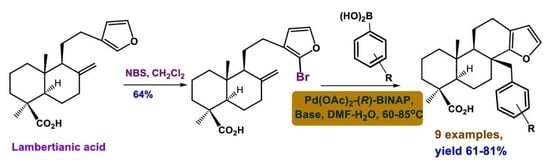
An Approach toward 17-Arylsubstituted Marginatafuran-Type Isospongian Diterpenoids via a Palladium-Catalyzed Heck–Suzuki Cascade Reaction of 16-Bromolambertianic Acid
Abstract
:1. Introduction
2. Results
3. Materials and Methods
3.1. General Information
3.2. Synthesis and Spectral Data of Compound (5), Tetracyclic Compounds (7a–j) and 16-Aryllambertianic Acid Derivatives (8b–d,j)
3.2.1. The Reaction of Lambertianic Acid (1) with N-Bromosuccinimide
3.2.2. Reaction of 16-Bromolambertianic Acid (5) with Phenylboronic Acids (6a–j)
- (a)
- A mixture of 2-bromofuran 5 (0.30 g, 0.76 mmol), arylboronic acid 6a–c (0.91 mmol), Pd(РPh3)4 (0.02 g, 0.02 mmol) and K2CO3 (0.38 g, 2.75 mmol) in DMF (3 mL) and H2O (1.5 mL) was stirred at 80–85 °C (bath) for 24 h under a stream of argon. After cooling, the stirred mixture was treated with diluted H2SO4 (0.1 mL in 1 mL of H2O) to pH 5 and extracted with CHCl3 (3 × 50 mL). The combined organic solution was washed with water (3 × 15 mL), dried over MgSO4, filtered and evaporated in vacuo. The residue was purified by column chromatography (eluent petroleum ether-Et2O, 2:1) to afford compounds 7a, 7b and 8b, or 7c and 8c, respectively.
- (b)
- A mixture of 2-bromofuran 5 (0.30 g, 0.76 mmol), (4-methoxyphenyl)boronic acid 6d (0.14 g, 0.91 mmol), Pd(OAc)2 (0.01 g, 0.04 mmol), P(Tol)3 (0.05 g, 0.15 mmol) and K2CO3 (0.38 g, 2.75 mmol) in DMF (3 mL) and H2O (1.5 mL) was stirred at 80–85 °C (bath) for 24 h under a stream of argon. After cooling, the stirred mixture was treated with diluted H2SO4 (0.1 mL in 1 mL of H2O) to pH 5 and extracted with CHCl3 (3 × 50 mL). The combined organic solution was washed with water (3 × 15 mL), dried over MgSO4 and evaporated in vacuo. The residue was purified by column chromatography (eluent petroleum ether-Et2O, 2:1) to afford compound 7d (0.06 g, 18%).
- (c)
- A mixture of 2-bromofuran 5 (0.30 g, 0.76 mmol), (4-methoxyphenyl)boronic acid 6d (0.14 g, 0.91 mmol), Pd(OAc)2 (0.01 g, 0.04 mmol), dppf (0.04 g, 0.08 mmol) and K2CO3 (0.38 g, 0.03 mmol) in DMF (3 mL) and H2O (1.5 mL) was stirred at 80–85 °C (bath) for 24 h under a stream of argon. After cooling, the stirred mixture was treated with diluted H2SO4 (0.1 mL in 1 mL of H2O) to pH 5 and extracted with CHCl3 (3 × 50 mL). The combined organic solution was washed with water (3 × 15 mL), dried over MgSO4 and evaporated in vacuo. The residue was purified by column chromatography (eluent petroleum ether-Et2O, 2:1) to give compounds 8d (0.06 g, 18%) and 7d (0.07 g, 22%).
- (d)
- A mixture of 2-bromofuran 5 (0.30 g, 0.76 mmol), (4-methoxyphenyl)boronic acid 3d (0.14 g, 0.91 mmol), Pd(OAc)2 (0.01 g, 0.04 mmol), TolBINAP (0.05 g, 0.08 mmol) and K2CO3 (0.38 g, 2.75 mmol) in DMF (3 mL) and H2O (1.5 mL) was stirred at 80–85 °C (bath) for 24 h under a stream of argon. After cooling, the stirred mixture was treated with diluted H2SO4 (0.1 mL in 1 mL of H2O) to pH 5 and extracted with CHCl3 (3 × 50 mL). The combined organic solution was washed with water (3 × 15 mL), dried over MgSO4 and evaporated in vacuo. The residue was purified by column chromatography (eluent petroleum ether-Et2O, 2:1) to give compounds 8d (0.04 g, 11%) and 7d (0.07 g, 22%).
