Controllable Assembly of Vanadium-Containing Polyoxoniobate-Based Materials and Their Electrocatalytic Activity for Selective Benzyl Alcohol Oxidation
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Methods
2.2. Synthesis of [Ni(en)2]5[PNb12O40(VO)5](OH)5·18H2O (1)
2.3. Synthesis of [Ni(en)3]5[PNb12O40(VO)2]∙17H2O (2)
2.4. Preparation of Working Electrode
2.5. Cyclic Voltammetry Experiments
2.6. Electrochemical Surface Area Experiments
2.7. Controlled Potential Electrolysis Experiments
3. Results and Discussion
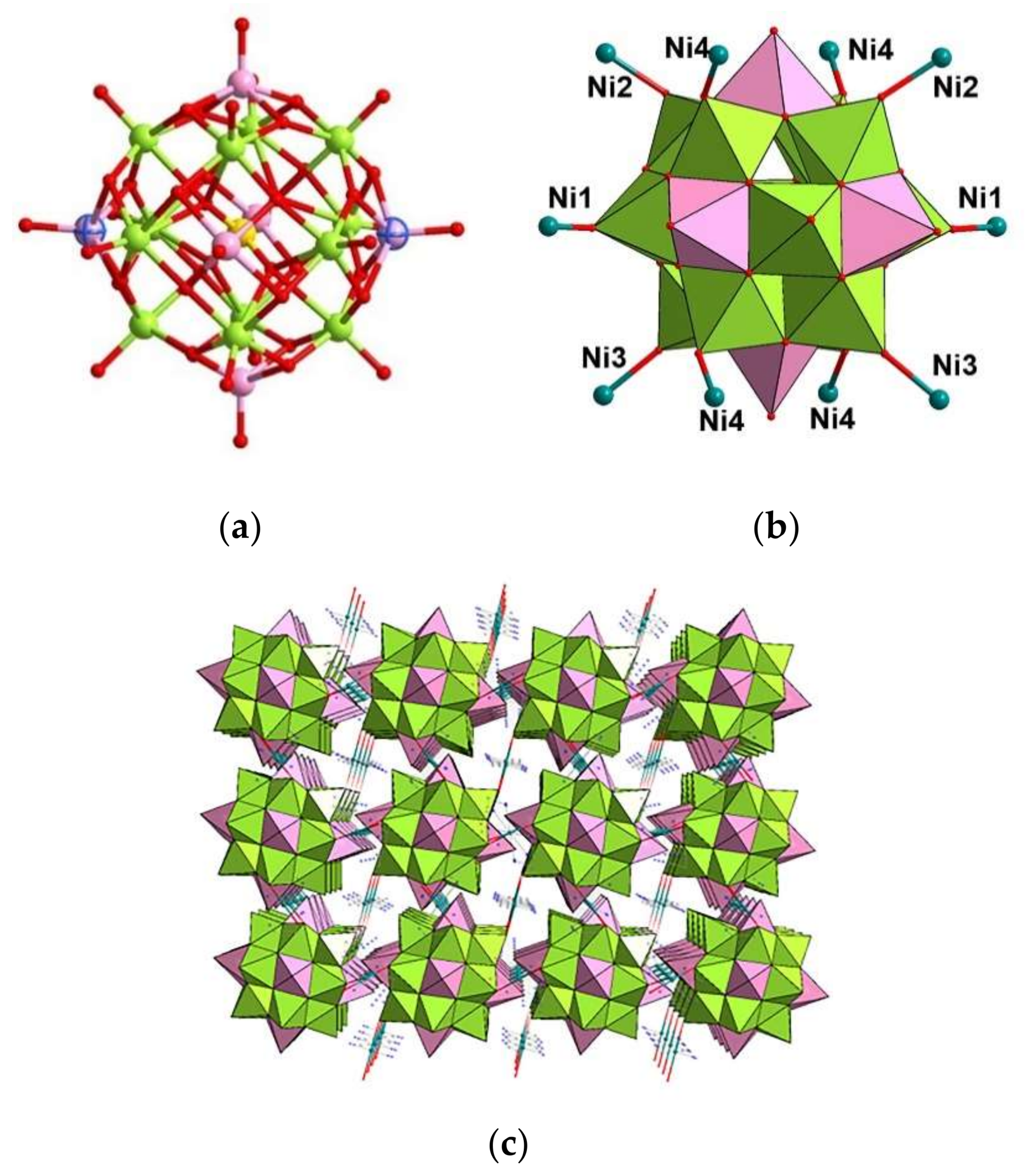
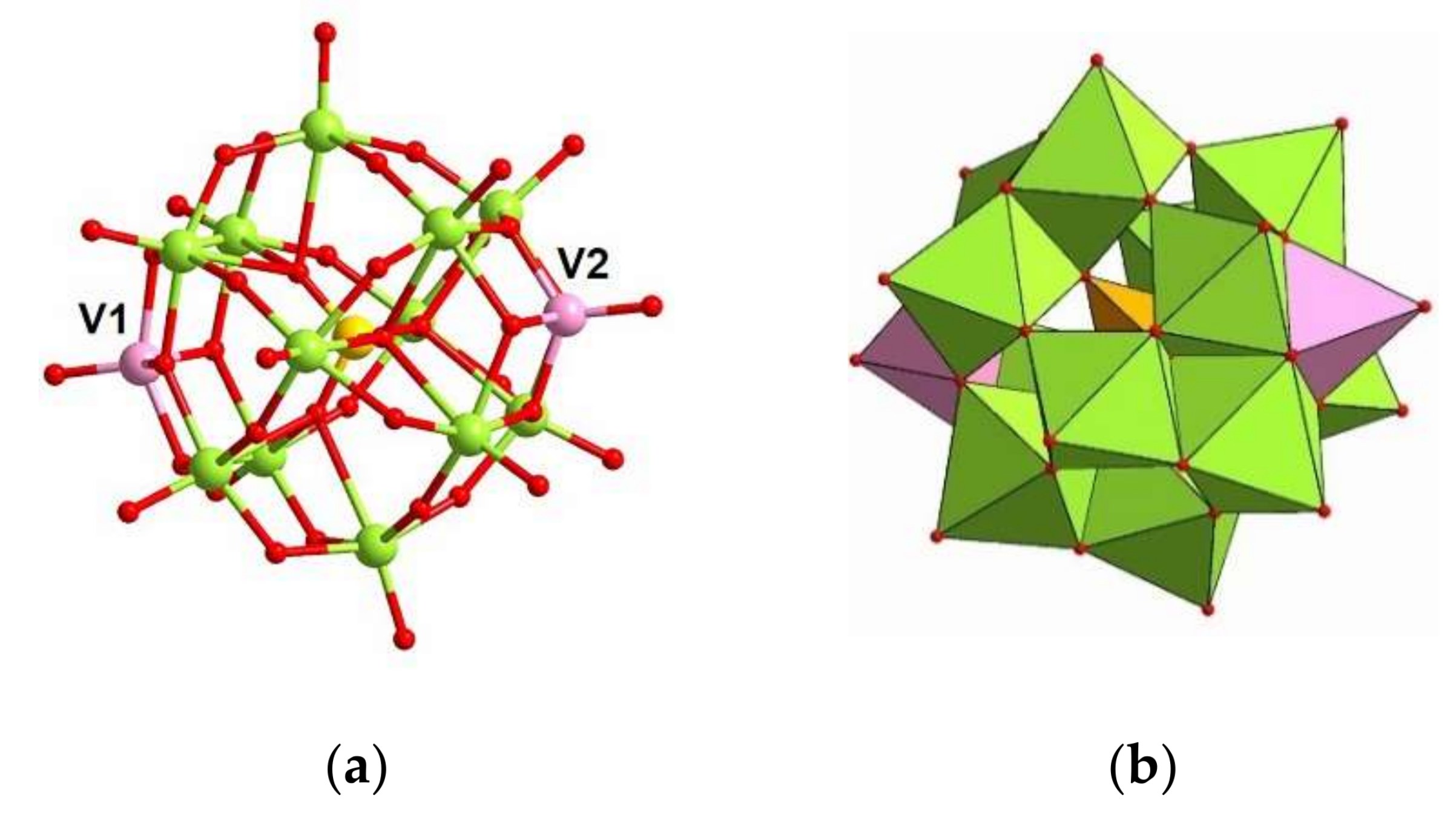
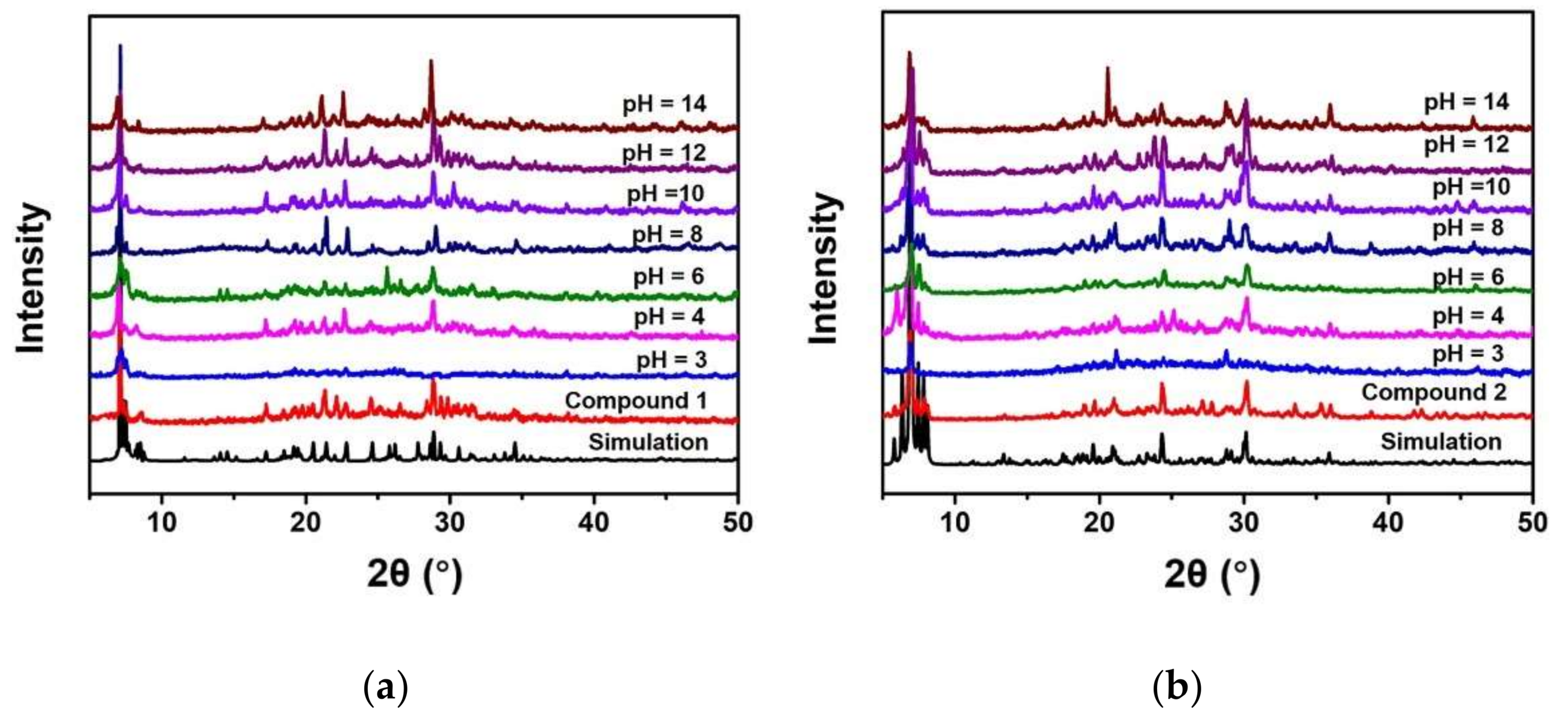
3.1. Synthesis and Structure
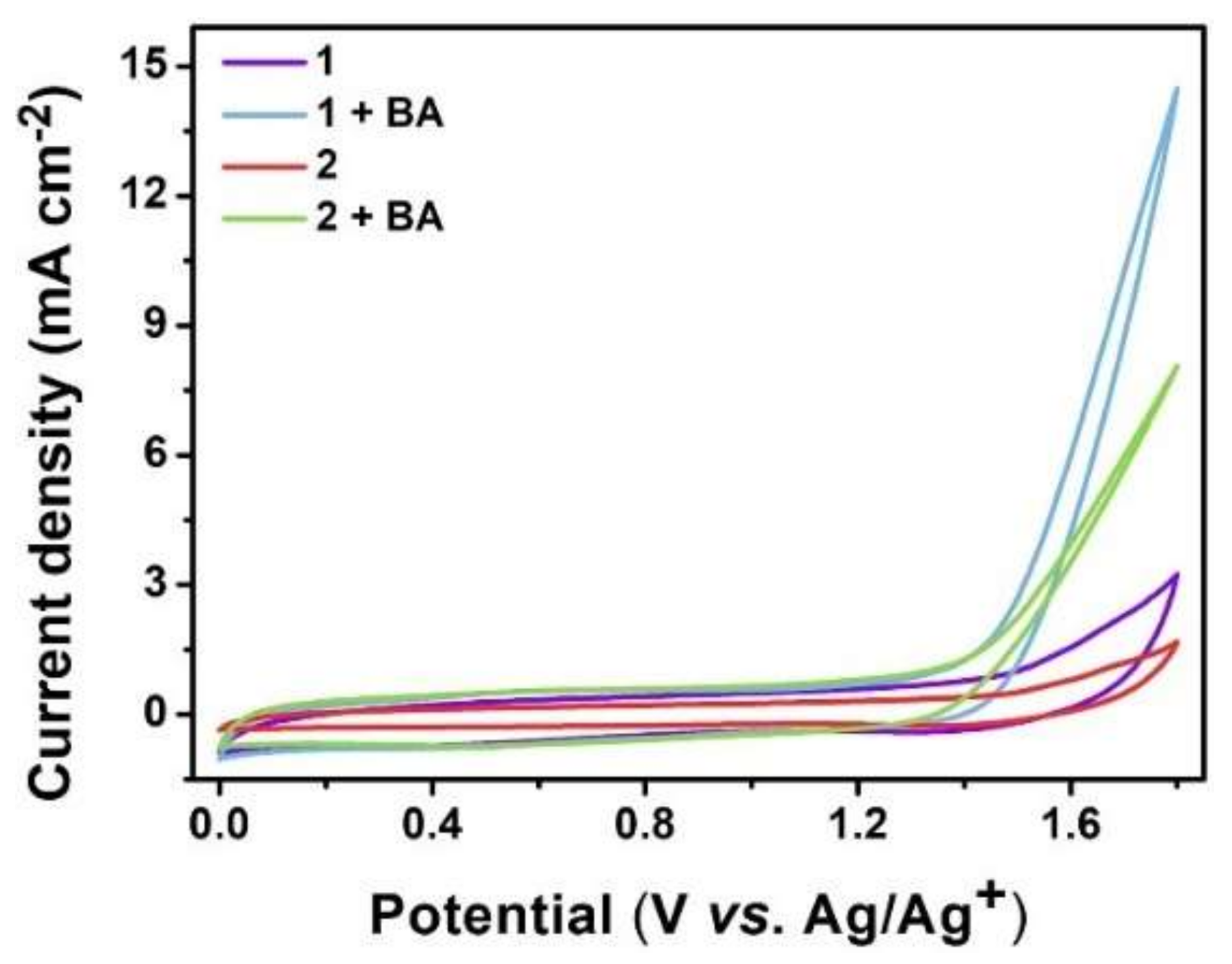
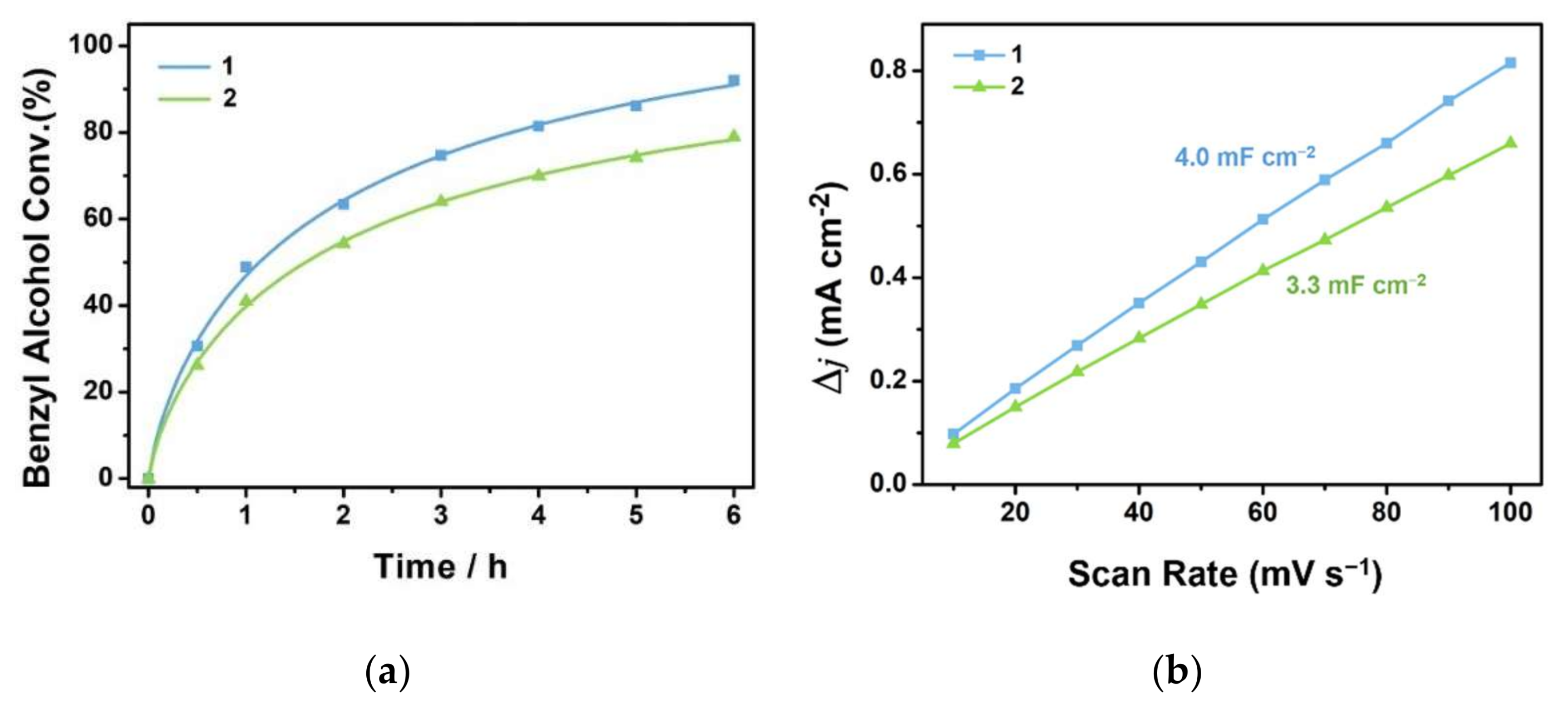
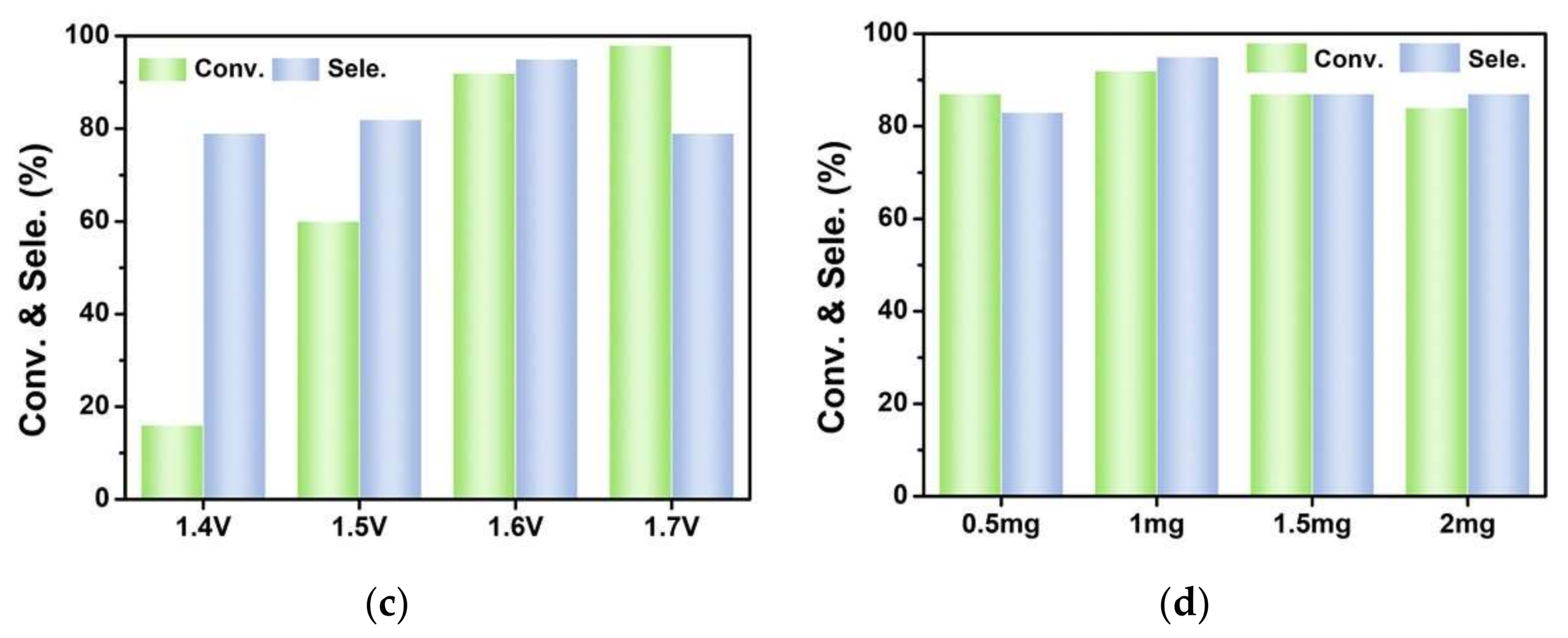
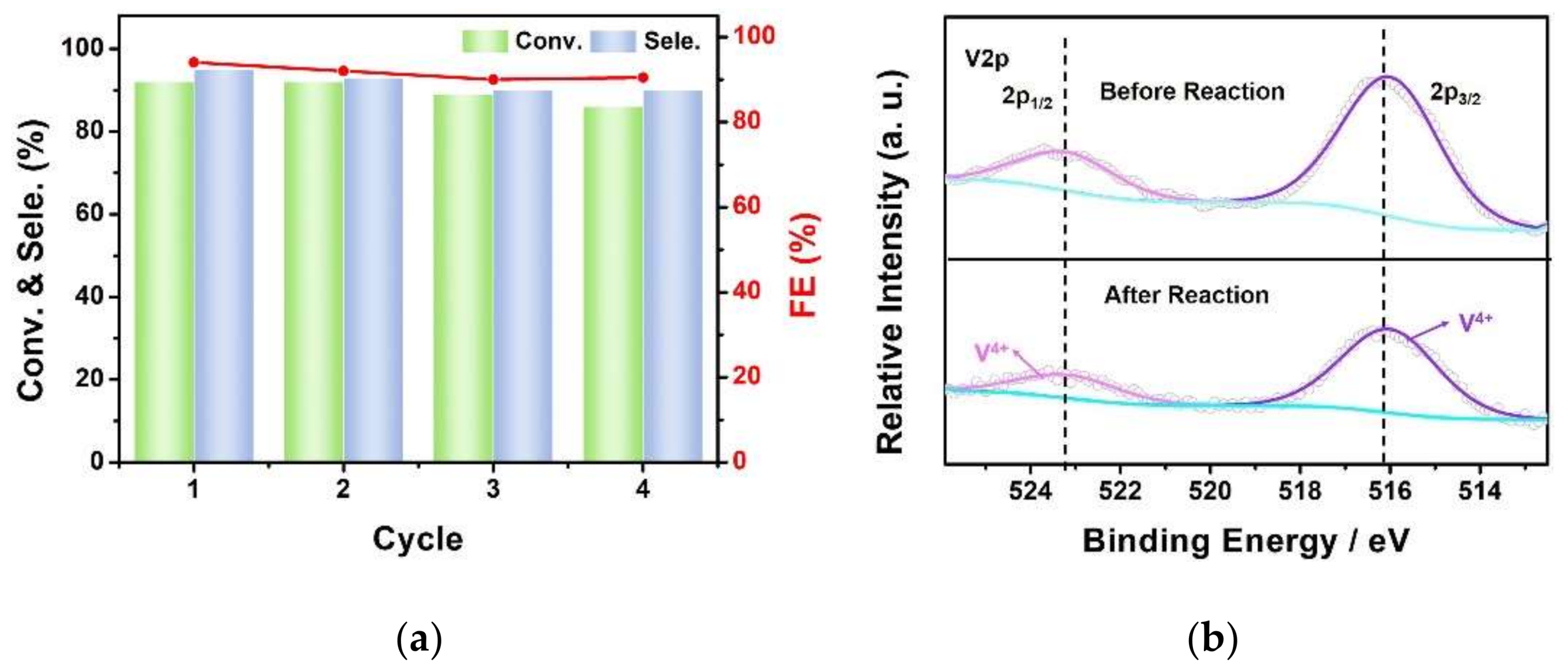
3.2. Electrocatalytic Selective Oxidation of Benzyl Alcohol
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Nyman, M.; Powers, C.R.; Bonhomme, F.; Alam, T.M.; Maginn, E.J.; Hobbs, D.T. Ion-Exchange Behavior of One-Dimensional Linked Dodecaniobate Keggin Ion Materials. Chem. Mater. 2008, 20, 2513–2521. [Google Scholar] [CrossRef]
- Zhao, H.; Li, Y.; Zhao, J.; Wang, L.; Yang, G. State-of-the-art Advances in the Structural Diversities and Catalytic Applications of Polyoxoniobate-based Materials. Coord. Chem. Rev. 2021, 443, 213966–213988. [Google Scholar] [CrossRef]
- Nyman, M. Polyoxoniobate Chemistry in the 21st Century. Dalton Trans. 2011, 40, 8049–8058. [Google Scholar] [CrossRef]
- Tsunashima, R.; Long, D.; Miras, H.N.; Gabb, D.; Pradeep, C.P.; Cronin, L. The Construction of High-nuclearity Isopolyoxoniobates with Pentagonal Building Blocks: [HNb27O76]16- and [H10Nb31O93(CO3)]23−. Angew. Chem. Int. Ed. 2010, 49, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Qin, C.; Su, Z.; Xing, Y.; Wang, X.; Shao, K.; Lan, Y.; Wang, E. Self-assembly and Photocatalytic Properties of Polyoxoniobates: {Nb24O72}, {Nb32O96}, and {K12Nb96O288} Clusters. J. Am. Chem. Soc. 2012, 134, 14004–14010. