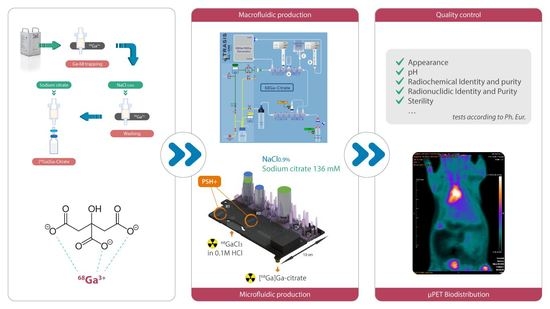
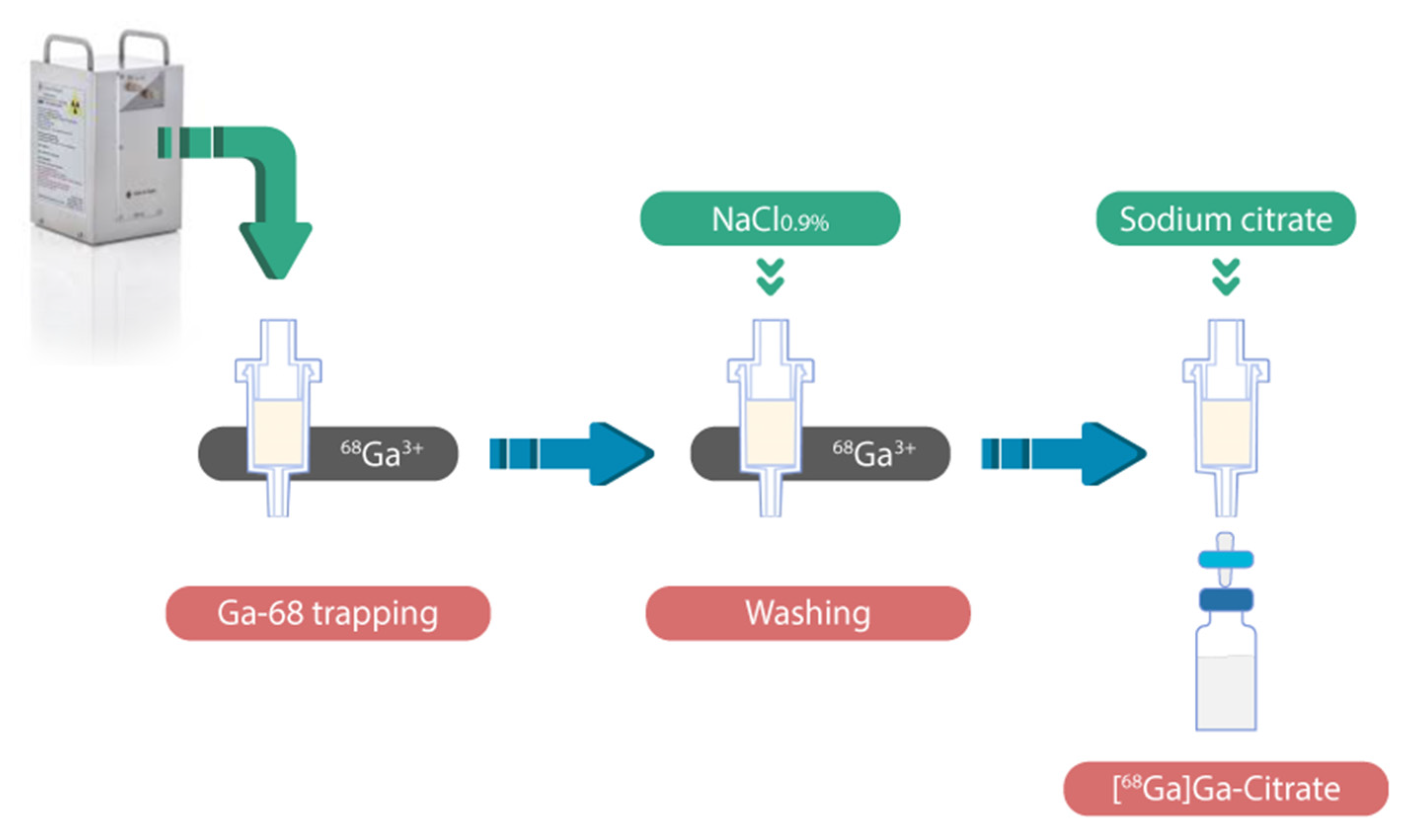
Fully Automated Macro- and Microfluidic Production of [68Ga]Ga-Citrate on mAIO® and iMiDEVTM Modules
Abstract
:1. Introduction
2. Results
2.1. Radiosynthesis of [68Ga]Ga-Citrate
2.1.1. Optimization of [68Ga]Ga-Citrate Radiosynthesis on mAIO®
- Determination of adapted cation exchange solid support
- Determination of optimal concentration of sodium citrate
- Radiosynthesis automation
2.1.2. Optimization of [68Ga]Ga-Citrate Radiosynthesis on iMiDEV™
- Automation of radiosynthesis
- Residual activity distribution
2.2. Quality Control of Metal Ions Impurities
2.3. Development of Quality Control Procedure
2.3.1. Radio-TLC
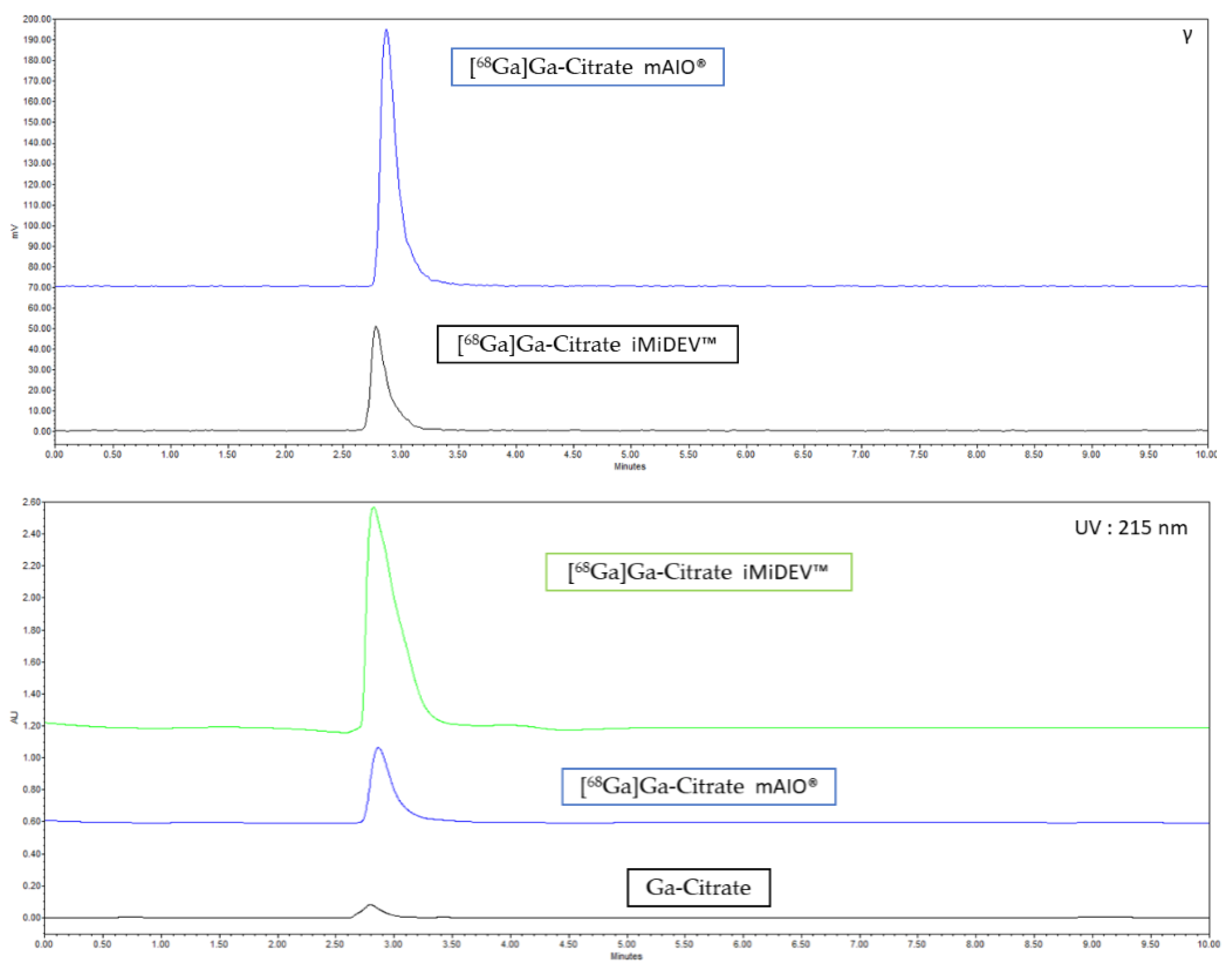
2.3.2. Radio-HPLC Identification Methods Development
2.4. Validation of [68Ga]Ga-Citrate Production
2.5. Evaluation of the Stability In Vitro
2.6. In Vitro Characterization
2.7. Physiological Biodistribution
3. Discussion
4. Materials and Methods
4.1. Radiosynthesis Process
4.2. Radiosynthesis of [68Ga]Ga-Citrate on mAIO® Module
4.3. Radiosynthesis of [68Ga]Ga-Citrate on iMiDEV™
4.4. Cerenkov Luminescence Imaging (CLI)
4.5. Quality Control Procedure
4.6. Quality Control of Metal Ions Impurities
4.7. In Vitro Stability
4.8. Serum Protein Binding (SPB) Evaluation
4.9. Blood Cell Binding
4.10. In Vivo Analysis of [68Ga]Ga-Citrate Biodistribution Using MicroPET
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Love, C.; Palestro, C.J. Nuclear medicine imaging of bone infections. Clin. Radiol. 2016, 71, 632–646. [Google Scholar] [CrossRef] [PubMed]
- Ayesa, S.L.; Schembri, G.P. Is 67gallium dead? A retrospective review of 67gallium imaging in a single tertiary referral centre. Nucl. Med. Commun. 2021, 42, 378–388. [Google Scholar] [CrossRef] [PubMed]
- Tseng, J.-R.; Chang, Y.-H.; Yang, L.-Y.; Wu, C.-T.; Chen, S.-Y.; Wan, C.-H.; Hsiao, I.-T.; Yen, T.-C. Potential usefulness of 68Ga-citrate PET/CT in detecting infected lower limb prostheses. EJNMMI Res. 2019, 9, 2. [Google Scholar] [CrossRef] [PubMed]
