Silibinin and Naringenin against Bisphenol A-Induced Neurotoxicity in Zebrafish Model—Potential Flavonoid Molecules for New Drug Design, Development, and Therapy for Neurological Disorders
Abstract
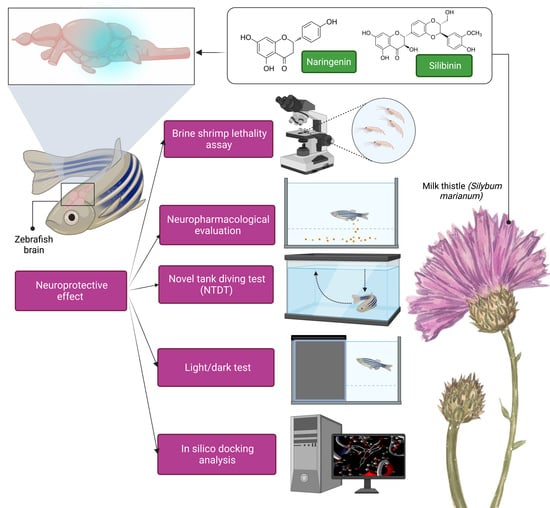
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Brine Shrimp Lethality Assay
2.3. Acute Toxicity Test
2.4. Neuropharmacological Evaluation
2.4.1. Novel Tank Diving Test (NTDT)
2.4.2. Light–Dark Test
2.5. In Silico Docking Analysis
Identification of Ligand and Protein
3. Results
3.1. Brine Shrimp Lethality Assay
3.2. Acute Toxicity Test
3.3. Neuropharmacological Evaluation in Zebrafish
3.3.1. Novel Tank Test: Silibinin and Naringenin Restores the Bottom Dwelling and Explorative Behaviour of Zebrafish following Co-Supplementation with BPA
3.3.2. Light/Dark Test
3.4. In Silico Docking Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vafeiadou, K.; Vauzour, D.; Lee, H.Y.; Rodriguez-Mateos, A.; Williams, R.J.; Spencer, J.P. The citrus flavanone naringenin inhibits inflammatory signaling in glial cells and protects against neuroinflammatory injury. Arch. Biochem. Biophys. 2009, 484, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Casadesus, G.; Shukitt-Hale, B.; Stellwagen, H.M.; Zhu, X.; Lee, H.G.; Smith, M.A.; Joseph, J.A. Modulation of hippocampal plasticity and cognitive behavior by short-term blueberry supplementation in aged rats. Nutr. Neurosci. 2004, 7, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Vafeiadou, K.; Vauzour, D.; Spencer, J.P. Neuroinflammation and its modulation by flavonoids. Endocrine, Metabolic & Immune Disorders-Drug Targets. Former. Curr. Drug Targets-Immune Endocr. Metab. Disord. 2007, 7, 211–224. [Google Scholar]
- Mehri, S.; Dadesh, Q.; Tabeshpour, J.; Hassani, F.V.; Karimi, G.; Hosseinzadeh, H. Evaluation of the neuroprotective effect of silymarin on acrylamide-induced neurotoxicity. Jundishapur. J. Nat. Pharm. Prod. 2016, 11, 55–95. [Google Scholar] [CrossRef]
- Madani, H.; Talebolhosseini, M.; Asgary, S.; Naderi, G.H. Hepatoprotective activity of Silybum marianum and Cichorium intybus against thioacetamide in rat. Pak. J. Nutr. 2008, 7, 172–176. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.I.; Narayan, M.; Barrett, J.S. Analysis and comparison of active constituents in commercial standardized silymarin extracts by liquid chromatography-electrospray ionization mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2007, 845, 9–103. [Google Scholar] [CrossRef] [PubMed]
- Federico, A.; Dallio, M.; Loguercio, C. Silymarin/silybin and chronic liver disease: A marriage of many years. Molecules 2017, 22, 191. [Google Scholar] [CrossRef] [Green Version]
- Bijak, M. Flavonolignans—Compounds not only for liver treatment. Pol. Merkur. Lekarski. 2017, 42, 34–37. [Google Scholar]
- Benavente-Garcia, O.; Castillo, J. Update on uses and properties of citrus flavonoids: New findings in anticancer, cardiovascular and anti-inflammatory activity. J. Agric. Food Chem. 2008, 56, 6185–6205. [Google Scholar] [CrossRef]
