Flavonoids as Potential Anti-Inflammatory Molecules: A Review
Abstract
:1. Introduction
2. Sources of Flavonoids
2.1. Flavones
2.2. Flavanols
2.3. Flavonols
2.4. Flavanones
2.5. Isoflavones
2.6. Anthocyanins
3. Biochemical Activities of Flavonoids
4. Bioavailability of Flavonoids
5. Role of Flavonoids as Anti-Inflammatory Agents
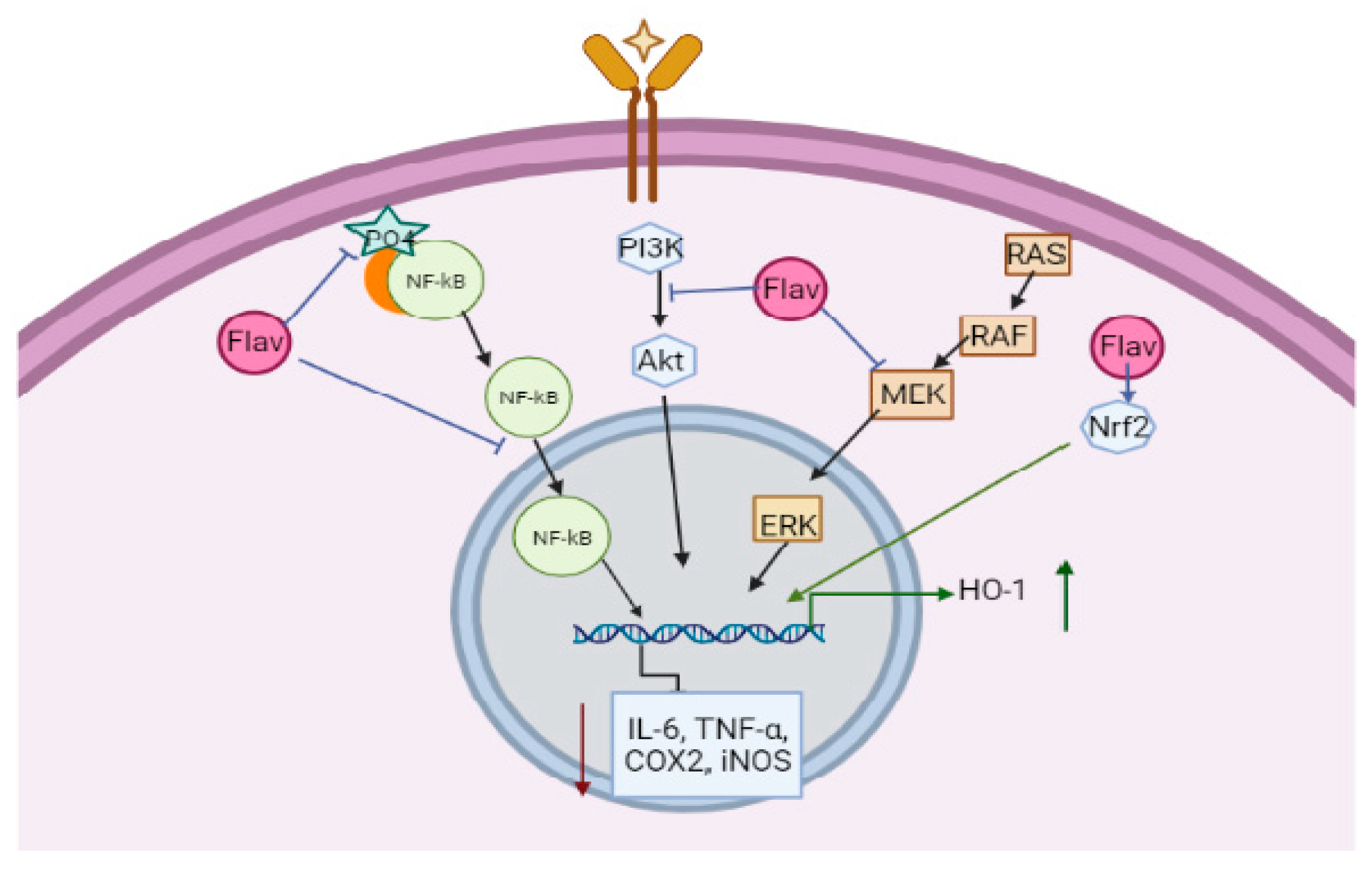
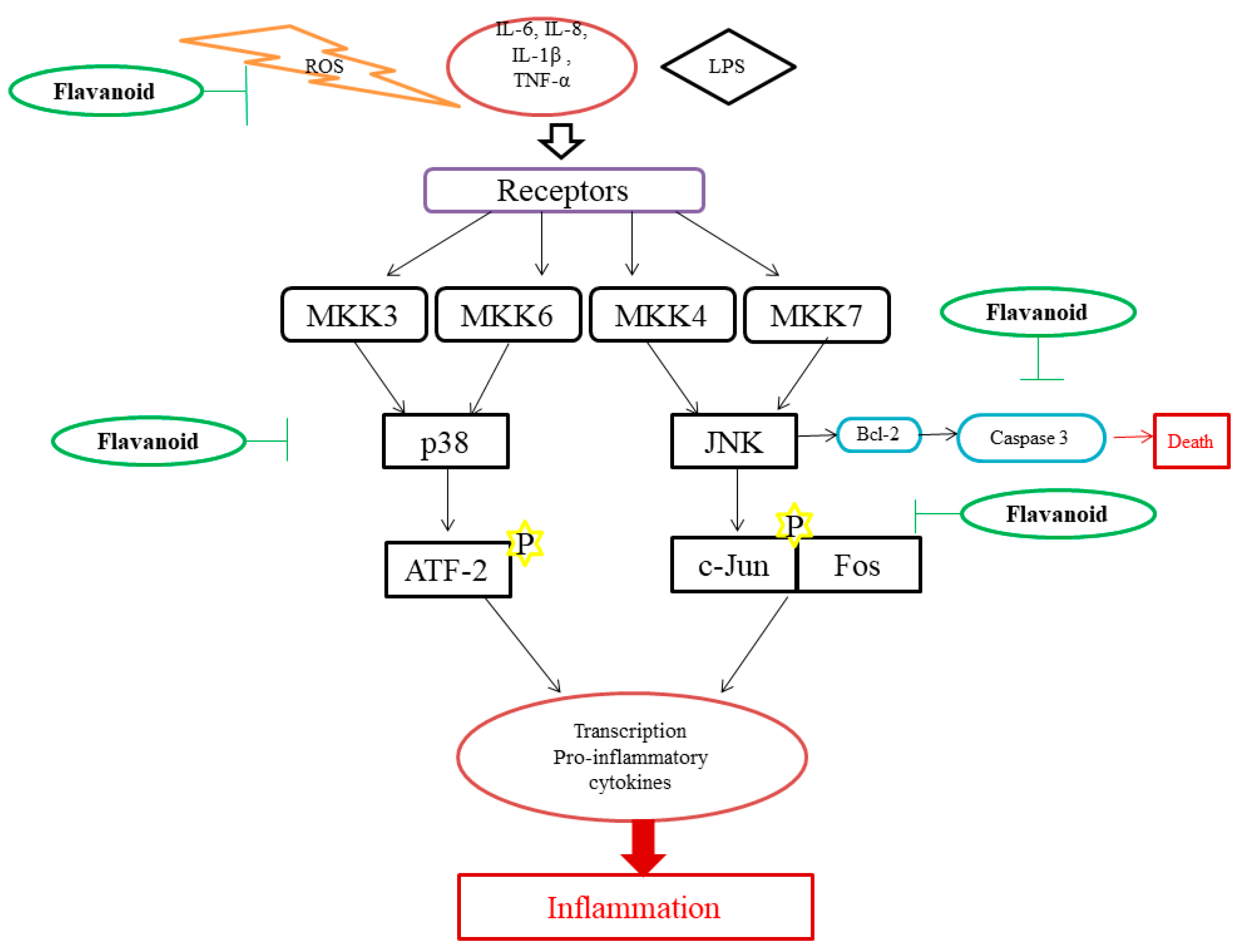
6. Mechanism of Action of Flavonoids
7. Effects of Flavonoids in Cardiovascular Disease
8. Effect of Flavonoids in Type 2 Diabetes
9. Effects of Flavonoids in Rheumatoid Arthritis (RA)
10. Effects of Flavonoids in Neurodegenerative Diseases
11. Effects of Flavonoids in Retinal Degeneration
12. Effects of Flavonoids in Inflammatory Bowel Disease (IBD)
13. Effect of Flavonoids in Cancer Treatment
14. Effects of Flavonoids in Obstructive Pulmonary Disorders
15. Effect of Flavonoids in Coronavirus Disease 2019 (COVID-19)
16. Flavonoids under Human Trials
17. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Serafini, M.; Peluso, I.; Raguzzini, A. Flavonoids as Anti-Inflammatory Agents. Proc. Nutr. Soc. 2010, 69, 273–278. [Google Scholar] [CrossRef] [Green Version]
- García-Lafuente, A.; Guillamón, E.; Villares, A.; Rostagno, M.A.; Martínez, J.A. Flavonoids as Anti-Inflammatory Agents: Implications in Cancer and Cardiovascular Disease. Inflamm. Res. 2009, 58, 537–552. [Google Scholar] [CrossRef]
- Lila, M.A. Anthocyanins and Human Health: An In Vitro Investigative Approach. J. Biomed. Biotechnol. 2004, 2004, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, C.R.; Carvalho, T.T.; Manchope, M.F.; Artero, N.A.; Rasquel-Oliveira, F.S.; Fattori, V.; Casagrande, R.; Verri, W.A., Jr. Therapeutic Potential of Flavonoids in Pain and Inflammation: Mechanisms of Action, Pre-Clinical and Clinical Data, and Pharmaceutical Development. Molecules 2020, 25, 762. [Google Scholar] [CrossRef] [Green Version]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.-H.; Jaremko, M. Important Flavonoids and Their Role as a Therapeutic Agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef]
- Crozier, A.; Jaganath, I.B.; Clifford, M.N. Dietary Phenolics: Chemistry, Bioavailability and Effects on Health. Nat. Prod. Rep. 2009, 26, 1001–1043. [Google Scholar] [CrossRef] [PubMed]
- Fattori, V.; Amaral, F.A.; Verri, W.A., Jr. Neutrophils and Arthritis: Role in Disease and Pharmacological Perspectives. Pharma. Res. 2016, 112, 84–98. [Google Scholar] [CrossRef]
- McDonald, B.; Pittman, K.; Menezes, G.B.; Hirota, S.A.; Slaba, I.; Waterhouse, C.C.M.; Beck, P.L.; Muruve, D.A.; Kubes, P. Intravascular Danger Signals Guide Neutrophils to Sites of Sterile Inflammation. Science 2010, 330, 362–366. [Google Scholar] [CrossRef] [PubMed]
- Leyva-López, N.; Gutierrez-Grijalva, E.P.; Ambriz-Perez, D.L.; Heredia, J.B. Flavonoids as Cytokine Modulators: A Possible Therapy for Inflammation-Related Diseases. Int. J. Mol. Sci. 2016, 17, 921. [Google Scholar] [CrossRef]
- Choy, K.W.; Murugan, D.; Leong, X.-F.; Abas, R.; Alias, A.; Mustafa, M.R. Flavonoids as Natural Anti-Inflammatory Agents Targeting Nuclear Factor-Kappa B (NFκB) Signaling in Cardiovascular Diseases: A Mini Review. Front. Pharmacol. 2019, 10, 1295. [Google Scholar] [CrossRef] [Green Version]
- Farzaei, M.H.; Singh, A.K.; Kumar, R.; Croley, C.R.; Pandey, A.K.; Coy-Barrera, E.; Patra, J.K.; Das, G.; Kerry, R.G.; Annunziata, G.; et al. Targeting Inflammation by Flavonoids: Novel Therapeutic Strategy for Metabolic Disorders. Int. J. Mol. Sci. 2019, 20, 4957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, R.H. Health-Promoting Components of Fruits and Vegetables in the Diet. Adv. Nutr. Int. Rev. J. 2013, 4, 384S–392S. [Google Scholar] [CrossRef] [PubMed]
- Janabi, A.H.W.; Kamboh, A.A.; Saeed, M.; BiBi, J.; Naveed, M.; Huixia, L. Flavonoid-rich foods (FRF): A promising nutraceutical approach against lifespan-shortening diseases. Iran. J. Basic Med. Sci. 2020, 23, 140–153. [Google Scholar] [CrossRef]
- Qiusheng, Z.; Xiling, S.; Xubo; Meng, S.; Changhai, W. Protective effects of luteolin-7-glucoside against liver injury caused by carbon tetrachloride in rats. Die Pharm. Int. J. Pharm. Sciences. 2004, 59, 286–288. [Google Scholar]
- Saleh, H.A.-R.; El-Nashar, Y.I.; Serag-El-Din, M.F.; Dewir, Y.H. Plant growth, yield and bioactive compounds of two culinary herbs as affected by substrate type. Sci. Hortic. 2019, 243, 464–471. [Google Scholar] [CrossRef]
