Catalytic Performance of One-Pot Synthesized Fe-MWW Layered Zeolites (MCM-22, MCM-36, and ITQ-2) in Selective Catalytic Reduction of Nitrogen Oxides with Ammonia
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physico-Chemical Properties of the Materials
2.1.1. Chemical Composition and Crystal Structure
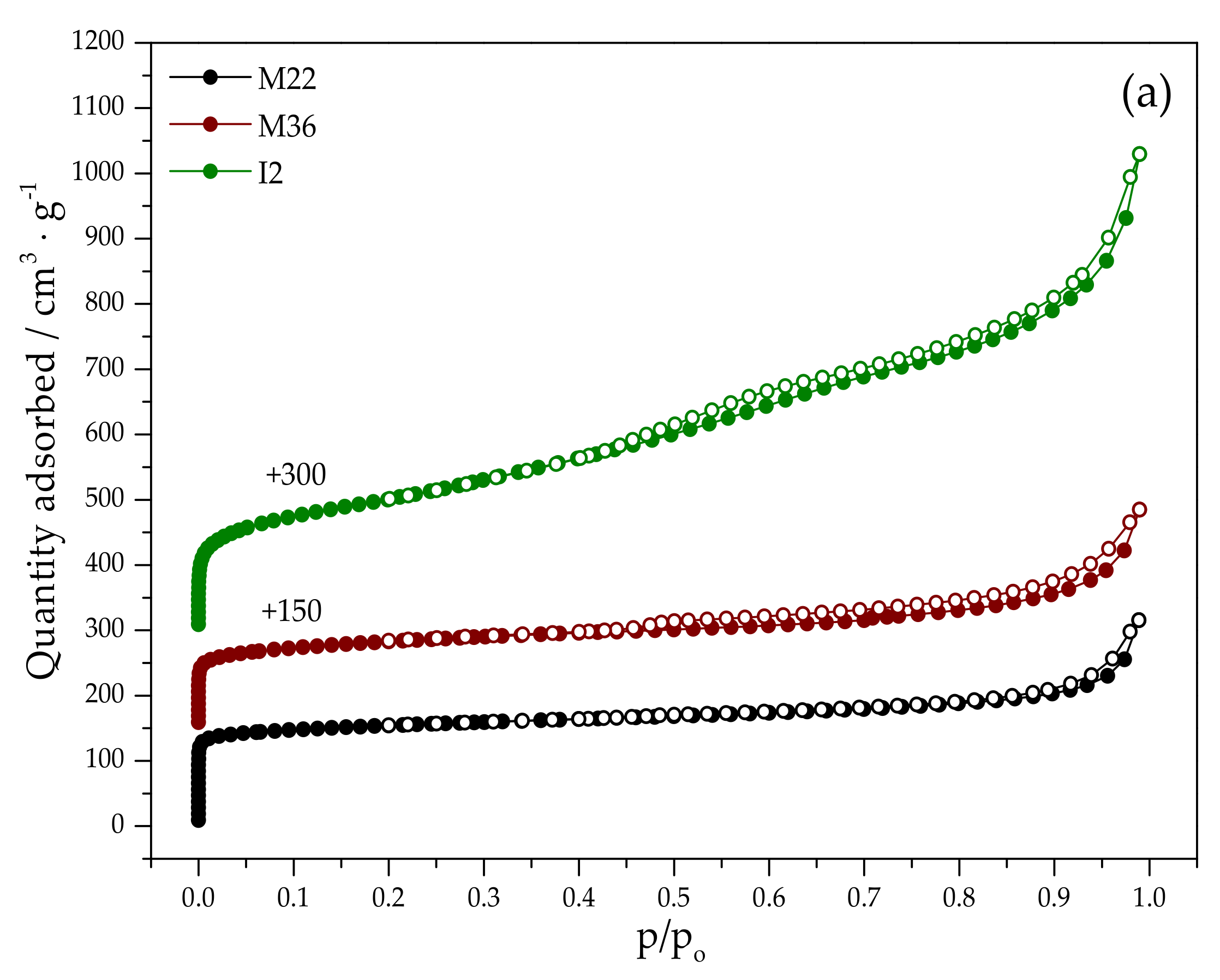
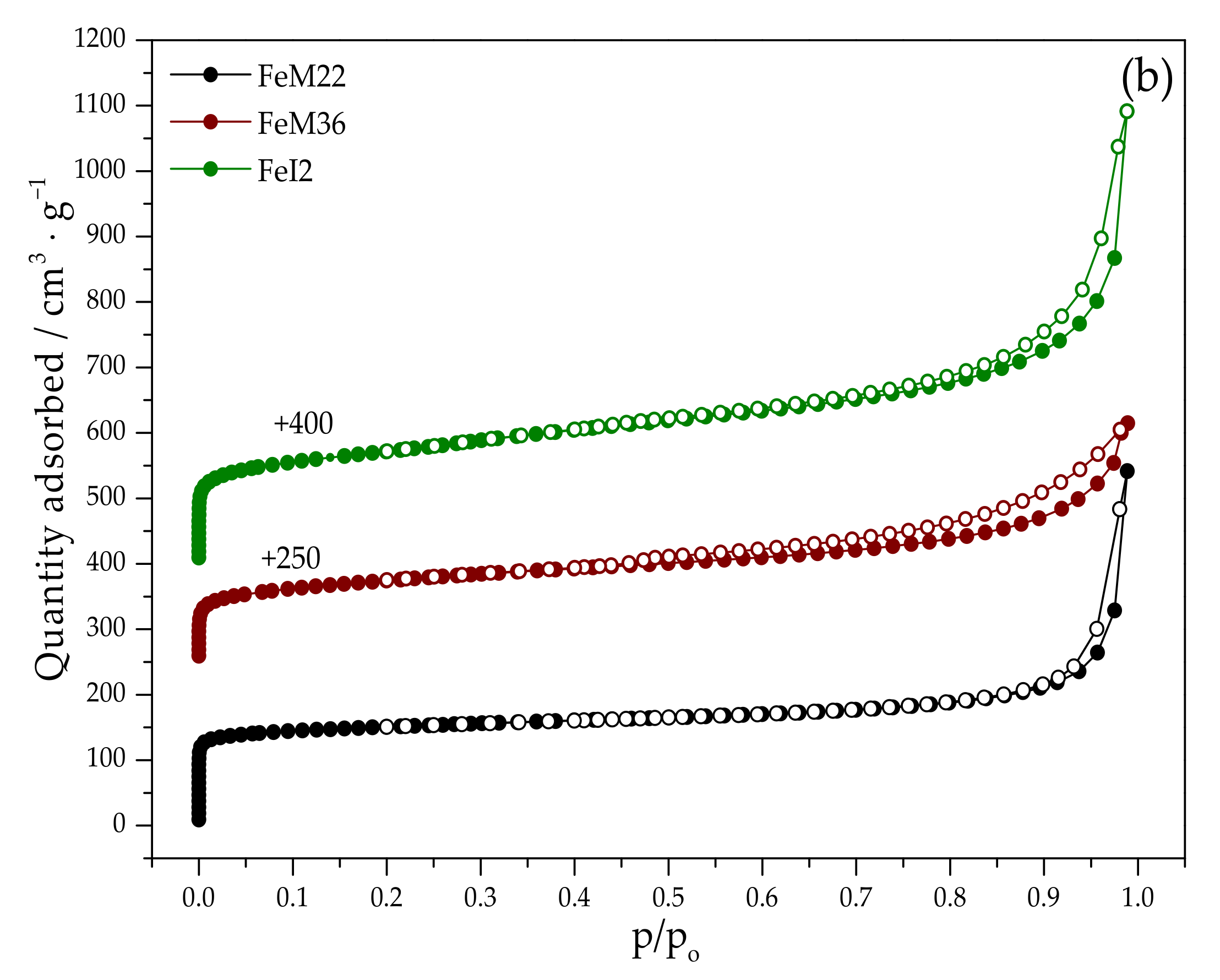
2.1.2. Textural Properties
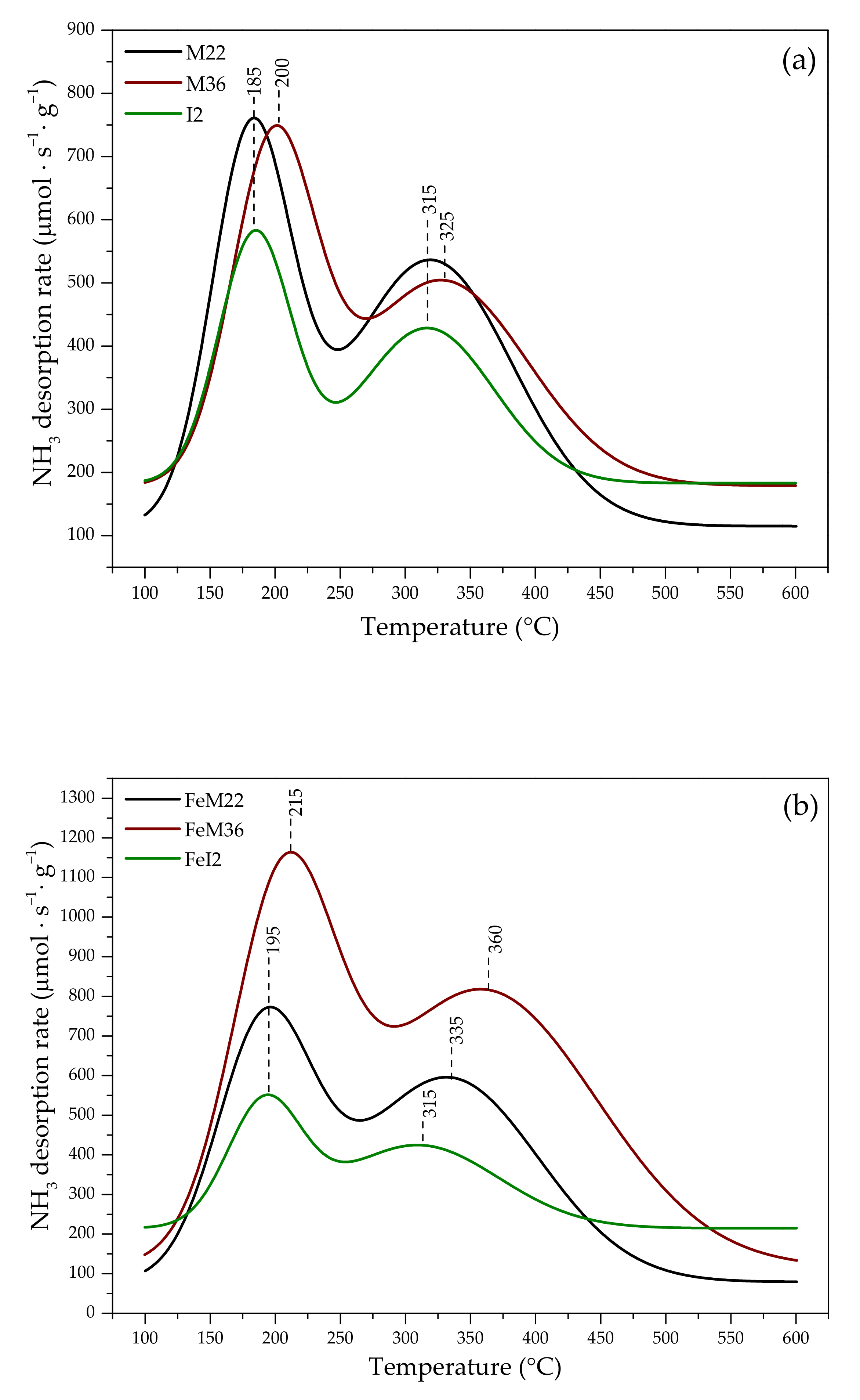
2.1.3. Acidity of the Catalysts
2.1.4. Characteristic Chemical Groups Present in the Materials
2.1.5. Speciation of Iron
2.2. Results of Catalytic Tests
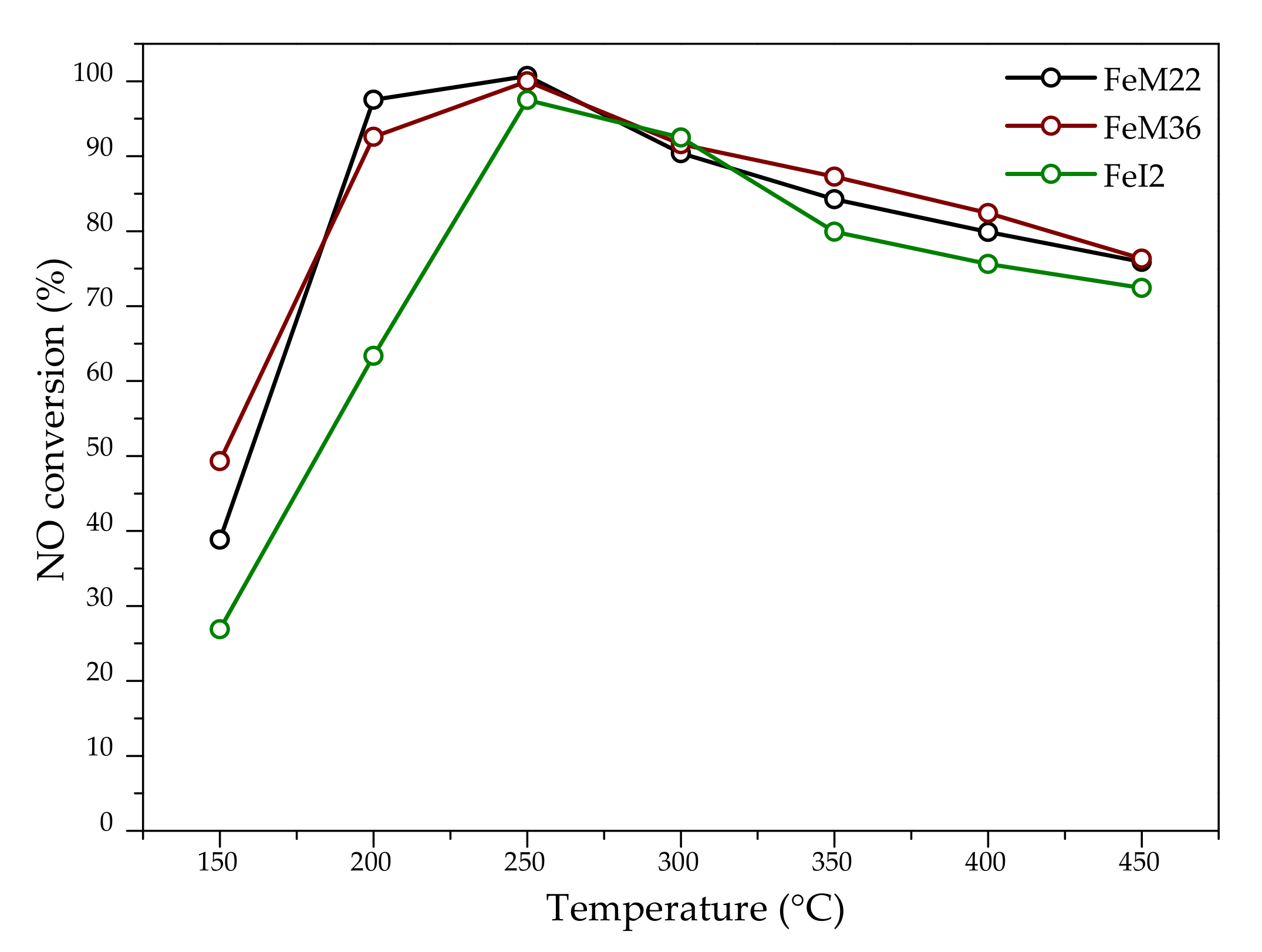
2.2.1. NO Conversion
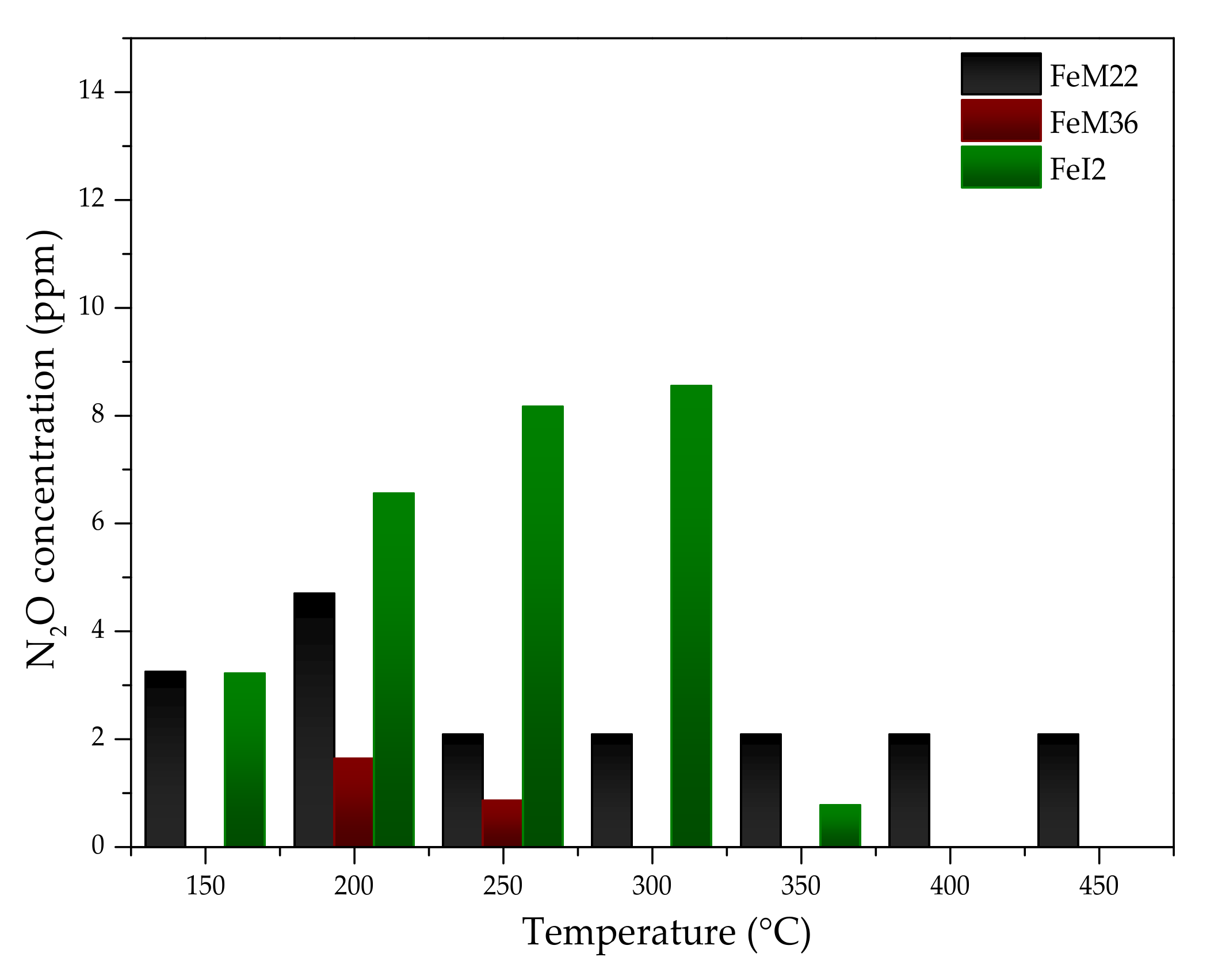
2.2.2. N2O Concentration
3. Materials and Methods
3.1. Preparation of the Materials
3.2. Characterization of the Materials
3.3. NH3-SCR Catalytic Tests
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Li, Y.; Li, L.; Yu, J. Applications of Zeolites in Sustainable Chemistry. Chem 2017, 3, 928–949. [Google Scholar] [CrossRef] [Green Version]
- Szerement, J.; Szatanik-Kloc, A.; Jarosz, R.; Bajda, T.; Mierzwa-Hersztek, M. Contemporary applications of natural and synthetic zeolites from fly ash in agriculture and environmental protection. J. Clean. Prod. 2021, 311, 127461. [Google Scholar] [CrossRef]
- Opanasenko, M.V.; Roth, W.J.; Čejka, J. Two-dimensional zeolites in catalysis: Current status and perspectives. Catal. Sci. Technol. 2016, 6, 2467–2484. [Google Scholar] [CrossRef]
- Grzybek, J.; Roth, W.J.; Gil, B.; Korzeniowska, A.; Mazur, M.; Čejka, J.; Morris, R.E. A new layered MWW zeolite synthesized with the bifunctional surfactant template and the updated classification of layered zeolite forms obtained by direct synthesis. J. Mater. Chem. A 2019, 7, 7701–7709. [Google Scholar] [CrossRef]
- Rutkowska, M.; Díaz, U.; Palomares, A.E.; Chmielarz, L. Cu and Fe modified derivatives of 2D MWW-type zeolites (MCM-22, ITQ-2 and MCM-36) as new catalysts for DeNOx process. Appl. Catal. B Environ. 2015, 168–169, 531–539. [Google Scholar] [CrossRef]
- Díaz, U. Layered Materials with Catalytic Applications Pillared and Delaminated Zeolites. Int. Sch. Res. Netw. 2012, 2012, 537164. [Google Scholar]
- Kikhtyanin, O.; Chlubná, P.; Jindrova, T.; Kubicka, D. Peculiar behavior of MWW materials aldol condensation of furfural and acetone - MCM-22 MCM-36. Dalt. Trans. 2014, 43, 10628. [Google Scholar] [CrossRef]
- Hao, J.; Wang, Y.; Liu, G.; Zhang, J.; Li, G.; Ma, X. Synthesis of ITQ-2 zeolites and catalytic performance in n-dodecane cracking. Chin. J. Chem. Eng. 2014, 22, 869–874. [Google Scholar] [CrossRef]
- Carriço, C.S.; Cruz, F.T.; Maurício, B.; Oliveira, D.S.; Pastore, H.O.; Andrade, H.M.C.; Mascarenhas, A.J.S. MWW-type catalysts for gas phase glycerol dehydration to acrolein. J. Catal. 2016, 334, 34–41. [Google Scholar] [CrossRef]
- Jankowska, A.; Kowalczyk, A.; Rutkowska, M.; Mozgawa, W.; Gil, B.; Chmielarz, L. Silica and silica-Titania intercalated MCM-36 modified with iron as catalysts for selective reduction of nitrogen oxides-The role of associated reactions. Catal. Sci. Technol. 2020, 10, 7940–7954. [Google Scholar] [CrossRef]