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Zhu, Z.; Wu, Y.; Qi, Y.; Li, X.; Zheng, S. Record High-Nuclearity Polyoxoniobates: Discrete Nanoclusters {Nb114}, {Nb81}, and {Nb52}, and Extended Frameworks Based on {Cu3Nb78} and {Cu4Nb78}. Angew. Chem. Int. Ed. 2017, 56, 16288–16292. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, X.; Qi, Y.; Yu, H.; Jin, L.; Zheng, S. {Nb288O768(OH)48(CO3)12}: A Macromolecular Polyoxometalate with Close to 300 Niobium Atoms. Angew. Chem. Int. Ed. 2018, 57, 8572–8576. [Google Scholar] [CrossRef]
- Nyman, M.; Bonhomme, F.; Alam, T.M.; Rodriguez, M.A.; Cherry, B.R.; Krumhansl, J.K.; Nenoff, T.M.; Sattler, A.M. A General Synthetic Procedure for Heteropolyniobates. Science 2002, 297, 996–998. [Google Scholar] [CrossRef]
- Zhang, Z.; Peng, J.; Shi, Z.; Zhou, W.; Khan, S.U.; Liu, H. Antimony-dependent Expansion for the Keggin Heteropolyniobate Family. Chem. Commun. 2015, 51, 3091–3093. [Google Scholar] [CrossRef] [Green Version]
- Son, J.; Casey, W.H. Reversible Capping/Uncapping of Phosphorous-centered Keggin-type Polyoxoniobate Clusters. Chem. Commun. 2015, 51, 1436–1438. [Google Scholar] [CrossRef] [Green Version]
- Guo, G.; Xu, Y.; Cao, J.; Hu, C. An Unprecedented Vanadoniobate Cluster with ‘Trans-vanadium’ Bicapped Keggin-type {VNb12O40(VO)2}. Chem. Commun. 2011, 47, 9411–9413. [Google Scholar] [CrossRef]
