Characterization of Natural Anthocyanin Indicator Based on Cellulose Bio-Composite Film for Monitoring the Freshness of Chicken Tenderloin
Abstract
:1. Introduction
2. Results and Discussion
2.1. HMB Film Characterization
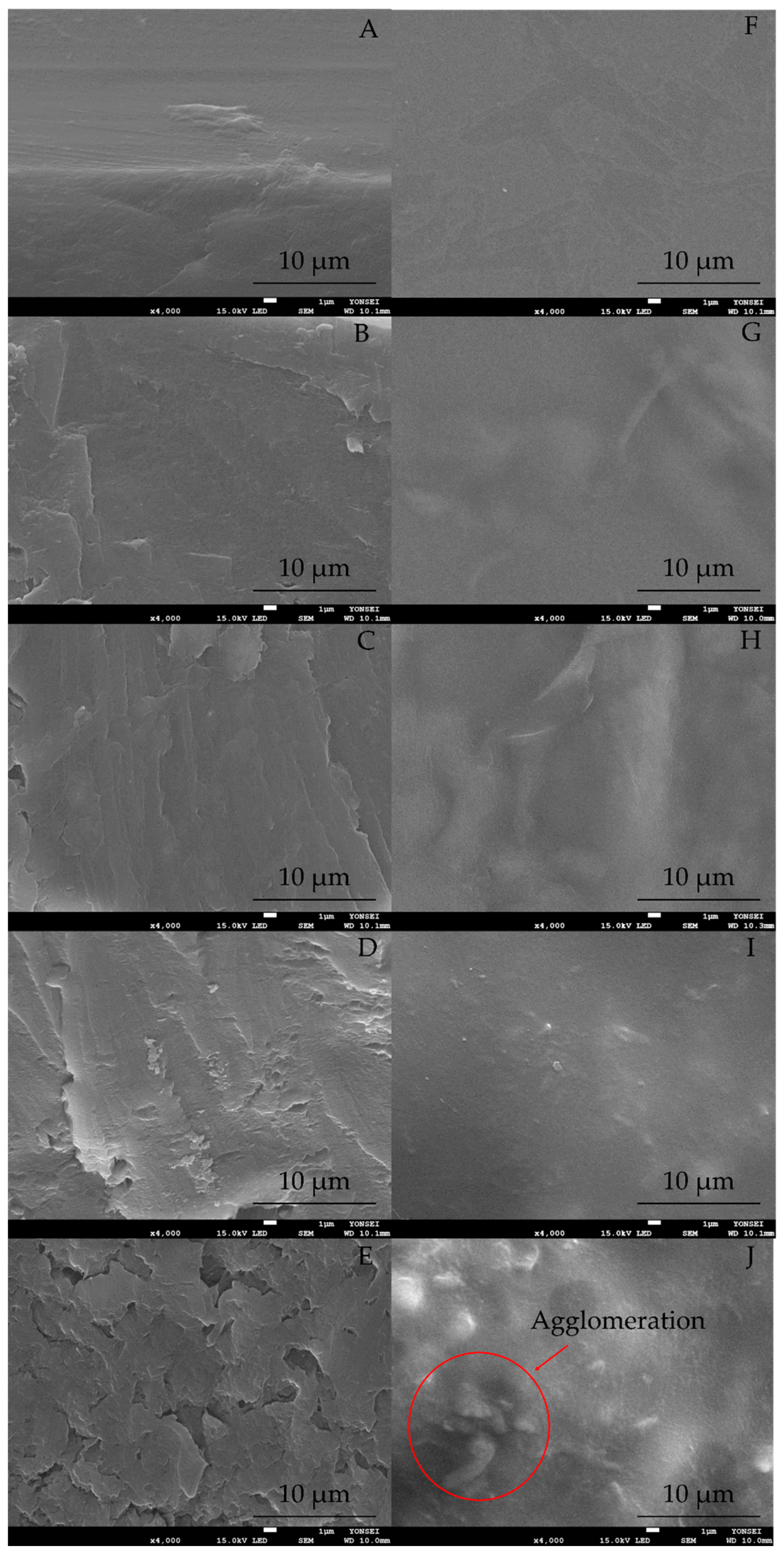
2.1.1. Morphological Analysis
2.1.2. Optical Properties
2.1.3. Physical Properties
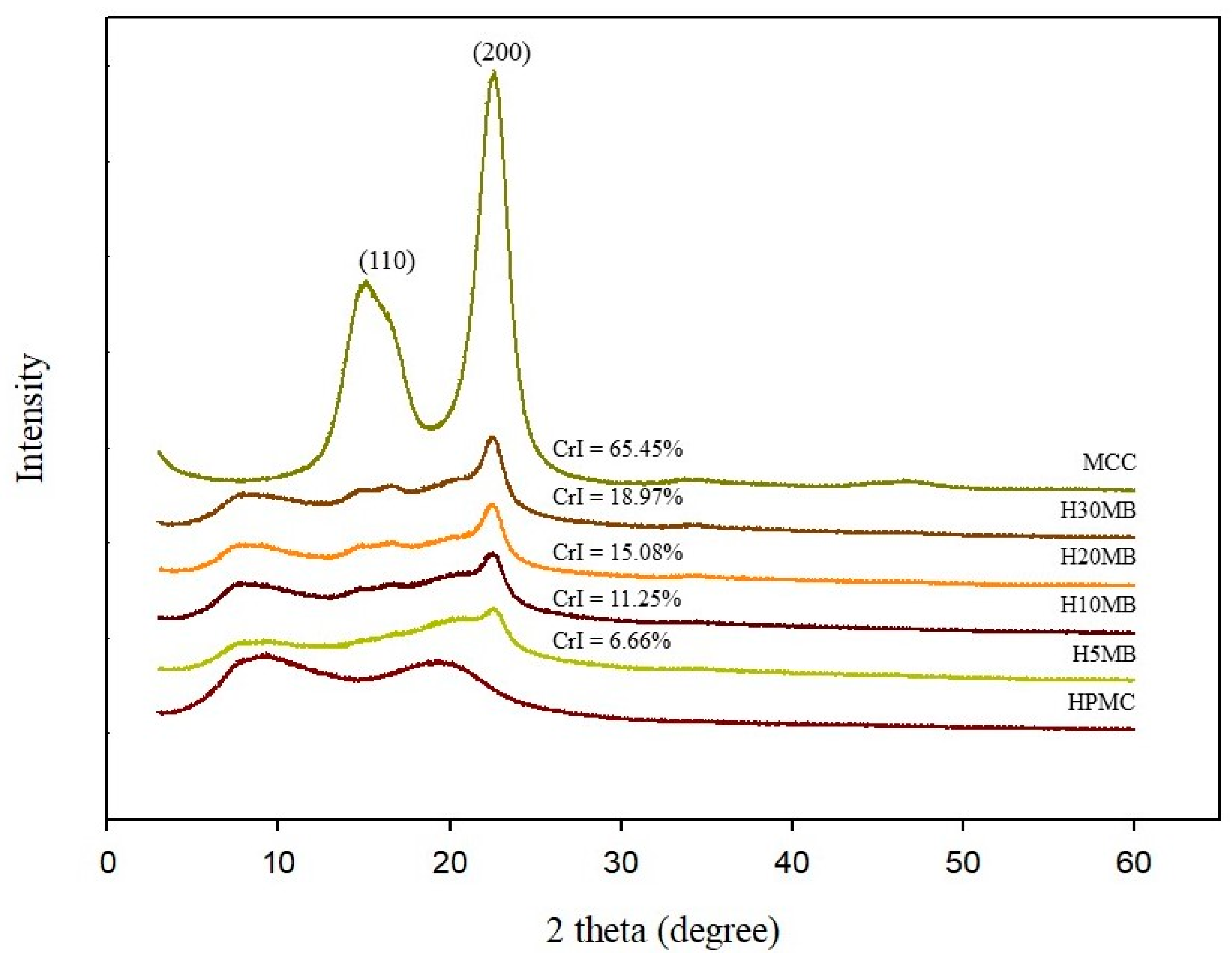
2.1.4. XRD Analysis
2.1.5. Thermal Properties
2.2. RA and RA-H20MB Indicator Charaterization
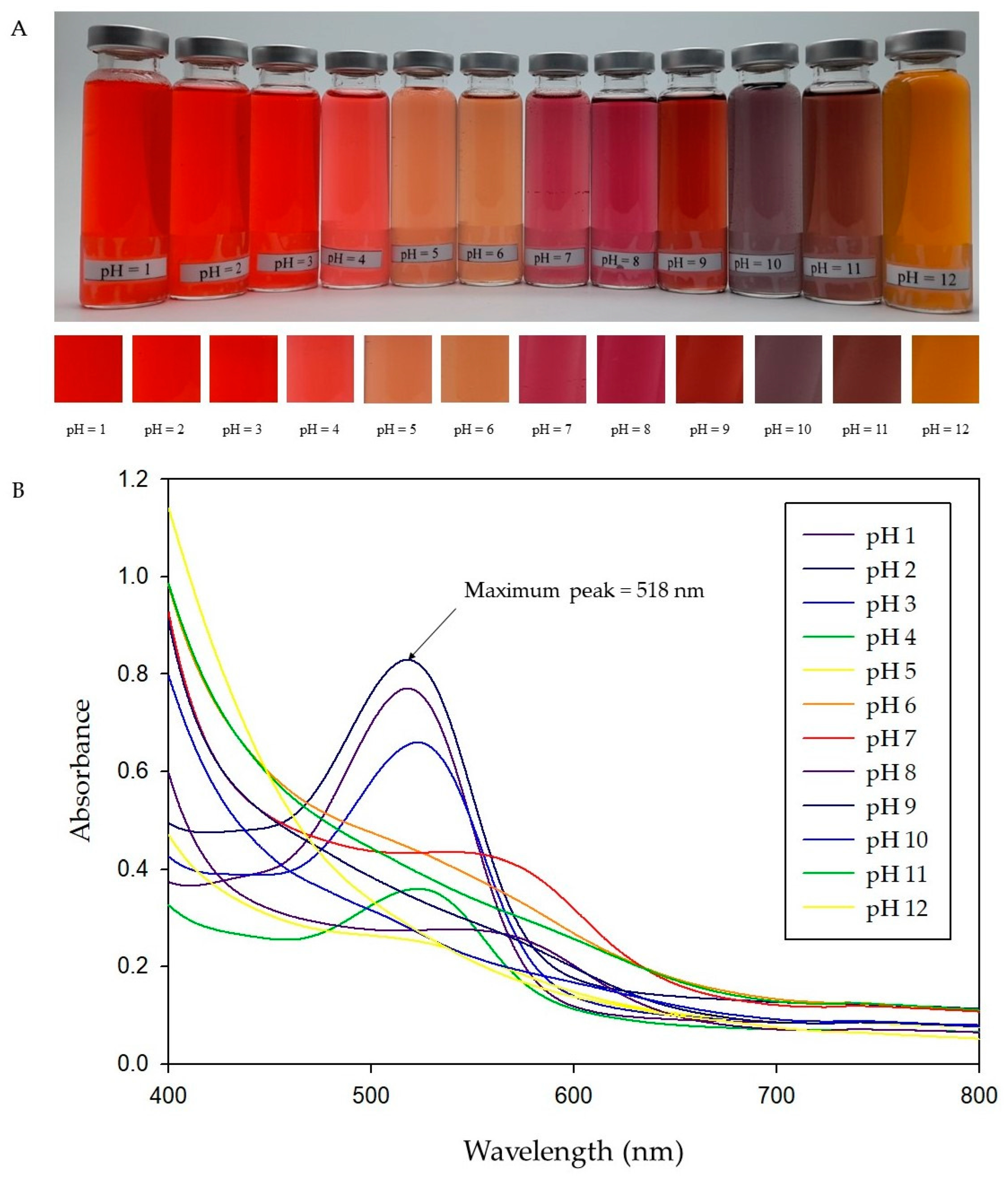
2.2.1. Color Response to pH of RA Anthocyanins
2.2.2. RA-H20MB Indicator
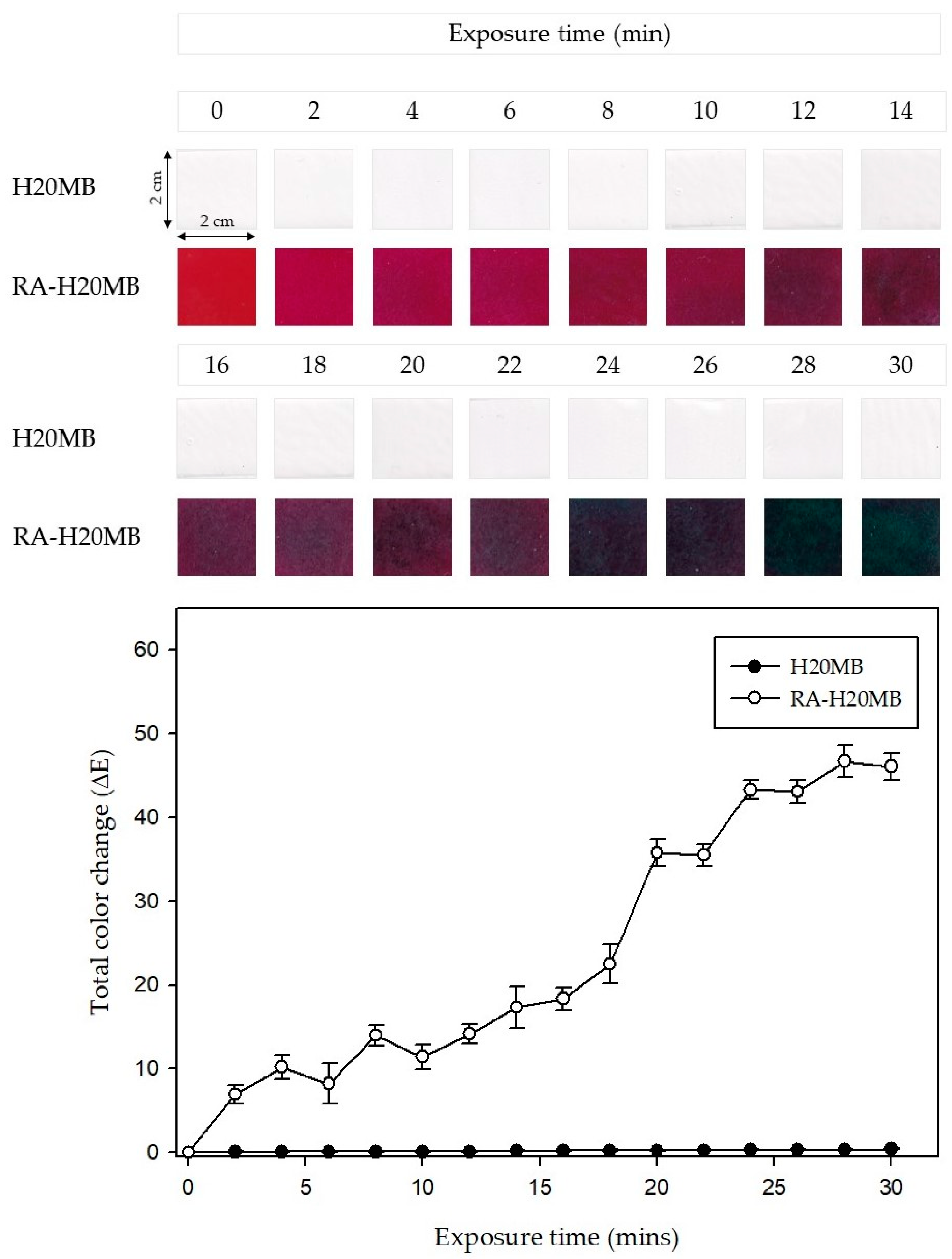
2.2.3. Application of RA-H20MB Indicator for Tracking Freshness of Chicken Tenderloin
3. Materials and Methods
3.1. HMB Film Prepalation
3.2. Charactrization of HMB
3.2.1. Field Emission Scanning Electron Microscopy (FE-SEM)
3.2.2. Mechanical Properties
3.2.3. Barrier Properties
3.2.4. Optical Property
3.2.5. X-ray Diffraction (XRD) Measurement
3.2.6. Thermal Properties
3.3. Application of HMB Film for Roselle Anthocyanin (RA) Indicator Fabrication
3.3.1. Extraction and Characterization of RA
3.3.2. Preparation of RA-HMB
3.3.3. Color Response of RA-HMB to Ammonia
3.3.4. Application of RA-HMB Indicator for Tracking Freshness of Chicken Tenderloin
3.4. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Lu, P.; Yang, Y.; Liu, R.; Liu, X.; Ma, J.; Wu, M.; Wang, S. Preparation of sugarcane bagasse nanocellulose hydrogel as a colourimetric freshness indicator for intelligent food packaging. Carbohydr. Polym. 2020, 249, 116831. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Xu, H.; McClements, D.J.; Chen, L.; Jiao, A.; Tian, Y.; Miao, M.; Jin, Z. Recent advances in intelligent food packaging materials: Principles, preparation and applications. Food Chem. 2021, 375, 131738. [Google Scholar] [CrossRef]
