Effects of Artonin E on Cell Growth Inhibition and Apoptosis Induction in Colon Cancer LoVo and HCT116 Cells
Abstract
:1. Introduction
2. Results
2.1. Artonin E Inhibited Cell Proliferation in LoVo and HCT116 Cells
2.2. Effect of Artonin E on Apoptosis Induction in LoVo and HCT116 Cells
2.3. Mitochondrial Membrane Potential (ΔΨm)
2.4. Cell Cycle Distribution
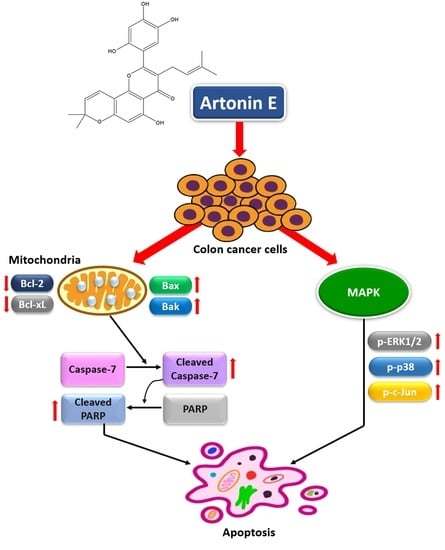
2.5. Expression of Bcl-2 Family, Caspase-7, and Cleaved-PARP in LoVo and HCT116 Cells
2.6. Effect of Artonin E on MAPK Protein Expression
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture
4.3. Cell Proliferation and Cell Viability Assays
4.4. Detection of Nuclear Morphological Changes
4.5. Detection of Mitochondrial Membrane Potential (ΔΨm)
4.6. Cell Cycle Analysis
4.7. Western Blot Analysis
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Roy, P.S.; Saikia, B.J. Cancer and cure: A critical analysis. Indian J. Cancer 2016, 53, 441–442. [Google Scholar]
- Farrell, C. Bone metastases: Assessment, management and treatment options. Br. J. Nurs. 2013, 22, S4–S11. [Google Scholar] [CrossRef]
- Franssen, L.C.; Lorenzi, T.; Burgess, A.E.F.; Chaplain, M.A.J. A Mathematical Framework for Modelling the Metastatic Spread of Cancer. Bull. Math. Biol. 2019, 81, 1965–2010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seyfried, T.N.; Huysentruyt, L.C. On the origin of cancer metastasis. Crit. Rev. Oncog. 2013, 18, 43–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anand, P.; Kunnumakkara, A.B.; Sundaram, C.; Harikumar, K.B.; Tharakan, S.T.; Lai, O.S.; Sung, B.; Aggarwal, B.B. Cancer is a preventable disease that requires major lifestyle changes. Pharm. Res. 2008, 25, 2097–2116. [Google Scholar] [CrossRef] [PubMed]
- Adjiri, A. DNA Mutations may not be the cause of cancer. Oncol. Ther. 2017, 5, 85–101. [Google Scholar] [CrossRef]
- Virani, S.; Bilheem, S.; Chansaard, W.; Chitapanarux, I.; Daoprasert, K.; Khuanchana, S. National and subnational population-based incidence of cancer in Thailand: Assessing Cancers with the Highest Burdens. Cancers 2017, 9, 108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishra, J.; Drummond, J.; Quazi, S.H.; Karanki, S.S.; Shaw, J.J.; Chen, B. Prospective of colon cancer treatments and scope for combinatorial approach to enhanced cancer cell apoptosis. Crit. Rev. Oncol. Hematol. 2013, 86, 232–250. [Google Scholar] [CrossRef] [Green Version]
- Goldar, S.; Khaniani, M.; Derakhshan, S.; Baradaran, B. Molecular mechanisms of apoptosis and roles in cancer development and treatment. Asian Pac. J. Cancer Prev. 2015, 16, 2129–2144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, P.; Lavery, A.; Turkington, R.C. An overview of acute gastrointestinal side effects of systemic anti-cancer therapy and their management. Best Pract. Res. Clin. Gastroenterol. 2020, 48, 101691. [Google Scholar] [CrossRef]