- Zhang, Y.; Shen, J.; Zheng, L.; Zhang, Z.; Li, Y.; Wang, E. Four Polyoxonibate-Based Inorganic–Organic Hybrids Assembly from Bicapped Heteropolyoxonibate with Effective Antitumor Activity. Cryst. Growth Des. 2013, 14, 110–116. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, X.; Lü, Y.; Li, G.; Xiao, L.; Cui, X.; Xu, J. New Organic–inorganic Hybrid Compounds Based on [SiNb12V2O42]12− with High Catalytic Activity for Styrene Epoxidation. Inorg. Chem. Front. 2017, 4, 1397–1404. [Google Scholar] [CrossRef]
- Hu, J.; Dong, J.; Huang, X.; Chi, Y.; Lin, Z.; Li, J.; Yang, S.; Ma, H.; Hu, C. Immobilization of Keggin Polyoxovanadoniobate in Crystalline Solids to Produce Effective Heterogeneous Catalysts towards Selective Oxidation of Benzyl-alkanes. Dalton Trans. 2017, 46, 8245–8251. [Google Scholar] [CrossRef]
- Li, S.; Ji, P.; Han, S.; Hao, Z.; Chen, X. Two Polyoxoniobates-based Ionic Crystals as Lewis Base Catalysts for Cyanosilylation. Inorg. Chem. Commun. 2020, 111, 107666. [Google Scholar] [CrossRef]
- Li, N.; Liu, Y.; Lu, Y.; He, D.; Liu, S.; Wang, X.; Li, Y.; Liu, S. An Arsenicniobate-based 3D Framework with Selective Adsorption and Anion-exchange Properties. New J. Chem. 2016, 40, 2220–2224. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, S.; Li, S.; Wang, X.; Jin, H.; Cui, J.; Liu, Y.; Liu, C. Hydrothermal Syntheses and Characterization of Two Multi-vanadium Capped Keggin Polyoxoniobates Derivatives. Chem. J. Chin. Univ. 2013, 34, 2046–2050. [Google Scholar] [CrossRef]
- Hu, J.; Wang, Y.; Zhang, X.; Chi, Y.; Yang, S.; Li, J.; Hu, C. Controllable Assembly of Vanadium-Containing Polyoxoniobate-Based Three-Dimensional Organic-Inorganic Hybrid Compounds and Their Photocatalytic Properties. Inorg. Chem. 2016, 55, 7501–7507. [Google Scholar] [CrossRef]
- Abramov, P.A.; Davletgildeeva, A.T.; Moroz, N.K.; Kompankov, N.B.; Santiago-Schubel, B.; Sokolov, M.N. Cation-Dependent Self-assembly of Vanadium Polyoxoniobates. Inorg. Chem. 2016, 55, 12807–12814. [Google Scholar] [CrossRef]