- Ghaani, M.; Cozzolino, C.A.; Castelli, G.; Farris, S. An overview of the intelligent packaging technologies in the food sector. Trends Food Sci. Technol. 2016, 51, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Moradi, M.; Tajik, H.; Almasi, H.; Forough, M.; Ezati, P. A novel pH-sensing indicator based on bacterial cellulose nanofibers and black carrot anthocyanins for monitoring fish freshness. Carbohydr. Polym. 2019, 222, 115030. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh-Sani, M.; Tavassoli, M.; Mohammadian, E.; Ehsani, A.; Khaniki, G.J.; Priyadarshi, R.; Rhim, J.-W. pH-responsive color indicator films based on methylcellulose/chitosan nanofiber and barberry anthocyanins for real-time monitoring of meat freshness. Int. J. Biol. Macromol. 2021, 166, 741–750. [Google Scholar] [CrossRef]
- Kang, S.; Wang, H.; Xia, L.; Chen, M.; Li, L.; Cheng, J.; Li, X.; Jiang, S.; Kang, S.; Wang, H.; et al. Colorimetric film based on polyvinyl alcohol/okra mucilage polysaccharide incorporated with rose anthocyanins for shrimp freshness monitoring. Carbohydr. Polym. 2020, 229, 115402. [Google Scholar] [CrossRef]
- Dong, H.; Ling, Z.; Zhang, X.; Zhang, X.; Ramaswamy, S.; Xu, F. Smart colorimetric sensing films with high mechanical strength and hydrophobic properties for visual monitoring of shrimp and pork freshness. Sens. Actuators B Chem. 2020, 309, 127752. [Google Scholar] [CrossRef]
- Saeidipour, F.; Mansourpour, Z.; Mortazavian, E.; Rafiee-Tehrani, N.; Rafiee-Tehrani, M. New comprehensive mathematical model for HPMC-MCC based matrices to design oral controlled release systems. Eur. J. Pharm. Biopharm. 2017, 121, 61–72. [Google Scholar] [CrossRef]
