Supramolecular Immobilization of Adamantyl and Carboxylate Modified N-Heterocyclic Carbene Ligand on Cucurbituril Substrates
Abstract
:1. Introduction
2. Experimental Section
2.1. General Synthetic Considerations
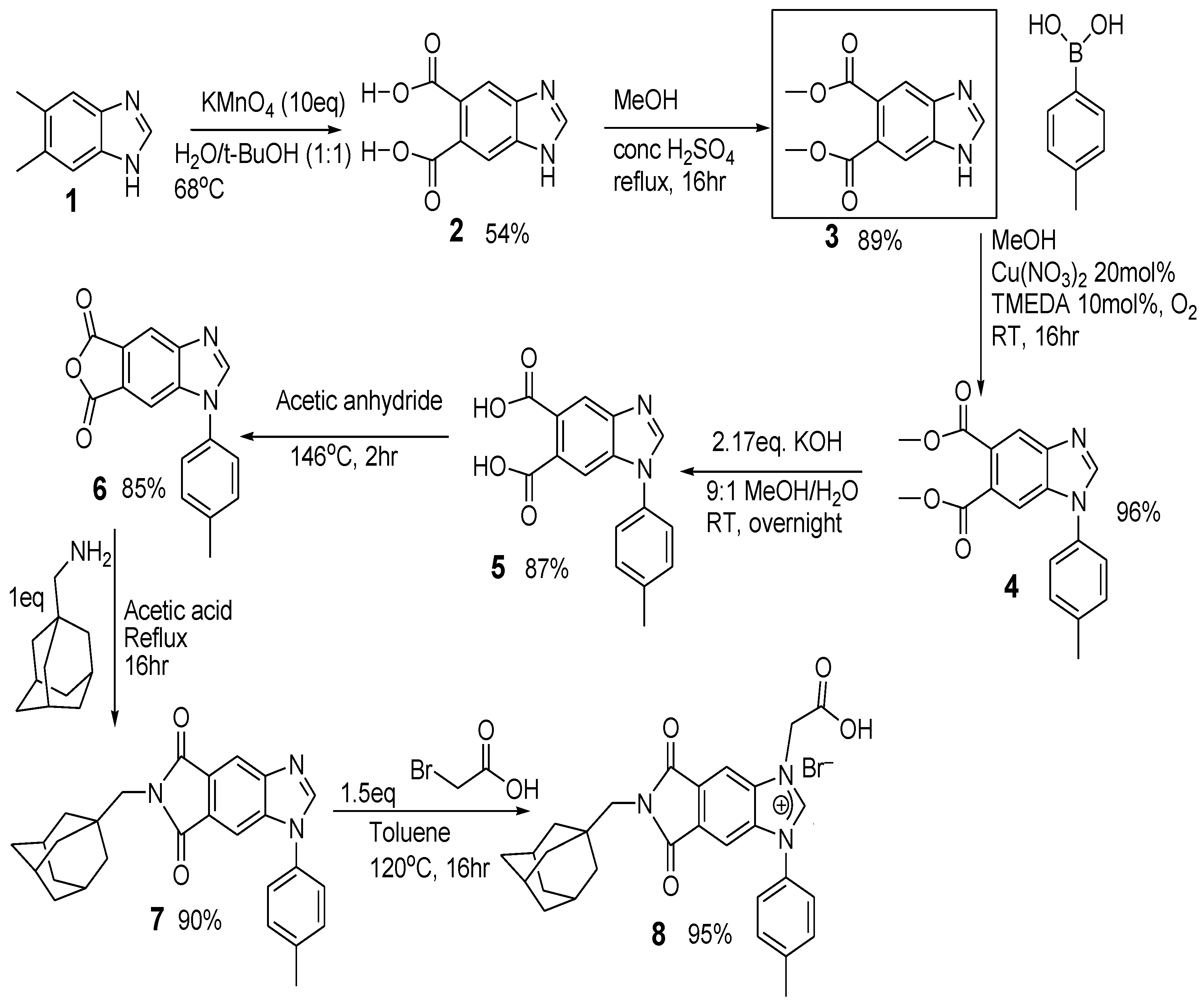
2.1.1. Synthesis of 1H-Benzo[d]imidazole-5,6-dicarboxylic acid (2)
2.1.2. Synthesis of Dimethyl 1H-Benzo[d]imidazole-5,6-dicarboxylate (3)
2.1.3. Synthesis of Dimethyl 1-p-Tolyl-1H-benzo[d]imidazole-5,6-dicarboxylate (4)
2.1.4. Synthesis of 1-p-Tolyl-1H-isobenzofuro[5,6-d]imidazole-5,7-dione (6)
2.1.5. Synthesis of 6-Adamantanemethyl-1-p-tolylimidazo [4,5-isoindole-5,7(1H,6H)-dione (7)
2.1.6. Synthesis of 8
2.2. Crystal Structure Data Acquisition
3. Results and Discussion
3.1. Design and Synthesis
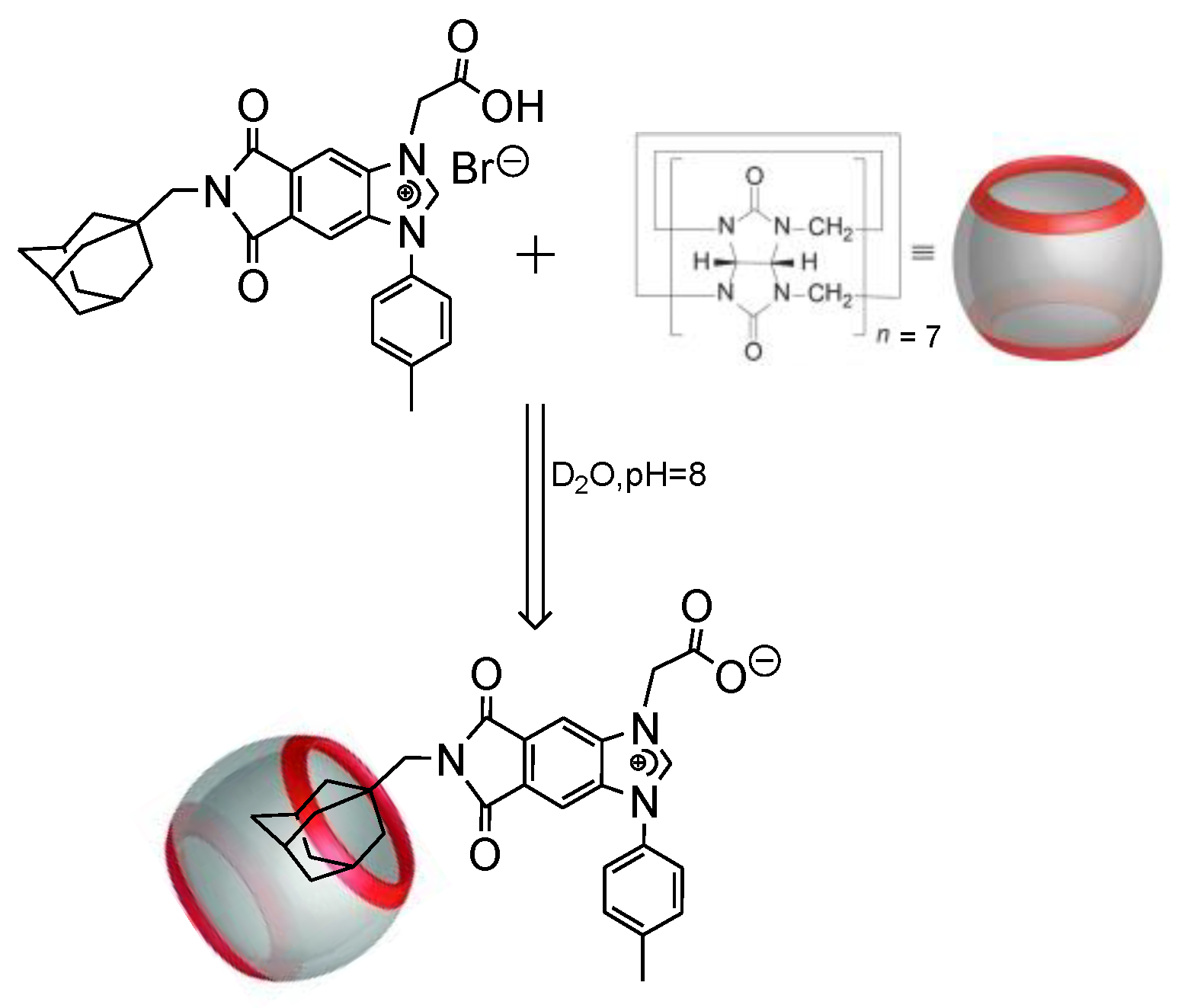
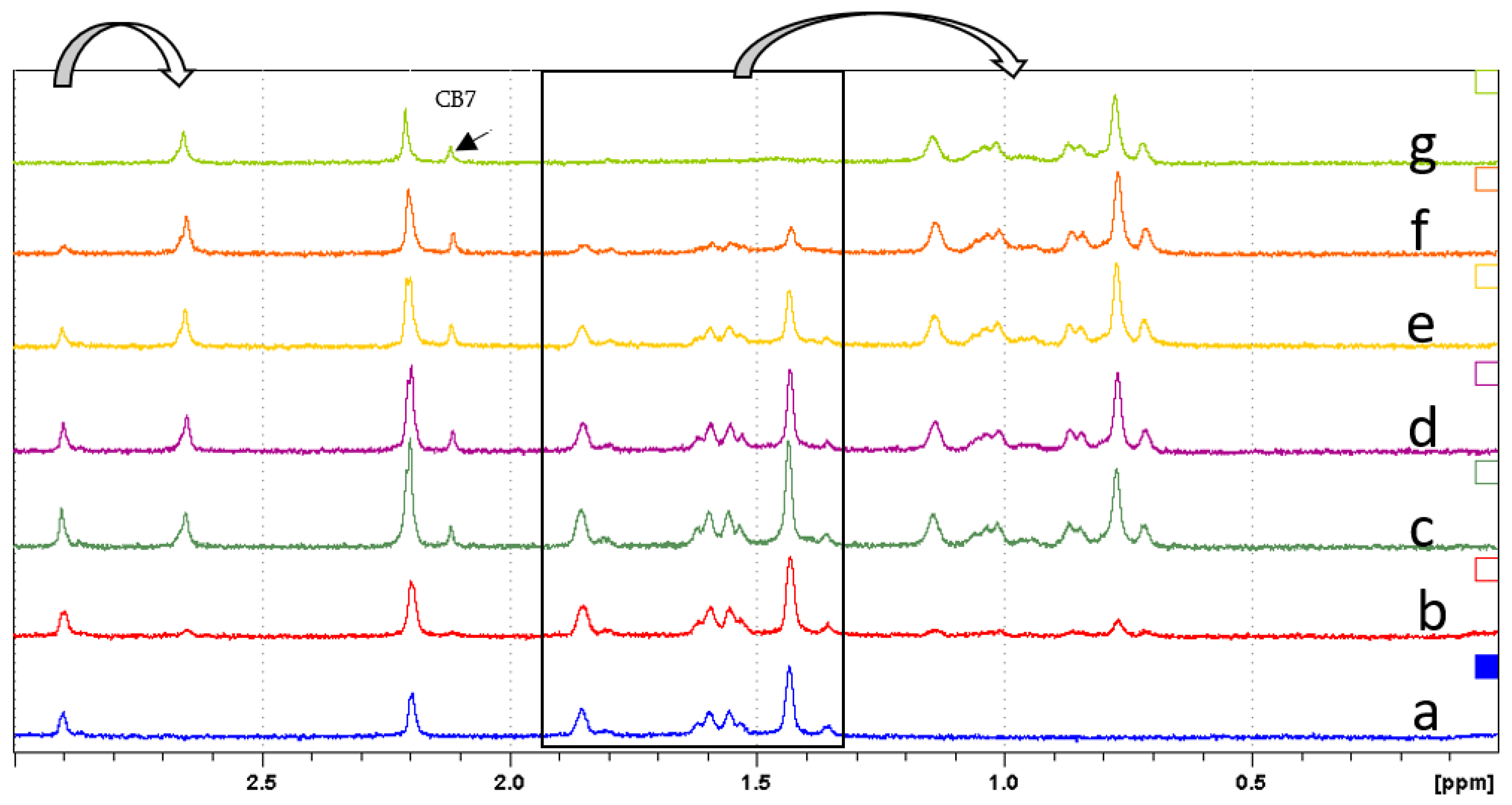
3.2. Host-Guest Complexation of 8 and CB7
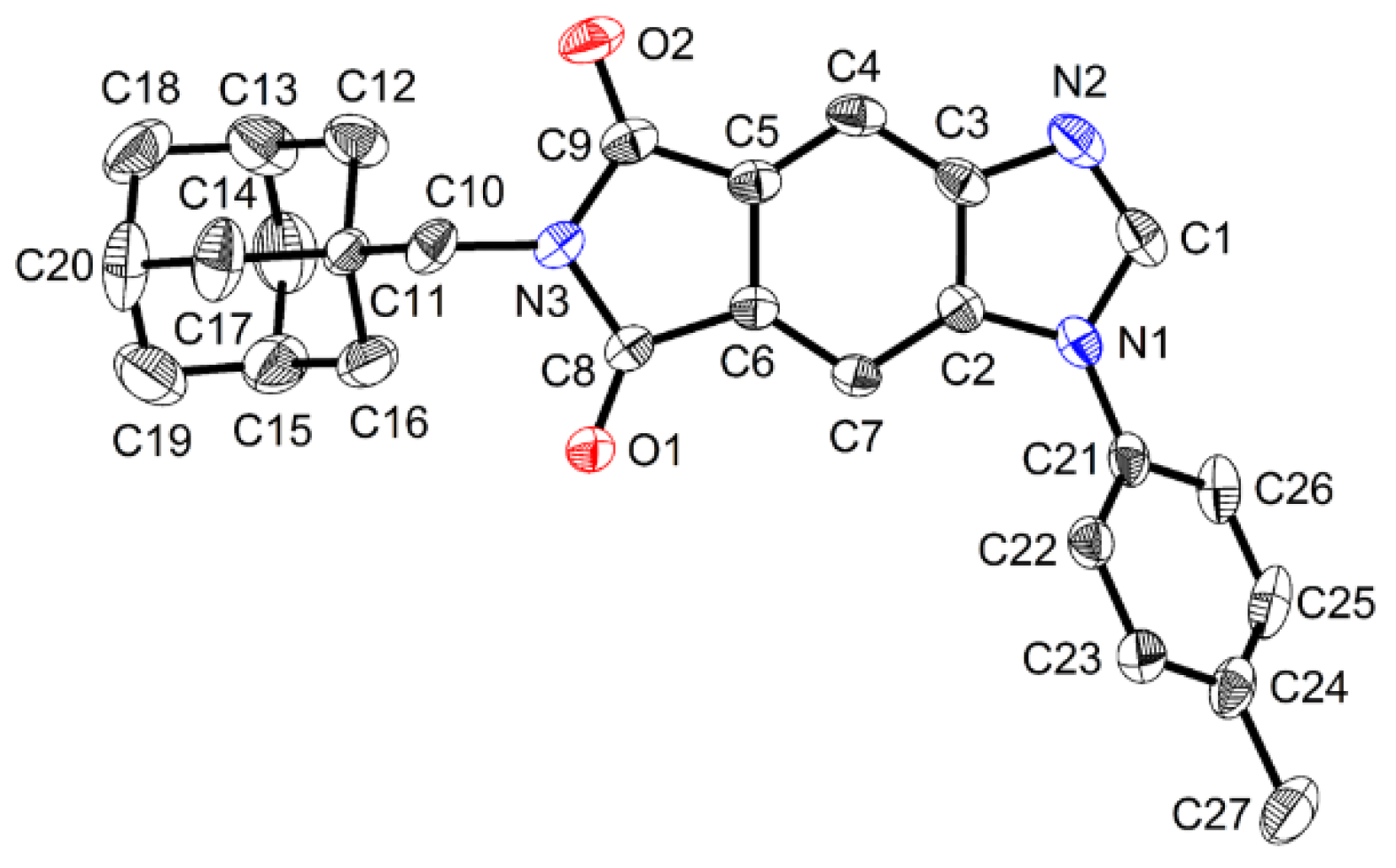
3.3. Solid State Analysis
4. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
| Ad | Adamantane |
| CB7 | Curcubit[7]uril |
| NHC | N-Heterocyclic Carbene |
| NMR | Nuclear Magnetic Resonances |
| TMED | Tetramethylethylenediamine |
| RBF | Round Bottom Flask |
References