- Huang, P.; Zhou, E.; Wang, X.; Sun, C.Y.; Wang, H.; Xing, Y.; Shao, K.; Su, Z. New Heteropolyniobates Based on a Bicapped Keggin-type {VNb14} Cluster with Selective Adsorption and Photocatalytic Properties. CrystEngComm 2014, 16, 9582–9585. [Google Scholar] [CrossRef]
- Shen, J.; Wu, Q.; Zhang, Y.; Zhang, Z.; Li, Y.; Lu, Y.; Wang, E. Unprecedented High-nuclear Transition-metal-cluster-substituted Heteropolyoxoniobates: Synthesis by {V8} Ring Insertion into the POM Matrix and Antitumor Activities. Chem. Eur. J. 2014, 20, 2840–2848. [Google Scholar] [CrossRef] [PubMed]
- Lan, Q.; Zhang, Z.; Li, Y.; Wang, E. Extended Structural Materials Composed of Transition-metal-substituted Arsenicniobates and Their Photocatalytic Activity. RSC Adv. 2015, 5, 44198–44203. [Google Scholar] [CrossRef]
- Yang, Z.; Shang, J.; Yang, Y.; Ma, P.; Niu, J.; Wang, J. Synthesis, Structures and Stability of Three V-substituted Polyoxoniobate Clusters Based on [TeNb9O33]17− Units. Dalton Trans. 2021, 50, 7610–7620. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Niu, H.; Niu, J. A Novel Lindqvist Type Polyoxoniobate Coordinated to Four Copper Complex Moieties: {Nb6O19[Cu(2,2′-bipy)]2[Cu(2,2′-bipy)2]2}·19H2O. Inorg. Chem. Commun. 2008, 11, 63–65. [Google Scholar] [CrossRef]
- Wang, J.; Niu, H.; Niu, J. Preparation, Crystal structure, and Characterization of an Inorganic–organic Hybrid Polyoxoniobate [Cu(en)2]3[Cu(en)2(H2O)]1.5[K0.5Nb24O72H14.5]2·25H2O. J. Chem. Sci. 2008, 120, 309–313. [Google Scholar] [CrossRef]
- Guo, G.; Xu, Y.; Hu, C. A Polyoxoniobate Based on Dimeric Hexanionbate: [Cu(en)2]4{[Nb6O19H2]K(H2O)5}2·(H2en)·17H2O. J. Coord. Chem. 2010, 63, 3137–3145. [Google Scholar] [CrossRef]
- Liang, Z.; Qiao, Y.; Li, M.; Ma, P.; Niu, J.; Wang, J. Two Synthetic Routes Generate Two Isopolyoxoniobates Based on {Nb16} and {Nb20}. Dalton Trans. 2019, 48, 17709–17712. [Google Scholar] [CrossRef]
