Migration Modeling as a Valuable Tool for Exposure Assessment and Risk Characterization of Polyethylene Terephthalate Oligomers
Abstract
:1. Introduction
2. Results and Discussion
2.1. PET Oligomers for Migration Modeling
2.2. Dependence of the Migration on the Diffusion Coefficient
2.3. Dependence of the Migration on the Partition Coefficient
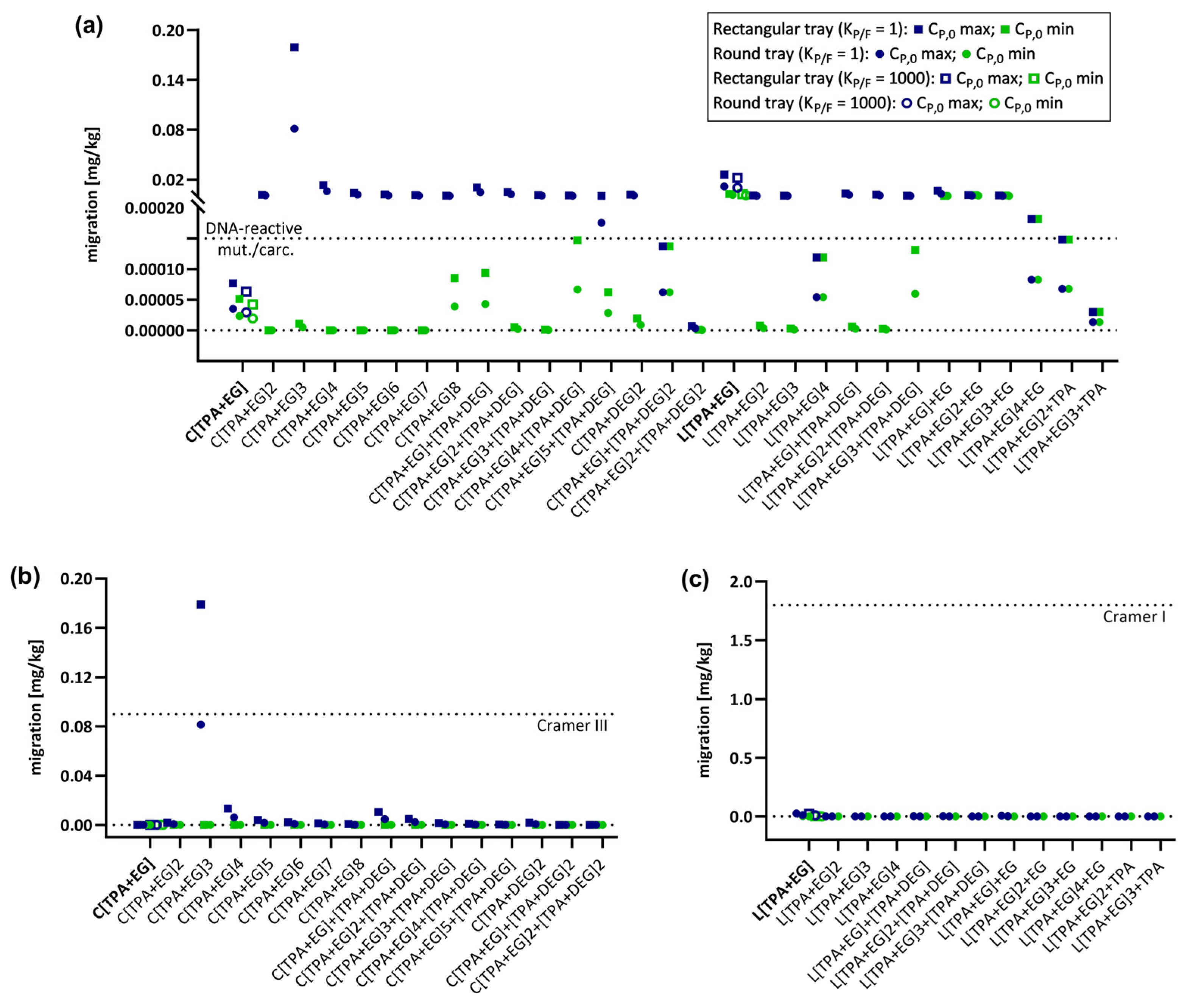
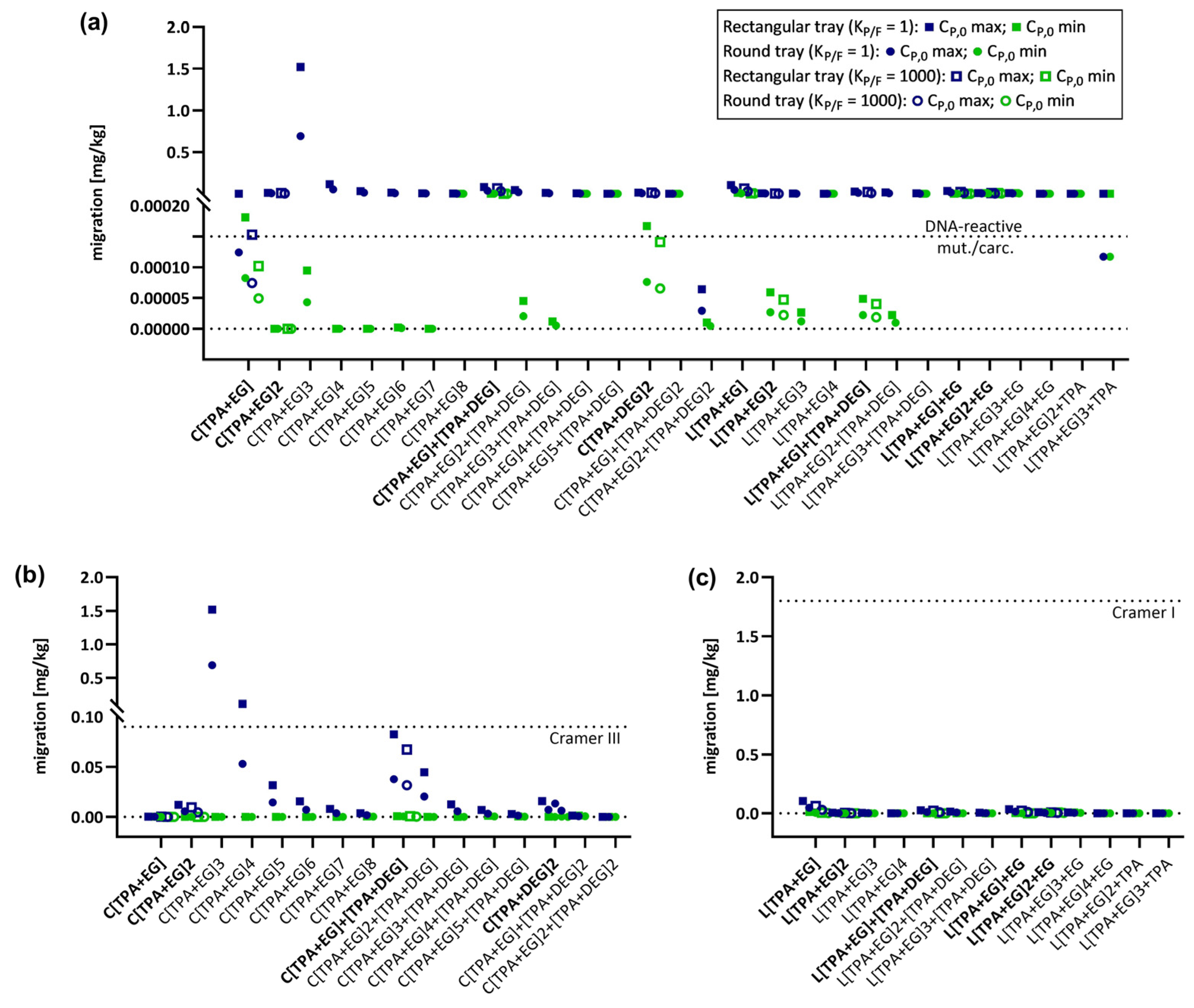
2.4. Migration Modeling of PET Oligomers
2.5. Modeling of CP,0 for PET Oligomers
3. Materials and Methods
3.1. Diffusion Modelling
3.2. Properties and Parameters Used for Migration Modeling
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Plastics Europe Plastics—The Facts 2021. Available online: https://plasticseurope.org/wp-content/uploads/2021/12/Plastics-the-Facts-2021-web-final.pdf (accessed on 29 October 2022).
- Welle, F. The facts about PET, European Federation of Bottled Water. 2018. Available online: https://www.petcore-europe.org/images/news/pdf/factsheet_the_facts_about_pet_dr_frank_welle_2018.pdf (accessed on 29 October 2022).
