Resistive Chemosensors for the Detection of CO Based on Conducting Polymers and Carbon Nanocomposites: A Review
Abstract
:1. Introduction
2. Chemosensors: An Overview on CO Detection
3. Sensing Principle
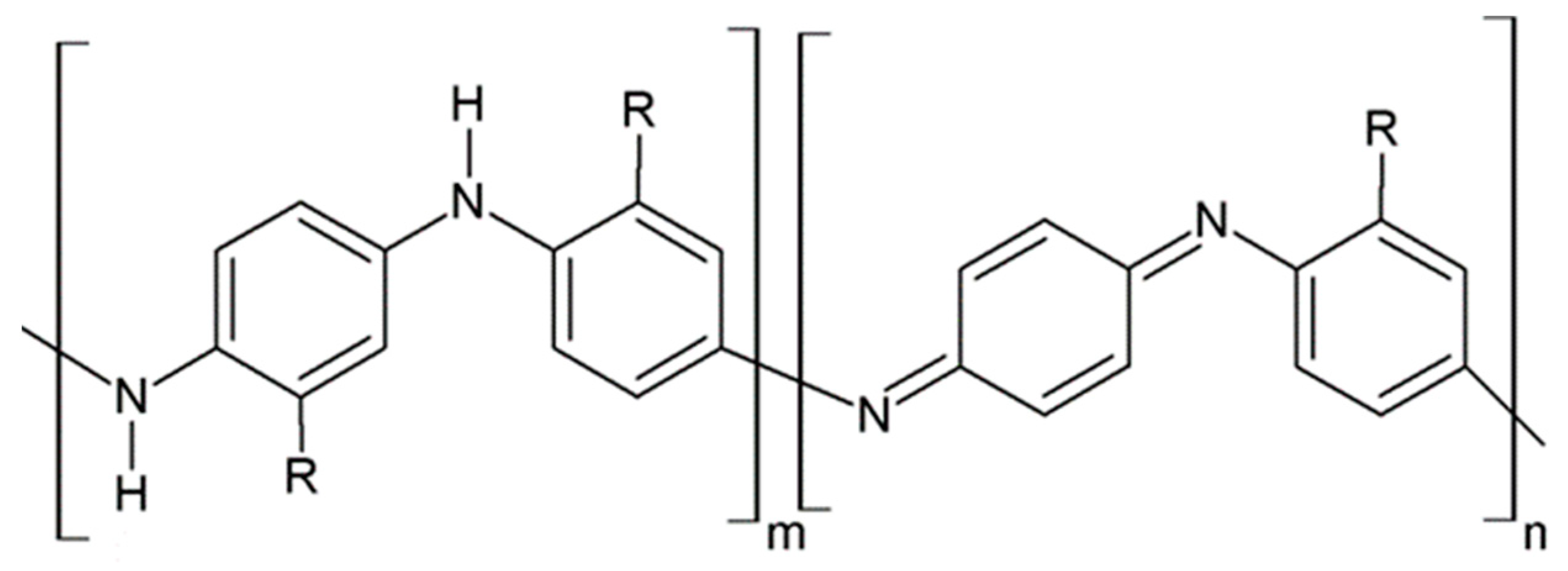
3.1. PANI Structure and Conductivity
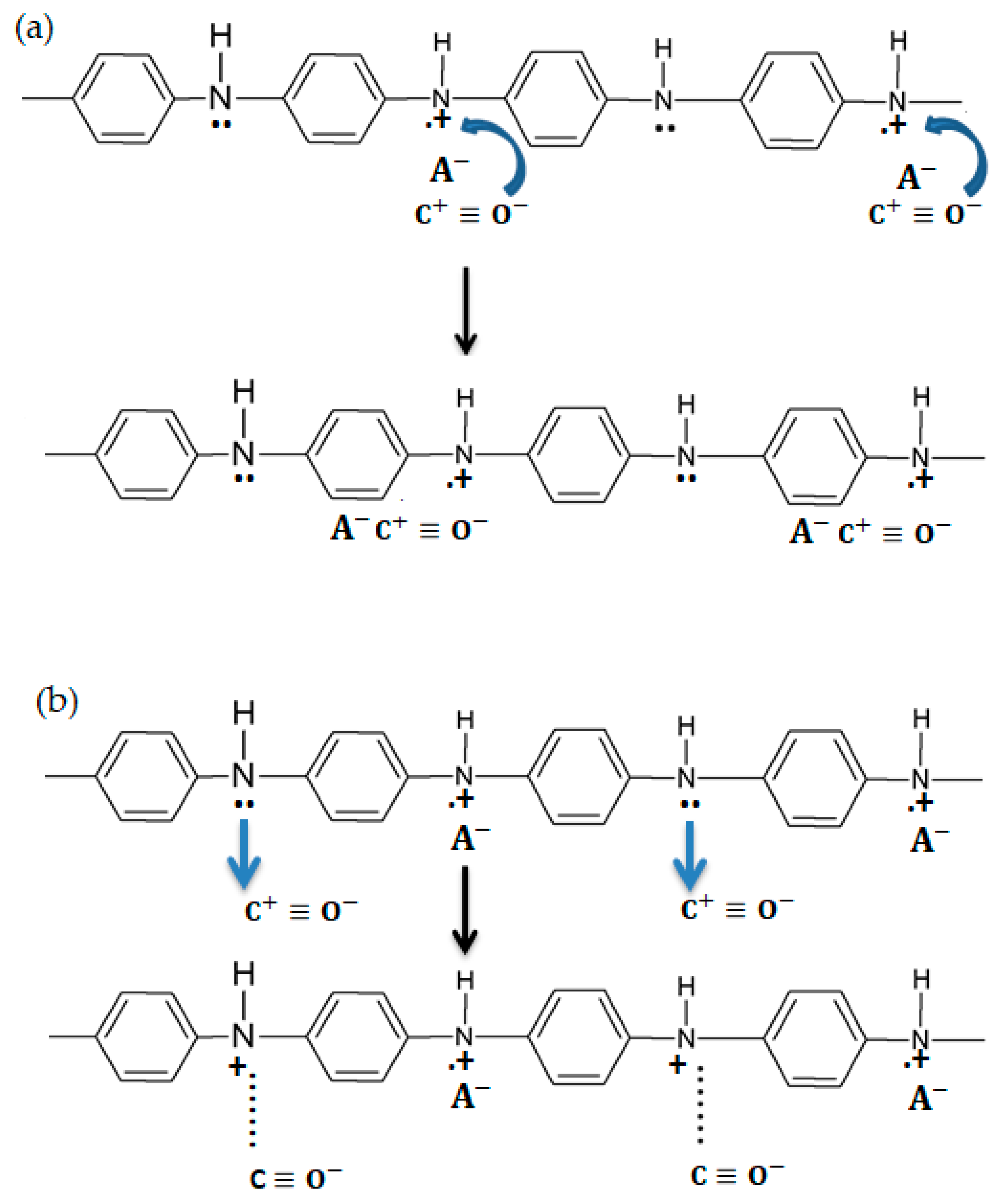
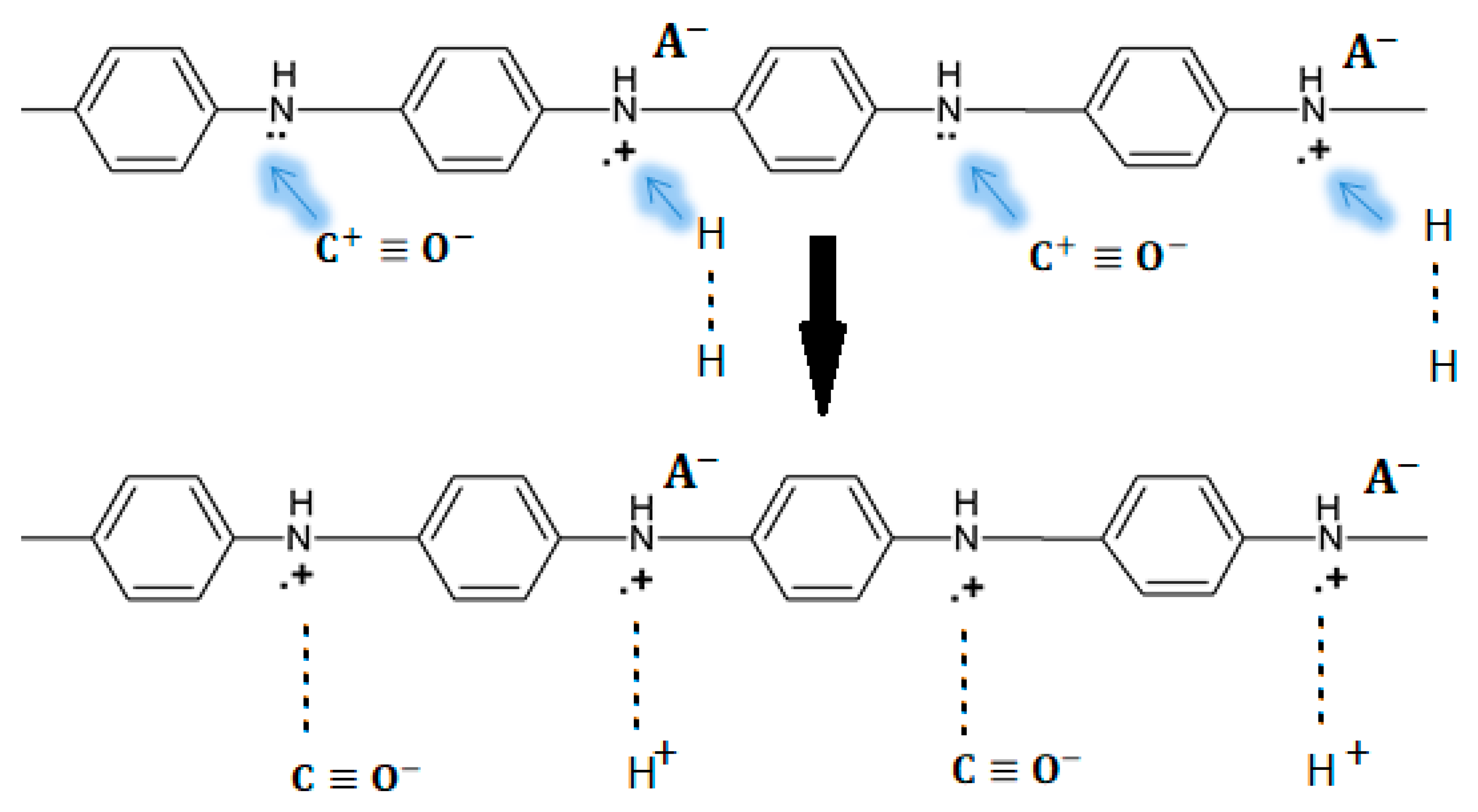
3.2. PANI Sensing Mechanism on CO Exposure
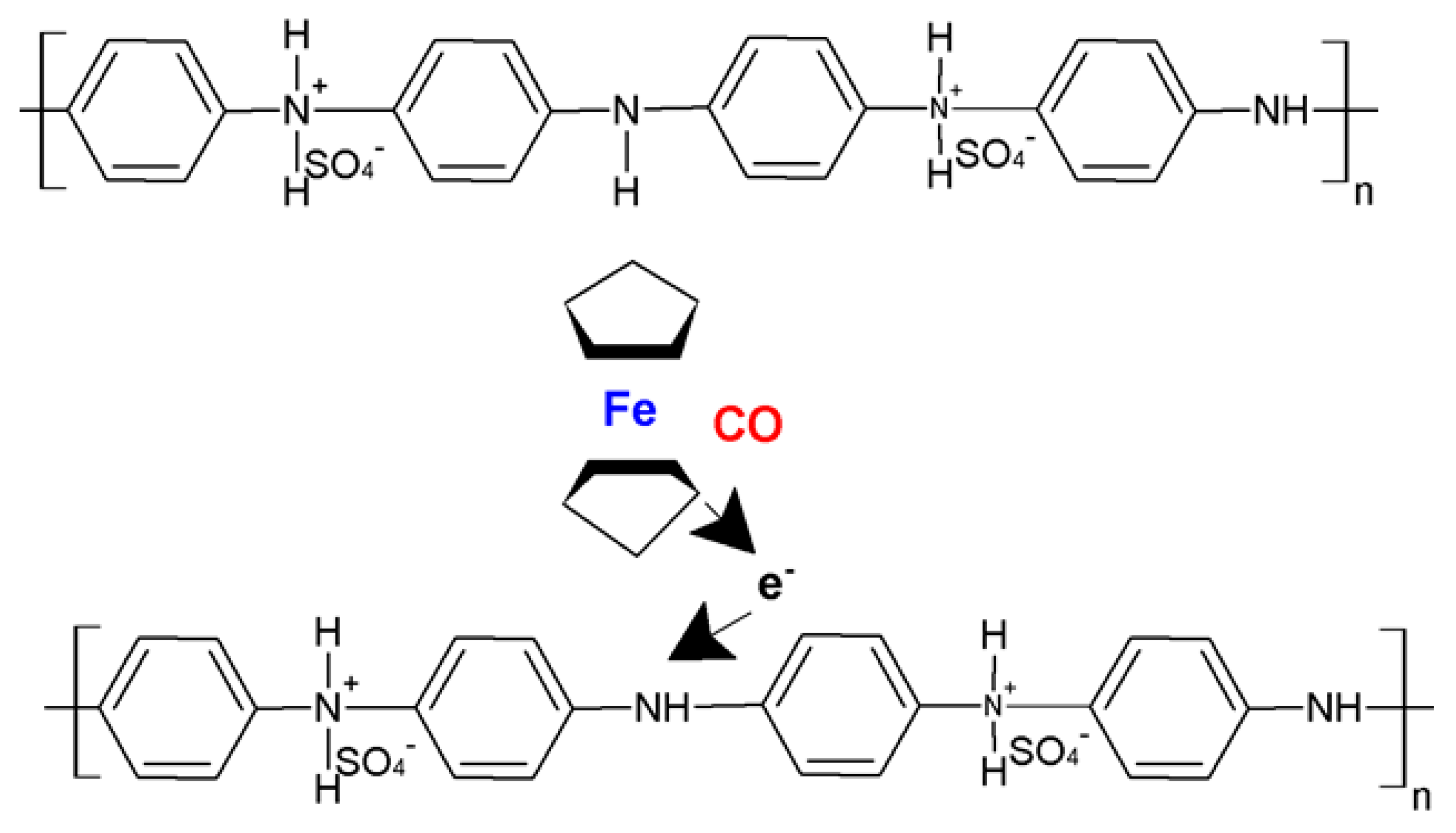
3.3. PANI–NCM Structure Sensing Mechanism on CO Exposure
3.4. PPy Structure and Conductivity
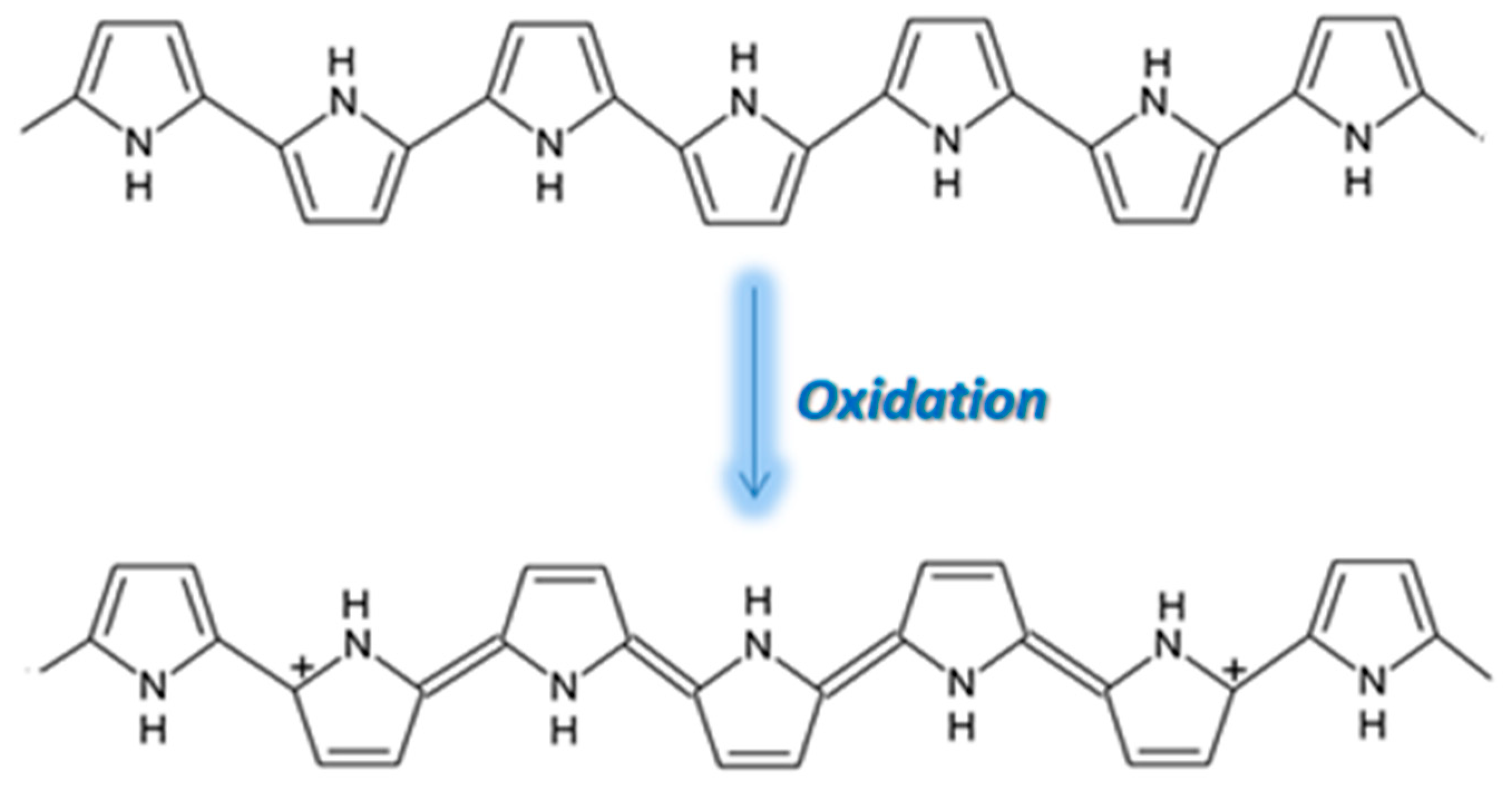
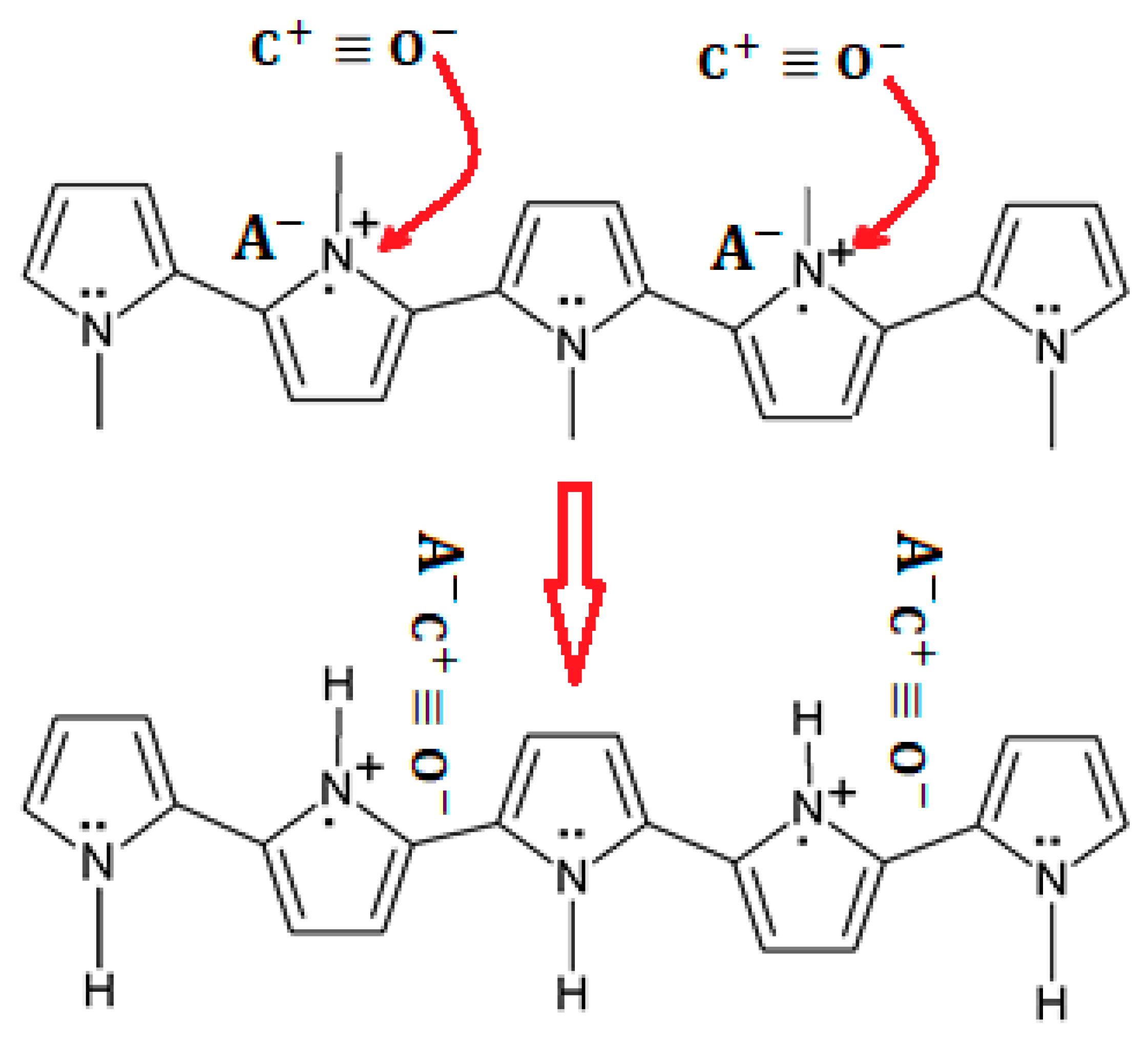
3.4.1. PPy Sensing Mechanism on CO Exposure
3.4.2. PPy-NCM Structure Sensing Mechanism on CO Exposure
3.5. PEDOT-Sensing Mechanism on CO Exposure
PEDOT-NCMs Structure Sensing Mechanism on CO Exposure
4. Formulation Strategies
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
References
- WHO. Air Quality Guidelines for Europe, 2nd ed.; WHO Regional Publications, European Series, no.91; World Health Organization Regional Office for Europe: Copenhagen, Denmark, 2000; p. 32. [Google Scholar]
- Hulanicki, A.; Glab, S.; Ingman, F.O.L.K.E. Chemical sensors: Definitions and classification. Pure Appl. Chem. 1991, 63, 1247–1250. [Google Scholar] [CrossRef]
- Masikini, M.; Chowdhury, M.; Nemraoui, O. Metal oxides: Application in exhaled breath acetone chemiresistive sensors. J. Electrochem. Soc. 2020, 167, 037537. [Google Scholar] [CrossRef] [Green Version]
- Neri, G. First fifty years of chemoresistive gas sensors. Chemosensors 2015, 3, 1–20. [Google Scholar] [CrossRef]
- Umar, A.; Ibrahim, A.A.; Algadi, H.; Albargi, H.; Alsairi, M.A.; Wang, Y.; Akbar, S. Supramolecularly assembled isonicotinamide/reduced graphene oxide nanocomposite for room-temperature NO2 gas sensor. Environ. Technol. Innov. 2022, 25, 102066. [Google Scholar] [CrossRef]
- Polyakov, M.; Ivanova, V.; Klyamer, D.; Köksoy, B.; Şenocak, A.; Demirbaş, E.; Basova, T. A Hybrid Nanomaterial Based on Single Walled Carbon Nanotubes Cross-Linked via Axially Substituted Silicon (IV) Phthalocyanine for Chemiresistive Sensors. Molecules 2020, 25, 2073. [Google Scholar] [CrossRef]
- Pandey, R.R.; Chusuei, C.C. Carbon Nanotubes, Graphene, and Carbon Dots as Electrochemical Biosensing Composites. Molecules 2021, 26, 6674. [Google Scholar] [CrossRef]
- Kong, J.; Franklin, N.R.; Zhou, C.; Chapline, M.G.; Peng, S.; Cho, K.; Dai, H. Nanotube molecular wires as chemical sensors. Science 2000, 287, 622–625. [Google Scholar] [CrossRef]
- Elhaes, H.; Fakhry, A.; Ibrahim, M. Carbon nano materials as gas sensors. Mater. Today Proc. 2016, 3, 2483–2492. [Google Scholar] [CrossRef]