- Li, C.; Zhao, D.; Li, N.; Ma, Y.; Wei, G.; Wang, G.; Zhang, D. Dimeric Dumbbell Architecture Based on PNb14 unit. Inorg. Chem. Commun. 2019, 102, 210–214. [Google Scholar] [CrossRef]
- Kinnan, M.K.; Creasy, W.R.; Fullmer, L.B.; Schreuder-Gibson, H.L.; Nyman, M. Nerve Agent Degradation with Polyoxoniobates. Eur. J. Inorg. Chem. 2014, 2014, 2361–2367. [Google Scholar] [CrossRef]
- Guo, W.; Lv, H.; Sullivan, K.P.; Gordon, W.O.; Balboa, A.; Wagner, G.W.; Musaev, D.G.; Bacsa, J.; Hill, C.L. Broad-Spectrum Liquid- and Gas-Phase Decontamination of Chemical Warfare Agents by One-Dimensional Heteropolyniobates. Angew. Chem. Int. Ed. 2016, 55, 7403–7407. [Google Scholar] [CrossRef]
- Dong, J.; Hu, J.; Chi, Y.; Lin, Z.; Zou, B.; Yang, S.; Hill, C.L.; Hu, C. A Polyoxoniobate-Polyoxovanadate Double-Anion Catalyst for Simultaneous Oxidative and Hydrolytic Decontamination of Chemical Warfare Agent Simulants. Angew. Chem. Int. Ed. 2017, 56, 4473–4477. [Google Scholar] [CrossRef]
- Zhen, N.; Dong, J.; Lin, Z.; Li, X.; Chi, Y.; Hu, C. Self-assembly of Polyoxovanadate-capped Polyoxoniobates and Their Catalytic Decontamination of Sulfur Mustard Simulants. Chem. Commun. 2020, 56, 13967–13970. [Google Scholar] [CrossRef]
- Macht, J.; Janik, M.J.; Neurock, M.; Iglesia, E. Catalytic Consequences of Composition in Polyoxometalate Clusters with Keggin Structure. Angew. Chem. Int. Ed. 2007, 46, 7864–7868. [Google Scholar] [CrossRef]
- Toma, F.M.; Sartorel, A.; Iurlo, M.; Carraro, M.; Parisse, P.; Maccato, C.; Rapino, S.; Gonzalez, B.R.; Amenitsch, H.; Da Ros, T.; et al. Efficient Water Oxidation at Carbon Nanotube-polyoxometalate Electrocatalytic Interfaces. Nat. Chem. 2010, 2, 826–831. [Google Scholar] [CrossRef]