- Corma, A.; Fornés, V.; Guil, J.M.; Pergher, S.; Maesen, T.L.M.; Buglass, J.G. Preparation, characterisation and catalytic activity of ITQ-2, a delaminated zeolite. Microporous Mesoporous Mater. 2000, 38, 301–309. [Google Scholar] [CrossRef]
- Corma, A.; Fornés, V.; Martínez-Triguero, J.; Pergher, S.B. Delaminated zeolites: Combining the benefits of zeolites and mesoporous materials for catalytic uses. J. Catal. 1999, 186, 57–63. [Google Scholar] [CrossRef]
- Wang, Y.; Yokoi, T.; Namba, S.; Kondo, J.N.; Tatsumi, T. MCM-22 for cracking of n-hexane. J. Catal. 2016, 333, 17–28. [Google Scholar] [CrossRef]
- Mauricio, B.; Andrade, H.M.C.; Mascarenhas, A.J.S. Oxidative dehydration of glycerol over alternative H, Fe-MCM-22 catalysts: Sustainable production of acrylic acid. Microporous Mesoporous Mater. 2019, 278, 366–377. [Google Scholar] [CrossRef]
- Rutkowska, M.; Jankowska, A.; Różycka-Dudek, E.; Dubiel, W.; Kowalczyk, A.; Piwowarska, Z.; Llopis, S.; Díaz, U.; Chmielarz, L. Modification of mcm-22 zeolite and its derivatives with iron for the application in N2O decomposition. Catalysts 2020, 10, 1139. [Google Scholar] [CrossRef]
- Chen, J.; Peng, G.; Zheng, W.; Zhang, W.; Guo, L.; Wu, X. Excellent performance of one-pot synthesized Fe-containing MCM-22 zeolites for the selective catalytic reduction of NO: Xwith NH3. Catal. Sci. Technol. 2020, 10, 6583–6598. [Google Scholar] [CrossRef]
- Bello, E.; Margarit, V.J.; Gallego, E.M.; Schuetze, F.; Hengst, C.; Corma, A.; Moliner, M. Deactivation and regeneration studies on Pd-containing medium pore zeolites as passive NOx adsorbers (PNAs) in cold-start applications. Microporous Mesoporous Mater. 2020, 302, 110222. [Google Scholar] [CrossRef]
- Skalska, K.; Miller, J.S.; Ledakowicz, S. Trends in NOx abatement: A review. Sci. Total Environ. 2010, 408, 3976–3989. [Google Scholar] [CrossRef]
- Dai, H.; Ma, D.; Zhu, R.; Sun, B.; He, J. Impact of control measures on nitrogen oxides, sulfur dioxide and particulate matter emissions from coal-fired power plants in Anhui Province, China. Atmosphere 2019, 10, 35. [Google Scholar] [CrossRef] [Green Version]
- Boningari, T.; Smirniotis, P.G. Impact of nitrogen oxides on the environment and human health: Mn-based materials for the NOx abatement. Curr. Opin. Chem. Eng. 2016, 13, 133–141. [Google Scholar] [CrossRef]
- Zyrkowski, M.; Motak, M.; Samojeden, B.; Szczepanek, K. Deactivation of V2O5-WO3/TiO2 DeNOx Catalyst under Commercial Conditions in Power Production Plant. Energies 2020, 13, 6200. [Google Scholar] [CrossRef]
- Szymaszek, A.; Samojeden, B.; Motak, M. The Deactivation of Industrial SCR Catalysts—A Short Review. Energies 2020, 13, 3870. [Google Scholar] [CrossRef]
- Saramok, M.; Szymaszek, A.; Inger, M.; Antoniak-jurak, K.; Samojeden, B.; Motak, M. Modified Zeolite Catalyst for a NOx Selective Catalytic Reduction Process in Nitric Acid Plants. Catalysts 2021, 11, 450. [Google Scholar] [CrossRef]
- Li, J.; Chang, H.; Ma, L.; Hao, J.; Yang, R.T. Low-temperature selective catalytic reduction of NOx with NH3 over metal oxide and zeolite catalysts—A review. Catal. Today 2011, 175, 147–156. [Google Scholar] [CrossRef]