- Segard, T.; Morandeau, L.M.J.A.; Dunne, M.L.; Robinson, J.O.; Murray, R.J.; Geelhoed, E.A.; Francis, R.J. Comparison between gallium-68 citrate positron emission tomography-computed tomography and gallium-67 citrate scintigraphy for infection imaging. Intern. Med. J. 2019, 49, 1016–1022. [Google Scholar] [CrossRef]
- Aghanejad, A.; Jalilian, A.R.; Ardaneh, K.; Bolourinovin, F.; Yousefnia, H.; Samani, A.B. Preparation and Quality Control of 68Ga-Citrate for PET Applications. Asia Ocean. J. Nucl. Med. Biol. 2015, 3, 99–106. [Google Scholar]
- Xu, T.; Zeng, Y.; Yang, H.; Cai, L.; Ding, H.; Liu, N.; Zhang, L.; Lei, L.; Qi, C.; Zhang, Y.; et al. Application of [68Ga]Ga-citrate PET/CT for Differentiating Prosthetic Joint Infection from Aseptic Loosening after Joint Replacement Surgery. Res. Sq. 2020. [Google Scholar]
- Vorster, M.; Maes, A.; van de Wiele, C.; Sathekge, M. 68Ga-citrate PET/CT in tuberculosis: A pilot study. Q. J. Nucl. Med. Mol. Imaging 2019, 63, 48–55. [Google Scholar] [CrossRef]
- Ankrah, A.O.; Lawal, I.O.; Boshomane, T.M.G.; Klein, H.C.; Ebenhan, T.; Dierckx, R.A.J.O.; Vorster, M.; Glaudemans, A.W.J.M.; Sathekge, M.M. Comparison of Fluorine(18)-fluorodeoxyglucose and Gallium(68)-citrate PET/CT in patients with tuberculosis. Nuklearmedizin 2019, 58, 371–378. [Google Scholar] [CrossRef]
- Vorster, M.; Maes, A.; van de Wiele, C.; Sathekge, M. Gallium-68 PET: A Powerful Generator-based Alternative to Infection and Inflammation Imaging. Semin. Nucl. Med. 2016, 46, 436–447. [Google Scholar] [CrossRef]
- Lankinen, P.; Noponen, T.; Autio, A.; Luoto, P.; Frantzèn, J.; Löyttyniemi, E.; Hakanen, A.J.; Aro, H.T.; Roivainen, A. A Comparative 68Ga-Citrate and 68Ga-Chloride PET/CT Imaging of Staphylococcus aureus Osteomyelitis in the Rat Tibia. Contrast Media Mol. Imaging 2018, 2018, 9892604. [Google Scholar] [CrossRef] [Green Version]