- Rahman, M.A.; Ramli, F.; Karimian, H.; Dehghan, F.; Nordin, N.; Ali, H.M. Artonin E induces apoptosis via mitochondrial dysregulation in SKOV-3 Ovarian cancer cells. PLoS ONE 2016, 11, e0151466. [Google Scholar] [CrossRef] [Green Version]
- Daus, M.; Chaithada, P.; Phongpaichit, S.; Watanapokasin, R.; Carroll, A.R.; Mahabusarakam, W. New prenylated dihydrochalcones from the leaves of Artocarpus elasticus. Phytochem. Lett. 2017, 19, 226–230. [Google Scholar] [CrossRef]
- Plaibua, K.; Pongrakhananon, V.; Chunhacha, P.; Sritularak, B.; Chanvorachote, P. Effects of artonin e on migration and in-vasion capabilities of human lung cancer cells. Anticancer Res. 2013, 33, 3078–3088. [Google Scholar]
- Duruisseaux, M.; Esteller, M. Lung cancer epigenetics: From knowledge to applications. Semin. Cancer Biol. 2018, 51, 116–128. [Google Scholar] [CrossRef] [PubMed]
- Etti, I.C.; Abdullah, R.; Kadir, A.; Hashim, N.M.; Yeap, S.K.; Imam, M.U. The molecular mechanism of the anticancer effect of Artonin E in MDA-MB 231 triple negative breast cancer cells. PLoS ONE 2017, 12, e0182357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cargnello, M.; Roux, P.P. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol. Mol. Biol. Rev. 2011, 75, 50–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sophonnithiprasert, T.; Mahabusarakam, W.; Watanapokasin, R. Artonin E sensitizes TRAIL-induced apoptosis by DR5 upregulation and cFLIP downregulation in TRAIL-refractory colorectal cancer LoVo cells. J. Gastrointest. Oncol. 2019, 10, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Camarillo, C.; Ocampo, E.A.; Casamichana, M.L.; Perez-Plasencia, C.; Alvarez-Sanchez, E.; Marchat, L.A. Protein kinases and transcription factors activation in response to UV-radiation of skin: Implications for carcinogenesis. Int. J. Mol. Sci. 2012, 13, 142–172. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, J.; Davis, B.; Kiechle, F. Hoechst 33342 induces apoptosis in HL-60 cells and inhibits topoisomerase I in vivo. Arch. Pathol. Lab. Med. 1999, 123, 921–927. [Google Scholar] [CrossRef]
- Bucevičius, J.; Lukinavičius, G.; Gerasimaitė, R. The use of Hoechst dyes for DNA staining and beyond. Chemosensors 2018, 6, 18. [Google Scholar] [CrossRef] [Green Version]
- Longley, D.; Johnston, P. Molecular mechanisms of drug resistance. J. Pathol. 2005, 205, 275–292. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, D.; Humblet, Y.; Siena, S.; Khayat, D.; Bleiberg, H.; Santoro, A. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N. Engl. J. Med. 2004, 351, 337–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bardelli, A.; Siena, S. Molecular mechanisms of resistance to cetuximab and panitumumab in colorectal cancer. J. Clin. Oncol. 2010, 28, 1254–1261. [Google Scholar] [CrossRef] [PubMed]
- Etti, I.C.; Rasedee, A.; Hashim, N.M.; Abdul, A.B.; Kadir, A.; Yeap, S.K.; Waziri, P.; Malami, I.; Lim, K.L.; Etti, C.J. Artonin E induces p53-independent G1 cell cycle arrest and apoptosis through ROS-mediated mitochondrial pathway and livin suppression in MCF-7 cells. Drug. Des. Dev. Ther. 2017, 20, 865–879. [Google Scholar] [CrossRef] [Green Version]