- Zhang, X.; Wang, B.; Lu, Y.; Xia, C.; Liu, J. Homogeneous and noncovalent immobilization of NHC-Cu catalyzed azide-alkyne cycloaddition reaction. Mol. Catal. 2021, 504, 111452. [Google Scholar] [CrossRef]
- Zhang, B.; Reek, J.N.H. Supramolecular Strategies for the Recycling of Homogeneous Catalysts. Chem. Asian J. 2021, 16, 3851–3863. [Google Scholar] [CrossRef]
- Peris, E. Smart N-Heterocyclic Carbene Ligands in Catalysis. Chem. Rev. 2018, 118, 9988–10031. [Google Scholar] [CrossRef] [PubMed]
- Keita, H. Synthesis and thermal characterization of solid state organic electrolytes for their potential lithium ion battery applications. Mater. Lett. X 2021, 12, 100093. [Google Scholar] [CrossRef]
- Wang, W.; Cui, L.; Sun, P.; Shi, L.; Yue, C.; Li, F. Reusable N-Heterocyclic Carbene Complex Catalysts and Beyond: A Per-spective on Recycling Strategies. Chem. Rev. 2018, 118, 9843–9929. [Google Scholar] [CrossRef]
- Sandeli, A.E.-K.; Boulebd, H.; Khiri-Meribout, N.; Benzerka, S.; Bensouici, C.; Özdemir, N.; Gürbüz, N.; Özdemir, I. New benzimidazolium N-Heterocyclic carbene precursors and their related Pd-NHC complex PEPPSI-type: Synthesis, structures, DFT calculations, biological activity, docking study, and catalytic application in the direct arylation. J. Mol. Struct. 2022, 1248, 131504. [Google Scholar] [CrossRef]