- Ferraz, C.R.; Carvalho, T.T.; Manchope, M.F.; Artero, N.A.; Rasquel-Oliveira, F.S.; Fattori, V.; Casagrande, R.; Verri, W.A. Therapeutic potential of flavonoids in pain and inflammation: Mechanisms of action, pre-clinical and clinical data and pharmaceutical development. Molecules 2020, 25, 762. [Google Scholar] [CrossRef] [Green Version]
- Bencan, Z.; Sledge, D.; Levin, E.D. Buspirone, chlordiazepoxide and diazepam effects in a zebrafish model of anxiety. Pharmacol. Biochem. Behav. 2009, 94, 75–80. [Google Scholar] [CrossRef] [Green Version]
- Pinho-Ribeiro, F.A.; Zarpelon, A.C.; Fattori, V.; Manchope, M.F.; Mizokami, S.S.; Casagrande, R.; Verri, W.A., Jr. Naringenin reduces inflammatory pain in mice. Neuropharmacology 2016, 105, 508–519. [Google Scholar] [CrossRef]
- Sun, Y.; Nakashima, M.N.; Takahashi, M.; Kuroda, N.; Nakashima, K. Determination of bisphenol A in rat brain by microdialysis and column switching high-performance liquid chromatography with fluorescence detection. Biomed. Chromatogr. 2002, 16, 319–326. [Google Scholar] [CrossRef]
- Murata, M.; Kang, J.H. Bisphenol A (BPA) and cell signaling pathways. Biotechnol. Adv. 2018, 36, 311–327. [Google Scholar] [CrossRef]
- Kalueff, A.V.; Echevarria, D.J.; Stewart, A.M. Gaining translational momentum: More zebrafish models for neuroscience research. Prog. Neuropsychopharmacol. Biol. Psychiatry 2014, 55, 1–6. [Google Scholar] [CrossRef]
- Stewart, A.M.; Braubach, O.; Spitsbergen, J.; Gerlai, R.; Kalueff, A.V. Zebrafish models for translational neuroscience research: From tank to bedside. Trends Neurosci. 2014, 37, 264–278. [Google Scholar] [CrossRef] [Green Version]
- Kabashi, E.; Brustein, E.; Champagne, N.; Drapeau, P. Zebrafish models for the functional genomics of neurogenetic disorders. Biochim. Biophys. Acta 2011, 1812, 335–345. [Google Scholar] [CrossRef] [Green Version]
- Wulliman, M.F.; Rupp, B.; Reichert, H. Neuroanatomy of the zebrafish brain: A topological Atlas. Trends Neurosci. 2012, 19, 101–115. [Google Scholar]
- Sakai, C.; Ijaz, S.; Hoffman, E.J. Zebrafish models of neurodevelopmental disorders: Past, present, and future. Front. Mol. Neurosci. 2018, 11, 294. [Google Scholar] [CrossRef] [Green Version]
- Kawato, S. Endocrine disrupters as disrupters of brain function: A neurosteroid viewpoint. Environ. Sci. 2004, 11, 1–4. [Google Scholar]
- Pradhan, L.K.; Sahoo, P.K.; Aparna, S.; Sargam, M.; Biswal, A.K.; Polai, O.; Chauhan, N.R.; Das, S.K. Suppression of bisphenol A-induced oxidative stress by taurine promotes neuroprotection and restores altered neurobehavioral response in zebrafish (Danio rerio). Environ. Toxicol. 2021, 36, 2342–2353. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, V.; Lodi, R.; Tonon, C.; D’Agata, V.; Sapienza, M.; Scapagnini, G.; Mangiameli, A.; Pennisi, G.; Stella, A.G.; Butterfield, D.A. Oxidative stress, mitochondrial dysfunction and cellular stress response in Friedreich’s ataxia. J. Neurol Sci. 2005, 233, 145–162. