Isolation and Identification of Bioactive Compounds from Bidens spp. Using HPLC-DAD and GC-MS Analysis and Their Biological Activity as Anticancer Molecules
Abstract
:1. Introduction
| Disorder | Plant Part | Dosage Form | Region/Country | References |
|---|---|---|---|---|
| B. pilosa | ||||
| Stomach ache | LE | Not stated | Africa | [11] |
| Colic | WP | Decoction | China, Africa | [11] |
| Catarrh | WP | Juice | Cuba | [12] |
| Diarrhea | LE, WP | Decoction | Uganda, Africa | [13] |
| Constipation | WP | Decoction | India | [14] |
| Dysentery | WP | Infusion | Africa | |
| Choleretic | WP | Decoction | America | [15] |
| Antirheumatic | RT, WP | Infusion | Hong Kong | [16] |
| Appendicitis | WP | Not stated | Hong Kong | [16] |
| Enteritis | WP | Decoction | China | [17] |
| Otitis | WP | Decoction | China, Africa | [18] |
| Gastritis | WP | Juice | Cuba | [19] |
| Diabetes | WP | Decoction | Taiwan, Cuba | [11] |
| Headache | WP | Decoction | Bafia, Cameroon | [11] |
| Diuretic | WP | Decoction | Central America | [20] |
| Hypotensive | WP | Juice | Cameroon | [21] |
| Fever | WP | Decoction | Not stated | [19] |
| Yellow Fever | LE, WP | Decoction | America | [13] |
| Acute hepatitis | WP | Decoction | Hong Kong | [22] |