- Fort, A.; Mugnaini, M.; Panzardi, E.; Lo Grasso, A.; Al Hamry, A.; Adiraju, A.; Kanoun, O. Modeling the Conductivity Response to NO2 Gas of Films Based on MWCNT Networks. Sensors 2021, 21, 4723. [Google Scholar] [CrossRef]
- Zhang, W.; Cao, S.; Wu, Z.; Zhang, M.; Cao, Y.; Guo, J.; Jia, D. High-performance gas sensor of polyaniline/carbon nanotube composites promoted by interface engineering. Sensors 2020, 20, 149. [Google Scholar] [CrossRef] [Green Version]
- Ma, X.; Gao, M.; He, X.; Li, G. Morphology tailoring of nano/micro-structured conductive polymers, composites and their applications in chemical sensors. Recent Pat. Nanotechnol. 2010, 4, 150–163. [Google Scholar] [CrossRef] [PubMed]
- Tonelli, D.; Scavetta, E.; Gualandi, I. Electrochemical deposition of nanomaterials for electrochemical sensing. Sensors 2019, 19, 1186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trakhtenberg, L.I.; Gerasimov, G.N.; Gromov, V.F.; Belysheva, T.V.; Ilegbusi, O.J. Gas semiconducting sensors based on metal oxide nanocomposites. J. Mater. Sci. Res. 2012, 1, 56. [Google Scholar] [CrossRef]
- Fine, G.F.; Cavanagh, L.M.; Afonja, A.; Binions, R. Metal oxide semi-conductor gas sensors in environmental monitoring. Sensors 2010, 10, 5469–5502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hojati, T.; Ebrahimi, M.; Afzalzadeh, R. Highly sensitive CO sensor based on ZnO/MWCNT nano sheet network grown via hydrothermal method. Mater. Chem. Phys. 2018, 207, 50–57. [Google Scholar] [CrossRef]
- Zhang, L.; Du, W.; Nautiyal, A.; Liu, Z.; Zhang, X. Recent progress on nanostructured conducting polymers and composites: Synthesis, application and future aspects. Sci. China Mater. 2018, 61, 303–352. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Ratinac, K.R.; Ringer, S.P.; Thordarson, P.; Gooding, J.J.; Braet, F. Carbon nanomaterials in biosensors: Should you use nanotubes or graphene? Angew. Chem. Int. Ed. 2010, 49, 2114–2138. [Google Scholar] [CrossRef]
- Basu, A.K.; Chauhan, P.S.; Awasthi, M.; Bhattacharya, S. α-Fe2O3 loaded rGO nanosheets based fast response/recovery CO gas sensor at room temperature. Appl. Surf. Sci. 2019, 465, 56–66. [Google Scholar] [CrossRef]
- Lakard, B.; Carquigny, S.; Segut, O.; Patois, T.; Lakard, S. Gas sensors based on electrodeposited polymers. Metals 2015, 5, 1371–1386. [Google Scholar] [CrossRef] [Green Version]
- Yoon, H. Current trends in sensors based on conducting polymer nanomaterials. Nanomaterials 2013, 3, 524–549. [Google Scholar] [CrossRef] [Green Version]
- Kumar, V.; Mirzaei, A.; Bonyani, M.; Kim, K.H.; Kim, H.W.; Kim, S.S. Advances in electrospun nanofiber fabrication for polyaniline (PANI)-based chemoresistive sensors for gaseous ammonia. TrAC-Trend Anal. Chem. 2020, 129, 115938. [Google Scholar] [CrossRef]