- Cory, S.; Adams, J.M. The Bcl2 family: Regulators of the cellular life-or-death switch. Nat. Rev.Cancer. 2002, 2, 647–656. [Google Scholar] [CrossRef]
- Lavrik, I.N.; Golks, A.; Krammer, P.H. Caspases: Pharmacological manipulation of cell death. J. Clin. Investig. 2005, 115, 2665–2672. [Google Scholar] [CrossRef]
- Innajak, S.; Tangchirakhaphan, S.; Nilwarangoon, S.; Tanjapatkul, N.; Mahabusarakam, W.; Watanapokasin, R. Anti-Proliferation and Apoptosis Induction in Epidermoid Carcinoma A431 Cells by Artonin E. J. Med. Assoc. Thail. 2017, 100, 54. [Google Scholar]
- Krishan, A. Rapid flow cytofluorometric analysis of mammalian cell cycle by propidium iodide staining. J. Cell. Biol. 1975, 66, 188–193. [Google Scholar] [CrossRef] [Green Version]
- Al-Zharani, M.; Nasr, F.A.; Alqahtani, A.S.; Cordero, M.A.W.; Alotaibi, A.A.; Bepari, A. In Vitro Cytotoxic Evaluation and Apoptotic Effects of Datura innoxia Grown in Saudi Arabia and Phytochemical Analysis. Appl. Sci. 2021, 11, 2864. [Google Scholar] [CrossRef]
- Guon, T.-E.; Chung, H.S. Induction of Apoptosis with Kigelia africanafruits in HCT116 Human Colon Cancer Cells via MAPKs Signaling Pathway. Nat. Prod. Sci. 2016, 22, 209–215. [Google Scholar] [CrossRef] [Green Version]
- Yu, S.W.; Wang, H.; Poitras, M.F.; Coombs, C.; Bowers, W.J.; Federoff, H.J.; Poirier, G.G.; Dawson, T.M.; Dawson, V.L. Mediation of poly (ADPribose) polymerase-1-dependent cell death by apoptosis-inducing factor. Science 2002, 297, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Yue, J.; López, J.M. Understanding MAPK Signaling Pathways in Apoptosis. Int. J. Mol. Sci. 2020, 21, 2346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tangchirakhaphan, S.; Innajak, S.; Nilwarangkoon, S.; Tanjapatkul, N.; Mahabusrakum, W.; Watanapokasin, R. Mechanism of apoptosis induction associated with ERK1/2 upregulation via goniothalamin in melanoma cells. Exp. Ther. Med. 2018, 15, 3052–3058. [Google Scholar] [CrossRef] [PubMed] [Green Version]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yangnok, K.; Innajak, S.; Sawasjirakij, R.; Mahabusarakam, W.; Watanapokasin, R. Effects of Artonin E on Cell Growth Inhibition and Apoptosis Induction in Colon Cancer LoVo and HCT116 Cells. Molecules 2022, 27, 2095. https://doi.org/10.3390/molecules27072095
Yangnok K, Innajak S, Sawasjirakij R, Mahabusarakam W, Watanapokasin R. Effects of Artonin E on Cell Growth Inhibition and Apoptosis Induction in Colon Cancer LoVo and HCT116 Cells. Molecules. 2022; 27(7):2095. https://doi.org/10.3390/molecules27072095
Chicago/Turabian StyleYangnok, Kanyaluck, Sukanda Innajak, Ratchawin Sawasjirakij, Wilawan Mahabusarakam, and Ramida Watanapokasin. 2022. "Effects of Artonin E on Cell Growth Inhibition and Apoptosis Induction in Colon Cancer LoVo and HCT116 Cells" Molecules 27, no. 7: 2095. https://doi.org/10.3390/molecules27072095