- (e)
- A mixture of 2-bromofuran 5 (0.30 g, 0.76 mmol), arylboronic acid 6d,i,j (0.91 mmol), Pd(OAc)2 (0.01 g, 0.04 mmol), (R)-BINAP (0.05 g, 0.08 mmol) (or (S)-BINAP, conditions e*), and K2CO3 (0.38 g, 2.75 mmol) in DMF (3 mL) and H2O (1.5 mL) was stirred at 80–85 °C (bath) for 24 h under a stream of argon. After cooling, the stirred mixture was treated with diluted H2SO4 (0.1 mL in 1 mL of H2O) to pH 5 and extracted with CHCl3 (3 × 50 mL). The combined organic solution was washed with water (3 × 15 mL), dried over MgSO4 and evaporated in vacuo. The residue was purified by column chromatography (eluent petroleum ether-Et2O, 2:1) to give compounds 7d,8d,7i,7j,8j.
- (f)
- A mixture of 2-bromofuran 5 (0.30 g, 0.76 mmol), arylboronic acid 6b–h (0.91 mmol), Pd(OAc)2 (0.01 g, 0.04 mmol), (R)-BINAP (0.05 g, 0.08 mmol) and K2CO3 (0.38 g, 2.75 mmol) in DMF (3 mL) and H2O (1.5 mL) was stirred at 60–65 °C (bath) for 24 h under a stream of argon. After cooling, the stirred mixture was treated with diluted H2SO4 (0.1 mL in 1 mL of H2O) to pH 5 and extracted with CHCl3 (3 × 50 mL). The combined organic solution was washed with water (3 × 15 mL), dried over MgSO4 and evaporated in vacuo. The residue was purified by column chromatography (eluent petroleum ether-Et2O, 2:1) to afford compound 7b,7c,7e–j,7d,8d.
- (g)
- A mixture of 2-bromofuran 5 (0.30 g, 0.76 mmol), (4-methoxyphenyl)boronic acid 6d (0.14g, 0.91 mmol), Pd(OAc)2 (0.01 g, 0.04 mmol), (R)-BINAP (0.05 g, 0.08 mmol) and Ce2CO3 (0.89 g, 2.74 mmol) in DMF (3 mL) and H2O (1.5 mL) was stirred at 60–65 °C (bath) for 24 h under a stream of argon. After cooling, the stirred mixture was treated with diluted H2SO4 (0.1 mL in 1 mL of H2O) to pH 5 and extracted with CHCl3 (3 × 50 mL). The combined organic solution was washed with water (3 × 15 mL), dried over MgSO4 and evaporated in vacuo. The residue was purified by column chromatography (eluent petroleum ether-Et2O, 2:1) to give compounds 7d,8d.
- (h)
- A mixture of 2-bromofuran 5 (0.30 g, 0.76 mmol), (4-methoxyphenyl)boronic acid 6d (0.14 g, 0.91 mmol), Pd(OAc)2 (0.01 g, 0.04 mmol), (R)-BINAP (0.05 g, 0.08 mmol) and K2CO3 (0.38 g, 2.75 mmol) in DMF (3 mL) and H2O (1.5 mL) was stirred at 40–45 °C (bath) for 24 h under a stream of argon. After cooling, the stirred mixture was treated with diluted H2SO4 (0.1 mL in 1 mL of H2O) to pH 5 and extracted with CHCl3 (3 × 50 mL). The organic layer was washed with water (3 × 15 mL), dried over MgSO4 and evaporated in vacuo. The residue was purified by column chromatography (eluent petroleum ether-Et2O, 2:1) to give unreacted 2-bromofuran 5 (0.21 g), compounds 7d (0.02 g, 6%) and 4d (0.02 g, 6%).
- (i)
- A mixture of 2-bromofuran 5 (0.30 g, 0.76 mmol), (4-methoxyphenyl)boronic acid 6d (0.14g, 0.91 mmol), Pd(OAc)2 (0.01 g, 0.04 mmol), (R)-BINAP (0.05 g, 0.08 mmol) and K2CO3 (0.38 g, 2.75 mmol) in CH3CN (3 mL) and H2O (1.5 mL) was stirred at 60–65 °C (bath) for 24 h under a stream of argon. After cooling, the stirred mixture was treated with diluted H2SO4 (0.1 mL in 1 mL of H2O) to pH 5 and extracted with CHCl3 (3 × 50 mL). The combined organic solution was washed with water (3 × 15 mL), dried over MgSO4 and evaporated in vacuo. The residue was purified by column chromatography (eluent petroleum ether-Et2O, 2:1) to give compounds 2 (0.13 g) and 7d (0.08 g, 24%).