- Deshmukh, K.; Basheer Ahamed, M.; Deshmukh, R.R.; Khadheer Pasha, S.K.; Bhagat, P.R.; Chidambaram, K. 3-Biopolymer composites with high dielectric performance: Interface engineering. In Biopolymer Composites in Electronics; Sadasivuni, K.K., Ponnamma, D., Kim, J., Cabibihan, J.-J., AlMaadeed, M.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 27–128. [Google Scholar] [CrossRef]
- Siepmann, J.; Peppas, N.A. Modeling of drug release from delivery systems based on hydroxypropyl methylcellulose (HPMC). Adv. Drug Deliv. Rev. 2012, 64, 163–174. [Google Scholar] [CrossRef]
- Bilbao-Sáinz, C.; Avena-Bustillos, R.J.; Wood, D.F.; Williams, T.G.; McHugh, T.H. Composite Edible Films Based on Hydroxypropyl Methylcellulose Reinforced with Microcrystalline Cellulose Nanoparticles. J. Agric. Food Chem. 2010, 58, 3753–3760. [Google Scholar] [CrossRef] [PubMed]
- Mathew, A.P.; Oksman, K.; Sain, M. Mechanical properties of biodegradable composites from poly lactic acid (PLA) and microcrystalline cellulose (MCC). J. Appl. Polym. Sci. 2005, 97, 2014–2025. [Google Scholar] [CrossRef]
- Siró, I.; Plackett, D. Microfibrillated cellulose and new nanocomposite materials: A review. Cellulose 2010, 17, 459–494. [Google Scholar] [CrossRef]
- Xian, X.; Wang, X.; Zhu, Y.; Guo, Y.; Tian, Y. Effects of MCC Content on the Structure and Performance of PLA/MCC Biocomposites. J. Polym. Environ. 2018, 26, 3484–3492. [Google Scholar] [CrossRef]
- Jridi, M.; Boughriba, S.; Abdelhedi, O.; Nciri, H.; Nasri, R.; Kchaou, H.; Kaya, M.; Sebai, H.; Zouari, N.; Nasri, M. Investigation of physicochemical and antioxidant properties of gelatin edible film mixed with blood orange (Citrus sinensis) peel extract. Food Packag. Shelf Life 2019, 21, 100342. [Google Scholar] [CrossRef]