- Kim, C.; Ondrusek, B.A.; Chung, H. Removable Water-Soluble Olefin Metathesis Catalyst via Host–Guest Interaction. Org. Lett. 2018, 20, 736–739. [Google Scholar] [CrossRef]
- Czarnocki, S.; Monsigny, L.; Sienkiewicz, M.; Kajetanowicz, A.; Grela, K. Ruthenium Olefin Metathesis Catalysts Featuring N-Heterocyclic Carbene Ligands Tagged with Isonicotinic and 4-(Dimethylamino)benzoic Acid Rests: Evaluation of a Modular Synthetic Strategy. Molecules 2021, 26, 5220. [Google Scholar] [CrossRef]
- Öztürk, B. Ammonium tagged Hoveyda-Grubbs catalysts immobilized on magnetically separable core-shell silica supports for ring-closing metathesis reactions. Microporous Mesoporous Mater. 2018, 267, 249–256. [Google Scholar] [CrossRef]
- Peris, E. Polyaromatic N-Heterocyclic carbene ligands and π-stacking. Catalytic consequences. Chem. Commun. 2016, 52, 5777–5787. [Google Scholar] [CrossRef] [Green Version]
- Chołuj, A.; Krzesiński, P.; Ruszczyńska, A.; Bulska, E.; Kajetanowicz, A.; Grela, K. Noncovalent Immobilization of Cationic Ruthenium Complex in a Metal–Organic Framework by Ion Exchange Leading to a Heterogeneous Olefin Metathesis Catalyst for Use in Green Solvents. Organometallics 2019, 38, 3397–3405. [Google Scholar] [CrossRef]
