Stability of Manganese(II)–Pyrazine, –Quinoxaline or –Phenazine Complexes and Their Potential as Carbonate Sequestration Agents
Abstract
:1. Introduction
2. Results and Discussion
2.1. Materials
2.2. Formation Constants of the Manganese(II) Complexes with Pyrazine, Quinoxaline or PhenaZine and Its Carbonate Complexes
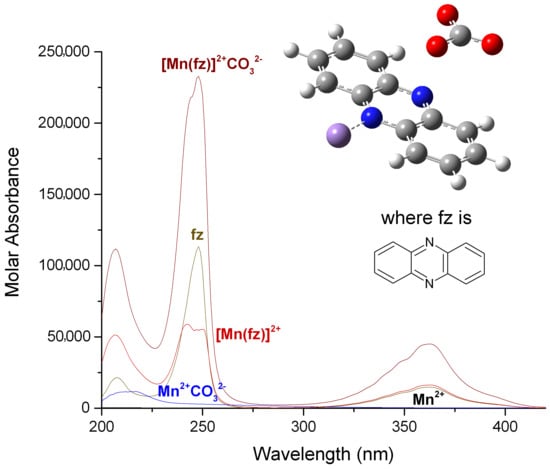
2.3. Distribution Curves of the Manganese(II) Complexes with Pyrazine, Quinoxaline and Phenazine and Its Carbonated Complexes
2.4. Far- and Mid-Infrared Spectra of the Complexes: [Mn(pz)]2+, [Mn(qx)]2+, [Mn(fz)]2+, [Mn(pz)]2+, [Mn(qx)]2+ and [Mn(fz)]2+
3. Materials and Methods
3.1. Materials, Physical Measurements and Methods
3.2. Equilibrium Studies of Manganese (II) with Pyrazine, Quinoxaline or Phenazine
3.3. Equilibrium Studies of Manganese (II) with and Pyrazine, Quinoxaline or Phenazine
3.4. Synthesis of the Complexes: [Mn(pz)]2+, [Mn(qx)]2+, [Mn(fz)]2+, [Mn(pz)]2+, [Mn(qx)]2+, [Mn(fz)]2+ and Its Far- and Mid-Infrared Spectrum
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Höök, M.; Tang, X. Depletion of fossil fuels and anthropogenic climate change—A review. Energy Policy 2013, 52, 797–809. [Google Scholar] [CrossRef] [Green Version]
- Gayathri, R.; Mahboob, S.; Govindarajan, M.; Al-Ghanim, K.A.; Ahmed, Z.; Al-Mulhm, N.; Vodovnik, M.; Vijayalakshmi, S. A review on biological carbon sequestration: A sustainable solution for a cleaner air environment, less pollution and lower health risks. J. King Saud Univ.–Sci. 2021, 33, 101282. [Google Scholar] [CrossRef]
- Yıldız, İ. 1.12 Fossil Fuels. In Comprehensive Energy Systems; Dincer, I., Ed.; Elsevier: Oxford, UK, 2018; pp. 521–567. [Google Scholar]
- Vaughan, A. Global warming is causing oceans to lose oxygen. New Sci. 2019, 244, 11. [Google Scholar] [CrossRef]
- Zeebe, R.E.; Zachos, J.C.; Caldeira, K.; Tyrrell, T. Oceans. Carbon emissions and acidification. Science 2008, 321, 51–52. [Google Scholar] [CrossRef] [PubMed]
- Reichle, D.E. Chapter 11—Anthropogenic alterations to the global carbon cycle and climate change. In The Global Carbon Cycle and Climate Change; Reichle, D.E., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 209–251. [Google Scholar]
- Zhao, B.; Tao, W.; Zhong, M.; Su, Y.; Cui, G. Process, performance and modeling of CO2 capture by chemical absorption using high gravity: A review. Renew. Sustain. Energy Rev. 2016, 65, 44–56. [Google Scholar] [CrossRef]
- Wolf, S.E.; Muller, L.; Barrea, R.; Kampf, C.J.; Leiterer, J.; Panne, U.; Hoffmann, T.; Emmerling, F.; Tremel, W. Carbonate-coordinated metal complexes precede the formation of liquid amorphous mineral emulsions of divalent metal carbonates. Nanoscale 2011, 3, 1158–1165. [Google Scholar] [CrossRef] [Green Version]