- Zhang, D.; Wu, Z.; Zong, X. Flexible and highly sensitive H2S gas sensor based on in-situ polymerized SnO2/rGO/PANI ternary nanocomposite with application in halitosis diagnosis. Sens. Actuator B Chem. 2019, 289, 32–41. [Google Scholar] [CrossRef]
- Sharma, H.J.; Salorkar, M.A.; Kondawar, S.B. H2 and CO gas sensor from SnO2/polyaniline composite nanofibers fabricated by electrospinning. Adv. Mater. Proc. 2017, 2, 61–66. [Google Scholar] [CrossRef] [Green Version]
- Misra, S.C.K.; Mathur, P.; Srivastava, B.K. Vacuum-deposited nanocrystalline polyaniline thin film sensors for detection of carbon monoxide. Sens. Actuator A Phys. 2004, 114, 30–35. [Google Scholar] [CrossRef]
- Dixit, V.; Misra, S.C.K.; Sharma, B.S. Carbon monoxide sensitivity of vacuum deposited polyaniline semiconducting thin films. Sens. Actuator B Chem. 2005, 104, 90–93. [Google Scholar] [CrossRef]
- Wanna, Y.; Srisukhumbowornchai, N.; Tuantranont, A.; Wisitsoraat, A.; Thavarungkul, N.; Singjai, P. The effect of carbon nanotube dispersion on CO gas sensing characteristics of polyaniline gas sensor. J. Nanosci. Nanotechnol. 2006, 6, 3893–3896. [Google Scholar] [CrossRef]
- Liu, C.; Noda, Z.; Sasaki, K.; Hayashi, K. Development of a polyaniline nanofiber-based carbon monoxide sensor for hydrogen fuel cell application. Int. J. Hydrog. Energy 2012, 37, 13529–13535. [Google Scholar] [CrossRef]
- Watcharaphalakorn, S.; Ruangchuay, L.; Chotpattananont, D.; Sirivat, A.; Schwank, J. Polyaniline/polyimide blends as gas sensors and electrical conductivity response to CO–N2 mixtures. Polym. Int. 2005, 54, 1126–1133. [Google Scholar] [CrossRef]
- Hejczyk, T.; Pustelny, T. Analysis of the Saw System with the PANI+ Nafion Sensing Structure for Detection of Low Concentration Carbon Monoxide. Arch. Acoust. 2020, 45, 681–686. [Google Scholar]
- Han, M.C.; Im, S.S. Electrical and structural analysis of conductive polyaniline/polyimide blends. J. Appl. Polym. Sci. 1999, 71, 2169–2178. [Google Scholar] [CrossRef]
- Wanna, Y.; Pratontep, S.; Wisitsoraat, A.; Tuantranont, A. Development of nanofibers composite Polyaniine/CNT fabricated by Electro spinning Technique for CO Gas Sensor. In Proceedings of the SENSORS, 2006 IEEE, Daegu, Korea, 22–25 October 2006; pp. 342–345. [Google Scholar]
- Roy, A.; Ray, A.; Sadhukhan, P.; Naskar, K.; Lal, G.; Bhar, R.; Sinha, C.; Das, S. Polyaniline-multiwalled carbon nanotube (PANI-MWCNT): Room temperature resistive carbon monoxide (CO) sensor. Synth. Met. 2018, 245, 182–189. [Google Scholar] [CrossRef]