- Peng, Q.; Zhao, X.; Chen, M.; Wang, J.; Cui, K.; Wei, X.; Hou, Z. Cationic Ru complexes anchored on POM via non-covalent interaction towards efficient transfer hydrogenation catalysis. Mol. Catal. 2022, 517, 112049. [Google Scholar] [CrossRef]
- Mizusaki, T.; Matsumoto, K.; Takeuchi, K.; Fukaya, N.; Takagi, Y.; Choi, J.-C. Direct Installation of a Silyl Linker on Ready-Made NHC Ligands: Immobilized NHC-Pd Complex for Buchwald–Hartwig Amination. Organometallics 2019, 38, 1872–1876. [Google Scholar] [CrossRef]
- Nahra, F.; Cazin, C.S.J. Sustainability in Ru- and Pd-based catalytic systems using N-Heterocyclic carbenes as ligands. Chem. Soc. Rev. 2021, 50, 3094–3142. [Google Scholar] [CrossRef]
- Assaf, K.I.; Nau, W.M. Cucurbiturils: From synthesis to high-affinity binding and catalysis. Chem. Soc. Rev. 2015, 44, 394–418. [Google Scholar] [CrossRef] [Green Version]
- Gandolfi, C.; Heckenroth, M.; Neels, A.; Laurenczy, G.; Albrecht, M. Chelating NHC Ruthenium(II) Complexes as Robust Homogeneous Hydrogenation Catalysts. Organometallics 2009, 28, 5112–5121. [Google Scholar] [CrossRef] [Green Version]