| Intestinal worms | LE | Decoction | Africa | [14] |
| Malaria | WP | Juice | China | [18] |
| Eye diseases | LE | Juice | Uganda | [13] |
| B. biternata | ||||
| Leprosy | LE | Not stated | India | [23] |
| Cuts and wounds | LE | Decoction | India | [23] |
| Nose bleeds | WP | Decoction | China | [23] |
| Gastric ulcers | LE | Maceration taken orally | Central America | [10] |
| Skin problems | WP | Topical application | Africa | [24] |
| Wounds | WP | Crushed herb | China | [24] |
| Snake bites | WP | Crushed herb | China | [23] |
| B. bipinnata L. | ||||
| Asthma | WP | Decoction | China | [25] |
| Colds | LE | Decoction | China | [25] |
| Fever | WP | Decoction | Not stated | [9] |
| Antimicrobial | AP | Decoction | Trinidad | [26] |
| Eye Diseases | LE | Juice | Uganda | [13] |
| Colds | LE, WP | Decoction | Uganda, China | [21] |
2. Material and Method
2.1. Formation of Crude Methanolic Plant Extract
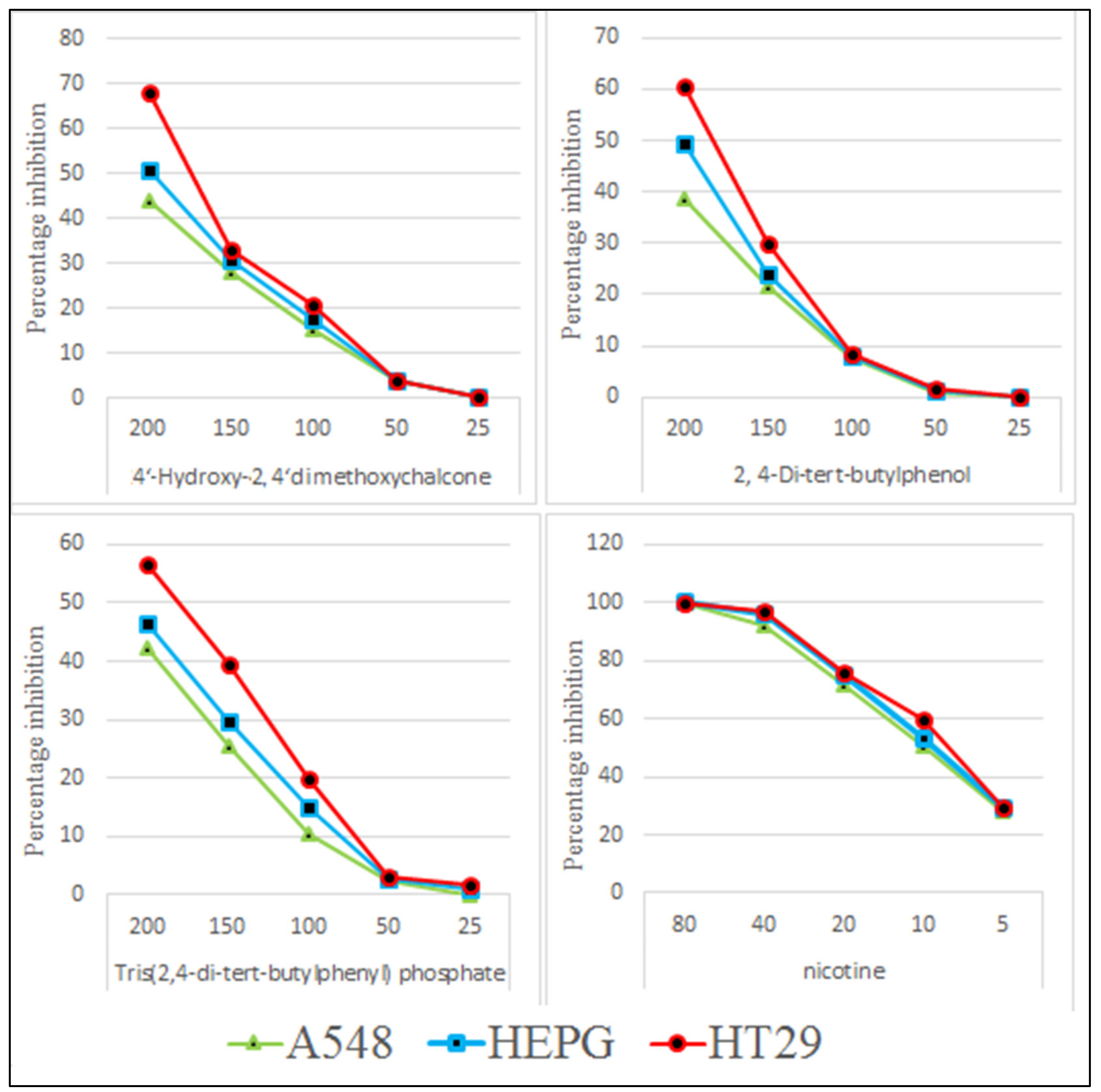
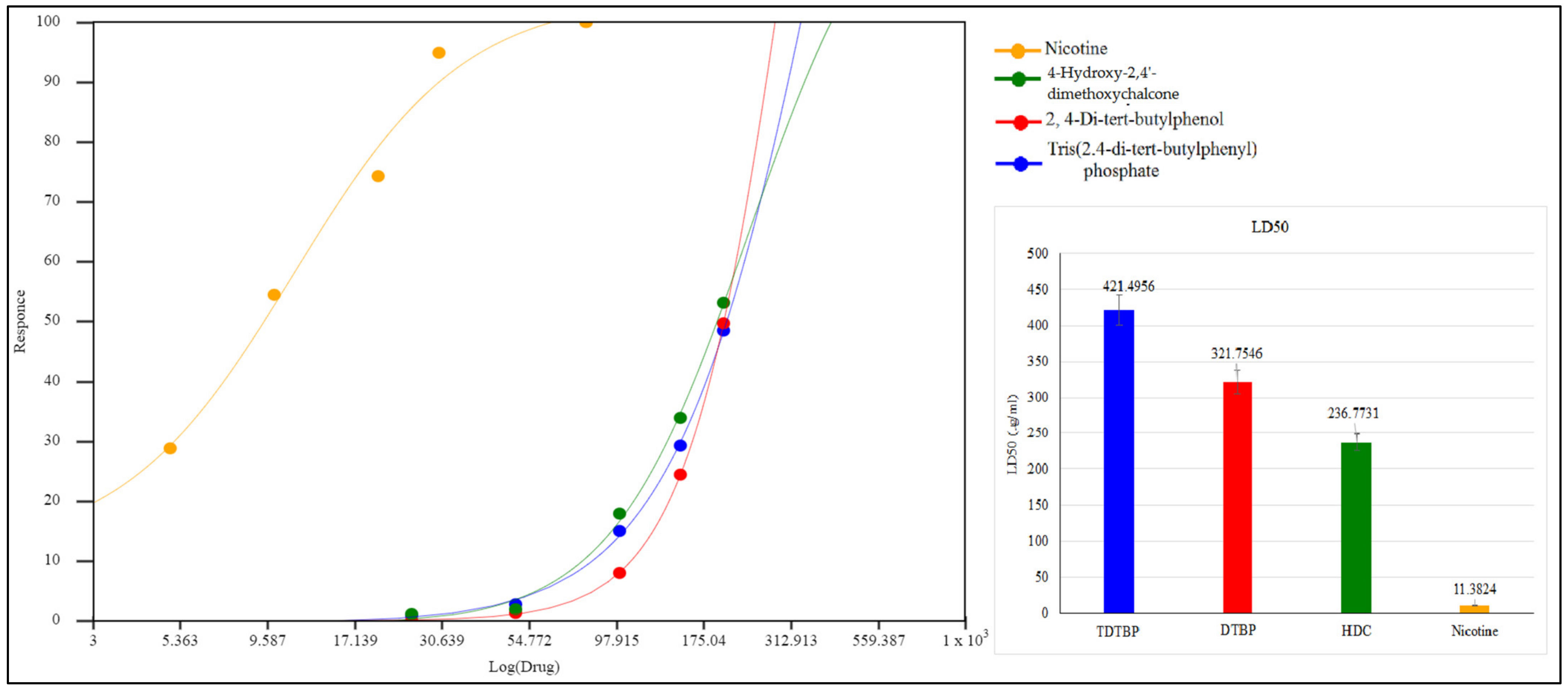
2.2. In-Vitro Cytotoxicity Assay
Thiazolyl Blue Tetrazolium Bromide (MTT)
2.3. In-Vivo Cytotoxicity Assay Caenorhabditis elegans
2.4. In Vivo Cytotoxicity Assay on Zebrafish
2.5. In Vivo Cytotoxicity Assay on Brine Shrimp
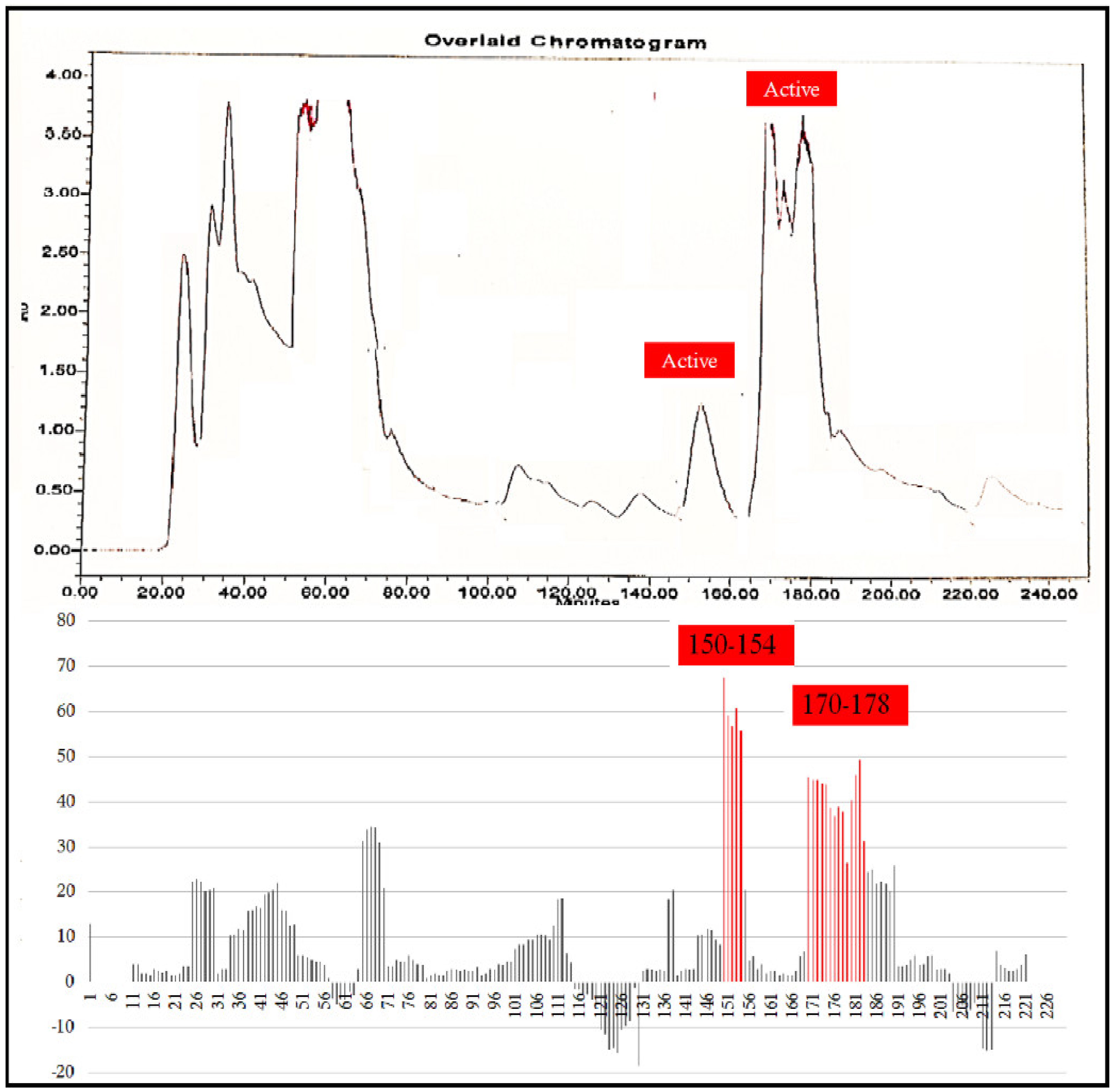