- Lago, J.H.G.; Toledo-Arruda, A.C.; Mernak, M.; Barrosa, K.H.; Martins, M.A.; Tibério, I.F.L.C.; Prado, C.M. Structure-Activity Association of Flavonoids in Lung Diseases. Molecules 2014, 19, 3570–3595. [Google Scholar] [CrossRef] [Green Version]
- Xie, Y.; Yang, W.; Tang, F.; Chen, X.; Ren, L. Antibacterial Activities of Flavonoids: Structure-Activity Relationship and Mechanism. Curr. Med. Chem. 2015, 22, 132–149. [Google Scholar] [CrossRef]
- Krishna, S.; Chandrasekaran, S.; Dhanasekar, D.; Perumal, A. GCMS analysis, antioxidant and antibacterial activities of ethanol extract of Anisomeles malabarica (L.) R.Br. ex. Sims leaves. Asian J. Pharm. Pharmacol. 2019, 5, 180–187. [Google Scholar] [CrossRef]
- Fidelis, Q.C.; Ribeiro, T.A.; Araújo, M.F.; De Carvalho, M.G. Ouratea genus: Chemical and pharmacological aspects. Rev. Bras. Farm. 2014, 24, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Yang, J. Brazil nuts and associated health benefits: A review. LWT 2009, 42, 1573–1580. [Google Scholar] [CrossRef]
- Ju, J.B.; Kim, J.S.; Choi, C.W.; Lee, H.K.; Oh, T.-K.; Kim, S.C. Comparison between ethanolic and aqueous extracts from Chinese juniper berries for hypoglycaemic and hypolipidemic effects in alloxan-induced diabetic rats. J. Ethnopharmacol. 2008, 115, 110–115. [Google Scholar] [CrossRef]
- Mascolo, N.; Autore, G.; Capasso, F.; Menghini, A.; Fasulo, M.P. Biological Screening of Italian Medicinal Plants for Anti-Inflammatory Activity. Phytother. Res. 1987, 1, 28–31. [Google Scholar] [CrossRef]
- Tunon, H.; Olavsdotter, C.; Bohlin, L. Evaluation of anti-inflammatory activity of some Swedish medicinal plants. Inhibition of prostaglandin biosynthesis and PAF-induced exocytosis. J. Ethnopharmacol. 1995, 48, 61–76. [Google Scholar] [CrossRef]
- Höferl, M.; Stoilova, I.; Schmidt, E.; Wanner, J.; Jirovetz, L.; Trifonova, D.; Krastev, L.; Krastanov, A. Chemical Composition and Antioxidant Properties of Juniper Berry (Juniperus Communis L.) Essential Oil. Action of the Essential Oil on the Anti-oxidant Protection of Saccharomyces Cerevisiae Model Organism. Antioxidants 2014, 3, 81–98. [Google Scholar] [CrossRef] [Green Version]
- Fierascu, I.; Ungureanu, C.; Avramescu, S.M.; Cimpeanu, C.; Georgescu, M.I.; Fierascu, R.C.; Ortan, A.; Sutan, A.N.; Anuta, V.; Zanfirescu, A.; et al. Genoprotective, antioxidant, antifungal and anti-inflammatory evaluation of hydroalcoholic extract of wild-growing Juniperus communis L. (Cupressaceae) native to Romanian southern sub-Carpathian hills. BMC Complement Altern. Med. 2018, 18, 3. [Google Scholar] [CrossRef] [Green Version]
- Thilakarathna, S.H.; Rupasinghe, H.P.V. Flavonoid Bioavailability and Attempts for Bioavailability Enhancement. Nutrients 2013, 5, 3367–3387. [Google Scholar] [CrossRef]
- Guazelli, C.F.S.; Fattori, V.; Colombo, B.B.; Georgetti, S.R.; Vicentini, F.T.M.C.; Casagrande, R.; Baracat, M.M.; Verri, W.A., Jr. Quercetin-Loaded Microcapsules Ameliorate Experimental Colitis in Mice by Anti-Inflammatory and Antioxidant Mechanisms. J. Nat. Prod. 2013, 76, 200–208. [Google Scholar] [CrossRef]
- Amanzadeh, E.; Esmaeili, A.; Abadi, R.E.N.; Kazemipour, N.; Pahlevanneshan, Z.; Beheshti, S. Quercetin conjugated with superparamagnetic iron oxide nanoparticles improves learning and memory better than free quercetin via interacting with proteins involved in LTP. Sci. Rep. 2019, 9, 6876. [Google Scholar] [CrossRef]
- Khan, A.W.; Kotta, S.; Ansari, S.H.; Sharma, R.K.; Ali, J. Self-nanoemulsifying drug delivery system (SNEDDS) of the poorly water-soluble grapefruit flavonoid Naringenin: Design, characterization, in vitro and in vivo evaluation. Drug Deliv. 2015, 22, 552–561. [Google Scholar] [CrossRef]
- Huang, M.; Su, E.; Zheng, F.; Tan, C. Encapsulation of flavonoids in liposomal delivery systems: The case of quercetin, kaempferol and luteolin. Food Funct. 2017, 8, 3198–3208. [Google Scholar] [CrossRef]
- Li, B.; Liu, H.; Amin, M.; Wegiel, L.A.; Taylor, L.S.; Edgar, K.J. Enhancement of naringenin solution concentration by solid dispersion in cellulose derivative matrices. Cellulose 2013, 20, 2137–2149. [Google Scholar] [CrossRef]
- Patra, A.; Satpathy, S.; Shenoy, A.K.; Bush, J.; Kazi, M.; Hussain, M.D. Formulation and evaluation of mixed polymeric micelles of quercetin for treatment of breast, ovarian, and multidrug resistant cancers. Int. J. Nanomed. 2018, 13, 2869–2881. [Google Scholar] [CrossRef] [Green Version]