- Ma, J.; Yu, L.; Chen, S.; Chen, W.; Wang, Y.; Guang, S.; Zhang, X.; Lu, W.; Wang, Y.; Bao, J. Structure-property evolution of poly(ethylene terephthalate) fibers in industrialized process under complex coupling of stress and temperature field. Macromolecules 2019, 52, 565–574. [Google Scholar] [CrossRef]
- Thoden van Velzen, E.U.; Brouwer, M.T.; Stärker, C.; Welle, F. Effect of recycled content and rPET quality on the properties of PET bottles, Part II: Migration. Packag. Technol. Sci. 2020, 33, 359–371. [Google Scholar] [CrossRef]
- Begley, T.H.; Hollifield, H.C. Evaluation of polyethylene terephthalate cyclic trimer migration from microwave food packaging using temperature-time profiles. Food Addit. Contam. 1990, 7, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Commission Regulation (EU) No 10/2011 on plastic materials and articles intended to come into contact with food. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2011:012:0001:0089:en:PDF (accessed on 29 October 2022).
- Brandsch, R.; Dequatre, C.; Mercea, P.; Milana, M.R.; Stoermer, A.; Trier, X.; Vitrac, O.; Schaefer, A.; Simoneau, C. Practical Guidelines on the Application of Migration Modelling for the Estimation of Specific Migration. 2015. Available online: https://op.europa.eu/en/publication-detail/-/publication/1b79bc61-97f6-11e5-983e-01aa75ed71a1/language-en (accessed on 29 October 2022).
- Grob, K.; Pfenninger, S.; Pohl, W.; Laso, M.; Imhof, D.; Rieger, K. European legal limits for migration from food packaging materials: 1. Food should prevail over simulants; 2. More realistic conversion from concentrations to limits per surface area. PVC cling films in contact with cheese as an example. Food Control 2007, 18, 201–210. [Google Scholar] [CrossRef]
- Ewender, J.; Welle, F. A new method for the prediction of diffusion coefficients in poly(ethylene terephthalate)—Validation data. Packag. Technol. Sci. 2022, 35, 405–413. [Google Scholar] [CrossRef]
- Begley, T.; Castle, L.; Feigenbaum, A.; Franz, R.; Hinrichs, K.; Lickly, T.; Mercea, P.; Milana, M.; O’Brien, A.; Rebre, S.; et al. Evaluation of migration models that might be used in support of regulations for food-contact plastics. Food Addit. Contam. 2005, 22, 73–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poças, M.F.; Oliveira, J.C.; Brandsch, R.; Hogg, T. Feasibility study on the use of probabilistic migration modeling in support of exposure assessment from food contact materials. Risk Anal. 2010, 30, 1052–1061. [Google Scholar] [CrossRef]
- Nerin, C.; Alfaro, P.; Aznar, M.; Domeño, C. The challenge of identifying non-intentionally added substances from food packaging materials: A review. Anal. Chim. Acta 2013, 775, 14–24. [Google Scholar] [CrossRef]
- Hoppe, M.; Fornari, R.; de Voogt, P.; Franz, R. Migration of oligomers from PET: Determination of diffusion coefficients and comparison of experimental versus modelled migration. Food Addit. Contam. Part A 2017, 34, 1251–1260. [Google Scholar] [CrossRef]
- Welle, F. A new method for the prediction of diffusion coefficients in poly(ethylene terephthalate). J. Appl. Polym. Sci. 2013, 129, 1845–1851. [Google Scholar] [CrossRef]
- Franz, R.; Gmeiner, M.; Gruner, A.; Kemmer, D.; Welle, F. Diffusion behaviour of the acetaldehyde scavenger 2-aminobenzamide in polyethylene terephthalate for beverage bottles. Food Addit. Contam. Part A 2016, 33, 364–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gehring, C.; Welle, F. Migration testing of polyethylene terephthalate: Comparison of regulated test conditions with migration into real food at the end of shelf life. Packag. Technol. Sci. 2018, 31, 771–780. [Google Scholar] [CrossRef]
- Paseiro-Cerrato, R.; Noonan, G.O.; Begley, T.H. Evaluation of long-term migration testing from can coatings into food simulants: Polyester coatings. J. Agric. Food Chem. 2016, 64, 2377–2385. [Google Scholar] [CrossRef] [PubMed]