- Leonard, N.J.; Kazmierczak, F.; Rykowski, A.Z. Convenient synthesis of linear benzopurines through a common intermediate. J. Org. Chem. 1987, 52, 2933–2935. [Google Scholar] [CrossRef]
- Shelton, K.L.; DeBord, M.A.; Wagers, P.O.; Southerland, M.R.; Williams, T.M.; Robishaw, N.K.; Shriver, L.P.; Tessier, C.A.; Panzner, M.J.; Youngs, W.J. Synthesis, anti-proliferative activity, SAR study, and preliminary in vivo toxicity study of substituted N,N′-bis(arylmethyl)benzimidazolium salts against a panel of non-small cell lung cancer cell lines. Bioorg. Med. Chem. 2017, 25, 421–439. [Google Scholar] [CrossRef] [Green Version]
- Wentzel, M.T.; Hewgley, J.B.; Kamble, R.M.; Wall, P.D.; Kozlowski, M.C. ChemInform Abstract: Copper-Catalyzed N-Arylation of Hindered Substrates under Mild Conditions. Adv. Synth. Catal. 2009, 351, 931–937. [Google Scholar] [CrossRef]
- Keita, H. Adamantane-Functionalized Phthalimide Scaffold: Pathways to Supramolecular Interactions and Drug Discovery. Organics 2021, 2, 388–394. [Google Scholar] [CrossRef]
- Viciu, M.S.; Stevens, E.D.; Petersen, J.L.; Nolan, S.P. N-Heterocyclic Carbene Palladium Complexes Bearing Carboxylate Ligands and Their Catalytic Activity in the Hydroarylation of Alkynes. Organometallics 2004, 23, 3752–3755. [Google Scholar] [CrossRef]