- Read, M. Flavonoids: Naturally occurring anti-inflammatory agents. Am. J. Pathol. 1995, 147, 235–237. [Google Scholar]
- Santangelo, C.; Varì, R.; Scazzocchio, B.; Di Benedetto, R.; Filesi, C.; Masella, R. Polyphenols, intracellular signalling and inflammation. Ann. Ist. Super Sanita 2007, 43, 394–405. [Google Scholar]
- Ferrali, M.; Signorini, C.; Caciotti, B.; Sugherini, L.; Ciccoli, L.; Giachetti, D.; Comporti, M. Protection against oxidative damage of erythrocyte membrane by the flavonoid quercetin and its relation to iron chelating activity. FEBS Lett. 1997, 416, 123–129. [Google Scholar] [CrossRef] [Green Version]
- Crespo, M.; Gálvez, J.; Cruz, T.; Ocete, M.; Zarzuelo, A. Anti-Inflammatory Activity of Diosmin and Hesperidin in Rat Colitis Induced by TNBS. Planta Med. 1999, 65, 651–653. [Google Scholar] [CrossRef]
- Nam, T.G.; Lim, T.-G.; Lee, B.H.; Lim, S.; Kang, H.; Eom, S.H.; Yoo, M.; Jang, H.W.; Kim, D.-O. Comparison of Anti-Inflammatory Effects of Flavonoid-Rich Common and Tartary Buckwheat Sprout Extracts in Lipopolysaccharide-Stimulated RAW 264.7 and Peritoneal Macrophages. Oxidative Med. Cell. Longev. 2017, 2017, 9658030. [Google Scholar] [CrossRef]
- Ortega, J.T.; Parmar, T.; Golczak, M.; Jastrzebska, B. Protective Effects of Flavonoids in Acute Models of Light-Induced Retinal Degeneration. Mol. Pharmacol. 2020, 99, 60–77. [Google Scholar] [CrossRef]
- Rates, S.M.K.; Schapoval, E.E.S.; Souza, L.; Henriques, A.T. Chemical Constituents and Pharmacological Activities ofPeschiera australis. Int. J. Pharmacogn. 1993, 31, 288–294. [Google Scholar] [CrossRef]
- Gomes, R.; Neto, A.; Melo, V.; Fernandes, V.; Dagrava, G.; Santos, W.; Pereira, P.S.; Couto, L.; Beleboni, R. Antinociceptive and anti-inflammatory activities of Tabernaemontana catharinensis. Pharm. Biol. 2009, 47, 372–376. [Google Scholar] [CrossRef] [Green Version]
- Marques, J.I.; Alves, J.S.F.; Torres-Rêgo, M.; Furtado, A.A.; Siqueira, E.M.D.S.; Galinari, E.; Araújo, D.F.D.S.; Guerra, G.C.B.; de Azevedo, E.P.; Fernandes-Pedrosa, M.D.F.; et al. Phytochemical Analysis by HPLC–HRESI-MS and Anti-Inflammatory Activity of Tabernaemontana catharinensis. Int. J. Mol. Sci. 2018, 19, 636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, J.; Lim, S.; Lee, S.; Choi, C.-I.; Kim, K. Antioxidant and Anti-Inflammatory Effects of White Mulberry (Morus alba L.) Fruits on Lipopolysaccharide-Stimulated RAW 264.7 Macrophages. Molecules 2021, 26, 920. [Google Scholar] [CrossRef] [PubMed]
- Cunha, T.M.; Roman-Campos, D.; Lotufo, C.M.; Duarte, H.L.; Souza, G.R.; Verri, W.A.; Funez, M.I.; Dias, Q.M.; Schivo, I.R.; Domingues, A.C.; et al. Morphine peripheral analgesia depends on activation of the PI3Kγ/AKT/nNOS/NO/KATP signaling pathway. Proc. Natl. Acad. Sci. USA 2010, 107, 4442–4447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sachs, D.; Cunha, F.Q.; Ferreira, S.H. Peripheral Analgesic Blockade of Hypernociception: Activation of Argi-nine/NO/CGMP/Protein Kinase G/ATP-Sensitive K+ Channel Pathway. Proc. Natl. Acad. Sci. USA 2004, 101, 3680–3685. [Google Scholar] [CrossRef] [Green Version]
- Gao, W.; Zan, Y.; Wang, Z.-J.J.; Hu, X.-Y.; Huang, F. Quercetin ameliorates paclitaxel-induced neuropathic pain by stabilizing mast cells, and subsequently blocking PKCε-dependent activation of TRPV1. Acta Pharmacol. Sin. 2016, 37, 1166–1177. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, T.T.; Mizokami, S.S.; Ferraz, C.R.; Manchope, M.F.; Borghi, S.M.; Fattori, V.; Calixto-Campos, C.; Camilios-Neto, D.; Casagrande, R.; Verri, W.A. The Granulopoietic Cytokine Granulocyte Colony-Stimulating Factor (G-CSF) Induces Pain: An-algesia by Rutin. Inflammopharmacology 2019, 27, 1285–1296. [Google Scholar] [CrossRef]
- Iio, M.; Ishimoto, S.; Nishida, Y.; Shiramizu, T.; Yunoki, H. Effects of Baicalein, a Flavonoid, and Other Anti-inflammatory Agents on Glyoxalase-I Activity. Agric. Biol. Chem. 1986, 50, 1073–1074. [Google Scholar] [CrossRef]
- Liskova, A.; Samec, M.; Koklesova, L.; Samuel, S.M.; Zhai, K.; Al-Ishaq, R.K.; Kubatka, P.; La Rocca, G.; Ullrich, D.; Mozos, I.; et al. Flavonoids against the SARS-CoV-2 induced inflammatory storm. Biomed. Pharmacother. 2021, 138, 111430. [Google Scholar] [CrossRef]
- Ruiz-Iglesias, P.; Estruel-Amades, S.; Camps-Bossacoma, M.; Massot-Cladera, M.; Franch, A.; Pérez-Cano, F.J.; Castell, M. Influence of Hesperidin on Systemic Immunity of Rats Following an Intensive Training and Exhausting Exercise. Nutrients 2020, 12, 1291. [Google Scholar] [CrossRef]