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, S.; Matsuzawa, D.; Ishii, D.; Tomizawa, H.; Sutoh, C.; Nakazawa, K.; Amano, K.; Sajiki, J.; Shimizu, E. Effects of perinatal exposure to low dose of bisphenol A on anxiety like behavior and dopamine metabolites in brain. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2012, 39, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Nakagami, A.; Negishi, T.; Kawasaki, K.; Imai, N.; Nishida, Y.; Ihara, T.; Kuroda, Y.; Yoshikawa, Y.; Koyama, T. Alterations in male infant behaviors towards its mother by prenatal exposure to bisphenol A in cynomolgus monkeys (Macaca fascicularis) during early suckling period. Psychoneuroendocrinology 2009, 34, 1189–1197. [Google Scholar] [CrossRef]
- Ryan, B.C.; Vandenbergh, J.G. Developmental exposure to environmental estrogens alters anxiety and spatial memory in female mice. Horm. Behav. 2006, 50, 85–93. [Google Scholar] [CrossRef]
- Ishido, M.; Masuo, Y.; Terasaki, M.; Morita, M. Rat hyperactivity by bisphenol A, but not by its derivatives, 3-hydroxybisphenol A or bisphenol A 3,4-quinone. Toxicol. Lett. 2011, 206, 300–305. [Google Scholar] [CrossRef]
- Martini, M.; Miceli, D.; Gotti, S.; Viglietti-Panzica, C.; Fissore, E.; Palanza, P.; Panzica, G. Effects of perinatal administration of bisphenol A on the neuronal nitric oxide synthase expressing system in the hypothalamus and limbic system of CD1 mice. J. Neuroendocrinol. 2010, 22, 1004–1012. [Google Scholar] [CrossRef]
- Miyagawa, K.; Narita, M.; Narita, M.; Akama, H.; Suzuki, T. Memory impairment associated with a dysfunction of the hippocampal cholinergic system induced by prenatal and neonatal exposures to bisphenol-A. Neurosci. Lett. 2007, 418, 236–241. [Google Scholar] [CrossRef]
- Lourenço, R.; Camilo, M.E. Taurine: A conditionally essential amino acid in humans? An overview in health and disease. Nutr. Hosp. 2002, 17, 262–270. [Google Scholar]
- Sarkar, P.; Basak, P.; Ghosh, S.; Kundu, M.; Sil, P.C. Prophylactic role of taurine and its derivatives against diabetes mellitus and its related complications. Food Chem. Toxicol. 2017, 110, 109–121. [Google Scholar] [CrossRef]
- Manfra, L.; Savorelli, F.; Pisapia, M.; Magaletti, E.; Cicero, A.M. Long-term lethal toxicity test with the crustacean Artemia franciscana. J. Vis. Exp. 2012, 14, e3790. [Google Scholar] [CrossRef] [Green Version]
- Déciga-Campos, M.; Rivero-Cruz, I.; Arriaga-Alba, M.; Castañeda-Corral, G.; AngelesLópez, G.E.; Navarrete, A.; Mata, R. Acute toxicity and mutagenic activity of Mexican plants used in traditional medicine. J. Ethnopharmacol. 2007, 110, 334–342. [Google Scholar] [CrossRef]
- OECD. Test No. 203: Fish, Acute Toxicity Test, OECD Guidelines for the Testing of Chemicals. Sec. 2; OECD Publishing: Paris, France, 2019. [Google Scholar]
- Vossen, L.E.; Brunberg, R.; Rådén, P.; Winberg, S.; Roman, E. The zebrafish multivariate concentric square field: A standardized test for behavioral profiling of zebrafish (Danio rerio). Front. Behav. Neurosci. 2022, 16, 744533. [Google Scholar] [CrossRef]
