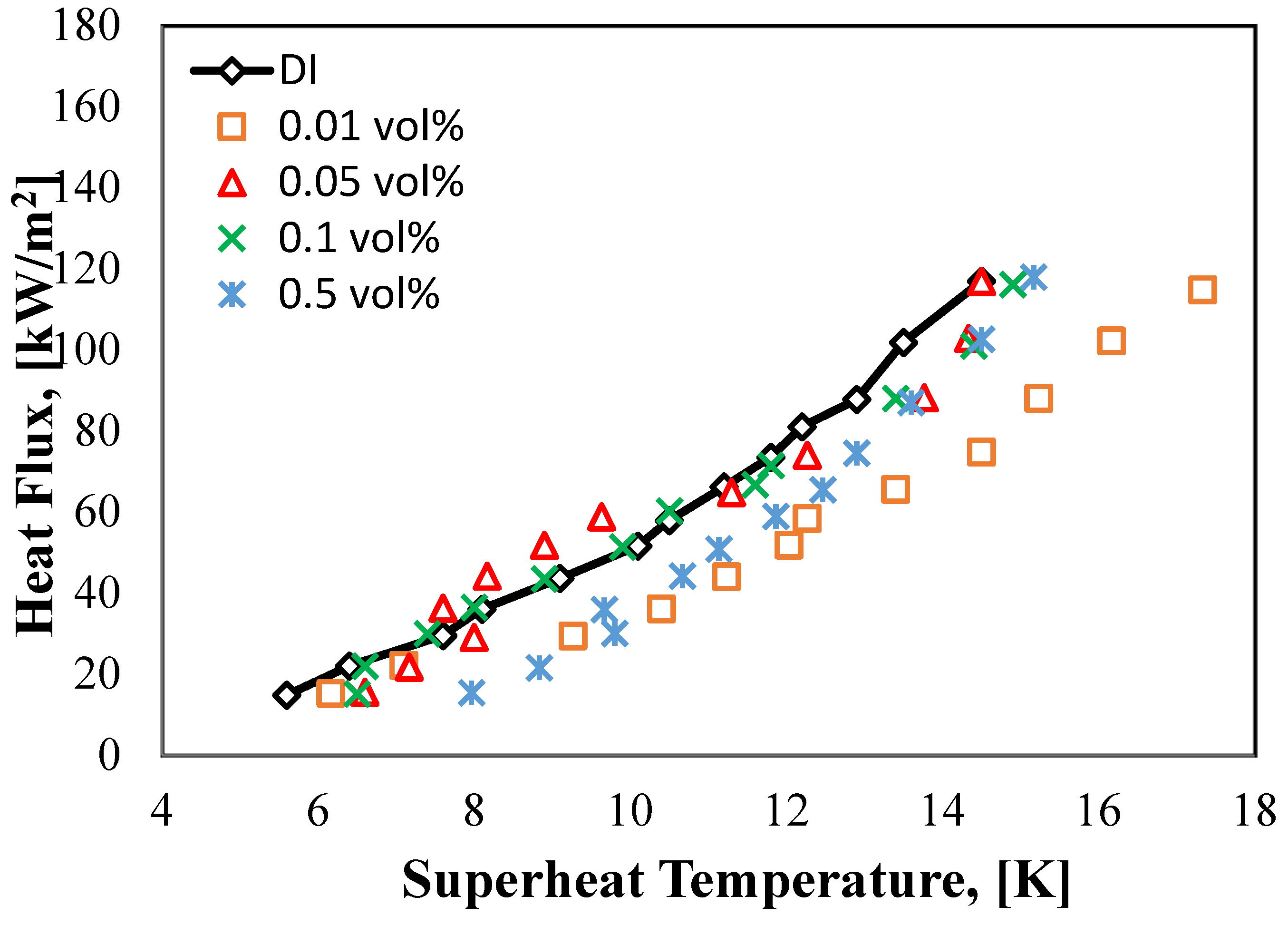
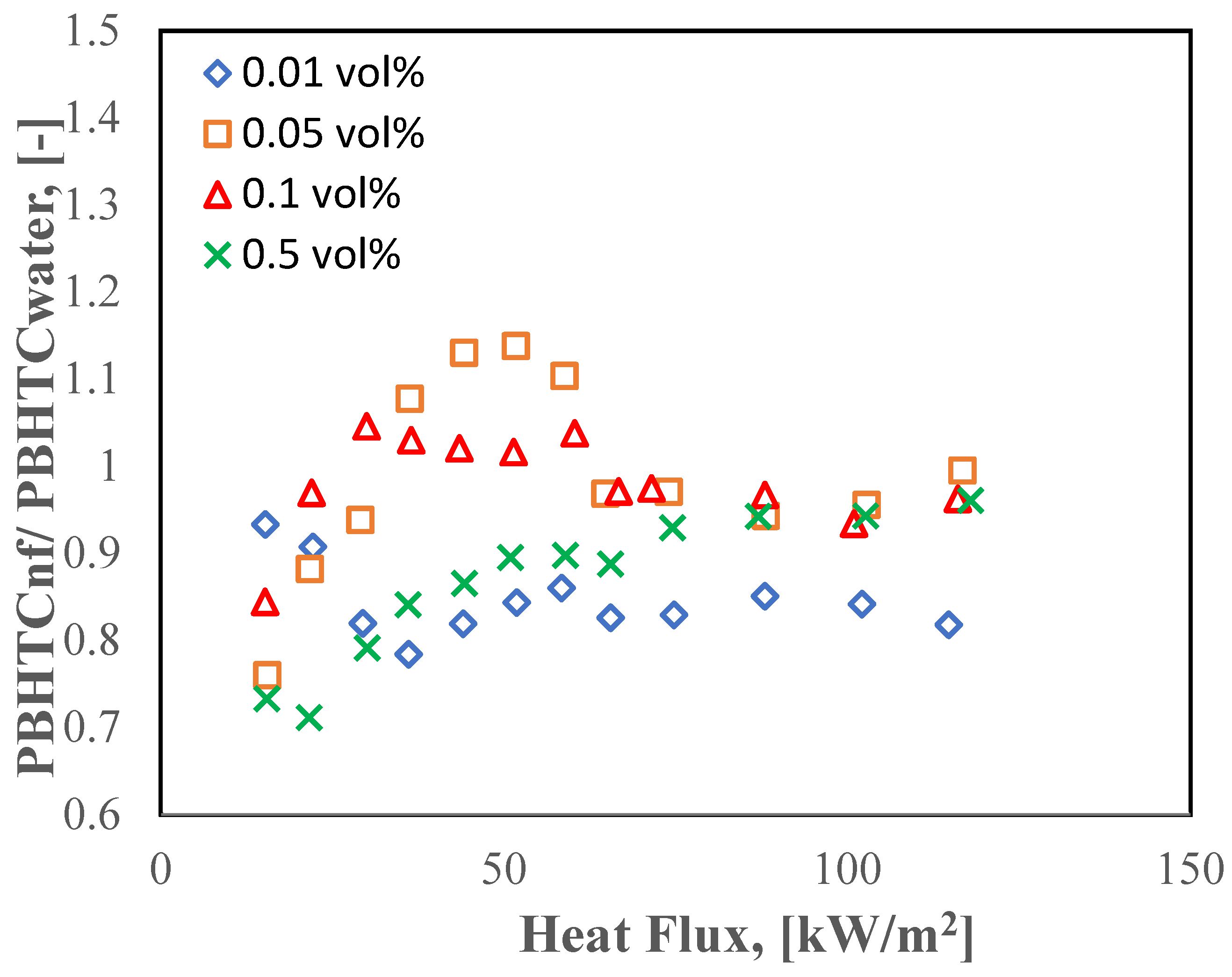
3.2. Pool Boiling of Halloysite Nanofluids
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
Nomenclature
| HNT | Halloysite nanotube |
| DI water | Deionized water |
| HT | Heat transfer |
| HTC | Heat transfer coefficient |
| MWCNT | Multi-walled carbon nanotube |
| PBHT | Pool boiling heat transfer |
| PBHTC | Pool boiling heat transfer coefficient |
| TC | Thermal conductivity |
| HF | Heat flux |
References
- Li, J.; Zhang, X.; Xu, B.; Yuan, M. Nanofluid research and applications: A review. Int. Commun. Heat Mass Transf. 2021, 127, 105543. [Google Scholar] [CrossRef]
- Ali, H.M.; Babar, H.; Shah, T.R.; Sajid, M.U.; Qasim, M.A.; Javed, S. Preparation Techniques of TiO2 Nanofluids and Challenges: A Review. Appl. Sci. 2018, 8, 587. [Google Scholar] [CrossRef] [Green Version]
- Muneeshwaran, M.; Srinivasan, G.; Muthukumar, P.; Wang, C.-C. Role of hybrid-nanofluid in heat transfer enhancement—A review. Int. Commun. Heat Mass Transf. 2021, 125, 105341. [Google Scholar] [CrossRef]
- Mashali, F.; Languri, E.M.; Davidson, J.; Kerns, D.; Johnson, W.; Nawaz, K.; Cunningham, G. Thermo-physical properties of diamond nanofluids: A review. Int. J. Heat Mass Transf. 2019, 129, 1123–1135. [Google Scholar] [CrossRef]
- Chakraborty, S.; Panigrahi, P.K. Stability of nanofluid: A review. Appl. Therm. Eng. 2020, 174, 115259. [Google Scholar] [CrossRef]
- Kumar, P.; Sarviya, R.M. Recent developments in preparation of nanofluid for heat transfer enhancement in heat exchangers: A review. Mater. Today Proc. 2021, 44, 2356–2361. [Google Scholar] [CrossRef]
- Wang, B.; Shih, T.-M.; Huang, J. Enhancing and attenuating heat transfer characteristics for circulating flows of nanofluids within rectangular enclosures. Int. Commun. Heat Mass Transf. 2020, 117, 104800. [Google Scholar] [CrossRef]
- Ajeeb, W.; Oliveira, M.S.; Martins, N.; Murshed, S.S. Forced convection heat transfer of non-Newtonian MWCNTs nanofluids in microchannels under laminar flow. Int. Commun. Heat Mass Transf. 2021, 127, 105495. [Google Scholar] [CrossRef]