- Kumar, V.; Boddeti, D.K. 68Ga-Radiopharmaceuticals for PET Imaging of Infection and Inflammation. In Theranostics, Gallium-68, and Other Radionuclides; Baum, R.P., Rösch, F., Eds.; Recent Results in Cancer Research; Springer: Berlin/Heidelberg, Germany, 2013; Volume 194, pp. 189–219. ISBN 978-3-642-27993-5. [Google Scholar]
- Nanni, C.; Errani, C.; Boriani, L.; Fantini, L.; Ambrosini, V.; Boschi, S.; Rubello, D.; Pettinato, C.; Mercuri, M.; Gasbarrini, A.; et al. 68Ga-Citrate PET/CT for Evaluating Patients with Infections of the Bone: Preliminary Results. J. Nucl. Med. 2010, 51, 1932–1936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tetikkurt, C.; Yanardag, H.; Sayman, B.H.; Bilir, M.; Tetikkurt, S.; Bilgic, S.; Kubat, B.; Bilgin, S. Diagnostic utility of 68Ga-citrate and 18FDG PET/CT in sarcoidosis patients. Monaldi Arch. Chest Dis. 2020, 90, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Behr, S.C.; Villanueva-Meyer, J.E.; Li, Y.; Wang, Y.-H.; Wei, J.; Moroz, A.; Lee, J.K.L.; Hsiao, J.C.; Gao, K.T.; Ma, W.; et al. Targeting iron metabolism in high-grade glioma with 68Ga-citrate PET/MR. JCI Insight 2018, 3, e93999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salomäki, S.P.; Kemppainen, J.; Hohenthal, U.; Luoto, P.; Eskola, O.; Nuutila, P.; Seppänen, M.; Pirilä, L.; Oksi, J.; Roivainen, A. Head-to-Head Comparison of 68Ga-Citrate and 18F-FDG PET/CT for Detection of Infectious Foci in Patients with Staphylococcus aureus Bacteraemia. Contrast Media Mol. Imaging 2017, 2017, 3179607. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Cai, J.; Yang, H.; Zhu, Z.; Li, F. 68Ga-citrate PET/CT is Valuable for Abdominal Lymphoma Imaging. Int. J. New Technol. Res. 2016, 2, 4. [Google Scholar]
- Rizzello, A.; Di Pierro, D.; Lodi, F.; Trespidi, S.; Cicoria, G.; Pancaldi, D.; Nanni, C.; Marengo, M.; Marzola, M.C.; Al-Nahhas, A.; et al. Synthesis and quality control of 68Ga citrate for routine clinical PET. Nucl. Med. Commun. 2009, 30, 542–545. [Google Scholar] [CrossRef]