- Ibrahim, M.; Mussulini, B.H.; Moro, L.; de Assis, A.M.; Rosemberg, D.B.; de Oliveira, D.L.; Rocha, J.B.; Schwab, R.S.; Schneider, P.H.; Souza, D.O.; et al. Anxiolytic effects of diphenyl diselenide on adult zebrafish in a novelty paradigm. Prog. NeuroPsychopharmacol. Biol. Psychiatry 2014, 54, 187–194. [Google Scholar] [CrossRef] [Green Version]
- Egan, R.J.; Bergner, C.L.; Hart, P.C.; Cachat, J.M.; Canavello, P.R.; Elegante, M.F.; Elkhayat, S.I.; Bartels, B.K.; Tien, A.K.; Tien, D.H.; et al. Understanding behavioral and physiological phenotypes of stress and anxiety in zebrafish. Behav. Brain Res. 2009, 205, 38–44. [Google Scholar] [CrossRef] [Green Version]
- Serra, E.L.; Medalha, C.C.; Mattioli, R. Natural preference of zebrafish (Danio rerio) for a dark environment. Braz. J. Med. Biol. Res. 1999, 32, 1551–1553. [Google Scholar] [CrossRef]
- Stewart, A.M.; Ullmann, J.F.; Norton, W.H.; Parker, M.O.; Brennan, C.H.; Gerlai, R.; Kalueff, A.V. Molecular psychiatry of zebrafish. Mol. Psychiatry 2015, 20, 2–17. [Google Scholar] [CrossRef] [Green Version]
- Bernstein, F.C.; Koetzle, T.F.; Williams, G.J.; Meyer, E.F.; Brice, M.D.; Rodgers, J.R.; Tasumi, M. The Protein Data Bank: A computer-based archival file for macromolecular structures. Arch. Biochem. Biophys. 1978, 185, 584–591. [Google Scholar] [CrossRef]
- Bolton, E.E.; Wang, Y.; Thiessen, P.A.; Bryant, S.H. PubChem: Integrated platform of small molecules and biological activities. Annu. Rep. Comput. Chem. 2008, 4, 217–241. [Google Scholar]
- Fernandes, V.; Sharma, D.; Kalia, K.; Tiwari, V. Neuroprotective effects of silibinin: An in silico and in vitro study. Int. J. Neurosci. 2018, 128, 935–945. [Google Scholar] [CrossRef]
- Van Reekum, R.; Black, S.; Conn, D. Cognition enhancing drugs in dementia: A guide to the near future. Can. J. Psych. 1997, 42 (Suppl. S1), 35–50. [Google Scholar]
- Lalwani, D.; Ruan, Y.; Taniyasu, S. Nationwide distribution and potential risk of bisphenol analogues in Indian waters. Ecotoxicol. Environ. Saf. 2020, 200, 110718. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, E.; Yamashita, N.; Taniyasu, S. Bisphenol A and other bisphenol analogues including BPS and BPF in surface water samples from Japan, China, Korea and India. Ecotoxicol. Environ. Saf. 2015, 122, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Moneim, A.M.; Al-Kahtani, M.A.; El-Kersh, M.A.; Al-Omair, M.A. Free radical-scavenging, anti-inflammatory/anti-fibrotic and hepatoprotective actions of Taurine and Silymarin against CCl4 induced rat liver damage. PLoS ONE 2015, 10, e0144509. [Google Scholar] [CrossRef] [Green Version]
- Meyer, B.N.; Ferrigni, N.R.; Putnam, J.E.; Jacobsen, L.B.; Nichols, D.J.; McLaughlin, J.L. Brine shrimp: A convenient general bioassay for active plant constituents. Planta Med. 1982, 45, 31–34. [Google Scholar] [CrossRef]