- Kim, I.; Dong, K.Y.; Ju, B.K.; Choi, H.H. Gas sensor for CO and NH3 using polyaniline/CNTs composite at room temperature. In Proceedings of the 10th IEEE International Conference on Nanotechnology, Ilsan, Korea, 17–20 August 2010; pp. 466–469. [Google Scholar]
- Savin, M.; Mihailescu, C.M.; Avramescu, V.; Dinulescu, S.; Firtat, B.; Craciun, G.; Moldovan, C. A New Hybrid Sensitive PANI/SWCNT/Ferrocene-Based Layer for a Wearable CO Sensor. Sensors 2021, 21, 1801. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Lu, Y.; Ma, Y.; Li, Y.; Du, F.; Chen, Y. Noncovalent nanohybrid of ferrocene with single-walled carbon nanotubes and its enhanced electrochemical property. Chem. Phys. Lett. 2006, 420, 416–420. [Google Scholar] [CrossRef]
- Guo, B.; Ma, Z.; Pan, L.; Shi, Y. Properties of conductive polymer hydrogels and their application in sensors. J. Polym. Sci. Pol. Phys. 2019, 57, 1606–1621. [Google Scholar] [CrossRef] [Green Version]
- Bhat, N.V.; Gadre, A.P.; Bambole, V.A. Investigation of electropolymerized polypyrrole composite film: Characterization and application to gas sensors. J. Appl. Polym. Sci. 2003, 88, 22–29. [Google Scholar] [CrossRef]
- Gustafsson, G.; Lundström, I.; Liedberg, B.; Wu, C.R.; Inganäs, O.; Wennerström, O. The interaction between ammonia and poly (pyrrole). Synth. Met. 1989, 31, 163–179. [Google Scholar] [CrossRef]
- Guernion, N.; Ewen, R.J.; Pihlainen, K.; Ratcliffe, N.M.; Teare, G.C. The fabrication and characterisation of a highly sensitive polypyrrole sensor and its electrical responses to amines of differing basicity at high humidities. Synth. Met. 2002, 126, 301–310. [Google Scholar] [CrossRef]
- Jang, C.; Park, J.K.; Yun, G.H.; Choi, H.H.; Lee, H.J.; Yook, J.G. Radio-frequency/microwave gas sensors using conducting polymer. Materials 2020, 13, 2859. [Google Scholar] [CrossRef]
- Farea, M.A.; Mohammed, H.Y.; Ingle, N.N.; Al-Gahouari, T.; Mahadik, M.M.; Bodkhe, G.A.; Shirsat, M.D. Carbon monoxide sensor based on polypyrrole–graphene oxide composite: A cost-effective approach. Appl. Phys. A 2021, 127, 681. [Google Scholar] [CrossRef]
- Liu, D.M.; Aguilar-Hernandez, J.; Potje-Kamloth, K.; Liess, H.D. A new carbon monoxide sensor using a polypyrrole film grown on an interdigital-capacitor substrate. Sens. Actuator B Chem. 1997, 41, 203–206. [Google Scholar] [CrossRef]
- Lee, J.J.; Yoo, D.; Park, C.; Kim, J.H.; Choi, H.H. Behavior of toxic gas sensor based on conducting polypyrrole. Macromol. Symp. 2015, 354, 280–286. [Google Scholar] [CrossRef]
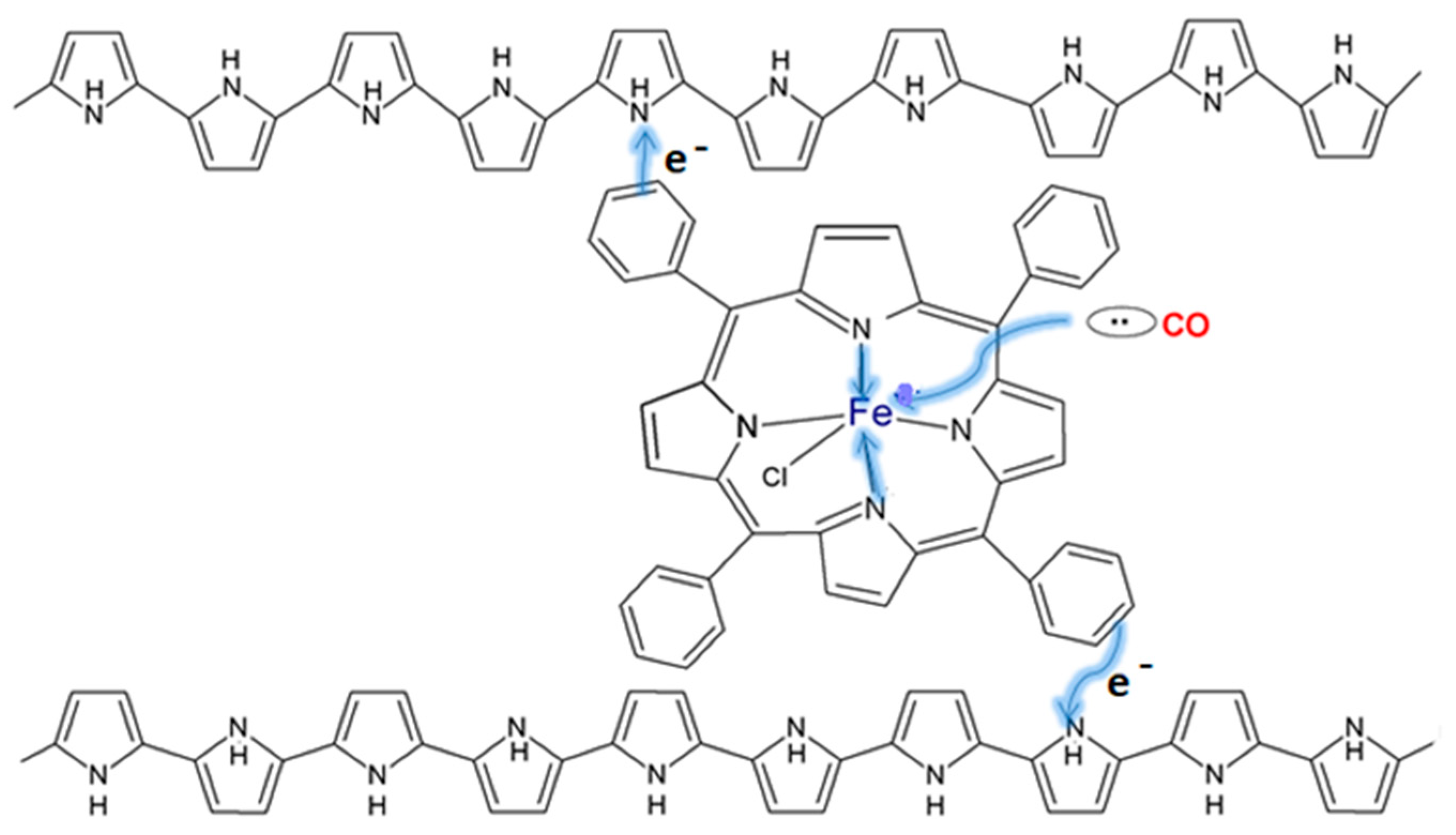
- Paul, S.; Amalraj, F.; Radhakrishnan, S. CO sensor based on polypyrrole functionalized with iron porphyrin. Synth. Met. 2009, 159, 1019–1023. [Google Scholar] [CrossRef]
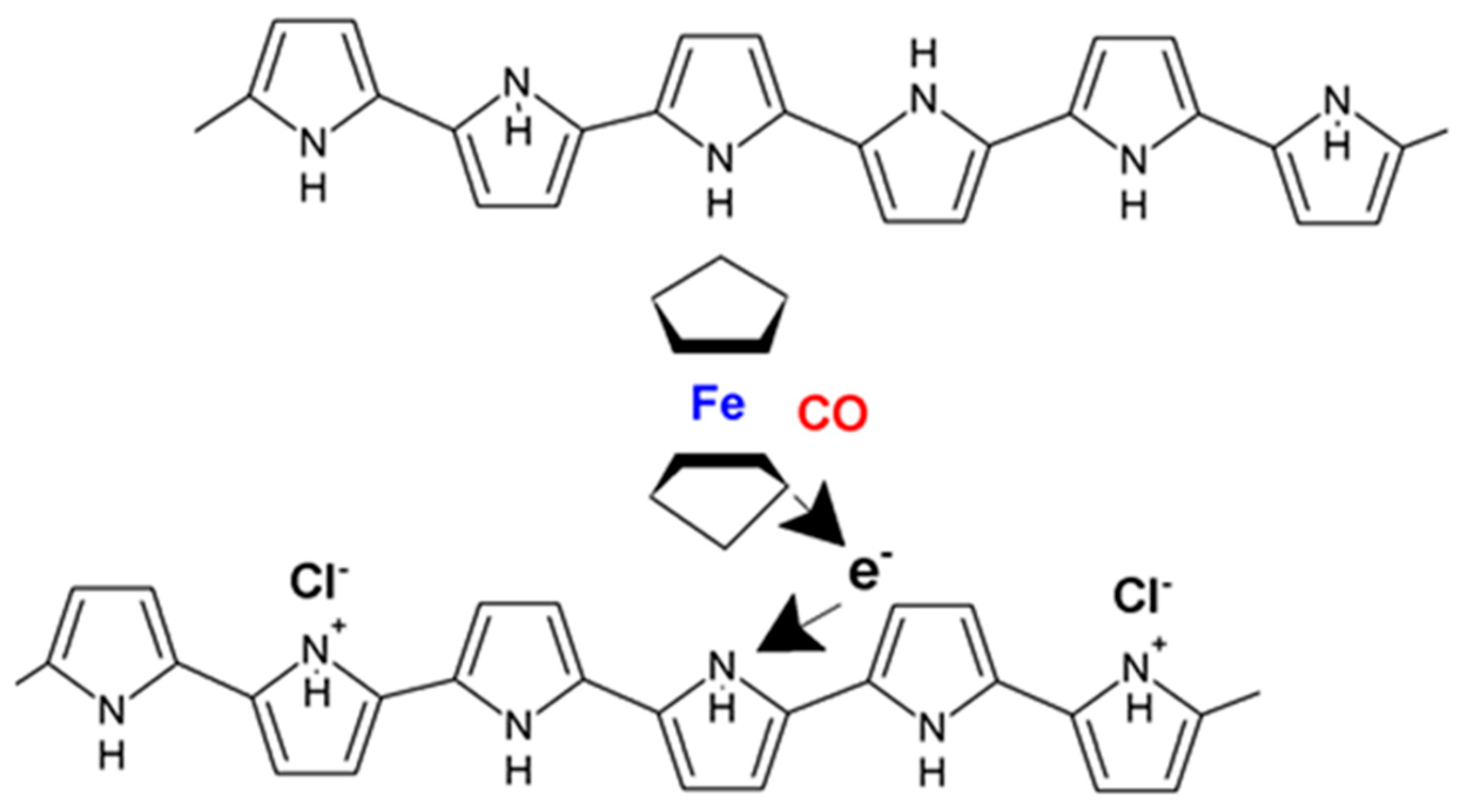