3.2.3. Spectral Data of Tetracyclic Compounds (7a–j) and 16-Aryllambertianic Acid Derivatives (8b–d,j)
3.3. Synthesis of 15-Substituted 16-Bromolambertianic Acid Derivatives Substituted (9–12)
3.4. Synthesis of 15-Substituted 16-Aryllambertianic Acid Derivatives (13a–c,14)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Gustafson, K.; Andersen, R.J.; Cun-Heng, H.; Clardy, J. Marginatafuran, a Furanoditerpene with a New Carbon Skeleton from the Dorid Nudibranch Cadlina luteomarginata. Tetrahedron Lett. 1985, 26, 2521–2524. [Google Scholar] [CrossRef]
- Faulkner, D.J.; Molinski, T.F.; Andersen, R.J.; Dumdei, E.J.; de Silva, E.D. Geographical variation in defensive chemicals from Pacific coast dorid nudibranchs and some related marine mollusks. Comp. Biochem. Physiol. C 1990, 97, 233–240. [Google Scholar] [CrossRef]
- Bobzin, S.C.; Faulkner, D.J. Diterpenes from the marine sponge Aplysilla polyrhaphis and the dorid nudibranch. Chromodoris norrisi. J. Org. Chem. 1989, 54, 3902–3907. [Google Scholar] [CrossRef]
- Tischler, M.; Andersen, R.J.; Choudhary, M.I.; Clardy, J. Terpenoids from the Sponge Aplysilla glacialis and Specimens of the Nudibranch Cadlina luteomarginata Found on the Sponge. J. Org. Chem. 1991, 56, 42–47. [Google Scholar] [CrossRef]
- Dumdei, E.J.; Kubanek, J.; Coleman, J.E.; Pika, J.; Andersen, R.J.; Steiner, J.R.; Clardy, J. New terpenoid metabolites from the skin extracts, an egg mass, and dietary sponges of the northeastern pacific dorid nudibranch Cadlina luteomarginata. Can. J. Chem. 1997, 75, 773–789. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.-N.; Wang, Q.; He, L.; Wang, X.; Li, W.-D.Z. Syntheses of perillene and natural congeners via Li2CuCl4-catalyzed cross-coupling reaction of allylic carbonates. Tetrahedron Lett. 2022, 90, 153610. [Google Scholar] [CrossRef]
- Nishizawa, M.; Yamada, H.; Hayashi, Y. Cyclization control of ambliofuran analog: Effective total synthesis of (±)-baiyunol. J. Org. Chem. 1987, 52, 4878–4884. [Google Scholar] [CrossRef]
- Pandey, U.C.; Sarmah, P.; Sharma, R.P. Polyene cyclization: Cyclization studies on an acyclic furanoditerpene and its epoxide. Tetrahedron 1984, 40, 3739–3748. [Google Scholar] [CrossRef]
- Zhao, J.-F.; Zhao, Y.-J.; Loh, T.-P. Indium tribromide-promoted arene-terminated epoxy olefin cyclization. Chem. Commun. 2008, 2008, 1353–1355. [Google Scholar] [CrossRef]
- Zoretic, P.A.; Shen, Z.; Wang, M.; Ribeiro, A.A. A biomimetic-like radical approach to furanoditerpenes. Tetrahedron Lett. 1995, 36, 2925–2928. [Google Scholar] [CrossRef]
- Gris, A.; Cabedo, N.; Navarro, I.; de Alfonso, I.; Agulló, C.; Abad-Somovilla, A. General diastereoselective synthetic approach toward isospongian diterpenes. Synthesis of (−)-marginatafuran, (−)-marginatone, and (−)-20-acetoxymarginatone. J. Org. Chem. 2012, 77, 5664–5680. [Google Scholar] [CrossRef] [PubMed]
- Kolympadi, M.; Liapis, M.; Ragoussis, V. Synthesis of the Marine furanoditerpene (−)-marginatone. Tetrahedron 2005, 61, 2003–2010. [Google Scholar] [CrossRef]
- Shul’ts, E.E.; Mironov, M.E.; Kharitonov, Y.V. Furanoditerpenoids of the Labdane Series: Occurrence in plants, total synthesis, several transformations, and biological activity. Chem. Nat. Compd. 2014, 50, 2–21. [Google Scholar] [CrossRef]