- Kang, O.H.; Choi, J.G.; Lee, J.H.; Kwon, D.Y. Luteolin isolated from the flowers of Lonicera japonica suppresses inflammatory mediator release by blocking NF-κB and MAPKs activation pathways in HMC-1 cells. Molecules 2010, 15, 385–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.C.; Chang, H.H.; Chung, Y.H.; Lee, T.Y. Andrographolide acts as an anti-inflammatory agent in LPS-stimulated RAW264. 7 macrophages by inhibiting STAT3-mediated suppression of the NF-κB pathway. J. Ethnopharmacol. 2011, 135, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, Y.; Chen, S.-S.; Zhang, L.; Wang, J.; Yang, Y.; Zhang, S.; Pan, Y.; Wang, Y.; Yang, L. Systems Pharmacology Dissection of the Anti-Inflammatory Mechanism for the Medicinal Herb Folium Eriobotryae. Int. J. Mol. Sci. 2015, 16, 2913–2941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hämäläinen, M.; Nieminen, R.; Vuorela, P.; Heinonen, M.; Moilanen, E. Anti-inflammatory effects of flavonoids: Genistein, kaempferol, quercetin, and daidzein inhibit STAT-1 and NF-κB activations, whereas flavone, isorhamnetin, naringenin, and pelargonidin inhibit only NF-κB activation along with their inhibitory effect on iNOS expression and NO production in activated macrophages. Mediat. Inflamm. 2007, 2007, 045673. [Google Scholar]
- Jiang, J.; Jia, Y.; Lu, X.; Zhang, T.; Zhao, K.; Fu, Z.; Pang, C.; Qian, Y. Vitexin suppresses RANKL-induced osteoclastogenesis and prevents lipopolysaccharide (LPS)-induced osteolysis. J. Cell. Physiol. 2019, 234, 17549–17560. [Google Scholar] [CrossRef] [PubMed]
- Borghi, S.M.; Carvalho, T.T.; Staurengo-Ferrari, L.; Hohmann, M.S.; Pinge-Filho, P.; Casagrande, R.; Verri, W.A., Jr. Vitexin inhibits inflammatory pain in mice by targeting TRPV1, oxidative stress, and cytokines. J. Nat. Prod. 2013, 76, 1141–1149. [Google Scholar] [CrossRef]
- Ye, M.; Wang, Q.; Zhang, W.; Li, Z.; Wang, Y.; Hu, R. Oroxylin A Exerts Anti-Inflammatory Activity on Lipopolysaccha-ride-Induced Mouse Macrophage via Nrf2/ARE Activation. Biochem. Cell Biol. 2014, 92, 337–348. [Google Scholar] [CrossRef]
- Chen, X.-L.; Dodd, G.; Thomas, S.; Zhang, X.; Wasserman, M.A.; Rovin, B.H.; Kunsch, C. Activation of Nrf2/ARE Pathway Protects Endothelial Cells from Oxidant Injury and Inhibits Inflammatory Gene Expression. Am. J. Physiol. Heart Circ. Phys Iol. 2006, 290, H1862–H1870. [Google Scholar] [CrossRef] [Green Version]
- Imam, F.; Al-Harbi, N.O.; Al-Harbia, M.M.; Korashy, H.M.; Ansari, M.A.; Sayed-Ahmed, M.M.; Nagi, M.N.; Iqbal, M.; Anwer, K.; Kazmi, I.; et al. Rutin attenuates carfilzomib-induced cardiotoxicity through inhibition of NF-κB, hypertrophic gene expression and oxidative stress. Cardiovasc. Toxicol. 2017, 17, 58–66. [Google Scholar] [CrossRef]
- Li, X.W.; Guo, B.; Shen, Y.Y.; Yang, J.R. Effect of chrysin on expression of NOX4 and NF-κB in right ventricle of monocrotaline-induced pulmonary arterial hypertension of rats. Yao Xue Xue Bao Acta Pharm. Sin. 2015, 50, 1128–1134. [Google Scholar]
- Yang, M.; Xiong, J.; Zou, Q.; Wang, D.-D.; Huang, C.-X. Chrysin attenuates interstitial fibrosis and improves cardiac function in a rat model of acute myocardial infarction. J. Mol. Histochem. 2018, 49, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Al-Ishaq, R.K.; Abotaleb, M.; Kubatka, P.; Kajo, K.; Büsselberg, D. Flavonoids and their anti-diabetic effects: Cellular mechanisms and effects to improve blood sugar levels. Biomolecules 2019, 9, 430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bule, M.; Abdurahman, A.; Nikfar, S.; Abdollahi, M.; Amini, M. Antidiabetic effect of quercetin: A systematic review and meta-analysis of animal studies. Food Chem. Toxicol. 2019, 125, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Ola, M.S.; Ahmed, M.M.; Ahmad, R.; Abuohashish, H.M.; Al-Rejaie, S.S.; Alhomida, A.S. Neuroprotective Effects of Rutin in Streptozotocin-Induced Diabetic Rat Retina. J. Mol. Neurosci. 2015, 56, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-L.; Gao, J.-M.; Xing, L.-Z. Therapeutic potential of Oroxylin A in rheumatoid arthritis. Int. Immunopharmacol. 2016, 40, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Park, W. Anti-inflammatory effects of oroxylin A on RAW 264.7 mouse macrophages induced with polyinosinic-polycytidylic acid. Exp. Ther. Med. 2016, 12, 151–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Yang, X.; Chu, Y.; Li, M. Identification of Baicalin as an Immunoregulatory Compound by Controlling TH17 Cell Differentiation. PLoS ONE 2011, 6, e17164. [Google Scholar] [CrossRef]