- Mohan, S.; Dinesha, P.; Kumar, S. NOx reduction behaviour in copper zeolite catalysts for ammonia SCR systems: A review. Chem. Eng. J. 2020, 384, 123253. [Google Scholar] [CrossRef]
- Liu, Q.; Bian, C.; Ming, S.; Guo, L.; Zhang, S.; Pang, L.; Liu, P.; Chen, Z.; Li, T. The opportunities and challenges of iron-zeolite as NH3-SCR catalyst in purification of vehicle exhaust. Appl. Catal. A Gen. 2020, 607, 117865. [Google Scholar] [CrossRef]
- Metkar, P.S.; Harold, M.P.; Balakotaiah, V. Experimental and kinetic modeling study of NH3-SCR of NOx on Fe-ZSM-5, Cu-chabazite and combined Fe- and Cu-zeolite monolithic catalysts. Chem. Eng. Sci. 2013, 87, 51–66. [Google Scholar] [CrossRef]
- Martín, N.; Vennestrøm, P.N.R.; Thøgersen, J.R.; Moliner, M.; Corma, A. Fe-Containing Zeolites for NH3-SCR of NOx: Effect of Structure, Synthesis Procedure, and Chemical Composition on Catalytic Performance and Stability. Chem.—A Eur. J. 2017, 23, 13404–13414. [Google Scholar] [CrossRef] [Green Version]
- Pereda-Ayo, B.; De La Torre, U.; Illán-Gómez, M.J.; Bueno-López, A.; González-Velasco, J.R. Role of the different copper species on the activity of Cu/zeolite catalysts for SCR of NOx with NH3. Appl. Catal. B Environ. 2014, 147, 420–428. [Google Scholar] [CrossRef] [Green Version]
- Metkar, P.S.; Harold, M.P.; Balakotaiah, V. Selective catalytic reduction of NOx on combined Fe- and Cu-zeolite monolithic catalysts: Sequential and dual layer configurations. Appl. Catal. B Environ. 2012, 111–112, 67–80. [Google Scholar] [CrossRef]
- Dash, A.K.; Mukherjee, D.; Dhulap, A.; Haider, S.; Kumar, D. Green chemistry appended synthesis, metabolic stability and pharmacokinetic assessment of medicinally important chromene dihydropyrimidinones. Bioorg. Med. Chem. Lett. 2019, 29, 126750. [Google Scholar] [CrossRef] [PubMed]
- Boroń, P.; Chmielarz, L.; Gurgul, J.; Łątka, K.; Gil, B.; Marszałek, B.; Dzwigaj, S. Influence of iron state and acidity of zeolites on the catalytic activity of FeHBEA, FeHZSM-5 and FeHMOR in SCR of NO with NH3 and N2O decomposition. Microporous Mesoporous Mater. 2015, 203, 73–85. [Google Scholar] [CrossRef]
- Rivas, F.C.; Rodríguez-Iznaga, I.; Berlier, G.; Ferro, D.T.; Concepción-Rosabal, B.; Petranovskii, V. Iron Modified Natural Zeolite as Sustainable Environmental Catalysts: Correlation between Iron Speciation and Catalytic Activity in NO-Reduction. Prime Arch. Chem. 2020, 1–28. [Google Scholar] [CrossRef]
- Brandenberger, S.; Kröcher, O.; Tissler, A.; Althoff, R. The determination of the activities of different iron species in Fe-ZSM-5 for SCR of NO by NH3. Appl. Catal. B Environ. 2010, 95, 348–357. [Google Scholar] [CrossRef]
- Bols, M.L.; Devos, J.; Rhoda, H.M.; Plessers, D.; Solomon, E.I.; Schoonheydt, R.A.; Sels, B.F.; Dusselier, M. Selective Formation of α-Fe(II) Sites on Fe-Zeolites through One-Pot Synthesis. J. Am. Chem. Soc. 2021, 143, 16243–16255. [Google Scholar] [CrossRef]
- Shishkin, A.; Kannisto, H.; Carlsson, P.A.; Härelind, H.; Skoglundh, M. Synthesis and functionalization of SSZ-13 as an NH3-SCR catalyst. Catal. Sci. Technol. 2014, 4, 3917–3926. [Google Scholar] [CrossRef] [Green Version]