- Müller, M.; Schröder, J.; Magg, C.; Seifert, K. Synthesis of (+)-coronarin E. Tetrahedron Lett. 1998, 39, 4655–4656. [Google Scholar] [CrossRef]
- Chernov, S.V.; Shul’ts, E.E.; Shakirov, M.M.; Tolstikov, G.A. Synthetic transformations of higher terpenoids: XII. Transformation of lambertianic acid into 14,16-epoxyabietane diterpenoids. Russ. J. Org. Chem. 2006, 42, 36–41. [Google Scholar] [CrossRef]
- Shults, E.E.; Velder, J.; Schmalz, H.-G.; Chernov, S.V.; Rybalova, T.V.; Gatilov, Y.V.; Henze, G.; Tolstikov, G.A.; Prokop, A. Gram-scale synthesis of pinusolide and evaluation of its antileukemic potential. Bioorg. Med. Chem. Lett. 2006, 16, 4228–4232. [Google Scholar] [CrossRef] [PubMed]
- Mironov, M.E.; Kharitonov, Y.V.; Shul’ts, E.E.; Shakirov, M.M.; Bagryanskaya, I.Y.; Tolstikov, G.A. Synthetic transformations of higher terpenoids. XXI. Preparation of phlomisoic acid and its N-containing derivatives. Chem. Nat. Compd. 2010, 46, 233–241. [Google Scholar] [CrossRef]
- Kharitonov, Y.V.; Shul’ts, E.E.; Shakirov, M.M.; Pokrovskii, M.A.; Pokrovskii, A.G.; Tolstikov, G.A. Synthetic transformation of higher terpenoids 31. Synthesis of 1,2,3-triazolyl-containing furan labdanoids and studies of their cytotoxic activity. Russ. Chem. Bull. 2013, 62, 2046–2055. [Google Scholar] [CrossRef]
- Mironov, M.E.; Pokrovsky, M.A.; Kharitonov, Y.V.; Shakirov, M.M.; Pokrovsky, A.G.; Shults, E.E. Furanolabdanoid–based 1,2,4-oxadiazoles: Synthesis and cytotoxic activity. ChemistrySelect 2016, 1, 417–424. [Google Scholar] [CrossRef]
- Kharitonov, Y.V.; Shul’ts, E.E.; Rybalova, T.V.; Pavlova, A.V.; Tolstikova, T.G. Synthetic Transformations of Higher Terpenoids. 40. Synthesis and Assessment of Analgesic Activity of N-Containing Derivatives of Lambertianic. Acid. Chem. Nat. Compd. 2021, 57, 879–886. [Google Scholar] [CrossRef]
- Grigg, R.; Sansano, J.M.; Santhakumar, V.; Sridharan, V.; Thangavelanthum, R.; Thornton-Pett, M.; Wilson, D. Palladium catalyzed tandem cyclisation-anion capture processes. Part 3. Organoboron anion transfer agents. Tetrahedron 1997, 53, 11803–11826. [Google Scholar] [CrossRef]
- Biemolt, J.; Ruijter, E. Advances in palladium catalyzed cascade cyclizations. Adv. Synth. Catal. 2018, 360, 3821–3871. [Google Scholar] [CrossRef]
- Ping, Y.; Li, Y.; Zhu, J.; Kong, W. Construction of quaternary stereocenters by palladium-catalyzed carbopalladation-initiated cascade reactions. Angew. Chem. Int. Ed. 2019, 58, 1562–1573. [Google Scholar] [CrossRef]
- Barbolla, I.; Sotomayor, N.; Lete, E. Carbopalladation/Suzuki coupling cascade for the generation of quaternary centers. Access to pyrrolo[1,2-b]isoquinolines. J. Org. Chem. 2019, 84, 10183–10196. [Google Scholar] [CrossRef]
- Jiang, Y.; McNamee, R.E.; Smith, P.J.; Sozanschi, A.; Tong, Z.; Anderson, E.A. Advances in polycyclization cascades in natural product synthesis. Chem Soc. Rev. 2021, 50, 58–71. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.E. Diastereoselective synthesis of tetrahydroquinolines via a palladium-catalyzed Heck–Suzuki cascade reaction. Tetrahedron Lett. 2012, 53, 2308–2311. [Google Scholar] [CrossRef]