- Evtushok, V.Y.; Suboch, A.N.; Podyacheva, O.Y.; Stonkus, O.A.; Zaikovskii, V.I.; Chesalov, Y.A.; Kibis, L.S.; Kholdeeva, O.A. Highly Efficient Catalysts Based on Divanadium-Substituted Polyoxometalate and N-Doped Carbon Nanotubes for Selective Oxidation of Alkylphenols. ACS Catal. 2018, 8, 1297–1307. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, J.; Jing, X.; Dong, J.; Liu, H.; Lv, H.; Chi, Y.; Hu, C. A Polyoxometalate@covalent Triazine Framework as a Robust Electrocatalyst for Selective Benzyl Alcohol Oxidation Coupled with Hydrogen Production. J. Mater. Chem. A 2021, 9, 6152–6159. [Google Scholar] [CrossRef]
- Li, Z.; Liu, C.; Geng, W.; Dong, J.; Chi, Y.; Hu, C. Electrocatalytic Ethylbenzene Valorization Using a Polyoxometalate@covalent Triazine Framework with Water as the Oxygen Source. Chem. Commun. 2021, 57, 7430–7433. [Google Scholar] [CrossRef]
- Filowitz, M.; Ho, R.K.C.; Klemperer, W.G.; Shum, W. 17O Nuclear Magnetic Resonance Spectroscopy of Polyoxometalates. 1. Sensitivity and Resolution. Inorg. Chem. 1979, 18, 93–103. [Google Scholar] [CrossRef]
- Dietl, N.; Hockendorf, R.F.; Schlangen, M.; Lerch, M.; Beyer, M.K.; Schwarz, H. Generation, Reactivity towards Hydrocarbons, and Electronic Structure of Heteronuclear Vanadium Phosphorous Oxygen Cluster Ions. Angew. Chem. Int. Ed. 2011, 50, 1430–1434. [Google Scholar] [CrossRef]
- Guilherme, L.R.; Massabni, A.C.; Dametto, A.C.; de Souza Correa, R.; De Araujo, A.S. Synthesis, Infrared Spectroscopy and Crystal Structure Determination of a New Decavanadate. J. Chem. Crystallogr. 2010, 40, 897–901. [Google Scholar] [CrossRef]
- Son, J.H.; Ohlin, C.A.; Johnson, R.L.; Yu, P.; Casey, W.H. A Soluble Phosphorus-centered Keggin Polyoxoniobate with Bicapping Vanadyl Groups. Chem. Eur. J. 2013, 19, 5191–5197. [Google Scholar] [CrossRef]