- Zhernosekov, K.P.; Filosofov, D.V.; Baum, R.P.; Aschoff, P.; Bihl, H.; Razbash, A.A.; Jahn, M.; Jennewein, M.; Rosch, F. Processing of Generator-Produced 68Ga for Medical Application. J. Nucl. Med. 2007, 48, 1741–1748. [Google Scholar] [CrossRef]
- Uğur, A.; Gültekin, A. Physiological Animal Imaging with 68Ga-Citrate. Curr. Radiopharm. 2021, 14, 51–56. [Google Scholar] [CrossRef]
- Jensen, S.B.; Nielsen, K.M.; Mewis, D.; Kaufmann, J. Fast and simple one-step preparation of 68Ga citrate for routine clinical PET. Nucl. Med. Commun. 2013, 34, 806–812. [Google Scholar] [CrossRef]
- Mirzaei, R.A.; Jalilian, A.; Akhlaghi, M.; Beiki, D. Production of 68Ga-citrate Based on a SnO2 Generator for Short-Term Turpentine Oil-Induced Inflammation Imaging in Rats. Curr. Radiopharm. 2016, 9, 208–214. [Google Scholar] [CrossRef]
- Reverchon, J.; Khayi, F.; Roger, M.; Moreau, A.; Kryza, D. Optimization of the radiosynthesis of [68Ga]Ga-PSMA-11 using a Trasis MiniAiO synthesizer: Do we need to heat and purify? Nucl. Med. Commun. 2020, 41, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, M.A.; Andersen, V.L.; Hendel, H.W.; Vriamont, C.; Warnier, C.; Masset, J.; Huynh, T.H.V. Automated synthesis of 68 Ga/177 Lu-PSMA on the Trasis miniAllinOne. J. Label. Compd. Radiopharm. 2020, 63, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Isal, S.; Pierson, J.; Imbert, L.; Clement, A.; Collet, C.; Pinel, S.; Veran, N.; Reinhard, A.; Poussier, S.; Gauchotte, G.; et al. PET imaging of 68Ga-NODAGA-RGD, as compared with 18F-fluorodeoxyglucose, in experimental rodent models of engrafted glioblastoma. EJNMMI Res. 2018, 8, 51. [Google Scholar] [CrossRef] [PubMed]
- Ovdiichuk, O.; Mallapura, H.; Pineda, F.; Hourtané, V.; Långström, B.; Halldin, C.; Nag, S.; Maskali, F.; Karcher, G.; Collet, C. Implementation of iMiDEVTM, a new fully automated microfluidic platform for radiopharmaceutical production. Lab. Chip 2021, 21, 2272–2282. [Google Scholar] [CrossRef] [PubMed]
- Levesque, N.; Girard, L.; Léger, J.; Dorval, M. Stability of Trisodium Citrate 4.0% and 46.7% in Polyvinyl Chloride Syringes. Can. J. Hosp. Pharm. 2001, 54, 5. [Google Scholar]