- Zhang, Z.M.; Xu, B.; Wu, L.; Wu, Y.; Qian, Y.; Zhou, L.; Liu, Y.; Zhang, J. Enantioselective dicarbofunctionalization of unactivated alkenes by palladium-catalyzed tandem Heck/Suzuki coupling reaction. Angew. Chem. Int. Ed. 2019, 58, 14653–14659. [Google Scholar] [CrossRef]
- Kong, W.; Wang, Q.; Zhu, J. Water as a hydride source in palladium-catalyzed enantioselective reductive Heck reactions. Angew. Chem. Int. Ed. 2017, 56, 3987–3991. [Google Scholar] [CrossRef]
- Zhang, Z.M.; Xu, B.; Wu, L.; Zhou, L.; Ji, D.; Liu, Y.; Li, Z.; Zhang, J. Palladium/XuPhos-catalyzed enantioselective carboiodination of olefin-tethered aryl iodides. J. Am. Chem. Soc. 2019, 141, 8110–8115. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, L.; Zhang, Z.-M.; Xu, B.; Liu, Y.; Zhang, J. Enantioselective difunctionalization of alkenes by a palladium-catalyzed Heck/borylation sequence. Chem. Sci. 2022, 13, 2021–2025. [Google Scholar] [CrossRef]
- Chernov, S.V.; Shul’ts, E.E.; Shakirov, M.M.; Tolstikov, G.A. Synthetic transformations of higher terpenoids: VII. Synthesis of tetrahydro-β-carbolines of the labdane series. Russ. J. Org. Chem. 2002, 38, 665–671. [Google Scholar] [CrossRef]
- Tolstikova, T.G.; Sorokina, N.V.; Dolgikh, M.P.; Chernov, S.V.; Kharitonov, Y.V.; Shul′ts, E.E.; Tolstikov, G.A. Neurotropic activity of lambertianic acid adducts with N-substituted maleinimides. Pharm. Chem. J. 2004, 38, 532–534. [Google Scholar] [CrossRef]
- Riley, A.P.; Day, V.W.; Prisinzano, T.E. Palladium-catalyzed transformations of salvinorin A, a neoclerodane diterpene from Salvia divinorum. Org. Lett. 2013, 15, 5936–5939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riley, A.P.; Groer, C.E.; Young, D.; Ewald, A.W.; Kivell, B.M.; Prisinzano, T.E. Synthesis and κ opioid receptor activity of furan-substituted salvinorin A analogues. J. Med. Chem. 2014, 57, 10464–10475. [Google Scholar] [CrossRef] [Green Version]
- Zapf, A.; Ehrentraut, A.; Beller, M. A new highly efficient catalyst system for the coupling of nonactivated and deactivated aryl chlorides with arylboronic acids. Angew. Chem. Int. Ed. 2000, 39, 4153–4155. [Google Scholar] [CrossRef]
- Martin, R.; Buchwald, S.L. Palladium-catalyzed Suzuki−Miyaura cross-coupling reactions employing dialkylbiaryl phosphine ligand. Acc. Chem Res. 2008, 41, 1461–1473. [Google Scholar] [CrossRef] [Green Version]
- Yin, J.; Buchwald, S.L. Pd-catalyzed intermolecular amidation of aryl halides: The discovery that xantphos can be trans-chelating in a palladium complex. J. Am. Chem. Soc. 2002, 124, 6043–6048. [Google Scholar] [CrossRef]
- Hall, D.G. Structure, properties, and preparation of boronic acid derivatives. In Boronic Acids; Hall, D.G., Ed.; Wiley-VCH: Weinheim, Germany, 2011; Volume 1, pp. 8–13. [Google Scholar]
- Petrone, D.A.; Malik, H.A.; Clemenceau, A.; Lautens, M. Functionalized chromans and isochromans via a diastereoselective Pd(0)-catalyzed carboiodination. Org. Lett. 2012, 14, 4806–4809. [Google Scholar] [CrossRef]