- Bastos, M.L.; Lima, M.R.; Conserva, L.M.; Andrade, V.S.; Rocha, E.M.; Lemos, R.P. Studies on the antimicrobial activity and brine shrimp toxicity of Zeyheria tuberculosa (Vell.) Bur. (Bignoniaceae) extracts and their main constituents. Ann. Clin. Microbiol. Antimicrob. 2009, 8, 16. [Google Scholar] [CrossRef] [Green Version]
- Maximino, C.; Puty, B.; Benzecry, R.; Araújo, J.; Lima, M.G.; de Jesus Oliveira Batista, E. Role of serotonin in zebrafish (Danio rerio) anxiety: Relationship with serotonin levels and effect of buspirone, WAY 100635, SB 224289, fluoxetine and para-chlorophenylalanine (pCPA) in two behavioral models. Neuropharmacology 2013, 71, 83–97. [Google Scholar] [CrossRef]
- Cachat, J.M.; Canavello, P.R.; Elegante, M.F.; Bartels, B.K.; Elkhayat, S.I.; Hart, P.C. Modeling stress and anxiety in zebrafish. In Zebrafish models in Neurobehavioral Research 2011; Humana Press: Totowa, NJ, USA, 2011; pp. 73–88. [Google Scholar]
- Sciacca, M.F.; Romanucci, V.; Zarrelli, A.; Monaco, I.; Lolicato, F.; Spinella, N. Inhibition of Aβ amyloid growth and toxicity by silybins: The crucial role of stereochemistry. ACS Chem. Neurosci. 2017, 8, 1767–1778. [Google Scholar] [CrossRef] [Green Version]
- Garikapati, D.R.; Shaik, P.B.; Penchalaiah, H. Evaluate neuroprotective effect of silibinin using chronic unpredictable stress (cus) model. Int. J. Physiol. Pathophysiol. Pharmacol. 2018, 10, 184–191. [Google Scholar]
- Amin, I.; Majid, S.; Farooq, A.; Wani, H.A.; Noor, F.; Khan, R. Naringenin (4,5,7-trihydroxyflavanone) as a potent neuroprotective agent: From chemistry to medicine. Stud. Natl. Prod. Chem. 2020, 65, 271–300. [Google Scholar]








| Test Substance | Silibinin and Naringenin |
|---|---|
| Test fish | Zebrafish (Danio rerio); Strain: wild-type, 4–6 months old |
| Test procedure | Static method |
| pH | 6.0 to 8.5 |
| Temperature | 21–25 °C |
| Control | A group exposed to reverse osmosis water serves as control |
| Test concentration | Silibinin/Naringenin (100 mg/L) for performing the limit test |
| Number of fish | 10 fish per concentration for the test sample and control |
| Observation | Fish behaviour was closely observed and the number of deaths was also recorded at intervals of 24, 48, 72, and 96 h. |
| Environmental Conditions | Temperature Was Maintained in the Range of 26 ± 1.5 °C, pH 7–8, Dissolved Oxygen Concentration of not less than 60%. |
| Housing | Zebrafish were housed in groups in a 40 L tank with dechlorinated water with constant aeration and filtration. |
| Diet & Water | Provided with commercially available flake food with Artemia salina (brine shrimp) nauplii and dechlorinated water. |
| Feeding | Brine shrimp nauplii provided once daily and flake food twice daily for one day before the start of the study |
| Test drug | Silibinin and naringenin. Test solutions freshly prepared prior to administration. |
| Inducing agent | Bisphenol A |
| Control | A control group was maintained |
| Concentration of test drug | 10 µM |
| Concentration of inducing agent | 17.52 µM |
| Treatment | For 21 consecutive days by tank water immersion |
| Evaluation | Novel tank test: on day 22 Light/dark test: on day 22 |