- Liang, G.; Mudawar, I. Review of single-phase and two-phase nanofluid heat transfer in macro-channels and micro-channels. Int. J. Heat Mass Transf. 2019, 136, 324–354. [Google Scholar] [CrossRef]
- Yang, Y.M.; Maa, J.R. Boiling of suspension of solid particles in water. Int. J. Heat Mass Transf. 1984, 27, 145–147. [Google Scholar] [CrossRef]
- Das, S.K.; Putra, N.; Roetzel, W. Pool boiling of nano-fluids on horizontal narrow tubes. Int. J. Multiph. Flow 2003, 29, 1237–1247. [Google Scholar] [CrossRef]
- Sarafraz, M.; Hormozi, F. Experimental investigation on the pool boiling heat transfer to aqueous multi-walled carbon nanotube nanofluids on the micro-finned surfaces. Int. J. Therm. Sci. 2016, 100, 255–266. [Google Scholar] [CrossRef]
- He, Y.; Li, H.; Hu, Y.; Wang, X.; Zhu, J. Boiling heat transfer characteristics of ethylene glycol and water mixture based ZnO nanofluids in a cylindrical vessel. Int. J. Heat Mass Transf. 2016, 98, 611–615. [Google Scholar] [CrossRef]
- Akbari, E.; Gheitaghy, A.M.; Saffari, H.; Hosseinalipour, S.M. Effect of silver nanoparticle deposition in re-entrant inclined minichannel on bubble dynamics for pool boiling enhancement. Exp. Therm. Fluid Sci. 2017, 82, 390–401. [Google Scholar] [CrossRef]
- Manetti, L.; Stephen, M.T.; Beck, P.A.; Cardoso, E.M. Evaluation of the heat transfer enhancement during pool boiling using low concentrations of Al2O3-water based nanofluid. Exp. Therm. Fluid Sci. 2017, 87, 191–200. [Google Scholar] [CrossRef] [Green Version]
- Salimpour, M.R.; Abdollahi, A.; Afrand, M. An experimental study on deposited surfaces due to nanofluid pool boiling: Comparison between rough and smooth surfaces. Exp. Therm. Fluid Sci. 2017, 88, 288–300. [Google Scholar] [CrossRef]
- Karimzadehkhouei, M.; Shojaeian, M.; Şendur, K.; Mengüç, M.P.; Koşar, A. The effect of nanoparticle type and nanoparticle mass fraction on heat transfer enhancement in pool boiling. Int. J. Heat Mass Transf. 2017, 109, 157–166. [Google Scholar] [CrossRef]
- Kole, M.; Dey, T. Investigations on the pool boiling heat transfer and critical heat flux of ZnO-ethylene glycol nanofluids. Appl. Therm. Eng. 2012, 37, 112–119. [Google Scholar] [CrossRef]