- Begley, T.H.; Hollifield, H.C. High-performance liquid chromatographic determination of migrating poly(ethylene terephthalate) oligomers in corn oil. J. Agric. Food Chem. 1990, 38, 145–148. [Google Scholar] [CrossRef]
- Omer, E.; Cariou, R.; Remaud, G.; Guitton, Y.; Germon, H.; Hill, P.; Dervilly-Pinel, G.; le Bizec, B. Elucidation of non-intentionally added substances migrating from polyester-polyurethane lacquers using automated LC-HRMS data processing. Anal. Bioanal. Chem. 2018, 410, 5391–5403. [Google Scholar] [CrossRef]
- Bauer, A.; Jesús, F.; Gómez Ramos, M.J.; Lozano, A.; Fernández-Alba, A.R. Identification of unexpected chemical contaminants in baby food coming from plastic packaging migration by high resolution accurate mass spectrometry. Food Chem. 2019, 295, 274–288. [Google Scholar] [CrossRef]
- Sapozhnikova, Y.; Nuñez, A.; Johnston, J. Screening of chemicals migrating from plastic food contact materials for oven and microwave applications by liquid and gas chromatography—Orbitrap mass spectrometry. J. Chromatogr. A 2021, 1651, 462261. [Google Scholar] [CrossRef]
- Schmid, P.; Welle, F. Chemical migration from beverage packaging materials—A review. Beverages 2020, 6, 37. [Google Scholar] [CrossRef]
- Bolognesi, C.; Castle, L.; Cravedi, J.-P.; Engel, K.-H.; Fowler, P.; Franz, R.; Grob, K.; Gürtler, R.; Husøy, T.; Mennes, W.; et al. Scientific Opinion on the safety assessment of the substance, furan-2, 5-dicarboxylic acid, CAS No 3238-40-2, for use in food contact materials. EFSA J. 2014, 12, 3866. [Google Scholar] [CrossRef] [Green Version]
- More, S.J.; Bampidis, V.; Benford, D.; Bragard, C.; Halldorsson, T.I.; Hernández-Jerez, A.F.; Hougaard Bennekou, S.; Koutsoumanis, K.P.; Machera, K.; Naegeli, H.; et al. Guidance on the use of the threshold of toxicological concern approach in food safety assessment. EFSA J. 2019, 17, 5708. [Google Scholar] [CrossRef]
- Kroes, R.; Renwick, A.G.; Feron, V.; Galli, C.L.; Gibney, M.; Greim, H.; Guy, R.H.; Lhuguenot, J.C.; van de Sandt, J.J.M. Application of the threshold of toxicological concern (TTC) to the safety evaluation of cosmetic ingredients. Food Chem. Toxicol. 2007, 45, 2533–2562. [Google Scholar] [CrossRef] [PubMed]
- Tsochatzis, E.D.; Alberto Lopes, J.; Kappenstein, O.; Tietz, T.; Hoekstra, E.J. Quantification of PET cyclic and linear oligomers in teabags by a validated LC-MS method-In silico toxicity assessment and consumer’s exposure. Food Chem. 2020, 317, 126427. [Google Scholar] [CrossRef] [PubMed]
- Poças, M.F.; Oliveira, J.C.; Oliveira, F.A.R.; Hogg, T. A Critical survey of predictive mathematical models for migration from packaging. Crit. Rev. Food Sci. Nutr. 2008, 48, 913–928. [Google Scholar] [CrossRef] [PubMed]
- Lickly, T.D.; Rainey, M.L.; Burgert, L.C.; Breder, C.V.; Borodinsky, L. Using a simple diffusion model to predict residual monomer migration—Considerations and limitations. Food Addit. Contam. 1997, 14, 65–74. [Google Scholar] [CrossRef]
- Limm, W.; Hollifield, H.C. Modelling of additive diffusion in polyolefins. Food Addit. Contam. 1996, 13, 949–967. [Google Scholar] [CrossRef]
- Baner, A.; Brandsch, J.; Franz, R.; Piringer, O. The application of a predictive migration model for evaluating the compliance of plastic materials with European food regulations. Food Addit. Contam. 1996, 13, 587–601. [Google Scholar] [CrossRef]
- Geueke, B.; Groh, K.J.; Maffini, M.V.; Martin, O.V.; Boucher, J.M.; Chiang, Y.-T.; Gwosdz, F.; Jieh, P.; Kassotis, C.D.; Łańska, P.; et al. Systematic evidence on migrating and extractable food contact chemicals: Most chemicals detected in food contact materials are not listed for use. Crit. Rev. Food Sci. Nutr. 2022, 1–11. [Google Scholar] [CrossRef]
- Food Packaging Forum Foundation, FCCmigex Database. 2022. Available online: https://www.foodpackagingforum.org/fccmigex (accessed on 24. December 2022).