- Hermawan, D.; Lai, T.K.; Jafarzadeh, S.; Gopakumar, D.A.; Hasan, M.; Owolabi, F.A.T.; Aprilia, N.A.S.; Rizal, S.; Khalil, H.P.S.A. Development of seaweed-based bamboo microcrystalline cellulose films intended for sustainable food packaging applications. BioResources 2019, 14, 3389–3410. [Google Scholar] [CrossRef]
- Hazrati, K.; Sapuan, S.; Zuhri, M.; Jumaidin, R. Preparation and characterization of starch-based biocomposite films reinforced by Dioscorea hispida fibers. J. Mater. Res. Technol. 2021, 15, 1342–1355. [Google Scholar] [CrossRef]
- Wang, X.; Tang, Y.; Zhu, X.; Zhou, Y.; Hong, X. Preparation and characterization of polylactic acid/polyaniline/nanocrystalline cellulose nanocomposite films. Int. J. Biol. Macromol. 2020, 146, 1069–1075. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Shi, Y.; Chen, G.; Cai, M. Enhanced mechanical and hydrophobic properties of composite cassava starch films with stearic acid modified MCC (microcrystalline cellulose)/NCC (nanocellulose) as strength agent. Int. J. Biol. Macromol. 2019, 142, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Bumbudsanpharoke, N.; Lee, W.; Chung, U.; Ko, S. Study of humidity-responsive behavior in chiral nematic cellulose nanocrystal films for colorimetric response. Cellulose 2018, 25, 305–317. [Google Scholar] [CrossRef]
- Terinte, N.; Ibbett, R.; Schuster, K.C. Overview on native cellulose and microcrystalline cellulose I structure studied by X-ray diffraction (WAXD): Comparison between measurement techniques. Lenzing. Ber. 2011, 89, 118–131. [Google Scholar]
- Hamdan, M.A.; Ramli, N.A.; Othman, N.A.; Amin, K.N.M.; Adam, F. Characterization and property investigation of microcrystalline cellulose (MCC) and carboxymethyl cellulose (CMC) filler on the carrageenan-based biocomposite film. Mater. Today Proc. 2021, 42, 56–62. [Google Scholar] [CrossRef]