- Kamel, M.S.; Lezsovits, F.; Abdollahi, A.; Izadi, M. Amelioration of pool boiling thermal performance in case of using a new hybrid nanofluid. Case Stud. Therm. Eng. 2021, 24, 100872. [Google Scholar] [CrossRef]
- Massaro, M.; Noto, R.; Riela, S. Past, Present and Future Perspectives on Halloysite Clay Minerals. Molecules 2020, 25, 4863. [Google Scholar] [CrossRef]
- Rawtani, D.; Phd, M.E.; Rawtani, D.; Agrawal, Y.K. Multifarious applications of halloysite nanotubes: A review Use of Halloysite Nanotube to overcome complexation of ciprofloxacin with iron through invitro approach View project Nanocomposite based luminescent paper sensor for on the spot trace level detec. Rev. Adv. Mater. Sci. 2012, 30, 282–295. [Google Scholar]
- Le Ba, T.; Mahian, O.; Wongwises, S.; Szilágyi, I.M. Review on the recent progress in the preparation and stability of graphene-based nanofluids. J. Therm. Anal. 2020, 142, 1145–1172. [Google Scholar] [CrossRef] [Green Version]
- Alberola, J.; Mondragón, R.; Juliá, J.; Hernández, L.; Cabedo, L. Characterization of halloysite-water nanofluid for heat transfer applications. Appl. Clay Sci. 2014, 99, 54–61. [Google Scholar] [CrossRef] [Green Version]
- Le Ba, T.; Alkurdi, A.Q.; Lukács, I.E.; Molnár, J.; Wongwises, S.; Gróf, G.; Szilágyi, I.M. A Novel Experimental Study on the Rheological Properties and Thermal Conductivity of Halloysite Nanofluids. Nanomaterials 2020, 10, 1834. [Google Scholar] [CrossRef] [PubMed]
- Kamel, M.S.; Lezsovits, F. Experimental Study on Pool Boiling Heat Transfer Performance of Magnesium Oxide Nanoparticles Based Water Nanofluid. Pollack Period. 2020, 15, 101–112. [Google Scholar] [CrossRef]
- El Haddar, A.; Elkhadir, G.; Azdimousa, A.; Fagel, N.; Hassani, I.-E.E.A.E.; El Ouahabi, M.; Iz-Eddine, E.A.E.H. Characterization of halloysite (North East Rif, Morocco): Evaluation of its suitability for the ceramics industry. Clay Miner. 2018, 53, 65–78. [Google Scholar] [CrossRef]
- Rohsenow, W.M. A Method of Correlating Heat Transfer Data for Surface Boiling of Liquids; Technical Report; MIT: Cambridge, MA, USA, 1952. [Google Scholar]