- Gao, S.; Lin, Y.; Jiao, X.; Sun, Y.; Luo, Q.; Zhang, W.; Li, D.; Yang, J.; Xie, Y. Partially Oxidized Atomic Cobalt Layers for Carbon Dioxide Electroreduction to Liquid Fuel. Nature 2016, 529, 68–71. [Google Scholar] [CrossRef]
- Zhong, W.; Liu, H.; Bai, C.; Liao, S.; Li, Y. Base-Free Oxidation of Alcohols to Esters at Room Temperature and Atmospheric Conditions using Nanoscale Co-Based Catalysts. ACS Catal. 2015, 5, 1850–1856. [Google Scholar] [CrossRef]
- Guo, Z.; Liu, B.; Zhang, Q.; Deng, W.; Wang, Y.; Yang, Y. Recent Advances in Heterogeneous Selective Oxidation Catalysis for Sustainable Chemistry. Chem. Soc. Rev. 2014, 43, 3480–3524. [Google Scholar] [CrossRef] [PubMed]
- Horn, E.J.; Rosen, B.R.; Chen, Y.; Tang, J.; Chen, K.; Eastgate, M.D.; Baran, P.S. Scalable and Sustainable Electrochemical Allylic C-H Oxidation. Nature 2016, 533, 77–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, Z.; Zheng, Y.; Wang, H.; Li, J.; Zhu, Q.; Wang, Y.; Ma, N.; Hu, G.; He, B.; Knop-Gericke, A.; et al. Engineering Interface with One-Dimensional Co3O4 Nanostructure in Catalytic Membrane Electrode: Toward an Advanced Electrocatalyst for Alcohol Oxidation. ACS Nano 2017, 11, 12365–12377. [Google Scholar] [CrossRef]
- Zheng, J.; Chen, X.; Zhong, X.; Li, S.; Liu, T.; Zhuang, G.; Li, X.; Deng, S.; Mei, D.; Wang, J. Hierarchical Porous NC@CuCo Nitride Nanosheet Networks: Highly Efficient Bifunctional Electrocatalyst for Overall Water Splitting and Selective Electrooxidation of Benzyl Alcohol. Adv. Funct. Mater. 2017, 27, 1704169–1704179. [Google Scholar] [CrossRef]
- Rafiee, M.; Konz, Z.M.; Graaf, M.D.; Koolman, H.F.; Stahl, S.S. Electrochemical Oxidation of Alcohols and Aldehydes to Carboxylic Acids Catalyzed by 4-Acetamido-TEMPO: An Alternative to “Anelli” and “Pinnick” Oxidations. ACS Catal. 2018, 8, 6738–6744. [Google Scholar] [CrossRef]