- Radhakrishnan, S.; Paul, S. Conducting polypyrrole modified with ferrocene for applications in carbon monoxide sensors. Sens. Actuator B Chem. 2007, 125, 60–65. [Google Scholar] [CrossRef]
- Paul, S.; Chavan, N.N.; Radhakrishnan, S. Polypyrrole functionalized with ferrocenyl derivative as a rapid carbon monoxide sensor. Synth. Met. 2009, 159, 415–418. [Google Scholar] [CrossRef]
- Naikoo, R.A.; Tomar, R. Fabrication of a novel Zeolite-X/Reduced graphene oxide/Polypyrrole nanocomposite and its role in sensitive detection of CO. Mater. Chem. Phys. 2018, 211, 225–233. [Google Scholar] [CrossRef]
- Javadpour, S.; Gharavi, A.; Feizpour, A.; Khanehzar, A.; Panahi, F. Morpholine doped poly (3,4-ethylenedioxy) thiophene–poly (styrenesulfonate) as a low temperature and quick carbon monoxide sensor. Sens. Actuator B Chem. 2009, 142, 152–158. [Google Scholar] [CrossRef]
- Zampetti, E.; Pantalei, S.; Muzyczuk, A.; Bearzotti, A.; De Cesare, F.; Spinella, C.; Macagnano, A. A high sensitive NO2 gas sensor based on PEDOT–PSS/TiO2 nanofibres. Sens. Actuator B Chem. 2013, 176, 390–398. [Google Scholar] [CrossRef]
- Lin, Y.; Huang, L.; Chen, L.; Zhang, J.; Shen, L.; Chen, Q.; Shi, W. Fully gravure-printed NO2 gas sensor on a polyimide foil using WO3-PEDOT: PSS nanocomposites and Ag electrodes. Sens. Actuator B Chem. 2015, 216, 176–183. [Google Scholar] [CrossRef]
- Pasha, A.; Khasim, S.; Khan, F.A.; Dhananjaya, N. Fabrication of gas sensor device using poly (3, 4-ethylenedioxythiophene)-poly (styrenesulfonate)-doped reduced graphene oxide organic thin films for detection of ammonia gas at room temperature. Iran. Polym. J. 2019, 28, 183–192. [Google Scholar] [CrossRef]
- Zhang, H.D.; Yan, X.; Zhang, Z.H.; Yu, G.F.; Han, W.P.; Zhang, J.C.; Long, Y.Z. Electrospun PEDOT: PSS/PVP nanofibers for CO gas sensing with quartz crystal microbalance technique. Int. J. Polym. Sci. 2016, 2016, 3021353. [Google Scholar] [CrossRef]
- Memarzadeh, R.; Javadpour, S.; Panahi, F.; Shim, Y.B. Low Temperature Carbon Monoxide Sensor Based on Co (salen) Doped PEDOT: PSS. In Proceedings of the 14th International Meeting on Chemical Sensors (IMCS), Nuremberg, Germany, 20–23 May 2012; pp. 1105–1108. [Google Scholar]
- Arabloo, F.; Javadpour, S.; Memarzadeh, R.; Panahi, F.; Emami, M.D.; Shariat, M.H. The interaction of carbon monoxide to Fe (III)(salen)-PEDOT: PSS composite as a gas sensor. Synth. Met. 2015, 209, 192–199. [Google Scholar] [CrossRef]
- Arabloo, F.; Memarzadeh, R.; Panahi, F.; Davazdahemami, M.; Javadpour, S.; Shariat, M.H. Low Temperature CO Sensor Based on PEDOT: PSS/Fe (II)(salen) Composite Thin Film. Adv. Proc. Mater. Eng. 2018, 12, 1–10. [Google Scholar]