- Yang, X.; Yang, J.; Zou, H. Baicalin Inhibits IL-17-Mediated Joint Inflammation in Murine Adjuvant-Induced Arthritis. Clin. Dev. Immunol. 2013, 2013, 268065. [Google Scholar] [CrossRef] [Green Version]
- Kelepouri, D.; Mavropoulos, A.; Bogdanos, D.P.; Sakkas, L.I. The Role of Flavonoids in Inhibiting Th17 Responses in Inflammatory Arthritis. J. Immunol. Res. 2018, 2018, 9324357. [Google Scholar] [CrossRef]
- Chi, L.; Gao, W.; Shu, X.; Lu, X. A Natural Flavonoid Glucoside, Icariin, Regulates Th17 and Alleviates Rheumatoid Arthritis in a Murine Model. Mediat. Inflamm. 2014, 2014, 392062. [Google Scholar] [CrossRef]
- Tanaka, S.; Furuya, K.; Yamamoto, K.; Yamada, K.; Ichikawa, M.; Suda, M.; Makabe, H. Procyanidin B2 gallates inhibit IFN-γ and IL-17 production in T cells by suppressing T-bet and RORγt expression. Int. Immunopharmacol. 2017, 44, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.-L.; Heo, Y.-J.; Park, M.-K.; Oh, H.-J.; Park, J.-S.; Woo, Y.-J.; Ju, J.-H.; Park, S.-H.; Kim, H.-Y.; Min, J.-K. Grape seed proanthocyanidin extract (GSPE) attenuates collagen-induced arthritis. Immunol. Lett. 2009, 124, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Uddin, S.; Kabir, T.; Niaz, K.; Jeandet, P.; Clément, C.; Mathew, B.; Rauf, A.; Rengasamy, K.R.; Sobarzo-Sánchez, E.; Ashraf, G.M.; et al. Molecular Insight into the Therapeutic Promise of Flavonoids against Alzheimer’s Disease. Molecules 2020, 25, 1267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Li, X.; Ciric, B.; Ma, C.-G.; Gran, B.; Rostami, A.; Zhang, G.-X. Therapeutic Effect of Baicalin on Experimental Au-toimmune Encephalomyelitis Is Mediated by SOCS3 Regulatory Pathway. Sci. Rep. 2015, 5, 17407. [Google Scholar] [CrossRef] [Green Version]
- Patel, R.; Varghese, J.F.; Singh, R.P.; Yadav, U.C.S. Induction of Endothelial Dysfunction by Oxidized Low-Density Lipopro-teins via Downregulation of Erk-5/Mef2c/KLF2 Signaling: Amelioration by Fisetin. Biochimie 2019, 163, 152–162. [Google Scholar] [CrossRef]
- Kwon, K.H.; Murakami, A.; Tanaka, T.; Ohigashi, H. Dietary rutin, but not its aglycone quercetin, ameliorates dextran sulfate sodium-induced experimental colitis in mice: Attenuation of pro-inflammatory gene expression. Biochem. Pharmacol. 2005, 69, 395–406. [Google Scholar] [CrossRef]
- Salaritabar, A.; Darvishi, B.; Hadjiakhoondi, F.; Manayi, A.; Sureda, A.; Nabavi, S.F.; Fitzpatrick, L.R.; Bishayee, A. Therapeutic potential of flavonoids in inflammatory bowel disease: A comprehensive review. World J. Gastroenterol. 2017, 23, 5097–5114. [Google Scholar] [CrossRef]
- Kubatka, P.; Mazurakova, A.; Samec, M.; Koklesova, L.; Zhai, K.; Al-Ishaq, R.; Kajo, K.; Biringer, K.; Vybohova, D.; Brockmueller, A.; et al. Flavonoids against non-physiologic inflammation attributed to cancer initiation, development, and progression—3PM pathways. EPMA J. 2021, 12, 559–587. [Google Scholar] [CrossRef]
- Hou, S.; Yuan, Q.; Yu, N.; Liu, B.; Huang, G.; Yuan, X. Cardamonin attenuates chronic inflammation and tumorigenesis in colon. Cell Cycle 2019, 18, 3275–3287. [Google Scholar] [CrossRef]
- Adebayo, A.H.; Tan, N.-H.; Akindahunsi, A.A.; Zeng, G.-Z.; Zhang, Y.-M. Anticancer and Antiradical Scavenging Activity of Ageratum Conyzoides L. (Asteraceae). Pharmacogn. Mag. 2010, 6, 62. [Google Scholar]
- Lin, Z.; Lin, Y.; Shen, J.; Jiang, M.; Hou, Y. Flavonoids in Ageratum conyzoides L. Exert Potent Antitumor Effects on Human Cervical Adenocarcinoma HeLa Cells In Vitro and In Vivo. BioMed Res. Int. 2020, 2020, 2696350. [Google Scholar] [CrossRef] [PubMed]
- Bayala, B.; Bassole, I.H.N.; Gnoula, C.; Nebie, R.; Yonli, A.; Morel, L.; Figueredo, G.; Nikiema, J.-B.; Lobaccaro, J.-M.A.; Simpore, J. Chemical Composition, Antioxidant, Anti-Inflammatory and Anti-Proliferative Activities of Essential Oils of Plants from Burkina Faso. PLoS ONE 2014, 9, e92122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kabala-Dzik, A.; Rzepecka-Stojko, A.; Kubina, R.; Iriti, M.; Wojtyczka, R.D.; Buszman, E.; Stojko, J. Flavonoids, Bioactive Components of Propolis, Exhibit Cytotoxic Activity and Induce Cell Cycle Arrest and Apoptosis in Human Breast Cancer Cells MDA-MB-231 and MCF-7: A Comparative Study. Cell Mol. Biol. 2018, 64, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Chen, S.; Wei, C.; Rankin, G.O.; Ye, X.; Chen, Y.C. Flavonoids from Chinese bayberry leaves induced apoptosis and G1 cell cycle arrest via Erk pathway in ovarian cancer cells. Eur. J. Med. Chem. 2018, 147, 218–226. [Google Scholar] [CrossRef] [Green Version]