- Andonova, S.; Tamm, S.; Montreuil, C.; Lambert, C.; Olsson, L. The effect of iron loading and hydrothermal aging on one-pot synthesized Fe/SAPO-34 for ammonia SCR. Appl. Catal. B Environ. 2016, 180, 775–787. [Google Scholar] [CrossRef]
- Martín, N.; Vennestrøm, P.N.R.; Thøgersen, J.R.; Moliner, M.; Corma, A. Iron-Containing SSZ-39 (AEI) Zeolite: An Active and Stable High-Temperature NH3-SCR Catalyst. ChemCatChem 2017, 9, 1754–1757. [Google Scholar] [CrossRef] [Green Version]
- Xiao, P.; Wang, Y.; Osuga, R.; Kondo, J.N.; Yokoi, T. One-pot synthesis of highly active Fe-containing MWW zeolite catalyst: Elucidation of Fe species and its impact on catalytic performance. Adv. Powder Technol. 2021, 32, 1070–1080. [Google Scholar] [CrossRef]
- Roth, W.J.; Dorset, D.L. Expanded view of zeolite structures and their variability based on layered nature of 3-D frameworks. Microporous Mesoporous Mater. 2011, 142, 32–36. [Google Scholar] [CrossRef]
- Xing, E.; Shi, Y.; Zheng, A.; Zhang, J.; Gao, X.; Liu, D.; Xin, M.; Xie, W.; Zhang, F.; Mu, X.; et al. Transformation from NaA to MCM-49 zeolite and its catalytic alkylation performance. Ind. Eng. Chem. Res. 2015, 54, 3123–3135. [Google Scholar] [CrossRef]
- Marosz, M.; Samojeden, B.; Kowalczyk, A.; Rutkowska, M.; Motak, M.; Díaz, U.; Palomares, A.E.; Chmielarz, L. MCM-22, MCM-36, and ITQ-2 zeolites with different Si/Al molar ratios as effective catalysts of methanol and ethanol dehydration. Materials 2020, 13, 2399. [Google Scholar] [CrossRef] [PubMed]
- He, Y.J.; Nivarthy, G.S.; Eder, F.; Seshan, K.; Lercher, J.A. Synthesis, characterization and catalytic activity of the pillared molecular sieve MCM-36. Microporous Mesoporous Mater. 1998, 25, 207–224. [Google Scholar] [CrossRef]
- Roth, W.J.; Gil, B.; Makowski, W.; Sławek, A.; Korzeniowska, A.; Grzybek, J.; Siwek, M.; Michorczyk, P. Framework-substituted cerium MCM-22 zeolite and its interlayer expanded derivative MWW-IEZ. Catal. Sci. Technol. 2016, 6, 2742–2753. [Google Scholar] [CrossRef]
- Margarit, V.J.; Martínez-Armero, M.E.; Navarro, M.T.; Martínez, C.; Corma, A. Direct Dual-Template Synthesis of MWW Zeolite Monolayers. Angew. Chem.—Int. Ed. 2015, 54, 13724–13728. [Google Scholar] [CrossRef] [Green Version]
- Alothman, Z.A. A Review: Fundamental Aspects of Silicate Mesoporous Materials. Materials 2012, 5, 2874–2902. [Google Scholar] [CrossRef] [Green Version]
- Schoonheydt, R.A.; Pinnavaia, T.O.M.; Lagaly, G.; Gangas, N.; Kiel, È.; Chemie, È.A. Pillared clays and pillared layered solids. History 1999, 71, 2367–2371. [Google Scholar] [CrossRef]
- Li, X.; Zhang, C.; Zhang, X.; Li, W.; Tan, P.; Ma, L.; Fang, Q.; Chen, G. Study on improving the SO2 tolerance of low-temperature SCR catalysts using zeolite membranes: NO/SO2 separation performance of aluminogermanate membranes. Chem. Eng. J. 2018, 335, 483–490. [Google Scholar] [CrossRef]
- Ostroumova, V.A.; Maksimov, A.L. MWW-Type Zeolites: MCM-22, MCM-36, MCM-49, and MCM-56 (A Review). Pet. Chem. 2019, 59, 788–801. [Google Scholar] [CrossRef]