- Jang, W.J.; Kang, B.; Lee, J.H.; Choi, Y.M.; Kim, C.; Yun, J. NHC-copper-thiophene-2-carboxylate complex for the hydroboration of terminal alkynes. Org. Biomol. Chem. 2019, 17, 5249–5252. [Google Scholar] [CrossRef] [PubMed]
- Endo, K.; Herbert, M.B.; Grubbs, R.H. Investigations into Ruthenium Metathesis Catalysts with Six-Membered Chelating NHC Ligands: Relationship between Catalyst Structure and Stereoselectivity. Organometallics 2013, 32, 5128–5135. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keita, H. Supramolecular Immobilization of Adamantyl and Carboxylate Modified N-Heterocyclic Carbene Ligand on Cucurbituril Substrates. Molecules 2022, 27, 1662. https://doi.org/10.3390/molecules27051662
Keita H. Supramolecular Immobilization of Adamantyl and Carboxylate Modified N-Heterocyclic Carbene Ligand on Cucurbituril Substrates. Molecules. 2022; 27(5):1662. https://doi.org/10.3390/molecules27051662
Chicago/Turabian StyleKeita, Hamidou. 2022. "Supramolecular Immobilization of Adamantyl and Carboxylate Modified N-Heterocyclic Carbene Ligand on Cucurbituril Substrates" Molecules 27, no. 5: 1662. https://doi.org/10.3390/molecules27051662