- Nagappan, A.; Lee, H.J.; Saralamma, V.V.G.; Park, H.S.; Hong, G.E.; Yumnam, S.; Raha, S.; Charles, S.N.; Shin, S.C.; Kim, E.H.; et al. Flavonoids Isolated from Citrus Platymamma Induced G2/M Cell Cycle Arrest and Apoptosis in A549 Human Lung Cancer Cells. Oncol. Lett. 2016, 12, 1394–1402. [Google Scholar] [CrossRef] [Green Version]
- Hong, S.H.; Cha, H.-J.; Hwang-Bo, H.; Kim, M.Y.; Kim, S.Y.; Ji, S.Y.; Cheong, J.; Park, C.; Lee, H.; Kim, G.-Y.; et al. Anti-Proliferative and pro-Apoptotic Effects of Licochalcone A through ROS-Mediated Cell Cycle Arrest and Apoptosis in Human Bladder Cancer Cells. Int. J. Mol. Sci. 2019, 20, 3820. [Google Scholar] [CrossRef] [Green Version]
- Ji, X.; Wei, X.; Qian, J.; Mo, X.; Kai, G.; An, F.; Lu, Y. 2′, 4′-Dihydroxy-6′-Methoxy-3′, 5′-Dimethylchalcone Induced Apoptosis and G1 Cell Cycle Arrest through PI3K/AKT Pathway in BEL-7402/5-FU Cells. Food Chem. Toxicol. 2019, 131, 110533. [Google Scholar] [CrossRef]
- Fan, J.-J.; Hsu, W.-H.; Lee, K.-H.; Chen, K.-C.; Lin, C.-W.; Lee, Y.-L.A.; Ko, T.-P.; Lee, L.-T.; Lee, M.-T.; Chang, M.-S.; et al. Dietary Flavonoids Luteolin and Quercetin Inhibit Migration and Invasion of Squamous Carcinoma through Reduction of Src/Stat3/S100A7 Signaling. Antioxidants 2019, 8, 557. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Huang, S.; Chen, C.-L.; Su, S.-B.; Fang, D.-D. Isoliquiritigenin Inhibits Ovarian Cancer Metastasis by Reversing Epithelial-to-Mesenchymal Transition. Molecules 2019, 24, 3725. [Google Scholar] [CrossRef] [Green Version]
- Weseler, A.R.; Geraets, L.; Moonen, H.J.J.; Manders, R.J.F.; van Loon, L.J.C.; Pennings, H.-J.; Wouters, E.F.M.; Bast, A.; Hageman, G.J. Poly (ADP-ribose) Polymerase-1–Inhibiting Flavonoids Attenuate Cytokine Release in Blood from Male Patients with Chronic Obstructive Pulmonary Disease or Type 2 Diabetes. J. Nutr. 2009, 139, 952–957. [Google Scholar] [CrossRef]
- Lu, Y.; Yu, T.; Liu, J.; Gu, L. Vitexin attenuates lipopolysaccharide-induced acute lung injury by controlling the Nrf2 pathway. PLoS ONE 2018, 13, e0196405. [Google Scholar] [CrossRef] [PubMed]
- Shen, R.; Deng, W.; Li, C.; Zeng, G. A natural flavonoid glucoside icariin inhibits Th1 and Th17 cell differentiation and ameliorates experimental autoimmune encephalomyelitis. Int. Immunopharmacol. 2015, 24, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Castilla, P.; Dávalos, A.; Teruel, J.L.; Cerrato, F.; Fernández-Lucas, M.; Merino, J.L.; Sánchez-Martín, C.C.; Ortuño, J.; A Lasunción, M. Comparative effects of dietary supplementation with red grape juice and vitamin E on production of superoxide by circulating neutrophil NADPH oxidase in hemodialysis patients. Am. J. Clin. Nutr. 2008, 87, 1053–1061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zern, T.L.; Wood, R.J.; Greene, C.; West, K.L.; Liu, Y.; Aggarwal, D.; Shachter, N.S.; Fernandez, M.L. Grape Polyphenols Exert a Cardioprotective Effect in Pre- and Postmenopausal Women by Lowering Plasma Lipids and Reducing Oxidative Stress. J. Nutr. 2005, 135, 1911–1917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizza, S.; Muniyappa, R.; Iantorno, M.; Kim, J.; Chen, H.; Pullikotil, P.; Senese, N.; Tesauro, M.; Lauro, D.; Cardillo, C.; et al. Citrus Polyphenol Hesperidin Stimulates Production of Nitric Oxide in Endothelial Cells while Improving Endothelial Function and Reducing Inflammatory Markers in Patients with Metabolic Syndrome. J. Clin. Endocrinol. Metab. 2011, 96, E782–E792. [Google Scholar] [CrossRef]
- Cospite, M. Double-blind, placebo-controlled evaluation of clinical activity and safety of Daflon 500 mg in the treatment of acute hemorrhoids. Angiology 1994, 45 Pt 2, 566–573. [Google Scholar]
- Meshikhes, A.-W.N. Efficacy of Daflon in the treatment of hemorrhoids. Saudi Med J. 2002, 23, 1496–1498. [Google Scholar]
- Bogdanski, P.; Suliburska, J.; Szulinska, M.; Stepien, M.; Pupek-Musialik, D.; Jabłecka, A. Green tea extract reduces blood pressure, inflammatory biomarkers, and oxidative stress and improves parameters associated with insulin resistance in obese, hypertensive patients. Nutr. Res. 2012, 32, 421–427. [Google Scholar] [CrossRef]