- Zhang, X.; Liu, F.; Payne, A.C.; Nickels, M.L.; Bellan, L.M.; Manning, H.C. High-Yielding Radiosynthesis of [68Ga]Ga-PSMA-11 Using a Low-Cost Microfluidic Device. Mol. Imaging Biol. 2020, 22, 1370–1379. [Google Scholar] [CrossRef]
- Garcia-Arguello, S.F.; Lopez-Lorenzo, B.; Ruiz-Cruces, R. Automated production of [68Ga]Ga-DOTANOC and [68Ga]Ga-PSMA-11 using a TRACERlab FXFN synthesis module. J. Label. Compd. Radiopharm. 2019, 62, 146–153. [Google Scholar] [CrossRef]
- Asti, M.; De Pietri, G.; Fraternali, A.; Grassi, E.; Sghedoni, R.; Fioroni, F.; Roesch, F.; Versari, A.; Salvo, D. Validation of 68Ge/68Ga generator processing by chemical purification for routine clinical application of 68Ga-DOTATOC. Nucl. Med. Biol. 2008, 35, 721–724. [Google Scholar] [CrossRef]
- Goldberg, R.N.; Kishore, N.; Lennen, R.M. Thermodynamic Quantities for the Ionization Reactions of Buffers. J. Phys. Chem. Ref. Data 2002, 31, 231–370. [Google Scholar] [CrossRef] [Green Version]
- Petrik, M.; Vlckova, A.; Novy, Z.; Urbanek, L.; Haas, H.; Decristoforo, C. Selected 68Ga-siderophores versus 68Ga-colloid and 68Ga-citrate: Biodistribution and small animal imaging in mice. Biomed. Pap. 2015, 159, 60–66. [Google Scholar] [CrossRef] [Green Version]
- Thackeray, J.T.; Bankstahl, J.P.; Wang, Y.; Korf-Klingebiel, M.; Walte, A.; Wittneben, A.; Wollert, K.C.; Bengel, F.M. Targeting post-infarct inflammation by PET imaging: Comparison of 68Ga-citrate and 68Ga-DOTATATE with 18F-FDG in a mouse model. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Kahn Ali, S.; Ait-Mohand, S.; Dumulon-Perreault, V.; Guérin, B. [68Ga]Ga-4HMSA a promising new PET tracer for imaging inflammation. EJNMMI Res. 2021, 11, 114. [Google Scholar] [CrossRef] [PubMed]
- European Pharmacopoeia, 10th ed.; Council of Europe: Strasbourg, France, 2020; Available online: https://www.edqm.eu/en/european-pharmacopoeia-ph-eur-10th-edition (accessed on 10 December 2021).