- Olewnik, E.; Garman, K.; Czerwiński, W. Thermal properties of new composites based on nanoclay, polyethylene and polypropylene. J. Therm. Anal. 2010, 101, 323–329. [Google Scholar] [CrossRef]
- Khouaja, A.; Koubaa, A.; Ben Daly, H. Dielectric properties and thermal stability of cellulose high-density polyethylene bio-based composites. Ind. Crop. Prod. 2021, 171, 113928. [Google Scholar] [CrossRef]
- Zhang, J.; Zou, X.; Zhai, X.D.; Huang, X.W.; Jiang, C.P.; Holmes, M. Preparation of an intelligent pH film based on biodegradable polymers and roselle anthocyanins for monitoring pork freshness. Food Chem. 2019, 272, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, G.; Chi, W.; Xu, S.; Wang, L. Developing a simultaneously antioxidant and pH-responsive κ-carrageenan/hydroxypropyl methylcellulose film blended with Prunus maackii extract. Int. J. Biol. Macromol. 2020, 155, 1393–1400. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Ding, H.; Chen, L.; Zhang, S.; Wu, P.; Xie, K.; Pan, Z.; Zhang, G.; Dai, G.; Wu, H.; et al. Characterization of chilled chicken spoilage using an integrated microbiome and metabolomics analysis. Food Res. Int. 2021, 144, 110328. [Google Scholar] [CrossRef]
- Balamatsia, C.C.; Patsias, A.; Kontominas, M.G.; Savvaidis, I.N. Possible role of volatile amines as quality-indicating metabolites in modified atmosphere-packaged chicken fillets: Correlation with microbiological and sensory attributes. Food Chem. 2007, 104, 1622–1628. [Google Scholar] [CrossRef]
- Baltić, T.; Ćirić, J.; Velebit, B.; Petronijević, R.; Lakićević, B.; Đorđević, V.; Janković, V. Changes in total viable count and TVB-N content in marinated chicken breast fillets during storage. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Zlatibor, Serbia, 1–4 October 2017; p. 012073. [Google Scholar]