- Ma, Y.; Xu, T.; Zhang, X.; Wang, J.; Xu, H.; Huang, W.; Zhang, H. Excellent adsorption performance and capacity of modified layered ITQ-2 zeolites for elemental mercury removal and recycling from flue gas. J. Hazard. Mater. 2022, 423, 127118. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Luo, J.; Gao, F.; Kamasamudram, K.; Currier, N.; Peden, C.H.F.; Yezerets, A. New insights into Cu/SSZ-13 SCR catalyst acidity. Part I: Nature of acidic sites probed by NH3 titration. J. Catal. 2017, 348, 291–299. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-González, L.; Rodríguez-Castellón, E.; Jiménez-López, A.; Simon, U. Correlation of TPD and impedance measurements on the desorption of NH3 from zeolite H-ZSM-5. Solid State Ionics 2008, 179, 1968–1973. [Google Scholar] [CrossRef]
- Liu, B.; Huo, H.; Meng, Q.; Gao, S. Synthesis of ITQ-2 zeolite under static conditions and its properties. Sci. China Ser. B Chem. 2006, 49, 148–154. [Google Scholar] [CrossRef]
- Kröcher, O.; Brandenberger, S. Active sites, deactivation and stabilization of Fe-ZSM-5 for the selective catalytic reduction (SCR) of NO with NH3. Chimia (Aarau) 2012, 66, 687–693. [Google Scholar] [CrossRef]
- Min, H.K.; Park, M.B.; Hong, S.B. Methanol-to-olefin conversion over H-MCM-22 and H-ITQ-2 zeolites. J. Catal. 2010, 271, 186–194. [Google Scholar] [CrossRef]
- Lacarriere, A.; Luck, F.; Świerczyński, D.; Fajula, F.; Hulea, V. Methanol to hydrocarbons over zeolites with MWW topology: Effect of zeolite texture and acidity. Appl. Catal. A Gen. 2011, 402, 208–217. [Google Scholar] [CrossRef]
- Høj, M.; Beier, M.J.; Grunwaldt, J.D.; Dahl, S. The role of monomeric iron during the selective catalytic reduction of NOx by NH3 over Fe-BEA zeolite catalysts. Appl. Catal. B Environ. 2009, 93, 166–176. [Google Scholar] [CrossRef]
- Song, S.; Wu, G.; Dai, W.; Guan, N.; Li, L. Al-free Fe-beta as a robust catalyst for selective reduction of nitric oxide by ammonia. Catal. Sci. Technol. 2016, 6, 8325–8335. [Google Scholar] [CrossRef]
- Thakkar, R.; Bandyopadhyay, R. Preparation, characterization, and post-synthetic modification of layered MCM-22 zeolite precursor. J. Chem. Sci. 2017, 129, 1671–1676. [Google Scholar] [CrossRef]
- Zaitan, H.; Bianchi, D.; Achak, O.; Chafik, T. A comparative study of the adsorption and desorption of o-xylene onto bentonite clay and alumina. J. Hazard. Mater. 2008, 153, 852–859. [Google Scholar] [CrossRef] [PubMed]
- Juybar, M.; Khanmohammadi Khorrami, M.; Bagheri Garmarudi, A.; Zandbaaf, S. Determination of acidity in metal incorporated zeolites by infrared spectrometry using artificial neural network as chemometric approach. Spectrochim. Acta—Part A Mol. Biomol. Spectrosc. 2020, 228, 117539. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Lingfa, P. Sodium bentonite and kaolin clays: Comparative study on their FT-IR, XRF, and XRD. Mater. Today Proc. 2020, 22, 737–742. [Google Scholar] [CrossRef]
- Favvas, E.P.; Tsanaktsidis, C.G.; Sapalidis, A.A.; Tzilantonis, G.T.; Papageorgiou, S.K.; Mitropoulos, A.C. Clinoptilolite, a natural zeolite material: Structural characterization and performance evaluation on its dehydration properties of hydrocarbon-based fuels. Microporous Mesoporous Mater. 2016, 225, 385–391. [Google Scholar] [CrossRef]