- Styring, P.; Quadrelli, E.A.; Armstrong, K. Carbon Dioxide Utilisation: Closing the Carbon Cycle; Elsevier Science: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Mazzotti, M.; Abanades, J.; Allam, R.; Lackner, K.S.; Meunier, F.; Rubin, E.; Sanchez, J.C.; Yogo, K.; Zevenhoven, R. Mineral carbonation and industrial uses of carbon dioxide. In IPCC Special Report on Carbon Dioxide Capture and Storage; Cambridge University Press: New York, NY, USA, 2005; pp. 319–338. [Google Scholar]
- Lackner, K.S. Carbonate Chemistry for Sequestering Fossil Carbon. Annu. Rev. Energy Environ. 2002, 27, 193–232. [Google Scholar] [CrossRef]
- Pingitore, N.E.; Eastman, M.P.; Sandidge, M.; Oden, K.; Freiha, B. The coprecipitation of manganese(II) with calcite: An experimental study. Mar. Chem. 1988, 25, 107–120. [Google Scholar] [CrossRef]
- Silva, A.M.; Cunha, E.C.; Silva, F.D.R.; Leão, V.A. Treatment of high-manganese mine water with limestone and sodium carbonate. J. Clean. Prod. 2012, 29–30, 11–19. [Google Scholar] [CrossRef] [Green Version]
- Wartel, M.; Skiker, M.; Auger, Y.; Boughriet, A. Interaction of manganese(II) with carbonates in seawater: Assessment of the solubility product of MnCO3 and Mn distribution coefficient between the liquid phase and CaCO3 Particles. Mar. Chem. 1990, 29, 99–117. [Google Scholar] [CrossRef]
- Namgung, S.; Guo, B.; Sasaki, K.; Lee, S.S.; Lee, G. Macroscopic and microscopic behaviors of Mn(II) (ad)sorption to goethite with the effects of dissolved carbonates under anoxic conditions. Geochim. Cosmochim. Acta 2020, 277, 300–319. [Google Scholar] [CrossRef]
- Maga, J.A.; Sizer, C.E. Pyrazines in foods. Review. J. Agric. Food Chem. 1973, 21, 22–30. [Google Scholar] [CrossRef]
- Adams, T.B.; Doull, J.; Feron, V.J.; Goodman, J.I.; Marnett, L.J.; Munro, I.C.; Newberne, P.M.; Portoghese, P.S.; Smith, R.L.; Waddell, W.J.; et al. The FEMA GRAS assessment of pyrazine derivatives used as flavor ingredients. Food Chem. Toxicol. 2002, 40, 429–451. [Google Scholar] [CrossRef]
- Pereira, J.A.; Pessoa, A.M.; Cordeiro, M.N.D.S.; Fernandes, R.; Prudêncio, C.; Noronha, J.P.; Vieira, M. Quinoxaline, its derivatives and applications: A State of the Art review. Eur. J. Med. Chem. 2015, 97, 664–672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pierson, L.S.; Pierson, E.A. Metabolism and function of phenazines in bacteria: Impacts on the behavior of bacteria in the environment and biotechnological processes. Appl. Microbiol. Biotechnol. 2010, 86, 1659–1670. [Google Scholar] [CrossRef] [Green Version]
- Krishnaiah, M.; de Almeida, N.R.; Udumula, V.; Song, Z.; Chhonker, Y.S.; Abdelmoaty, M.M.; do Nascimento, V.A.; Murry, D.J.; Conda-Sheridan, M. Synthesis, biological evaluation, and metabolic stability of phenazine derivatives as antibacterial agents. Eur. J. Med. Chem. 2018, 143, 936–947. [Google Scholar] [CrossRef]
- Wei, T.-B.; Yong, B.-R.; Dang, L.-R.; Zhang, Y.-M.; Yao, H.; Lin, Q. A simple water-soluble phenazine dye for colorimetric/fluorogenic dual-mode detection and removal of Cu2+ in natural water and plant samples. Dye. Pigment. 2019, 171, 107707. [Google Scholar] [CrossRef]