- Ramezani, Z.; Zarei, M.; Raminnejad, N. Comparing the effectiveness of chitosan and nanochitosan coatings on the quality of refrigerated silver carp fillets. Food Control 2015, 51, 43–48. [Google Scholar] [CrossRef]
- Gutiérrez, T.J. Surface and nutraceutical properties of edible films made from starchy sources with and without added blackberry pulp. Carbohydr. Polym. 2017, 165, 169–179. [Google Scholar] [CrossRef]
- Çam, M.; Hışıl, Y.; Durmaz, G. Classification of eight pomegranate juices based on antioxidant capacity measured by four methods. Food Chem. 2009, 112, 721–726. [Google Scholar] [CrossRef]
- Jiang, G.; Hou, X.; Zeng, X.; Zhang, C.; Wu, H.; Shen, G.; Li, S.; Luo, Q.; Li, M.; Liu, X.; et al. Preparation and characterization of indicator films from carboxymethyl-cellulose/starch and purple sweet potato (Ipomoea batatas (L.) lam) anthocyanins for monitoring fish freshness. Int. J. Biol. Macromol. 2020, 143, 359–372. [Google Scholar] [CrossRef]
- Talukder, S.; Mendiratta, S.; Kumar, R.; Agrawal, R.; Soni, A.; Luke, A.; Chand, S. Jamun fruit (Syzgium cumini) skin extract based indicator for monitoring chicken patties quality during storage. J. Food Sci. Technol. 2020, 57, 537–548. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Baek, S.-K.; Song, K.B. Physical and antioxidant properties of alginate films prepared from Sargassum fulvellum with black chokeberry extract. Food Packag. Shelf Life 2018, 18, 157–163. [Google Scholar] [CrossRef]
- Nogueira, G.F.; Fakhouri, F.M.; Velasco, J.I.; de Oliveira, R.A. Active edible films based on arrowroot starch with microparticles of blackberry pulp obtained by freeze-drying for food packaging. Polymers 2019, 11, 1382. [Google Scholar] [CrossRef] [Green Version]