- Hayashi, S.; Sasaki, N.; Yamazoe, S.; Tsukuda, T. Superior Base Catalysis of Group 5 Hexametalates [M6O19]8− (M = Ta, Nb) over Group 6 Hexametalates [M6O19]2− (M = Mo, W). J. Phys. Chem. C 2018, 122, 29398–29404. [Google Scholar] [CrossRef]
- Hayashi, S.; Yamazoe, S.; Tsukuda, T. Base Catalytic Activity of [Nb10O28]6−: Effect of Countercations. J. Phys. Chem. C 2020, 124, 10975–10980. [Google Scholar] [CrossRef]


 | ||||
|---|---|---|---|---|
| Entry | Cat. | Conv. (%) b | Sele. (%) b | FE (%) |
| 1 | - | 42 | 73 | 66 |
| 2 | Compound 1 | 92 | 95 | 93 |
| 3 | Compound 2 | 79 | 90 | 84 |
| 4 | Ni(en)3Cl2·H2O | 48 | 69 | 70 |
| 5 | K7H[Nb6O19]·13H2O | 72 | 79 | 76 |
| 6 | [N(CH3)4]10H5[PNb12O40]·30.5H2O | 70 | 78 | 76 |
| 7 | K6[V10O28]·10H2O | 83 | 76 | 78 |
| 8 | [N(CH3)4]9[PNb12O40(VO)2]·19H2O | 74 | 89 | 82 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Zhen, N.; Liu, C.; Zhang, D.; Dong, J.; Chi, Y.; Hu, C. Controllable Assembly of Vanadium-Containing Polyoxoniobate-Based Materials and Their Electrocatalytic Activity for Selective Benzyl Alcohol Oxidation. Molecules 2022, 27, 2862. https://doi.org/10.3390/molecules27092862
Li X, Zhen N, Liu C, Zhang D, Dong J, Chi Y, Hu C. Controllable Assembly of Vanadium-Containing Polyoxoniobate-Based Materials and Their Electrocatalytic Activity for Selective Benzyl Alcohol Oxidation. Molecules. 2022; 27(9):2862. https://doi.org/10.3390/molecules27092862
Chicago/Turabian StyleLi, Xiaoxia, Ni Zhen, Chengpeng Liu, Di Zhang, Jing Dong, Yingnan Chi, and Changwen Hu. 2022. "Controllable Assembly of Vanadium-Containing Polyoxoniobate-Based Materials and Their Electrocatalytic Activity for Selective Benzyl Alcohol Oxidation" Molecules 27, no. 9: 2862. https://doi.org/10.3390/molecules27092862