- Gorenflo, D.; Kenning, D. H2 Pool Boiling. VDI Heat Atlas 2009, 757–792. [Google Scholar] [CrossRef]
- Suriyawong, A.; Wongwises, S. Nucleate pool boiling heat transfer characteristics of TiO2–water nanofluids at very low concentrations. Exp. Therm. Fluid Sci. 2010, 34, 992–999. [Google Scholar] [CrossRef]
- Das, S.K.; Putra, N.; Roetzel, W. Pool boiling characteristics of nano-fluids. Int. J. Heat Mass Transf. 2003, 46, 851–862. [Google Scholar] [CrossRef]
- Kathiravan, R.; Kumar, R.; Gupta, A.; Chandra, R. Characterization and Pool Boiling Heat Transfer Studies of Nanofluids. J. Heat Transf. 2009, 131, 081902. [Google Scholar] [CrossRef]
- Shiro, N. The maximum and minimum values of the heat q transmitted from metal to boiling water under atmospheric pressure. Int. J. Heat Mass Transf. 1984, 27, 959–970. [Google Scholar] [CrossRef]
- Zhao, T.; Chen, J.; Chen, Y.; Zhang, Y.; Peng, J. Study on synergistic enhancement of oil recovery by halloysite nanotubes and glucose-based surfactants. J. Dispers. Sci. Technol. 2021, 42, 934–946. [Google Scholar] [CrossRef]
- Kim, S.; Bang, I.; Buongiorno, J.; Hu, L. Surface wettability change during pool boiling of nanofluids and its effect on critical heat flux. Int. J. Heat Mass Transf. 2007, 50, 4105–4116. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le Ba, T.; Baqer, A.; Saad Kamel, M.; Gróf, G.; Odhiambo, V.O.; Wongwises, S.; Ferenc, L.; Szilágyi, I.M. Experimental Study of Halloysite Nanofluids in Pool Boiling Heat Transfer. Molecules 2022, 27, 729. https://doi.org/10.3390/molecules27030729
Le Ba T, Baqer A, Saad Kamel M, Gróf G, Odhiambo VO, Wongwises S, Ferenc L, Szilágyi IM. Experimental Study of Halloysite Nanofluids in Pool Boiling Heat Transfer. Molecules. 2022; 27(3):729. https://doi.org/10.3390/molecules27030729
Chicago/Turabian StyleLe Ba, Thong, Ahmed Baqer, Mohammed Saad Kamel, Gyula Gróf, Vincent Otieno Odhiambo, Somchai Wongwises, Lezsovits Ferenc, and Imre Miklós Szilágyi. 2022. "Experimental Study of Halloysite Nanofluids in Pool Boiling Heat Transfer" Molecules 27, no. 3: 729. https://doi.org/10.3390/molecules27030729