- Mohammadalinejhad, S.; Almasi, H.; Moradi, M. Immobilization of Echium amoenum anthocyanins into bacterial cellulose film: A novel colorimetric pH indicator for freshness/spoilage monitoring of shrimp. Food Control 2020, 113, 107169. [Google Scholar] [CrossRef]
- Zepon, K.M.; Martins, M.M.; Marques, M.S.; Heckler, J.M.; Morisso, F.D.P.; Moreira, M.G.; Ziulkoski, A.L.; Kanis, L.A. Smart wound dressing based on κ–carrageenan/locust bean gum/cranberry extract for monitoring bacterial infections. Carbohydr. Polym. 2019, 206, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Yun, D.; Cai, H.; Liu, Y.; Xiao, L.; Song, J.; Liu, J. Development of active and intelligent films based on cassava starch and Chinese bayberry (Myrica rubra Sieb. et Zucc.) anthocyanins. RSC Adv. 2019, 9, 30905–30916. [Google Scholar] [CrossRef] [Green Version]
- Ebrahimi Tirtashi, F.; Moradi, M.; Tajik, H.; Forough, M.; Ezati, P.; Kuswandi, B. Cellulose/chitosan pH-responsive indicator incorporated with carrot anthocyanins for intelligent food packaging. Int. J. Biol. Macromol. 2019, 136, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Koosha, M.; Hamedi, S. Intelligent Chitosan/PVA nanocomposite films containing black carrot anthocyanin and bentonite nanoclays with improved mechanical, thermal and antibacterial properties. Prog. Org. Coat. 2019, 127, 338–347. [Google Scholar] [CrossRef]
- Prietto, L.; Mirapalhete, T.C.; Pinto, V.Z.; Hoffmann, J.F.; Vanier, N.L.; Lim, L.-T.; Dias, A.R.G.; da Rosa Zavareze, E. pH-sensitive films containing anthocyanins extracted from black bean seed coat and red cabbage. LWT 2017, 80, 492–500. [Google Scholar] [CrossRef]
- Jamróz, E.; Kulawik, P.; Guzik, P.; Duda, I. The verification of intelligent properties of furcellaran films with plant extracts on the stored fresh Atlantic mackerel during storage at 2 °C. Food Hydrocoll. 2019, 97, 105211. [Google Scholar] [CrossRef]