- Lesht, D.; Bauman, J.E. Thermodynamics of the manganese(II) bicarbonate system. Inorg. Chem. 1978, 17, 3332–3334. [Google Scholar] [CrossRef]
- Kan, A.T.; Fu, G.; Tomson, M.B. Effect of Methanol on Carbonate Equilibrium and Calcite Solubility in a Gas/Methanol/Water/Salt Mixed System. Langmuir 2002, 18, 9713–9725. [Google Scholar] [CrossRef]
- Mayer, U. Solvent effects on ion-pair equilibria. Coord. Chem. Rev. 1976, 21, 159–179. [Google Scholar] [CrossRef]
- Inada, Y.; Hayashi, H.; Sugimoto, K.-i.; Funahashi, S. Solvation Structures of Manganese(II), Iron(II), Cobalt(II), Nickel(II), Copper(II), Zinc(II), and Gallium(III) Ions in Methanol, Ethanol, Dimethyl Sulfoxide, and Trimethyl Phosphate As Studied by EXAFS and Electronic Spectroscopies. J. Phys. Chem. A 1999, 103, 1401–1406. [Google Scholar] [CrossRef]
- Machura, B.; Palion, J.; Mroziński, J.; Kalińska, B.; Amini, M.; Najafpour, M.; Kruszynski, R. Manganese(II) complexes of 2,3,5,6-tetra-(2-pyridyl)pyrazine—Syntheses, crystal structures, spectroscopic, magnetic and catalytic properties. Polyhedron 2013, 53, 132. [Google Scholar] [CrossRef]
- Manson, J.L.; Huang, Q.-z.; Lynn, J.W.; Koo, H.-J.; Whangbo, M.-H.; Bateman, R.; Otsuka, T.; Wada, N.; Argyriou, D.N.; Miller, J.S. Long-Range Magnetic Order in Mn[N(CN)2]2(pyz) {pyz = pyrazine}. Susceptibility, Magnetization, Specific Heat, and Neutron Diffraction Measurements and Electronic Structure Calculations. J. Am. Chem. Soc. 2001, 123, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Huo, L.H.; Gao, S.; Ng, S.W. Hexaaqua-manganese(II) tetra-aqua-bis(2-amino-pyrazine-κN)manganese(II) disulfate dihydrate. Acta Crystallogr. Sect. E Struct. Rep. Online 2009, 65, m1504. [Google Scholar] [CrossRef] [PubMed]
- Polunin, R.A.; Evstifeev, I.S.; Cador, O.; Golhen, S.; Gavrilenko, K.S.; Lytvynenko, A.S.; Efimov, N.N.; Minin, V.V.; Bogomyakov, A.S.; Ouahab, L.; et al. Versatile Reactivity of MnII Complexes in Reactions with N-Donor Heterocycles: Metamorphosis of Labile Homometallic Pivalates vs. Assembling of Endurable Heterometallic Acetates. Molecules 2021, 26, 1021. [Google Scholar] [CrossRef]
- Mikata, Y.; Murakami, K.; Ochi, A.; Nakagaki, F.; Naito, K.; Matsumoto, A.; Mitsuhashi, R.; Mikuriya, M. Conversion of (µ-OH)2Mn2(II,II) complex to (µ-O)2Mn2(III,III) core supported by a quinoxaline-based tetranitrogen ligand. Inorg. Chim. Acta 2020, 509, 119688. [Google Scholar] [CrossRef]
- Ayllón, J.A.; Santos, I.C.; Henriques, R.T.; Almeida, M.; Alcácer, L.; Duarte, M.T. Synthesis of tris(quinoxaline-2,3-dithiolato)manganese(IV) and its reaction with [Cu(CH3COO)2H2O]2. Crystal structure of [MnII(DMF)4(H2O)2][CuIII(qdt)2]2. Polyhedron 1998, 17, 4023–4031. [Google Scholar] [CrossRef]