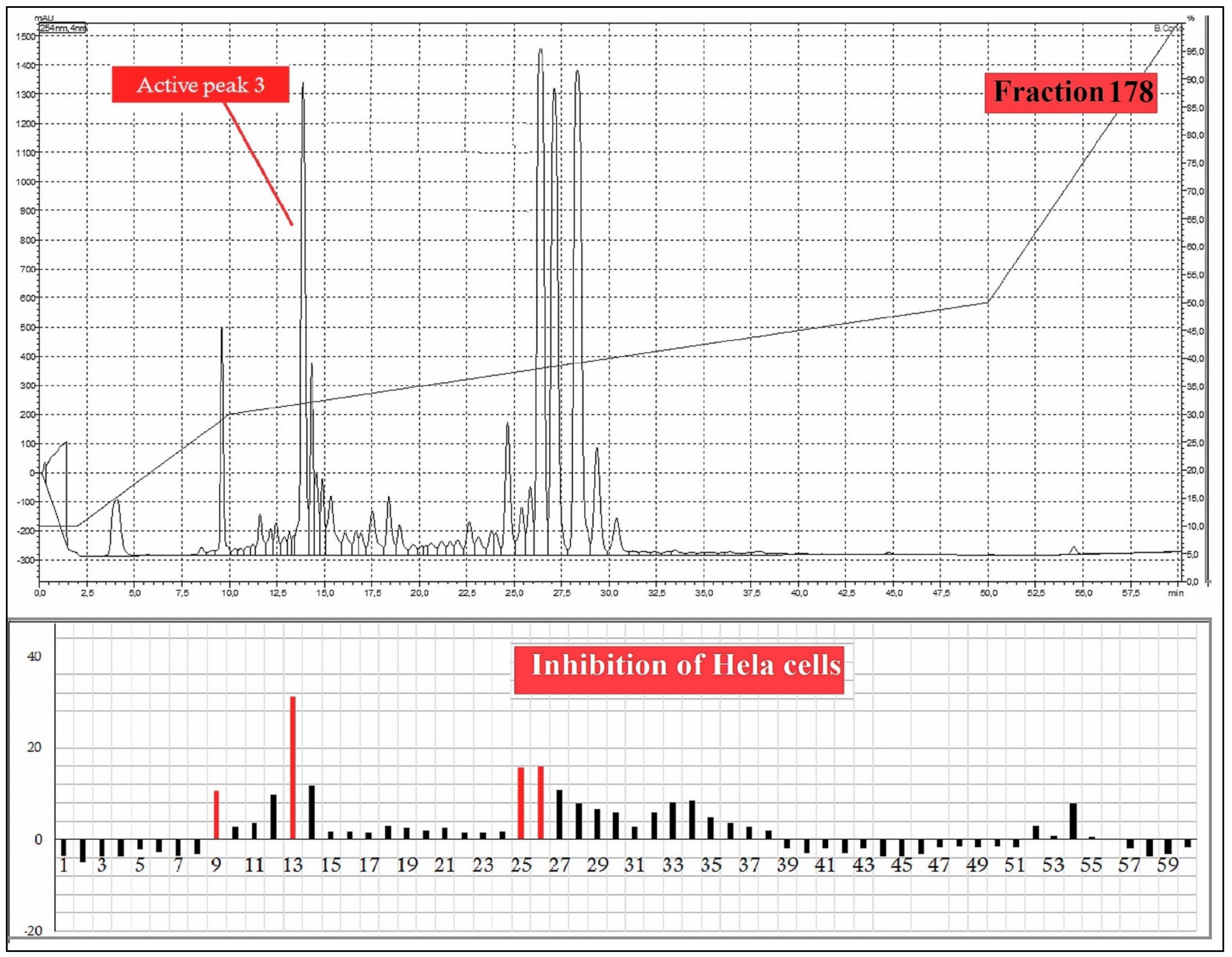
2.6. Bioassay-Guided Purification
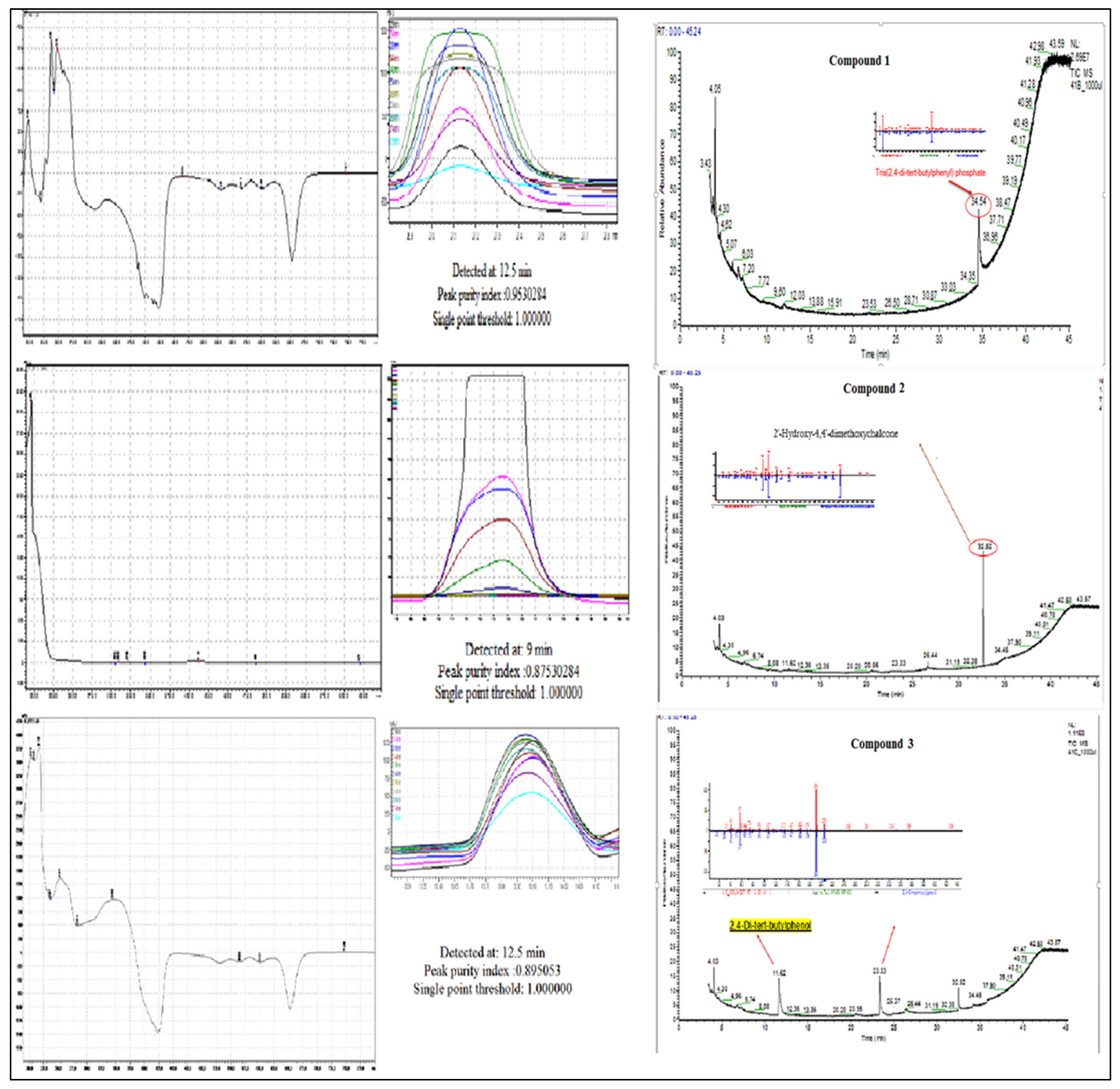
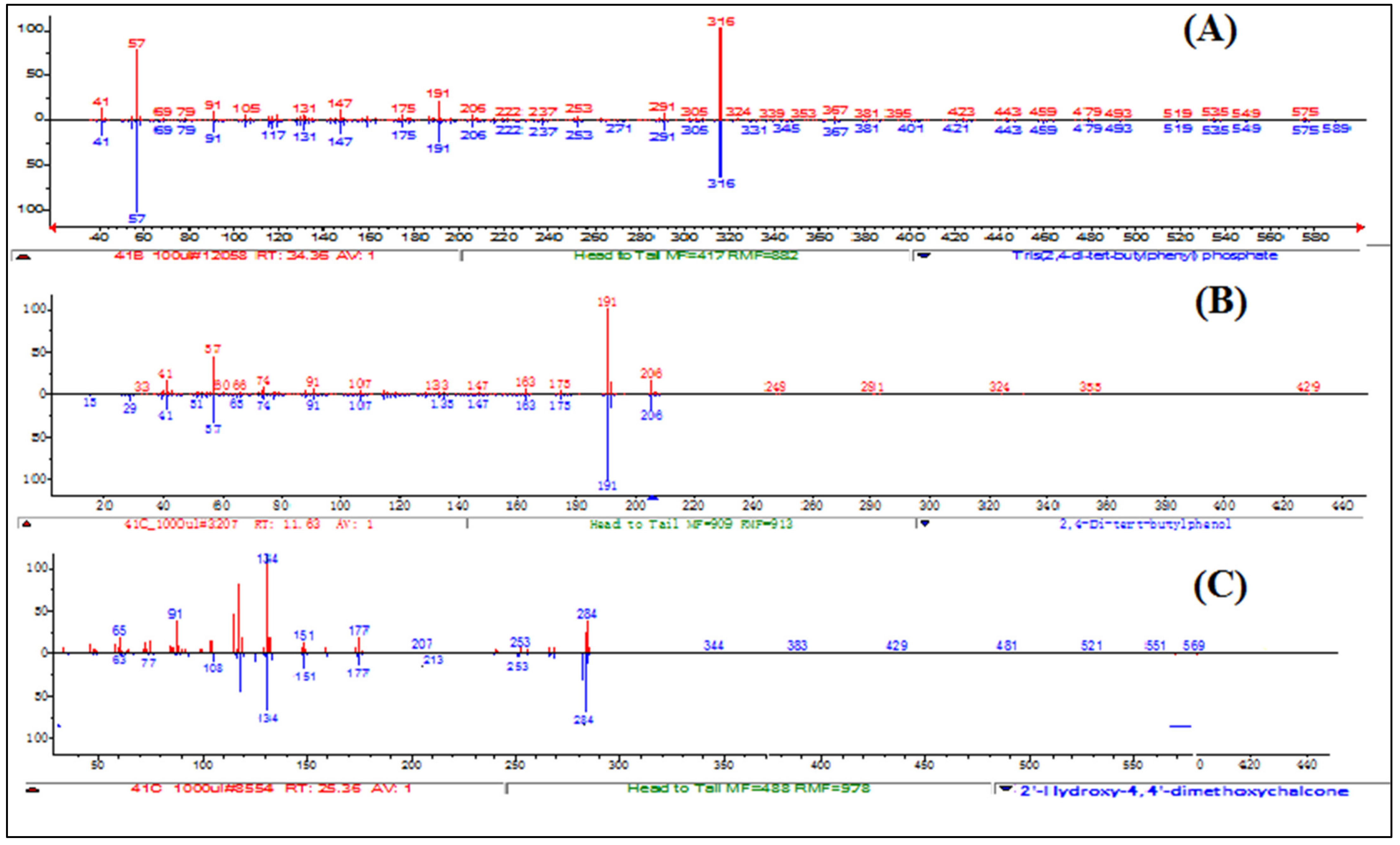
2.7. Identification of Isolated Compound
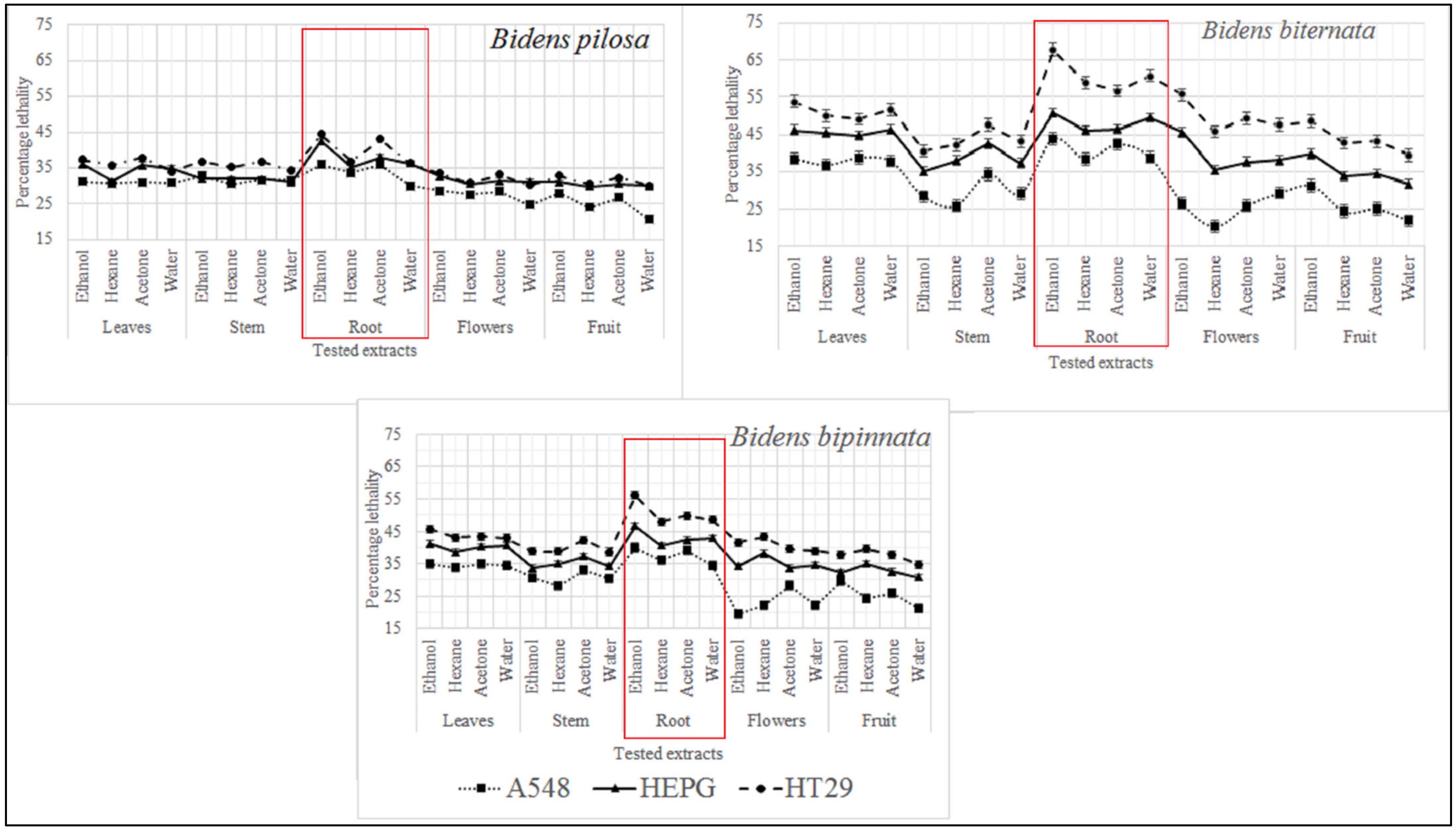
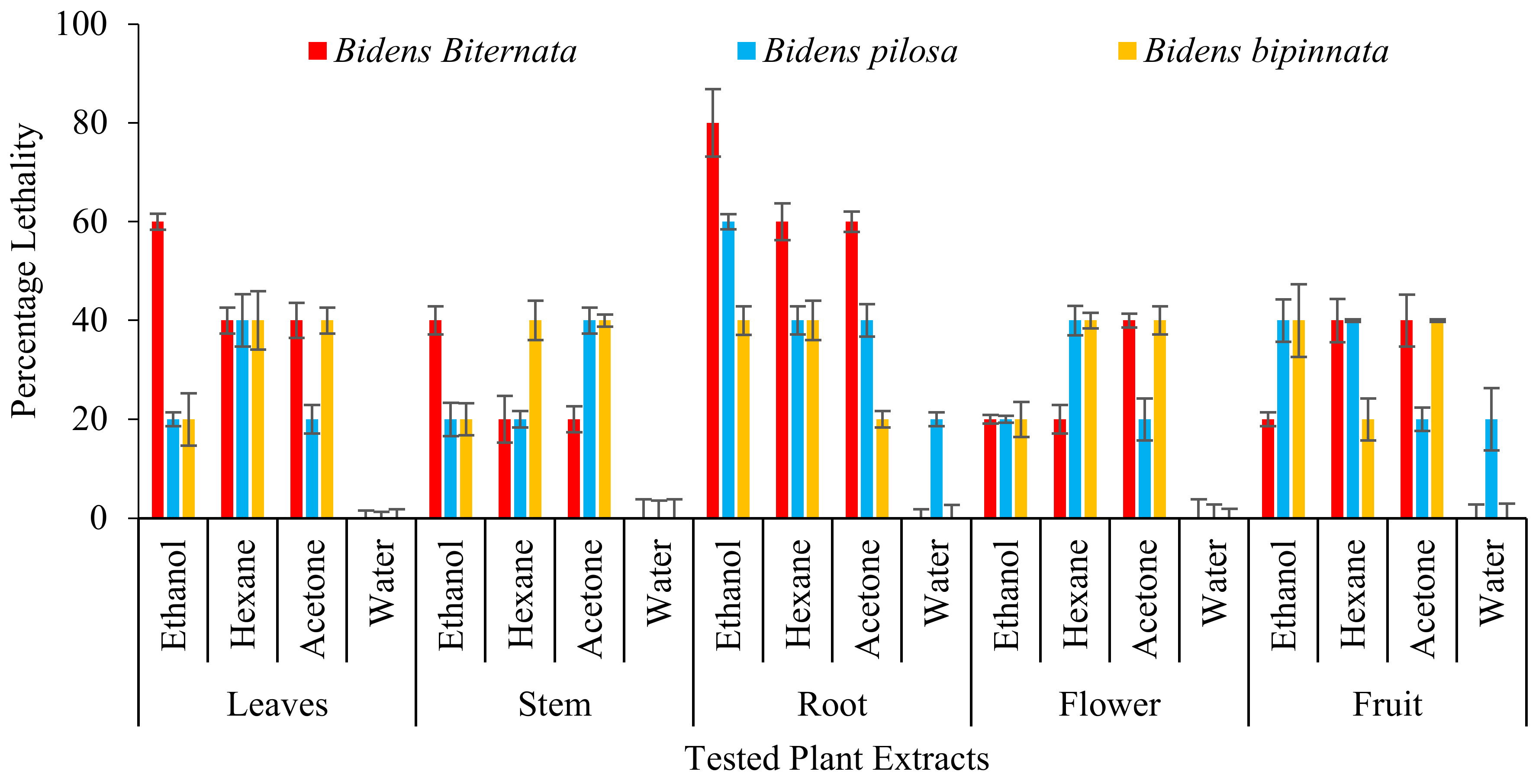
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly) phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxidants 2013, 18, 1818–1892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Efferth, T.; Oesch, F. Repurposing of plant alkaloids for cancer therapy: Pharmacology and toxicology. In Seminars in Cancer Biology; Elsevier: Amsterdam, The Netherlands, 2021; pp. 143–163. [Google Scholar]
- Zahara, K.; Bibi, Y.; Arshad, M.; Kaukab, G.; Ayoubi, S.A.; Qayyum, A. In-vitro examination and isolation of antidiarrheal compounds using five bacterial strains from invasive species Bidens bipinnata L. Saudi J. Biol. Sci. 2022, 29, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Maarfia, S.; Zellagui, A. Study of Essential Oils and Phenolic Compounds Their Changes and Anticancer Activity in Some Species Belonging to Asteraceae and Lamiaceae Families. 2019. Available online: http://bib.univ-oeb.dz:8080/jspui/handle/123456789/9129 (accessed on 6 March 2022).