- Onida, B.; Geobaldo, F.; Testa, F.; Aiello, R.; Garrone, E. H-bond formation and proton transfer in H-MCM-22 zeolite as compared to H-ZSM-5 and H-MOR: An FTIR study. J. Phys. Chem. B 2002, 106, 1684–1690. [Google Scholar] [CrossRef]
- Onida, B.; Geobaldo, F.; Testa, F.; Crea, F.; Garrone, E. FTIR investigation of the interaction at 77 K of diatomic molecular probes on MCM-22 zeolite. Microporous Mesoporous Mater. 1999, 30, 119–127. [Google Scholar] [CrossRef]
- Kessouri, A.; Boukoussa, B.; Bengueddach, A.; Hamacha, R. Synthesis of iron-MFI zeolite and its photocatalytic application for hydroxylation of phenol. Res. Chem. Intermed. 2018, 44, 2475–2487. [Google Scholar] [CrossRef]
- Kumar, M.S.; Schwidder, M.; Grünert, W.; Brückner, A. On the nature of different iron sites and their catalytic role in Fe-ZSM-5 DeNOx catalysts: New insights by a combined EPR and UV/VIS spectroscopic approach. J. Catal. 2004, 227, 384–397. [Google Scholar] [CrossRef]
- Bordiga, S.; Buzzoni, R.; Geobaldo, F.; Lamberti, C.; Giamello, E.; Zecchina, A.; Leofanti, G.; Petrini, G.; Tozzola, G.; Vlaic, G. Structure and reactivity of framework and extraframework iron in Fe-silicalite as investigated by spectroscopic and physicochemical methods. J. Catal. 1996, 158, 486–501. [Google Scholar] [CrossRef]
- Yang, S.; Xiong, S.; Liao, Y.; Xiao, X.; Qi, F.; Peng, Y.; Fu, Y.; Shan, W.; Li, J.; Fu, Y.; et al. Mechanism of N2O Formation during the Low-Temperature Selective Catalytic Reduction of NO with NH3 over Mn−Fe Spinel. Environ. Sci. Technol. 2014, 48, 10354–10362. [Google Scholar] [CrossRef]
- Testa, F.; Crea, F.; Diodati, G.D.; Pasqua, L.; Aiello, R.; Terwagne, G.; Lentz, P.; Nagy, J.B. Synthesis and characterization of Fe- and [Fe, Al] -MCM-22 zeolites. Procedia Eng. 2012, 32, 191–197. [Google Scholar] [CrossRef]
- Gao, F.; Zheng, Y.; Kukkadapu, R.K.; Wang, Y.; Walter, E.D.; Schwenzer, B.; Szanyi, J.; Peden, C.H.F. Iron Loading Effects in Fe/SSZ-13 NH3-SCR Catalysts: Nature of the Fe Ions and Structure-Function Relationships. ACS Catal. 2016, 6, 2939–2954. [Google Scholar] [CrossRef]
- Grzybek, J.; Gil, B.; Roth, W.J.; Skoczek, M.; Kowalczyk, A.; Chmielarz, L. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy Characterization of Co and Fe-MCM-56 catalysts for NH3-SCR and N2O decomposition: An in situ FTIR study. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 196, 281–288. [Google Scholar] [CrossRef]
- Gurgul, J.; Ła̧tka, K.; Hnat, I.; Rynkowski, J.; Dzwigaj, S. Identification of iron species in FeSiBEA by DR UV-vis, XPS and Mössbauer spectroscopy: Influence of Fe content. Microporous Mesoporous Mater. 2013, 168, 1–6. [Google Scholar] [CrossRef]
- Pérez-Ramírez, J.; Groen, J.C.; Brückner, A.; Kumar, M.S.; Bentrup, U.; Debbagh, M.N.; Villaescusa, L.A. Evolution of isomorphously substituted iron zeolites during activation: Comparison of Fe-beta and Fe-ZSM-5. J. Catal. 2005, 232, 318–334. [Google Scholar] [CrossRef]
- Brandenberger, S.; Kröcher, O.; Tissler, A.; Althoff, R.; Brandenberger, S.; Kro, O.; Tissler, A. The State of the Art in Selective Catalytic Reduction of NOx by Ammonia Using Metal - Exchanged Zeolite Catalysts The State of the Art in Selective Catalytic Reduction of NOx by Ammonia Using Metal-Exchanged Zeolite Catalysts. Catal. Rev. 2008, 50, 492–531. [Google Scholar] [CrossRef]