- Shavandi, M.; Moini, A.; Shakiba, Y.; Mashkorinia, A.; Dehghani, M.; Assar, S.; Kiani, A. Silymarin (Livergol®) Decreases Disease Activity Score in Patients with Rheumatoid Arthritis: A Non-randomized Single-arm Clinical Trial. Iran. J. Allergy Asthma Immunol. 2017, 16, 99–106. [Google Scholar]
- Feragalli, B.; Dugall, M.; Luzzi, R.; Ledda, A.; Hosoi, M.; Belcaro, G.; Cesarone, M.R. Pycnogenol®: Supplementary Manage-ment of Symptomatic Osteoarthritis with a Patch. An Observational Registry Study. Minerva Endocrinol. 2019, 44, 97–101. [Google Scholar] [CrossRef]
- Cisár, P.; Jány, R.; Waczulíková, I.; Sumegová, K.; Muchová, J.; Vojtaššák, J.; Duraćková, Z.; Lisy, M.; Rohdewald, P. Effect of pine bark extract (Pycnogenol®) on symptoms of knee osteoarthritis. Phytother. Res. 2008, 22, 1087–1092. [Google Scholar] [CrossRef] [PubMed]
- Awan, F.T.; Jones, J.A.; Maddocks, K.; Poi, M.; Grever, M.R.; Johnson, A.; Byrd, J.C.; Andritsos, L.A. A phase 1 clinical trial of flavopiridol consolidation in chronic lymphocytic leukemia patients following chemoimmunotherapy. Ann. Hematol. 2016, 95, 1137–1143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohno, Y.; Wakai, K.; Genka, K.; Ohmine, K.; Kawamura, T.; Tamakoshi, A.; Aoki, R.; Senda, M.; Hayashi, Y.; Nagao, K.; et al. Tea Consumption and Lung Cancer Risk: A Case-Control Study in Okinawa, Japan. Jpn. J. Cancer Res. 1995, 86, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- Bonner, M.R.; Rothman, N.; Mumford, J.L.; He, X.; Shen, M.; Welch, R.; Yeager, M.; Chanock, S.; Caporaso, N.; Lan, Q. Green Tea Consumption, Genetic Susceptibility, PAH-Rich Smoky Coal, and the Risk of Lung Cancer. Mutat. Res. Toxicol. Environ. Mutagen. 2005, 582, 53–60. [Google Scholar] [CrossRef]
- Javadi, F.; Ahmadzadeh, A.; Eghtesadi, S.; Aryaeian, N.; Zabihiyeganeh, M.; Foroushani, A.R.; Jazayeri, S. The Effect of Quercetin on Inflammatory Factors and Clinical Symptoms in Women with Rheumatoid Arthritis: A Double-Blind, Randomized Controlled Trial. J. Am. Coll. Nutr. 2017, 36, 9–15. [Google Scholar] [CrossRef]
- Tabak, C.; Arts, I.C.W.; Smit, H.A.; Heederik, D.; Kromhout, D. Chronic Obstructive Pulmonary Disease and Intake of Catechins, Flavonols, and Flavones: The MORGEN Study. Am. J. Respir. Crit. Care Med. 2001, 164, 61–64. [Google Scholar] [CrossRef] [PubMed]


| Flavonoids | Subtypes | Mol. Wt g/mol | Structure | Source | Reference |
|---|---|---|---|---|---|
| Flavanones | Naringenin | 272.25 | C15H12O5  | Citrus fruits | [1] |
| Taxifolin | 304.25 | C15H12O7 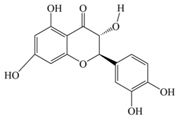 | |||
| Eriodictyol | 288.25 | C15H12O6 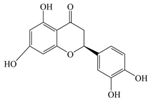 | |||
| Hesperetin | 302.28 | C16H14O6 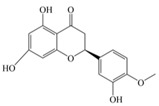 | |||
| Flavones | Apigenin | 270.24 | C15H10O5 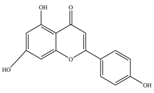 | Sweet red pepper, parsley, chamomile, celery, mint, and Ginkgo biloba | [11] |
| Wogonin | 284.26 | C16H12O5 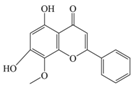 | |||
| Luteolin | 286.24 | C15H10O6 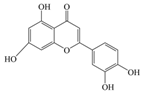 | |||
| Isoflavones | Genistein | 270.24 | C15H10O5 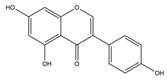 | Tofu, roasted soya nuts, miso | [11] |
| Daidzein | 254.24 | C15H10O4 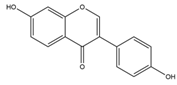 | |||
| Glycetein | 284.26 | C16H12O5 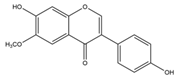 | |||
| Flavonols | Kaempferol | 286.24 | C15H10O6  | Saffron, lettuce, tomatoes, apples, grapes, berries, onions, kale, red wine, and tea | |
| Myricetin | 318.23 | C15H10O8  | |||
| Fisetin | 286.24 | C15H10O6  | |||
| Silymarin | 482.4 | C25H22O10 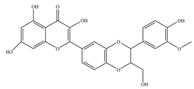 | |||
| Rutin | 610.5 | C27H30O16 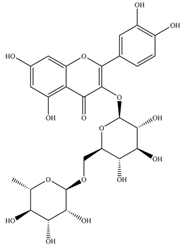 | |||
| Isorhamnetin | 316.26 | C16H12O7 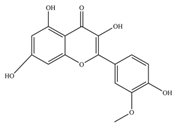 | |||
| Quercetin | 302.23 | C15H10O7  | |||
| Flavanols | Catechin | 290.27 | C15H14O6 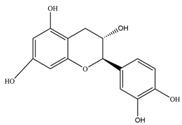 | Black and green tea and fruits such as bananas, peaches, blueberries, apples, and pears | [11] |
| Gallocatechin | 306.27 | C15H14O7  | |||
| Epicatechin | 290.27 | C15H14O6 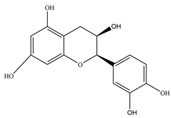 | |||
| Epigallocatechin | 306.27 | C15H14O7 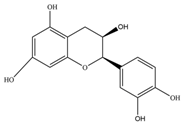 | |||
| Epicatechingallate | 442.4 | C22H18O10 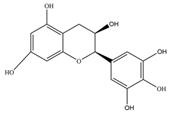 | |||