- Matos, M.J.; Santana, L.; Uriarte, E.; Abreu, O.A.; Molina, E.; Yordi, E.G. Coumarins—An important class of phytochemicals. Phytochem.-Isol. Charact. Role Hum. Health 2015, 25, 113–140. [Google Scholar]
- Yang, M.-T.; Lin, Y.-X.; Yang, G.; Kuo, T.-F.; Liang, Y.-C.; Lee, T.-H.; Chang, C.L.-T.; Yang, W.-C. Functional and Mechanistic Studies of Two Anti-coccidial Herbs, Bidens pilosa and Artemisia indica. Planta Med. 2021. [Google Scholar] [CrossRef] [PubMed]
- Deba, F.; Xuan, T.D.; Yasuda, M.; Tawata, S. Chemical composition and antioxidant, antibacterial and antifungal activities of the essential oils from Bidens pilosa Linn. var. Radiata. Food Control 2008, 19, 346–352. [Google Scholar] [CrossRef]
- Yuan, L.p.; Chen, F.h.; Ling, L.; Bo, H.; Chen, Z.w.; Li, F.; Zhong, M.m.; Xia, L.j. Protective effects of total flavonoids of Bidens bipinnata L. against carbon tetrachloride-induced liver fibrosis in rats. J. Pharm. Pharmacol. 2008, 60, 1393–1402. [Google Scholar] [CrossRef]
- Sukumaran, P.; Nair, A.G.; Chinmayee, D.M.; Mini, I.; Sukumaran, S.T. Phytochemical Investigation of Bidens biternata (Lour.) Merr. and Sheriff.—A Nutrient-Rich Leafy Vegetable from Western Ghats of India. Appl. Biochem. Biotechnol. 2012, 167, 1795–1801. [Google Scholar] [CrossRef]
- Bartolome, A.P.; Villaseñor, I.M.; Yang, W.-C. Bidens pilosa L. (Asteraceae): Botanical properties, traditional uses, phytochemistry, and pharmacology. Evid.-Based Complement. Altern. Med. 2013, 2013, 340215. [Google Scholar] [CrossRef] [Green Version]
- Pérez, M.; Boffill, M.A.; Morón, F.J.; Sueiro, M.L.; Marrero, E.; Betancourt, E. Ethnopharmacological and preclinical study of diuretic activity inmedicinal and food plants used by cuban population. Emir. J. Food Agric. 2011, 23, 214–221. [Google Scholar]
- Orwa, J.A.; Jondiko, I.; Minja, R.J.; Bekunda, M. The use of Toddalia asiatica (L) Lam. (Rutaceae) in traditional medicine practice in East Africa. J. Ethnopharmacol. 2008, 115, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Grierson, D.; Afolayan, A. Antibacterial activity of some indigenous plants used for the treatment of wounds in the Eastern Cape, South Africa. J. Ethnopharmacol. 1999, 66, 103–106. [Google Scholar] [CrossRef]
- Pachter, L.M.; Cloutier, M.M.; Bernstein, B.A. Ethnomedical (folk) remedies for childhood asthma in a mainland Puerto Rican community. Arch. Pediatr. Adolesc. Med. 1995, 149, 982–988. [Google Scholar] [CrossRef]
- Zahara, K.; Bibi, Y.; Masood, S.; Nisa, S.; Qayyum, A.; Ishaque, M.; Shahzad, K.; Ahmed, W.; Shah, Z.H.; Alsamadany, H.; et al. Using HPLC–DAD and GC–MS analysis isolation and identification of anticandida compounds from Gui Zhen Cao Herbs (Genus Bidens): An important Chinese medicinal formulation. Molecules 2021, 26, 5820. [Google Scholar] [CrossRef]
- Anyinam, C. Ecology and ethnomedicine: Exploring links between current environmental crisis and indigenous medical practices. Soc. Sci. Med. 1995, 40, 321–329. [Google Scholar] [CrossRef]
- Romero-Benavides, J.C.; Ruano, A.L.; Silva-Rivas, R.; Castillo-Veintimilla, P.; Vivanco-Jaramillo, S.; Bailon-Moscoso, N. Medicinal plants used as anthelmintics: Ethnomedical, pharmacological, and phytochemical studies. Eur. J. Med. Chem. 2017, 129, 209–217. [Google Scholar] [CrossRef]
- Fornet, A.R. Ethnobotany in HolguÃn, Cuba: Cultural and natural heritage to be preserved. Rev. Cuba. Plantas Med. 2018, 22. [Google Scholar]
- Svetaz, L.; Zuljan, F.; Derita, M.; Petenatti, E.; Tamayo, G.; Cáceres, A.; Cechinel Filho, V.; Giménez, A.; Pinzón, R.; Zacchino, S.A. Value of the ethnomedical information for the discovery of plants with antifungal properties. A survey among seven Latin American countries. J. Ethnopharmacol. 2010, 127, 137–158. [Google Scholar] [CrossRef] [PubMed]
- Nole, T.; Lionel, T.; Cedrix, T.; Gabriel, A. Ethnomedical and ethnopharmacological study of plants used for potential treatments of diabetes and arterial hypertension by indigenous people in three phytogeographic regions of Cameroon. Diabetes Case Rep. 2016, 1, 2. [Google Scholar] [CrossRef] [Green Version]
- Fabricant, D.S.; Farnsworth, N.R. The value of plants used in traditional medicine for drug discovery. Environ. Health Perspect. 2001, 109, 69–75. [Google Scholar]
- Zahara, K.; Bibi, Y.; Tabassum, S.; Mudrikah; Bashir, T.; Haider, S.; Araa, A.; Ajmal, M. A review on pharmacological properties of Bidens biternata: A potential nutraceutical. Asian Pac. J. Trop. Dis. 2015, 5, 595–599. [Google Scholar] [CrossRef]
- Sharma, A.; Bargali, K.; Pande, N. The allelopathic potential of bryophyte extract on seed germination and seedling growth of Bidens biternata. Nat. Sci. 2009, 7, 30–38. [Google Scholar]
- Wang, R.; Yan, W.; Quan, G.; Liu, S.; Zhang, J. Effects of light intensity on morphology and physiology of exotic invasive Bidens pilosa L. and non-invasive congener Bidens bipinnata L. Allelopath. J. 2017, 42, 157–168. [Google Scholar] [CrossRef]
- Lans, C. Comparison of plants used for skin and stomach problems in Trinidad and Tobago with Asian ethnomedicine. J. Ethnobiol. Ethnomed. 2007, 3, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ismail, N.; Ab Ghani, N.; Rusli, S.; Abdullah, N.; Awang, R. Cytotoxicity assessment of zirconia-reinforced experimental nanohybrid dental composite using MTT assay. Ann. Rom. Soc. Cell Biol. 2021, 2021, 14878–14886. [Google Scholar]