- Melián-Cabrera, I.; Espinosa, S.; Groen, J.C.; Linden, B.V.D.; Kapteijn, F.; Moulijn, J.A. Utilizing full-exchange capacity of zeolites by alkaline leaching: Preparation of Fe-ZSM5 and application in N2O decomposition. J. Catal. 2006, 238, 250–259. [Google Scholar] [CrossRef] [Green Version]
- Melián-Cabrera, I.; Espinosa, S.; Mentruit, C.; Kapteijn, F.; Moulijn, J.A. Alkaline leaching for synthesis of improved Fe-ZSM5 catalysts. Catal. Commun. 2006, 7, 100–103. [Google Scholar] [CrossRef] [Green Version]
- Jiang, T.; Lobo, R.F. On the Mechanism of Ammonia SCR over Cu- and Fe-Containing Zeolite Catalysts. Springer: New York, NY, USA, 2018. [Google Scholar]
- Forzatti, P.; Nova, I.; Tronconi, E. New “enhanced NH3-SCR” reaction for NOx emission control. Ind. Eng. Chem. Res. 2010, 49, 10386–10391. [Google Scholar] [CrossRef]
- Li, J.; Li, S. A DFT study toward understanding the high activity of Fe-Exchanged zeolites for the “fast” selective catalytic reduction of nitrogen oxides with ammonia. J. Phys. Chem. C 2008, 112, 16938–16944. [Google Scholar] [CrossRef]
- Pérez Vélez, R.; Ellmers, I.; Huang, H.; Bentrup, U.; Schünemann, V.; Grünert, W.; Brückner, A. Identifying active sites for fast NH3-SCR of NO/NO2 mixtures over Fe-ZSM-5 by operando EPR and UV-vis spectroscopy. J. Catal. 2014, 316, 103–111. [Google Scholar] [CrossRef]
- Chu, C.T.; Chang, C.D. Isomorphous substitution in zeolite frameworks. 1. Acidity of surface hydroxyls in [B]-, [Fe]-, [Ga]-, and [Al]-ZSM-5. J. Phys. Chem. 1985, 89, 1569–1571. [Google Scholar] [CrossRef]
- Luo, J.; Kamasamudram, K.; Currier, N.; Yezerets, A. NH3-TPD methodology for quantifying hydrothermal aging of Cu/SSZ-13 SCR catalysts. Chem. Eng. Sci. 2018, 190, 60–67. [Google Scholar] [CrossRef]
- Broclawik, E.; Góra, A.; Najbar, M. The role of tungsten in formation of active sites for no SCR on the V-W-O catalyst surface - Quantum chemical modeling (DFT). J. Mol. Catal. A Chem. 2001, 166, 31–38. [Google Scholar] [CrossRef]
- Zhou, H.; Ge, M.Y.; Wu, S.; Ye, B.; Su, Y. Iron based monolithic catalysts supported on Al2O3, SiO2, and TiO2: A comparison for NO reduction with propane. Fuel 2018, 220, 330–338. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, J.; Ma, G.; Wang, J.; Lin, J.; Wang, H.; Zhang, C.; Ding, M. Effect of iron loading on acidity and performance of Fe/HZSM-5 catalyst for direct synthesis of aromatics from syngas. Fuel 2018, 228, 1–9. [Google Scholar] [CrossRef]
- Zheng, M.; Zhou, N.; He, S.; Chang, F.; Zhong, J.; Xu, S.; Wang, Z.; Liu, T. Nitrous oxide (N2O) emissions from a pilot-scale oxidation ditch under different COD/N ratios, aeration rates and two shock-load conditions. J. Environ. Manage. 2021, 280, 111657. [Google Scholar] [CrossRef]
- Devadas, M.; Kröcher, O.; Elsener, M.; Wokaun, A.; Söger, N.; Pfeifer, M.; Demel, Y.; Mussmann, L. Influence of NO2 on the selective catalytic reduction of NO with ammonia over Fe-ZSM5. Appl. Catal. B Environ. 2006, 67, 187–196. [Google Scholar] [CrossRef]