- Anjabin, N.; Arabloo, F.; Javadpour, S. Modeling the CO gas response of PEDOT: PSS/Fe (salen) thin film for a gas sensor. Sci. Iran. Trans. C. 2020, 27, 1227–1233. [Google Scholar]
- Kim, H.; Jang, Y.; Lee, G.W.; Yang, S.Y.; Jung, J.; Oh, J. Tunable chemical grafting of three-dimensional poly (3, 4-ethylenedioxythiophene)/poly (4-styrenesulfonate)-multiwalled carbon nanotubes composite with faster charge-carrier transport for enhanced gas sensing performance. Sensors 2020, 20, 2470. [Google Scholar] [CrossRef]
- Lee, K.; Cho, K.H.; Ryu, J.; Yun, J.; Yu, H.; Lee, J.; Jang, J. Low-cost and efficient perovskite solar cells using a surfactant-modified polyaniline: Poly (styrenesulfonate) hole transport material. Electrochim. Acta 2017, 224, 600–607. [Google Scholar] [CrossRef]
- Huang, H.; Yao, J.; Chen, H.; Zeng, X.; Chen, C.; She, X.; Li, L. Facile preparation of halloysite/polyaniline nanocomposites via in situ polymerization and layer-by-layer assembly with good supercapacitor performance. J. Mater. Sci. 2016, 51, 4047–4054. [Google Scholar] [CrossRef]
- Liu, Q.; Wu, J.; Lan, Z.; Zheng, M.; Yue, G.; Lin, J.; Huang, M. Preparation of PAA-g-PEG/PANI polymer gel electrolyte and its application in quasi solid state dye-sensitized solar cells. Polym. Eng. Sci. 2015, 55, 322–326. [Google Scholar] [CrossRef]
- Lee, K.; Yu, H.; Lee, J.W.; Oh, J.; Bae, S.; Kim, S.K.; Jang, J. Efficient and moisture-resistant hole transport layer for inverted perovskite solar cells using solution-processed polyaniline. J. Mater. Chem. C 2018, 6, 6250–6256. [Google Scholar] [CrossRef]
- Han, H.; Lee, J.S.; Cho, S. Comparative studies on two-electrode symmetric supercapacitors based on polypyrrole: Poly (4-styrenesulfonate) with different molecular weights of poly (4-styrenesulfonate). Polymers 2019, 11, 232. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; Yang, Y.; Zhang, Z.; Yan, J.; Lin, Z.; Guo, X. Bifacial quasi-solid-state dye-sensitized solar cells with poly (vinyl pyrrolidone)/polyaniline transparent counter electrode. Nano Energy 2016, 26, 123–130. [Google Scholar] [CrossRef]
- Phongphut, A.; Sriprachuabwong, C.; Wisitsoraat, A.; Tuantranont, A.; Prichanont, S.; Sritongkham, P. A disposable amperometric biosensor based on inkjet-printed Au/PEDOT-PSS nanocomposite for triglyceride determination. Sens. Actuator B Chem. 2013, 178, 501–507. [Google Scholar] [CrossRef]
- Biswas, S.; Jeong, J.; Shim, J.W.; Kim, H. Improved charge transport in PANI: PSS by the uniform dispersion of silver nanoparticles. Appl. Surf. Sci. 2019, 483, 819–826. [Google Scholar] [CrossRef]
- Wenseleers, W.; Vlasov, I.I.; Goovaerts, E.; Obraztsova, E.D.; Lobach, A.S.; Bouwen, A. Efficient isolation and solubilization of pristine single-walled nanotubes in bile salt micelles. Adv. Funct. Mater. 2004, 14, 1105–1112. [Google Scholar] [CrossRef]