- Abid, M.; Hasun, S. Synthesis and characterization of manganese(II), cobalt(II), nickel(II), copper(II) and zinc(II) complexes with new Schiff base derived from 6,7-dimethyl-quinoxaline-2,3(1H,4H)-dione and thiosemicarbazide. Eur. J. Chem. 2015, 6, 44–47. [Google Scholar]
- Xu, M.-L.; Sun, S.-B.; Li, X.-Y.; Che, G.-B. Dichlorido(dipyrido[3,2-a:2′,3′-c]phenazine)manganese(II). Acta Crystallogr. Sect. E 2009, 65, m136. [Google Scholar] [CrossRef] [Green Version]
- Che, G.B.; Wang, J.; Liu, C.B.; Li, X.Y.; Liu, B. A one-dimensional chain structure based on unusual tetranuclear manganese(II) clusters. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 2008, 64, m362–m364. [Google Scholar] [CrossRef]
- Gans, P.; Sabatini, A.; Vacca, A. HypSpec 2008 Software; Protonic Software: Leeds, UK; Florence, Italy, 2008. [Google Scholar]
- Rocha, J.; Poneti, G.; Ferreira, J.; Ribeiro, R.; Nunes, F. Spectroscopic, Electrochemical, Magnetic and Structural Investigations of Dimanganese-(II/II) and Mixed-Valence-(II/III)-Tetraiminodiphenolate Complexes. J. Braz. Chem. Soc. 2014, 25, 1528–1535. [Google Scholar] [CrossRef]
- Melník, M.; Segľa, P.; Tatarko, M. New Trends in Coordination, Bioinorganic and Applied Inorganic Chemistry: XXIII. In Book of Abstracts, Program, Proceedings of the International Conference on Coordination and Bioinorganic Chemistry; Slovak University of Technology Press: Bratislava, Slovak, 2011. [Google Scholar]
- Hirt, R.C.; Schmitt, R.G. Observed n—π* Bands in the Ultraviolet Absorption Solution Spectra of Amino Asymmetric Triazines. J. Chem. Phys. 1955, 23, 600. [Google Scholar] [CrossRef]
- Halverson, F.; Hirt, R.C. Near Ultraviolet Solution Spectra of the Diazines. J. Chem. Phys. 1951, 19, 711–718. [Google Scholar] [CrossRef]
- Ito, M.; Shimada, R.; Kuraishi, T.; Mizushima, W. Ultraviolet Absorption of Pyrazine Vapor Due to n-π Transition. J. Chem. Phys. 1957, 26, 1508–1515. [Google Scholar] [CrossRef]
- Köttelwesch, H.; Schleitzer-Rust, E. Mn Manganese: Coordination Compounds 5; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Vinson, M.; Arvidson, R.; Luttge, A. Kinetic inhibition of calcite (104) dissolution by aqueous manganese(II). J. Cryst. Growth 2007, 307, 116–125. [Google Scholar] [CrossRef]
- Achelle, S.; Baudequin, C.; Plé, N. Luminescent materials incorporating pyrazine or quinoxaline moieties. Dye. Pigment. 2013, 98, 575–600. [Google Scholar] [CrossRef] [Green Version]
- Armentano, D.; de Munno, G.; Guerra, F.; Faus, J.; Lloret, F.; Julve, M. 2,2′-Bipyrimidine- and 2,3-bis(2-pyridyl)pyrazine-containing manganese(ii) compounds: Structural and magnetic properties. Dalton Trans. 2003, 24, 4626–4634. [Google Scholar] [CrossRef]
- Hague, D.N.; Martin, S.R. Kinetics of ternary complex formation between manganese(II) species and 2,2′-bipyridine. J. Chem. Soc. Dalton Trans. 1974, 3, 254–258. [Google Scholar] [CrossRef]
- Sanyal, S.G.; Modak, M.A.; Mudi, A.K. Studies on Manganese (II), Iron(II), Cobalt(II), Nickel(II) & Copper(II) Complexes with Pyrazine Amides. Indian J. Chem. 1982, 21A, 1044–1048. [Google Scholar]