- Grossale, A.; Nova, I.; Tronconi, E. Study of a Fe-zeolite-based system as NH3-SCR catalyst for diesel exhaust aftertreatment. Catal. Today 2008, 136, 18–27. [Google Scholar] [CrossRef]
- Sahu, P.; Eniyarppu, S.; Ahmed, M.; Sharma, D.; Sakthivel, A. Cerium ion-exchanged layered MCM-22: Preparation, characterization and its application for esterification of fatty acids. J. Porous Mater. 2018, 25, 999–1005. [Google Scholar] [CrossRef]
- Lim, J.B.; Cha, S.H.; Hong, S.B. Direct N2O decomposition over iron-substituted small-pore zeolites with different pore topologies. Appl. Catal. B Environ. 2019, 243, 750–759. [Google Scholar] [CrossRef]
- Corma, A.; Corell, C. Synthesis and characterization of the MCM-22 zeolite. Zeolites 1995, 15, 2–8. [Google Scholar] [CrossRef]
- Corma, A.; Fornes, V.; Pergher, S.B.; Maesen, T.L.M.; Buglass, J.G. Delaminated zeolite precursors as selective acidic catalysts. Nature 1998, 396, 353–356. [Google Scholar] [CrossRef]
- Rouquerol, J.; Llewellyn, P.; Rouquerol, F. Is the BET equation applicable to microporous adsorbents ? Stud. Surf. Sci. Catal. 2007, 160, 49–56. [Google Scholar]





| Sample Code | Si (wt.%) | Al (wt.%) | Fe (wt.%) | Si/Al | Si/Fe |
|---|---|---|---|---|---|
| M22 | 33.23 | 1.41 | - | 25 | - |
| M36 | 33.98 | 1.16 | - | 28 | - |
| I2 | 41.02 | 1.46 | - | 27 | - |
| FeM22 | 36.58 | 1.17 | 4.78 | 29 | 15 |
| FeM36 | 34.02 | 1.16 | 5.02 | 28 | 13 |
| FeI2 | 36.38 | 1.17 | 4.78 | 29 | 15 |
| Sample Code | SBET a (m2·g−1) | Smicro b (m2·g−1) | Sext b (m2·g−1) | Vtotal c (cm3·g−1) | Vmicro b (cm3·g−1) | Vmeso+macro d (cm3·g−1) |
|---|---|---|---|---|---|---|
| M22 | 590 | 338 | 158 | 0.431 | 0.169 | 0.262 |
| M36 | 569 | 434 | 141 | 0.480 | 0.172 | 0.308 |
| I2 | 539 | 375 | 164 | 0.450 | 0.183 | 0.267 |
| FeM22 | 438 | 381 | 77 | 0.482 | 0.185 | 0.297 |
| FeM36 | 716 | 209 | 507 | 0.740 | 0.162 | 0.578 |
| FeI2 | 550 | 350 | 201 | 0.635 | 0.174 | 0.461 |
| Sample Code | Concentration of Acid Sites (μmol·g−1) | ||
|---|---|---|---|
| Weak Sites | Strong Sites | Total Amount of Sites | |
| M22 | 761 | 535 | 1296 |
| M36 | 774 | 596 | 1370 |
| I2 | 747 | 504 | 1251 |
| FeM22 | 1158 | 813 | 1971 |
| FeM36 | 580 | 427 | 1007 |
| FeI2 | 546 | 421 | 967 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szymaszek-Wawryca, A.; Díaz, U.; Samojeden, B.; Motak, M. Catalytic Performance of One-Pot Synthesized Fe-MWW Layered Zeolites (MCM-22, MCM-36, and ITQ-2) in Selective Catalytic Reduction of Nitrogen Oxides with Ammonia. Molecules 2022, 27, 2983. https://doi.org/10.3390/molecules27092983
Szymaszek-Wawryca A, Díaz U, Samojeden B, Motak M. Catalytic Performance of One-Pot Synthesized Fe-MWW Layered Zeolites (MCM-22, MCM-36, and ITQ-2) in Selective Catalytic Reduction of Nitrogen Oxides with Ammonia. Molecules. 2022; 27(9):2983. https://doi.org/10.3390/molecules27092983
Chicago/Turabian StyleSzymaszek-Wawryca, Agnieszka, Urbano Díaz, Bogdan Samojeden, and Monika Motak. 2022. "Catalytic Performance of One-Pot Synthesized Fe-MWW Layered Zeolites (MCM-22, MCM-36, and ITQ-2) in Selective Catalytic Reduction of Nitrogen Oxides with Ammonia" Molecules 27, no. 9: 2983. https://doi.org/10.3390/molecules27092983