| S.no | Concentration (µg/mL) | Percentage Death of Nauplii following 24 h of Exposure | ||
|---|---|---|---|---|
| Silibinin | Naringenin | Potassium Dichromate | ||
| 1 | 0.1 | 21.68 ± 1.66 | 19.00 ± 2.64 | 25.00 ± 2.88 |
| 2 | 1.0 | 26.66 ± 1.45 | 30.34 ± 2.72 | 36.35 ± 1.85 |
| 3 | 10.0 | 33.33 ± 2.33 | 44.00 ± 1.52 | 59.33 ± 1.66 |
| 4 | 100.0 | 40.66 ± 1.66 | 52.23 ± 1.34 | 78.65 ± 1.46 |
| 5 | 1000.0 | 54.00 ± 1.54 | 77.66 ± 1.60 | 99.00 ± 1.15 |
| Log (Inhibitor) vs. Response | |||
|---|---|---|---|
| Best-Fit Values | Silibinin | Naringenin | Potassium Dichromate |
| Bottom | 80.11 | 53.00 | 94.00 |
| Top | 28.66 | 26.03 | 29.21 |
| LogLC50 | 1.959 | 1.534 | 1.219 |
| LC50 | 91.34 | 35.10 | 13.15 |
| Span | −52.32 | −26.96 | −64.79 |
| Compound | Exposure Concentration | Mortality | Abnormal Changes | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 24 h | 48 h | 72 h | 96 h | Swimming | LORF | LOE | Pigmentation | Others | ||
| Signs | ||||||||||
| Blank | - | - | - | - | - | - | - | - | - | - |
| Control (reverse osmosis water) | - | - | - | - | - | - | - | - | - | - |
| Silibinin | 100 mg/L | - | - | - | - | - | - | - | - | - |
| Naringenin | 100 mg/L | - | - | - | - | - | - | - | - | - |
| Compound | Acetylcholinesterase | Butyrylcholinesterase | ||||
|---|---|---|---|---|---|---|
| MolDock Score | Rerank Score | HBond | MolDock Score | Rerank Score | HBond | |
| Donepezil | −146.449 | −115.75 | 0 | - | - | - |
| Rivastigmine | - | - | - | −114.933 | −82.351 | −1.2642 |
| Silibinin | −164.255 | −17.442 | −12.855 | −156.414 | −39.779 | −10.363 |
| Naringenin | −126.023 | −17.442 | −12.855 | −114.323 | −89.786 | −9.9648 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thayumanavan, G.; Jeyabalan, S.; Fuloria, S.; Sekar, M.; Ravi, M.; Selvaraj, L.K.; Bala, L.; Chidambaram, K.; Gan, S.H.; Rani, N.N.I.M.; et al. Silibinin and Naringenin against Bisphenol A-Induced Neurotoxicity in Zebrafish Model—Potential Flavonoid Molecules for New Drug Design, Development, and Therapy for Neurological Disorders. Molecules 2022, 27, 2572. https://doi.org/10.3390/molecules27082572
Thayumanavan G, Jeyabalan S, Fuloria S, Sekar M, Ravi M, Selvaraj LK, Bala L, Chidambaram K, Gan SH, Rani NNIM, et al. Silibinin and Naringenin against Bisphenol A-Induced Neurotoxicity in Zebrafish Model—Potential Flavonoid Molecules for New Drug Design, Development, and Therapy for Neurological Disorders. Molecules. 2022; 27(8):2572. https://doi.org/10.3390/molecules27082572
Chicago/Turabian StyleThayumanavan, Geethanjali, Srikanth Jeyabalan, Shivkanya Fuloria, Mahendran Sekar, Monica Ravi, Logesh Kumar Selvaraj, Logeshwari Bala, Kumarappan Chidambaram, Siew Hua Gan, Nur Najihah Izzati Mat Rani, and et al. 2022. "Silibinin and Naringenin against Bisphenol A-Induced Neurotoxicity in Zebrafish Model—Potential Flavonoid Molecules for New Drug Design, Development, and Therapy for Neurological Disorders" Molecules 27, no. 8: 2572. https://doi.org/10.3390/molecules27082572