- Ge, W.; Zhao, Y.; Kong, X.; Sun, H.; Luo, M.; Yao, M.; Wei, B.; Ji, S. Combining salicylic acid and trisodium phosphate alleviates chilling injury in bell pepper (Capsicum annuum L.) through enhancing fatty-acid desaturation efficiency and water retention. Food Chem. 2020, 327, 127057. [Google Scholar] [CrossRef] [PubMed]




| Sample | Thickness (μm) | Tensile (MPa) | Elongation (%) | OTR (cc2/m2/day) | WVTR (g/m2/day) |
|---|---|---|---|---|---|
| HPMC | 74.59 ± 1.48 ns | 24.08 ± 3.51 a | 33.19 ± 3.19 c | 51.97 ± 1.35 d | 48.17 ± 3.97 bc |
| H5MB | 73.62 ± 2.38 ns | 28.17 ± 3.73 ab | 28.17 ± 2.79 bc | 20.37 ± 1.02 c | 45.00 ± 1.37 ab |
| H10MB | 75.84 ± 3.64 ns | 31.34 ± 2.86 bc | 25.23 ± 3.41 ab | 14.00 ± 1.49 b | 43.31 ± 2.19 ab |
| H20MB | 74.48 ± 3.48 ns | 35.99 ± 1.88 c | 24.96 ± 2.37 ab | 6.85 ± 0.47 a | 42.16 ± 3.21 a |
| H30MB | 76.98 ± 3.57 ns | 26.12 ± 2.84 ab | 21.29 ± 3.36 a | 22.44 ± 0.96 c | 51.93 ± 1.33 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boonsiriwit, A.; Itkor, P.; Sirieawphikul, C.; Lee, Y.S. Characterization of Natural Anthocyanin Indicator Based on Cellulose Bio-Composite Film for Monitoring the Freshness of Chicken Tenderloin. Molecules 2022, 27, 2752. https://doi.org/10.3390/molecules27092752
Boonsiriwit A, Itkor P, Sirieawphikul C, Lee YS. Characterization of Natural Anthocyanin Indicator Based on Cellulose Bio-Composite Film for Monitoring the Freshness of Chicken Tenderloin. Molecules. 2022; 27(9):2752. https://doi.org/10.3390/molecules27092752
Chicago/Turabian StyleBoonsiriwit, Athip, Pontree Itkor, Chanutwat Sirieawphikul, and Youn Suk Lee. 2022. "Characterization of Natural Anthocyanin Indicator Based on Cellulose Bio-Composite Film for Monitoring the Freshness of Chicken Tenderloin" Molecules 27, no. 9: 2752. https://doi.org/10.3390/molecules27092752