- Gagman, H.A.; Him, N.A.I.I.N.; Ahmad, H.; Sulaiman, S.F.; Zakaria, R.; Termizi, F.H.M. In Vitro Efficacy of Aqueous and Methanol Extract of Cassia siamea against the Motility of Caenorhabditis elegans. Trop. Life Sci. Res. 2020, 31, 145–159. [Google Scholar] [CrossRef]
- Van Dyck, A.; Bollaerts, I.; Beckers, A.; Vanhunsel, S.; Glorian, N.; van Houcke, J.; van Ham, T.J.; De Groef, L.; Andries, L.; Moons, L. Müller glia–myeloid cell crosstalk accelerates optic nerve regeneration in the adult zebrafish. Glia 2021, 69, 1444–1463. [Google Scholar] [CrossRef]
- Sasidharan, R.; Gerstein, M. Protein fossils live on as RNA. Nature 2008, 453, 729–731. [Google Scholar] [CrossRef]
- Van Wyk, B.-E.; Wink, M. Medicinal Plants of the World; CABI: Wallingford, UK, 2018. [Google Scholar]
- Pengelly, A. The Constituents of Medicinal Plants; CABI: Wallingford, UK, 2021. [Google Scholar]
- Karagöz, A.; Turgut-Kara, N.; Çakır, Ö.; Demirgan, R.; Arı, Ş. Cytotoxic activity of crude extracts from Astragalus chrysochlorus (Leguminosae). Biotechnol. Biotechnol. Equip. 2007, 21, 220–222. [Google Scholar] [CrossRef]
- Nemati, F.; Dehpouri, A.A.; Eslami, B.; Mahdavi, V.; Mirzanejad, S. Cytotoxic properties of some medicinal plant extracts from Mazandaran, Iran. Iran. Red Crescent Med. J. 2013, 15, e8871. [Google Scholar] [CrossRef] [Green Version]
- Di Paolo, C.; Seiler, T.-B.; Keiter, S.; Hu, M.; Muz, M.; Brack, W.; Hollert, H. The value of zebrafish as an integrative model in effect-directed analysis—A review. Environ. Sci. Eur. 2015, 27, 8. [Google Scholar] [CrossRef] [Green Version]
- Ganguly, P.; Breen, A.; Pillai, S.C. Toxicity of nanomaterials: Exposure, pathways, assessment, and recent advances. ACS Biomater. Sci. Eng. 2018, 4, 2237–2275. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-Y.; Chen, G.-R.; Pan, C.-X.; Deng, Z.-Y.; Ge, J.-F.; Li, N.; Chen, F.-H. Polyacetylenes from Bidens bipinnata L. and their biological activities. Phytochem. Lett. 2014, 7, 198–201. [Google Scholar] [CrossRef]
- Mei, W.-L.; Zeng, Y.-B.; Liu, J.; Dai, H.-F. GC-MS analysis of volatile constituents from five different kinds of Chinese eaglewood. J. Chin. Med. Mater. 2007, 30, 551–555. [Google Scholar]
- Liu, H. Study on the Chemical Composition Analysis and ISSR Molecular Marker of Chimonanthus. Master’s Thesis, Zhejiang Forestry University, Hangzhou, China, 2013. [Google Scholar]
- Gao, Y.; Guo, L.; Meng, X.; Zhang, L.; Yang, G. The optimal GC-MS analysis of essential oil (fresh, dried and bud) and aroma enhanced by β-glucosidase on Aesculus chinensis flowers. Prod. Specif. Importing China 2018, 5, 1–4. [Google Scholar]
- Chen, H.; Peng, Y.; Liu, G.; Li, H.; Gao, L.; Zhan, N.; Xie, Y. Dynamic changes of volatile components from developing seeds of Plukenetia volubilis. Sci. Silvae Sin. 2018, 54, 157–168. [Google Scholar]
- Ndiege, M.L.; Kengara, F.; Maiyoh, G.K. Characterization of Phenolic Compounds from Leaf Extract of Bidens pilosa Linn. Var. Radiata. South Asian Res. J. Nat. Prod. 2021, 4, 44–58. [Google Scholar]
- Freitas, S.; Costa, S.; Azevedo, C.; Carvalho, G.; Freire, S.; Barbosa, P.; Velozo, E.; Schaer, R.; Tardy, M.; Meyer, R. Flavonoids inhibit angiogenic cytokine production by human glioma cells. Phytother. Res. 2011, 25, 916–921. [Google Scholar] [CrossRef]
- Afrin, N.S.; Hossain, M.A.; Saha, K. Phytochemical screening of plant extracts and GC-MS analysis of n-Hexane soluble part of crude chloroform extract of Cuscuta reflexa (Roxb.). J. Pharmacogn. Phytochem. 2019, 8, 560–564. [Google Scholar]
- Saxena, S.; Rao, P. GC-MS screening of bioactive constituents and antioxidant profiling in an invasive weed, Malvastrum coromandelianum (L.) Garcke. Pharma Innov. 2018, 7, 738–746. [Google Scholar]
- Ding, Y.; Nguyen, H.T.; Kim, S.I.; Kim, H.W.; Kim, Y.H. The regulation of inflammatory cytokine secretion in macrophage cell line by the chemical constituents of Rhus sylvestris. Bioorgan. Med. Chem. Lett. 2009, 19, 3607–3610. [Google Scholar] [CrossRef] [PubMed]
- Nair, R.V.; Jayasree, D.V.; Biju, P.G.; Baby, S. Anti-inflammatory and anticancer activities of erythrodiol-3-acetate and 2,4-di-tert-butylphenol isolated from Humboldtia unijuga. Nat. Prod. Res. 2020, 34, 2319–2322. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zahara, K.; Bibi, Y.; Masood, S.; Nisa, S.; Sher, A.; Ali, N.; Kumar, S.; Qayyum, A.; Ahmed, W.; Sami, R.; et al. Isolation and Identification of Bioactive Compounds from Bidens spp. Using HPLC-DAD and GC-MS Analysis and Their Biological Activity as Anticancer Molecules. Molecules 2022, 27, 1927. https://doi.org/10.3390/molecules27061927
Zahara K, Bibi Y, Masood S, Nisa S, Sher A, Ali N, Kumar S, Qayyum A, Ahmed W, Sami R, et al. Isolation and Identification of Bioactive Compounds from Bidens spp. Using HPLC-DAD and GC-MS Analysis and Their Biological Activity as Anticancer Molecules. Molecules. 2022; 27(6):1927. https://doi.org/10.3390/molecules27061927
Chicago/Turabian StyleZahara, Kulsoom, Yamin Bibi, Saadia Masood, Sobia Nisa, Ahmad Sher, Naushad Ali, Sunjeet Kumar, Abdul Qayyum, Waseem Ahmed, Rokayya Sami, and et al. 2022. "Isolation and Identification of Bioactive Compounds from Bidens spp. Using HPLC-DAD and GC-MS Analysis and Their Biological Activity as Anticancer Molecules" Molecules 27, no. 6: 1927. https://doi.org/10.3390/molecules27061927