- Sobral, D.V.; Fuscaldi, L.L.; Durante, A.C.R.; Rangel, M.G.; Oliveira, L.R.; Mendonça, F.F.; Miranda, A.C.C.; Cabeza, J.M.; Montor, W.R.; Cabral, F.R.; et al. Radiochemical and biological properties of peptides designed to interact with EGF receptor: Relevance for glioblastoma. Nucl. Med. Biol. 2020, 88, 14–23. [Google Scholar] [CrossRef] [PubMed]





| Type of Cartridge | Trapping Efficiency (%) | Recovery Efficiency (%) |
|---|---|---|
| WCX | 3.66 ± 0.5 | ND |
| Accell plus CM | 0.32 ± 0.2 | ND |
| MCX | 99.50 ± 0.1 | 96.13 ± 0.2 |
| PS-H+ | 99.23 ± 0.1 | 88.92 ± 0.8 |
| AG 50W-X8 * | 99.70 ± 0.1 | 22.75 ± 0.5 |
| SCX Maxi-Clean | 99.60 ± 0.4 | 24.04 ± 1.1 |
| Type of Cartridge | Eluent | RCY (%) | RCP (%) |
|---|---|---|---|
| PS-H+ | ACD formula A | 53.0 ± 11.9 | 100 |
| Sodium Citrate 74.8 mM | 71.1 ± 2.7 | 100 | |
| Sodium Citrate 136 mM | 88.2 ± 0.7 | 99.9 ± 0.1 | |
| MCX | ACD formula A | 90.4 ± 1.0 | 100 |
| Sodium Citrate 74.8 mM | 94.7 ± 0.5 | 100 | |
| Sodium Citrate 136 mM | 95.5 ± 0.4 | 100 |
| Type of Cartridge | Trapping R1 (%) | Trapping R3 (%) | Recovery R1 (%) | Recovery R3 (%) |
|---|---|---|---|---|
| MCX | 95.3 ± 2.7 (n = 3) | 96.2 ± 2.8 (n = 3) | 89.3 ± 0.8 (n = 2) | 86.4 ± 0.2 (n = 2) |
| PS-H+ | 98.8 ± 1.1 (n = 9) | 98.9 ± 0.4 (n = 5) | 90.5 ± 2.6 (n = 7) | 88.5 ± 6.3 (n = 4) |
| Chamber | PS-H+ | MCX | ||||
|---|---|---|---|---|---|---|
| RCY (dc), % | Residual Activity on the Cassette (dc), % | RCP, % | RCY (dc), % | Residual Activity on the Cassette (dc), % | RCP, % | |
| R1 | 90.1 ± 3.1 (n = 6) | 9.2 ± 2.7 (n = 6) | 98.7 ± 1.2 (n = 6) | 84.9 ± 4.8 (n = 2) | 10.2 ± 0.3 (n = 2) | 98.8 ± 3.2 (n = 2) |
| R3 | 87.5 ± 5.5 (n = 5) | 10.3 ± 5.8 (n = 5) | 99.3 ± 1.0 (n = 5) | 80.7 ± 0.5 (n = 2) | 12.7 ± 0.3 (n = 2) | 89.5 ± 8.5 (n = 2) |
| Metal | Blank HCl 0.1 M/136 mM Sodium Citrate µg/L | 68Ge/68Ga Generator GalliaPharm µg/L | [68Ga]Ga-Citrate mAIO® µg/L | [68Ga]Ga-Citrate iMiDEV™ µg/L |
|---|---|---|---|---|
| Zn | 7.45/12.43 | 39,010.8 ± 2378.4 | 8046.9 ± 3687.3 | 235.9 ± 38.8 |
| Fe | 2.23/33.03 | 22.4 ± 0.1 | 85.4 ± 53.6 | 128.3 ± 18.0 |
| References | Stationary Phase /Mobile Phase | 68Ga3+ (Rf) | [68Ga]Ga-Citrate (Rf) |
|---|---|---|---|
| Xu et al. [6] | Whatman/ Methanol/Ammonium acetate 10% (1:1) | 0.1 | 0.2 |
| Jensen et al. [20] | iTLC-SG/ Sodium acetate (1.36 g) + acetic acid (0.58 mL) in 100 mL Water | 0.1 | 0.9 |