| Epigallocatechingallate(EGCG) | 458.4 | C22H18O11 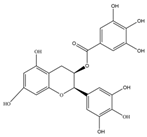 | |||
| Procyanidin | 594.5 | C30H26O13  | |||
| Anthocyanin | Cyanidin | 287.24 | C15H11O6+ 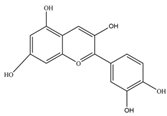 | Merlot grapes, red grapes, raspberries, strawberries, blueberries, cranberries, bilberries, and blackberries | [11] |
| Pelargonidin | 271.24 | C15H11O5+ 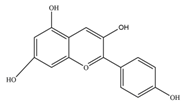 | |||
| Malvidin | 331.3 | C17H15O7+ 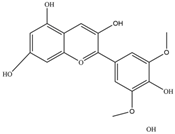 | |||
| Delphinidin | 338.69 | C15H11ClO7 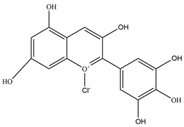 | |||
| Peonidin | 301.27 | C16H13O6+ 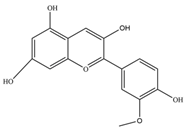 |
| Food/Dietary Source | Flavonoid | Quantity in mg L−1 (Approximately) | Reference |
|---|---|---|---|
| Green Tea | Gallocatechin B | 383 | [6] |
| Epicatechin | 738 | ||
| Epigallocatechin | 1565 | ||
| Epicatechin-3-O-gallate | 361 | ||
| Kaempferol-O-glucoside | 102 | ||
| Quercetin 3-O-glucoside | 185 | ||
| Black Tea | Quercetin 3-O-glucoside | 119 | [6] |
| Kaempferol-O-glucoside | 69 | ||
| Red Wine | Catechin | 41 | [6] |
| Epicatechin | 29 | ||
| Anthocyanins | 22 | ||
| Leek | Kaempferol | 10-60 * | [6] |
| Onions | Anthocyanins | 250 * | [6] |
| Potatoes | Anthocyanins | 16300 * | [13] |
| Apples | Flavanols | 91.7 * | [14] |
| Lemons | Flavanones | 498.1 * | [14] |
| Flavonoids | Activity | Cells/Animal Model Used | Reference |
|---|---|---|---|
| Quercetin | Cyclooxygenase 2 inhibition | Rat peritoneal macrophages | [1] |
| Inducible NO synthase inhibition | LPS/cytokine treated macrophages/cell lines | [1] | |
| Inhibiting MAPK, AP-1 DNA binding | LPS treated RAW cells. | [1] | |
| Extracellular signal-regulated kinase and p38 kinase inhibition | LPS treated RAW cells. | [1] | |
| Ob-Ra (leptin receptor), ERK1/2 phosphorylation, NF-κB, and TNF-α suppression | Leptin-induced human umbilical vein endothelial cells (HUVECS) | [11] | |
| Lysosomal enzyme reduction | Human polymorphonuclear leukocytes | [2] | |
| Neutrophils degranulation inhibition | Human neutrophils | [2] | |
| Kaempferol | Cyclooxygenase 2 | Rat peritoneal macrophages | [1] |
| NF-κB inhibition | LPS treated macrophages | [1] | |
| Apigenin | Inducible NO synthase inhibition | LPS/cytokine treated macrophages/cell lines | [1] |
| NF-κB inhibition, TLR-4, Myeloid differentiation primary response 88 (MyD88), pI-κB-α reduction | LPS treated macrophages | [1,11] | |
| Cyclooxygenase 2 | LPS treated macrophages | [2] | |
| Luteolin | Inducible NO synthase inhibition | LPS/cytokine treated macrophages/cell lines | [1] |
| NF-κB inhibition | Murine macrophages RAW 264.7 | [2] | |
| TNF-α, IL-6 inhibition | IL-1_-induced human synovial sarcoma cells (SW982) | [10] | |
| Luteolin-8-C-fucopyraNOSide (LU8C-FP) | IL-6 reduction | Phorbol-12-myristate-13-acetate plus calciumIonophore (PMACI)-stimulated THP-1 cells, human leukaemia monocytic cell lines. | [10] |
| Genistein | IL-6, TNF-α, IL-1β, IL-2, LTB4 inhibition | LPS treated Human blood monocyte | [1,2] |
| NF-κB inhibition | LPS treated macrophages | [1] | |
| Cyclooxygenase 2 | LPS treated macrophages | [2] | |
| Epigallocatechin | NF-κB inhibition | LPS treated macrophages | [1] |
| Silybin | TNF-α inhibition | LPS treated RAW cells. | [1] |
| Rutin | nuclear factor E2-related factor (Nrf) activation and NF-κB inhibition | Human embryonic kidney reporter cell line | [11] |
| Wogonin | Cyclooxygenase 2 | LPS treated macrophages | [2] |
| TNF-α inhibition | LPS treated RAW cells. | [2] | |
| Fisetin | TNF-α, IL-1β, IL-6, IL-8 reduction | Phorbol-12-myristate-13-acetate plus calciumIonophore (PMACI)-stimulated human mast cells | [10] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Khayri, J.M.; Sahana, G.R.; Nagella, P.; Joseph, B.V.; Alessa, F.M.; Al-Mssallem, M.Q. Flavonoids as Potential Anti-Inflammatory Molecules: A Review. Molecules 2022, 27, 2901. https://doi.org/10.3390/molecules27092901
Al-Khayri JM, Sahana GR, Nagella P, Joseph BV, Alessa FM, Al-Mssallem MQ. Flavonoids as Potential Anti-Inflammatory Molecules: A Review. Molecules. 2022; 27(9):2901. https://doi.org/10.3390/molecules27092901
Chicago/Turabian StyleAl-Khayri, Jameel M., Gandasi Ravikumar Sahana, Praveen Nagella, Biljo V. Joseph, Fatima M. Alessa, and Muneera Q. Al-Mssallem. 2022. "Flavonoids as Potential Anti-Inflammatory Molecules: A Review" Molecules 27, no. 9: 2901. https://doi.org/10.3390/molecules27092901