- Gans, P.; Sabatini, A.; Vacca, A. Investigation of equilibria in solution. Determination of equilibrium constants with the HYPERQUAD suite of programs. Talanta 1996, 43, 1739–1753. [Google Scholar] [CrossRef]
- Gans, P.; Sabatini, A.; Vacca, A. Determination of equilibrium constants from spectrophometric data obtained from solutions of known pH: The program pHab. Ann. Chim. 1999, 89, 45–49. [Google Scholar]
- Alderighi, L.; Gans, P.; Ienco, A.; Peters, D.; Sabatini, A.; Vacca, A. Hyperquad simulation and speciation (HySS): A utility program for the investigation of equilibria involving soluble and partially soluble species. Coord. Chem. Rev. 1999, 184, 311–318. [Google Scholar] [CrossRef]









| Solution Composition | [TL] Range from 35 to 671 and 71 to 1412 µmol L−1 [TM] Constant at 348 and 696 µmol L−1 | ||
|---|---|---|---|
| Ionic strength, electrolyte | Not used | ||
| pH range | Not used | ||
| Experimental method | Spectrophotometric titration | ||
| Temperature | 298 K | ||
| Total number of data points | Mn complexation: 40 solution spectra | ||
| Method of calculation | HypSpec | ||
| Species | Equilibrium | Log β | σ |
| [Mn(pz)]2+ | Mn2+ + pz ⇌ [Mn(pz)]2+ | log β110 = 4.6 ± 0.1 | 0.0036 |
| Solution composition | [TL] range from 6.14 to 167.81 and 15.36 to 245.85 µmol L−1 [TM] constant at 79.68 and 159.36 µmol L−1 | ||
| Ionic strength, electrolyte | Not used | ||
| pH range | Not used | ||
| Experimental method | Spectrophotometric titration | ||
| Temperature | 298 K | ||
| Total number of data points | Mn complexation: 36 solution spectra | ||
| Method of calculation | HypSpec | ||
| Species | Equilibrium | Log β | σ |
| [Mn(qx)]2+ | Mn2+ + qx ⇌ [Mn(qx)]2+ | log β110 = 5.9 ± 0.1 | 0.0278 |
| Solution composition | [TL] range from 1.78 to 33.74 and 3.55 to 71.03 µmol L−1 [TM] constant at 17.53 and 35.06 µmol L−1 | ||
| Ionic strength, electrolyte | Not used | ||
| pH range | Not used | ||
| Experimental method | Spectrophotometric titration | ||
| Temperature | 298 K | ||
| Total number of data points | Mn complexation: 40 solution spectra | ||
| Method of calculation | HypSpec | ||
| Species | Equilibrium | Log β | σ |
| [Mn(fz)]2+ | Mn2+ + fz ⇌ [Mn(fz)]2+ | log β110 = 6.0 ± 0.1 | 0.0161 |
| Solution Composition | [TCO3] Range from 133.8 to 602.5 µmol L−1 [TM] Constant at 334.7 µmol L−1 | ||
|---|---|---|---|
| Ionic strength, electrolyte | Not used | ||
| pH range | Not used | ||
| Experimental method | Spectrophotometric titration | ||
| Temperature | 298 K | ||
| Total number of data points | Mn complexation: 10 solution spectra | ||
| Method of calculation | HypSpec | ||
| Species | Equilibrium | Log β | σ |
| [Mn]2+ | Mn2+ + CO32− ⇌ [Mn]2+ | log β110 = 5.1 ± 0.1 | 0.0817 |