- Moore, V.C.; Strano, M.S.; Haroz, E.H.; Hauge, R.H.; Smalley, R.E.; Schmidt, J.; Talmon, Y. Individually suspended single-walled carbon nanotubes in various surfactants. Nano Lett. 2003, 3, 1379–1382. [Google Scholar] [CrossRef]
- Vaisman, L.; Marom, G.; Wagner, H.D. Dispersions of surface-modified carbon nanotubes in water-soluble and water-insoluble polymers. Adv. Funct. Mater. 2006, 16, 357–363. [Google Scholar] [CrossRef]
- Smith, R.J.; Lotya, M.; Coleman, J.N. The importance of repulsive potential barriers for the dispersion of graphene using surfactants. New J. Phys. 2010, 12, 125008. [Google Scholar] [CrossRef]
- Tkalya, E.E.; Ghislandi, M.; de With, G.; Koning, C.E. The use of surfactants for dispersing carbon nanotubes and graphene to make conductive nanocomposites. Curr. Opin. Colloid 2012, 17, 225–232. [Google Scholar] [CrossRef]


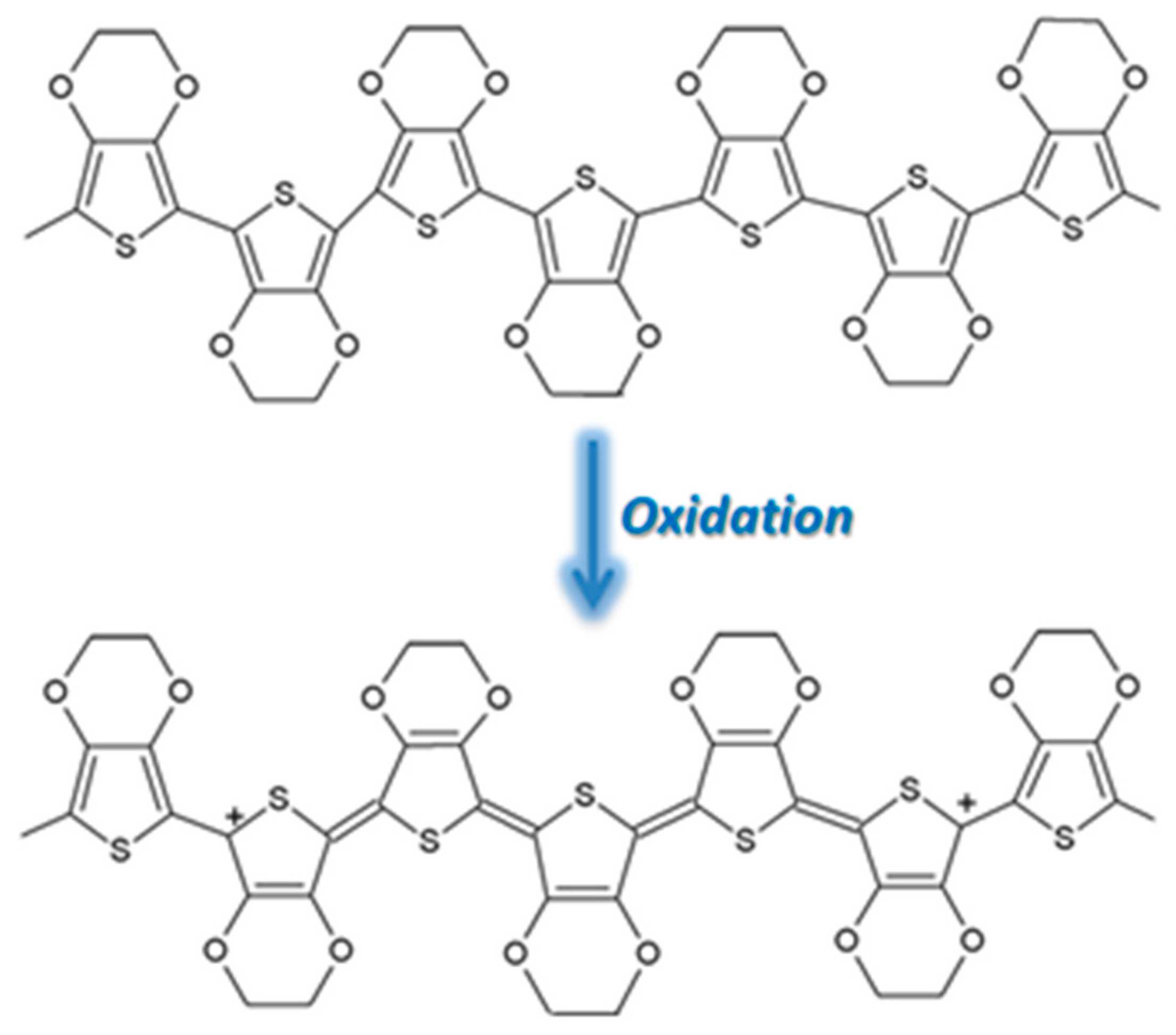
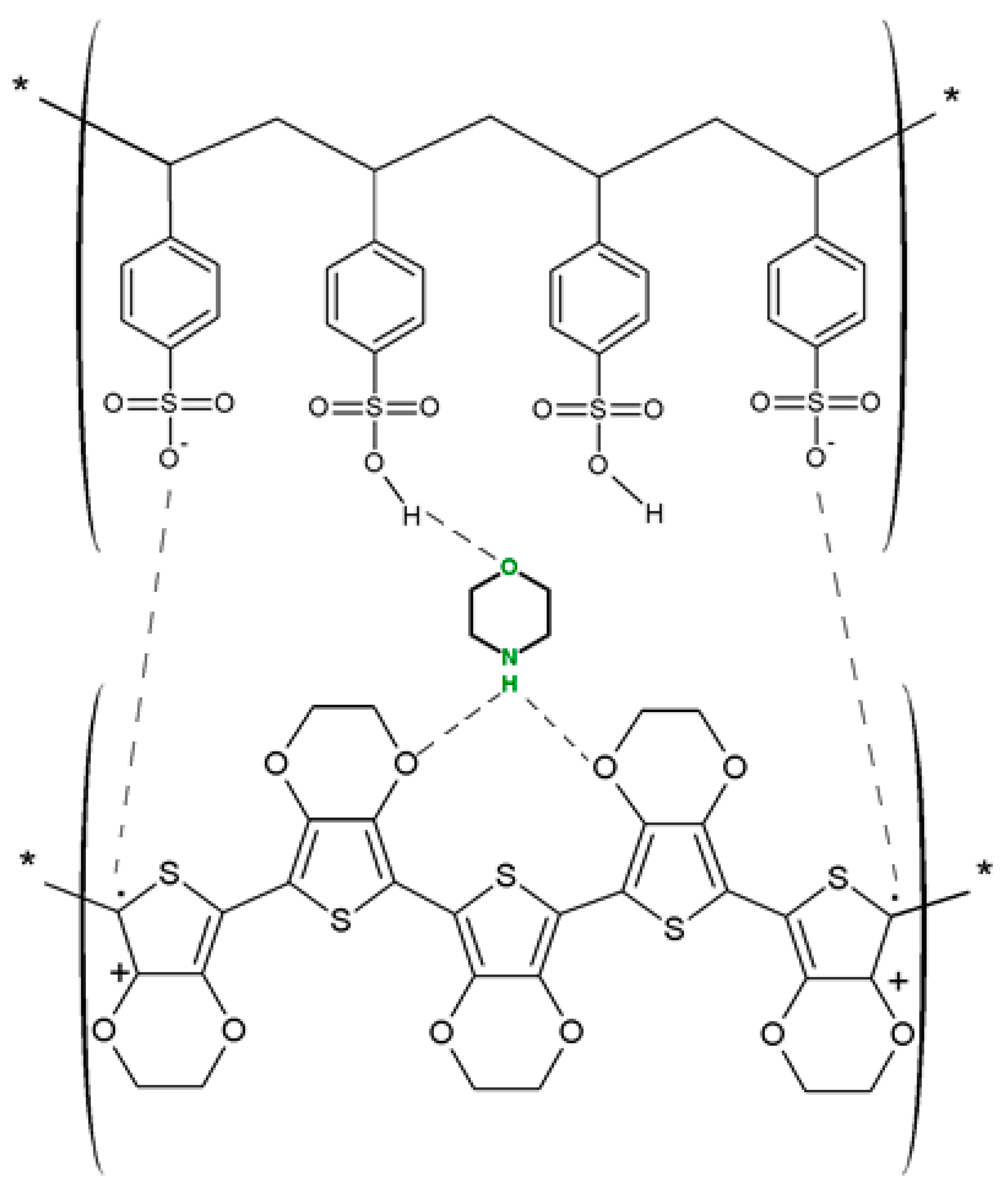

| Name of CP and NCMs | Abbreviation | Chemical Structures | |
|---|---|---|---|
| Polyaniline | PANI | 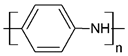 (a) | |
| PANI:PSS/SWCNTs |  |  | |
| (a) | (b) | ||
 (c) | |||
| PANI:PSS/MWCNTs |  |  | |
| (a) | (b) | ||
 (d) | |||
| PANI:PSS/SWCNT/Fc |  |  | |
| (a) | (b) | ||
 |  | ||
| (c) | (e) | ||
| PANI/rGO |  |  | |
| (a) | (f) | ||
| Poly(3,4-ethylenedioxy)thiophene | PEDOT | 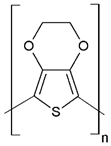 (g) | |
| PEDOT:PEG/SWCNTs |  | 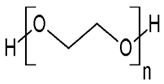 | |
| (g) | (h) | ||
 | |||
| (c) | |||
| PEDOT:PSS/MWCNTs |  |  | |
| (g) | (b) | ||
 | |||
| (d) | |||
| PEDOT/rGO |  |  | |
| (g) | (f) | ||
| Polydiphenylamine | PDPA/MWCNTs |  (i)  (d) | |
| Poly(dyallyldimethyl-ammonium chloride) | PDDA/MWCNTs |  |  |
| (j) | (d) | ||
| Polypyrrole | PPy |  (k) | |
| PPy/GO or rGO |  | 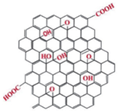 | |
| (k) | (l) | ||
 | |||
| (f) | |||
| Sensing Materials | Doping Agents | Concentration Range (ppm) | Response Time | Sensitivity | Operating Temp (°C) | Response Formula | References |
|---|---|---|---|---|---|---|---|
| Nanocrystalline PANI | HCl | 0.02–30 ppm | 8–10 s | 400–600 | RT | S * = Ie/Io, | [25] |
| PANI | HCl/Fe and Al | 0–150 ppm | 5 s | 800 | RT | S = (Ie − Io)/Io | [26] |
| PANI | Maleic acid | 100–500 ppm | 1.1 min | 0.01—0.03 | RT | [27] | |
| PANI | CSA ** | 1–100 ppm | - | −24% | - | [28] | |
| PANI horizontal nanofiber | HCl | 1–100 ppm | - | −18% for 1ppm | - | [28] | |
| PANI/PI | CSA HNO3 | 1–1000 ppm 1–1000 ppm | 36.8 min for 1000 ppm | 0.338cm−1; 1.040 S·cm−1 | 25 °C–55 °C at 1 atm | *** | [29] |
| PANI/ Nafion | NA | 0–20 ppm | 5 ppm in synthetic air | 34 °C; 42 °C | ΔF [Hz] | [30] |