| Mirzaie et al. [21] | Whatman/ Sodium acetate (1.5 g) + acetic acid (0.58 mL) in 100 mL Water | 0.2/0.95 | 0.95 |
| Aghanajab et al. [5] | Whatman/ Acetone/glacial acetic acid (3:1) | 0.3 | 1 |
| Rizetto et al. [17] | iTLC-SG/ Methanol/ acetic acid (9:1) | 0 | 1 |
| - | iTLC-SG/ ACD | 1 | 1 |
| Parameter | Method (Criteria of Acceptance) | mAIO® Production | iMiDEV™ Production |
|---|---|---|---|
| Radiosynthesis time | - | 11 min from SOE | 10 min from SOS |
| Radiosynthesis Yield | - | 96.1 ± 0.5 % | 90.1 ± 2.6% |
| Appearance | Visual inspection (colourless) | passed | passed |
| pH | pH strips (4–8) | 6 | 7 |
| Radiochemical purity | Radio-TLC (>95%) | 99.8 ± 0.1% | 98.5 ± 1.3% |
| Radionuclide purity | Gamma-spectrometry (511 keV +/− 10%) | 532 ± 3 keV | 532 ± 3 keV |
| Radionuclide identity | Half-life (62–74 min) | 69.3 min | 69.9 min |
| Bacterial endotoxins | LAL test (UE < 75/mL) | <1.5 UE/mL | <1.5 UE/mL |
| Sterility | sterile | sterile |
| Organs | SUVmean/bw [68Ga]Ga-Citrate mAIO® | SUVmean/bw [68Ga]Ga-Citrate iMiDEV™ |
|---|---|---|
| Liver | 0.95 ± 0.31 | 0.83 ± 0.18 |
| Heart | 2.61 ± 1.24 | 2.40 ± 0.46 |
| Left Kidney | 1.07 ± 0.29 | 0.97 ± 0.05 |
| Right Kidney | 1.23 ± 0.30 | 1.13 ± 0.06 |
| Bladder | 2.30 ± 1.56 | 1.66 ± 1.14 |
| Bone Marrow | 1.20 ± 0.35 | 1.10 ± 0.29 |
| Bone | 0.88 ± 0.23 | 0.76 ± 0.24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ovdiichuk, O.; Roeder, E.; Billotte, S.; Veran, N.; Collet, C. Fully Automated Macro- and Microfluidic Production of [68Ga]Ga-Citrate on mAIO® and iMiDEVTM Modules. Molecules 2022, 27, 994. https://doi.org/10.3390/molecules27030994
Ovdiichuk O, Roeder E, Billotte S, Veran N, Collet C. Fully Automated Macro- and Microfluidic Production of [68Ga]Ga-Citrate on mAIO® and iMiDEVTM Modules. Molecules. 2022; 27(3):994. https://doi.org/10.3390/molecules27030994
Chicago/Turabian StyleOvdiichuk, Olga, Emilie Roeder, Sébastien Billotte, Nicolas Veran, and Charlotte Collet. 2022. "Fully Automated Macro- and Microfluidic Production of [68Ga]Ga-Citrate on mAIO® and iMiDEVTM Modules" Molecules 27, no. 3: 994. https://doi.org/10.3390/molecules27030994
APA StyleOvdiichuk, O., Roeder, E., Billotte, S., Veran, N., & Collet, C. (2022). Fully Automated Macro- and Microfluidic Production of [68Ga]Ga-Citrate on mAIO® and iMiDEVTM Modules. Molecules, 27(3), 994. https://doi.org/10.3390/molecules27030994