| Solution composition | [TCO3] range from 30.18 to 301.88 µmol L−1 and 66.94 to 669.4 µmol L−1 [TL] constant at 349.44 and 700 µmol L−1 [TM] constant at 159.2 and 334 µmol L−1 | ||
| Ionic strength, electrolyte | Not used | ||
| pH range | Not used | ||
| Experimental method | Spectrophotometric titration | ||
| Temperature | 298 K | ||
| Total number of data points | Mn complexation: 20 solution spectra | ||
| Method of calculation | HypSpec | ||
| Species | Equilibrium | Log β | σ |
| [Mn(pz)]2+ | Mn2+ + pz + ⇌ [Mn(pz)]2+ | log β110 = 9.8 ± 0.1 | 0.1224 |
| Solution composition | [TCO3] range from 7.54 to 75.4 µmol L−1 and 16.0 to 160.0 µmol L−1 [TL] constant at 61.44 and 122.88 µmol L−1 [TM] constant at 31.84 and 80.0 µmol L−1 | ||
| Ionic strength, electrolyte | Not used | ||
| pH range | Not used | ||
| Experimental method | Spectrophotometric titration | ||
| Temperature | 298 K | ||
| Total number of data points | Mn complexation: 20 solution spectra | ||
| Method of calculation | HypSpec | ||
| Species | Equilibrium | Log β | σ |
| [Mn(qx)]2+ | Mn2+ + qx + ⇌ [Mn(qx)]2+ | log β110 = 11.7 ± 0.1 | 0.0266 |
| Solution composition | [TCO3] range from 0.94 to 8.49 µmol L−1 and 7.54 to 37.7 µmol L−1 [TL] constant at 17.76 and 35.52 µmol L−1 [TM] constant at 9.55 and 17.6 µmol L−1 | ||
| Ionic strength, electrolyte | Not used | ||
| pH range | Not used | ||
| Experimental method | Spectrophotometric titration | ||
| Temperature | 298 K | ||
| Total number of data points | Mn complexation: 19 solution spectra | ||
| Method of calculation | HypSpec | ||
| Species | Equilibrium | Log β | σ |
| [Mn(fz)]2+ | Mn2+ + fz + ⇌ [Mn(fz)]2+ | log β110 = 12.7 ± 0.1 | 0.0492 |
| Complex | Signal of Ring Vibration, cm−1 | ν(NH), cm−1 | ν(Mn–N), cm−1 | ν(Mn–O), cm−1 |
|---|---|---|---|---|
| [Mn(pz)]2+ | 1600 and 1200 [44] | 3330 cm−1 [41,45] | 250 cm−1 [41] | 400 and 300 cm−1 [41] |
| [Mn(qx)]2+ | ||||
| [Mn(fz)]2+ | 3290 cm−1 [41,45] | |||
| [Mn(pz)]2+ | 3330 cm−1 [41,45] | |||
| [Mn(qx)]2+ | ||||
| [Mn(fz)]2+ | 3290 cm−1 [41,45] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Segoviano-Garfias, J.J.N.; Zanor, G.A.; Ávila-Ramos, F.; Bivián-Castro, E.Y. Stability of Manganese(II)–Pyrazine, –Quinoxaline or –Phenazine Complexes and Their Potential as Carbonate Sequestration Agents. Molecules 2022, 27, 1648. https://doi.org/10.3390/molecules27051648
Segoviano-Garfias JJN, Zanor GA, Ávila-Ramos F, Bivián-Castro EY. Stability of Manganese(II)–Pyrazine, –Quinoxaline or –Phenazine Complexes and Their Potential as Carbonate Sequestration Agents. Molecules. 2022; 27(5):1648. https://doi.org/10.3390/molecules27051648
Chicago/Turabian StyleSegoviano-Garfias, José J. N., Gabriela A. Zanor, Fidel Ávila-Ramos, and Egla Yareth Bivián-Castro. 2022. "Stability of Manganese(II)–Pyrazine, –Quinoxaline or –Phenazine Complexes and Their Potential as Carbonate Sequestration Agents" Molecules 27, no. 5: 1648. https://doi.org/10.3390/molecules27051648