| Sensing Materials | Doping Agents | Concentration (ppm) | Response Time | Sensitivity | Operating Temp (°C) | Response Formula | References |
|---|---|---|---|---|---|---|---|
| PANI/PVA/fiber/CNTs | MA | 100 ppm–500 ppm | NA | 1.5–3.5 | RT | [33] | |
| PANI/dispersed CNTs | MA | 100–1000 ppm | 0.6 min | 0.04–0.12 | RT | [27] | |
| PANI/SWCNTs | HCl | 5 ppm; 80 ppm | NA | NA | RT | [34] | |
| PANI/MWCNT | HCl | 500–1000 ppm | 76 s | 6.8–26.7% | RT | [31] | |
| PANI:PSS/SWCNT/Fc | H2SO4 | 0–300 ppm | 33 s for >100 ppm 30 s for <100ppm | 6–55% | RT | [35] |
| Sensing Materials | Concentration Range (ppm) | Response Time | Sensitivity | Operating Temp (°C) | Response Formula | References |
|---|---|---|---|---|---|---|
| PPy | 100–500 ppm | 8–10 s | 6.5% for 500 ppm | 300 °C | [43] | |
| PPy:PSS | 9 ppm | - | ≈1% for 9 ppm | RT | [44] | |
| PPy-FeTPPCl | 100–300 ppm | 500 s | 12% for 100 ppm | RT | [45] | |
| PPy-Fc | 300 ppm | t50 = 96s | 25.8% for 300 ppm | RT | [46] | |
| PPy-Fc derivates | 300 ppm | t50 = 0.43 s | 12% for 300 ppm | RT | [47] |
| Sensing Materials | Concentration Range (ppm) | Response Time | Sensitivity | Operating Temp (°C) | Response Formula | References |
|---|---|---|---|---|---|---|
| Zeolite-X/rGO/PPy | 5–1000 | 303 s–600 s | 14.9–77.4% | RT | [48] | |
| PPy/rGO | 50–300 | 89s | 45% | RT | [42] |
| Sensing Materials | Concentration Range (ppm) | Response Time | Sensitivity | Operating Temp (°C) | Response Formula | References |
|---|---|---|---|---|---|---|
| PEDOT:PSS/ Morpholine | - | 5 s | - | Vacuum/mixing air and CO | Percent of resistance variation relative to the base (in vacuum) resistance of thin film | [49] |
| PEDOT:PSS/Co (salen) | - | - | - | RT | [54] | |
| PEDOT:PSS/Fe (salen) | 10–100 ppm | 38 s | 1.50 | RT | [55] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Savin, M.; Mihailescu, C.-M.; Moldovan, C.; Grigoroiu, A.; Ion, I.; Ion, A.C. Resistive Chemosensors for the Detection of CO Based on Conducting Polymers and Carbon Nanocomposites: A Review. Molecules 2022, 27, 821. https://doi.org/10.3390/molecules27030821
Savin M, Mihailescu C-M, Moldovan C, Grigoroiu A, Ion I, Ion AC. Resistive Chemosensors for the Detection of CO Based on Conducting Polymers and Carbon Nanocomposites: A Review. Molecules. 2022; 27(3):821. https://doi.org/10.3390/molecules27030821
Chicago/Turabian StyleSavin, Mihaela, Carmen-Marinela Mihailescu, Carmen Moldovan, Alexandru Grigoroiu, Ion Ion, and Alina Catrinel Ion. 2022. "Resistive Chemosensors for the Detection of CO Based on Conducting Polymers and Carbon Nanocomposites: A Review" Molecules 27, no. 3: 821. https://doi.org/10.3390/molecules27030821