- Klok, D.A.; Shakirov, M.M.; Grishko, V.V.; Raldugin, V.A. Vilsmeier-Haack reaction of methyl lambertianate and non-sensitized photocyclization of the resulting product. Russ. Chem. Bull. 1995, 44, 2412–2414. [Google Scholar] [CrossRef]
- Antonioletti, R.; D’Auria, M.; De Mico, A.; Piancatelli, G.; Scettri, A. Photochemical synthesis of 3- and 5-aryl-2-furyl derivatives. J. Chem. Soc. Perkin Trans. 1985, 1285–1288. Available online: https://pubs.rsc.org/en/content/articlelanding/1985/p1/p19850001285 (accessed on 29 March 2022). [CrossRef]
- Kharitonov, Y.V.; Shults, E.E.; Shakirov, M.M.; Bagryanskaya, I.Y.; Tolstikov, G.A. Synthetic transformations of higher terpenoids: XXII. Reactions of lambertianic acid derivatives with organozinc reagents obtained from ethyl bromoalkanoates. Russ. J. Org. Chem. 2010, 46, 1339–1347. [Google Scholar] [CrossRef]







| Entry | Arylboronic Acid | Conditions | Reaction Conditions | Yield [‡] % | ||||
|---|---|---|---|---|---|---|---|---|
| Catalyst | Base | T, °C | Solvent | 7 | 8 | |||
| 1 | 6b | a | Pd(PPh3)4 | K2CO3 | 80–85 | DMF-Н2О | 23 (7b) | 45 (8b) |
| 2 | 6c | a | Pd(PPh3)4 | K2CO3 | 80–85 | DMF-Н2О | 40 (7c) | 20 (8c) |
| 3 | 6d | a | Pd(PPh3)4 | K2CO3 | 80–85 | DMF-Н2О | - | - |
| 4 | 6d | a | Pd(PPh3)4 | K2CO3 | 95–100 | DMF-Н2О | - | - |
| 5 | 6d | Pd(OAc)2-(1-Adm)2PBn | K2CO3 | 80–85 | DMF-Н2О | - | - | |
| 6 | 6d | Pd(OAc)2-XPhos | K2CO3 | 80–85 | DMF-Н2О | - | - | |
| 7 | 6d | b | Pd(OAc)2-P(Tol)3 | K2CO3 | 80–85 | DMF-Н2О | 18 (7d) | - |
| 8 | 6d | c | Pd(OAc)2-dppf | K2CO3 | 80–85 | DMF-Н2О | 22 (7d) | 18 (8d) |
| 9 | 6d | d | Pd(OAc)2-TolBINAP | K2CO3 | 80–85 | DMF-Н2О | 22 (7d) | 11 (8d) |
| 10 | 6d | e | Pd(OAc)2-(R)-BINAP | K2CO3 | 80–85 | DMF-Н2О | 38 (7d) | 38 (8d) |
| 11 | 6d | e* | Pd(OAc)2-(S)-BINAP | K2CO3 | 80–85 | DMF-Н2О | 35 (7d) | 8 (8d) |
| 12 | 6d | f | Pd(OAc)2-(R)-BINAP | K2CO3 | 60–65 | DMF-Н2О | 56 (7d) | 28 (8d) |
| 13 | 6d | g | Pd(OAc)2-(R)-BINAP | Cs2CO3 | 60–65 | DMF-Н2О | 35 (7d) | 21 (8d) |
| 14 [§] | 6d | h | Pd(OAc)2-(R)-BINAP | K2CO3 | 40–45 | DMF-Н2О | 6 (7d) | 6 (8d) |
| 15 [⊥] | 6d | i | Pd(OAc)2-(R)-BINAP | K2CO3 | 60–65 | СH3CN-H2О | 24 (7d) | - |
| 16 | 6b | f | Pd(OAc)2-(R)-BINAP | K2CO3 | 60–65 | DMF-Н2О | 52 (7b) | trace |
| 17 | 6c | f | Pd(OAc)2-(R)-BINAP | K2CO3 | 60–65 | DMF-Н2О | 68 (7c) | trace |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kharitonov, Y.V.; Shults, E.E. An Approach toward 17-Arylsubstituted Marginatafuran-Type Isospongian Diterpenoids via a Palladium-Catalyzed Heck–Suzuki Cascade Reaction of 16-Bromolambertianic Acid. Molecules 2022, 27, 2643. https://doi.org/10.3390/molecules27092643
Kharitonov YV, Shults EE. An Approach toward 17-Arylsubstituted Marginatafuran-Type Isospongian Diterpenoids via a Palladium-Catalyzed Heck–Suzuki Cascade Reaction of 16-Bromolambertianic Acid. Molecules. 2022; 27(9):2643. https://doi.org/10.3390/molecules27092643
Chicago/Turabian StyleKharitonov, Yurii V., and Elvira E. Shults. 2022. "An Approach toward 17-Arylsubstituted Marginatafuran-Type Isospongian Diterpenoids via a Palladium-Catalyzed Heck–Suzuki Cascade Reaction of 16-Bromolambertianic Acid" Molecules 27, no. 9: 2643. https://doi.org/10.3390/molecules27092643