- Güçlü, G.; Yalçinyuva, T.; Özgümüş, S.; Orbay, M. Hydrolysis of waste polyethylene terephthalate and characterization of products by differential scanning calorimetry. Thermochim. Acta 2003, 404, 193–205. [Google Scholar] [CrossRef]
- Molinspiration Online Tool. Available online: https://www.molinspiration.com/cgi-bin/properties (accessed on 29 October 2022).
- Schreier, V.N.; Appenzeller-Herzog, C.; Brüschweiler, B.J.; Geueke, B.; Wilks, M.F.; Simat, T.J.; Schilter, B.; Smieško, M.; Muncke, J.; Odermatt, A.; et al. Evaluating the food safety and risk assessment evidence-base of polyethylene terephthalate oligomers: Protocol for a systematic evidence map. Environ. Int. 2022, 167, 107387. [Google Scholar] [CrossRef]
- Welle, F. Food law compliance of poly(ethylene terephthalate) (PET) food packaging materials. In Food Additives and Packaging; Komoprasert, V., Turowski, P., Eds.; Food and Drug Administration: Silver Spring, MD, USA, 2014; pp. 167–195. [Google Scholar] [CrossRef]
- Simoneau, C. Applicability of Generally Recognised Diffusion Models for the Estimation of Specific Migration in Support of EU Directive 2002/72/EC, Publications Office of the European Union. 2010. Available online: https://data.europa.eu/doi/10.2788/85958 (accessed on 29 October 2022).
- Piringer, O.G. Evaluation of plastics for food packaging. Food Addit. Contam. 1994, 11, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Brandsch, R.; Pemberton, M.; Schuster, D.; Welle, F. Impact of partitioning in short-term food contact applications focused on polymers in support of migration modelling and exposure risk assessment. Molecules 2022, 27, 121. [Google Scholar] [CrossRef] [PubMed]
- FoGe Forschungsgemeinschaft für Verpackungs- und Lebensmitteltechnik e.V. Machbarkeitsstudie für ein neues Bewertungsverfahren von migrierfähigen Packstoffkomponenten hinsichtlich der Verbraucherexposition am Beispiel von PET Getränkeflaschen. 2007. Available online: http://www.foge-ev.de/uploads/FoGe_Exposition_PET.pdf (accessed on 29 October 2022).
- Jickells, S.M.; Gramshaw, J.W.; Gilbert, J.; Castle, L. Migration into food during microwave and conventional oven heating. In Food and Packaging Interactions II; Risch, S.J., Hotchkiss, J.H., Eds.; ACS Symposium Series 473; ACS Publications: Washington, DC, USA, 1991; ISBN 9780841221222. [Google Scholar] [CrossRef]
- Ubeda, S.; Aznar, M.; Nerín, C. Determination of oligomers in virgin and recycled polyethylene terephthalate (PET) samples by UPLC-MS-QTOF. Anal. Bioanal. Chem. 2018, 410, 2377–2384. [Google Scholar] [CrossRef] [PubMed]
- Alberto Lopes, J.; Tsochatzis, E.D.; Karasek, L.; Hoekstra, E.J.; Emons, H. Analysis of PBT and PET cyclic oligomers in extracts of coffee capsules and food simulants by a HPLC-UV/FLD method. Food Chem. 2021, 345, 128739. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.J.; Lee, K.T. Determination of monomers and oligomers in polyethylene terephthalate trays and bottles for food use by using high performance liquid chromatography-electrospray ionization-mass spectrometry. Polym. Test. 2012, 31, 490–499. [Google Scholar] [CrossRef]
- Dulio, V.; Po, R.; Borrelli, R.; Guarini, A.; Santini, C. Characterization of low-molecular-weight oligomers in recycled poly(ethylene terephthalate). Die Angew. Makromol. Chem. 1995, 225, 109–122. [Google Scholar] [CrossRef]
- Mutsuga, M.; Tojima, T.; Kawamura, Y.; Tanamoto, K. Survey of formaldehyde, acetaldehyde and oligomers in polyethylene terephthalate food-packaging materials. Food Addit. Contam. 2007, 22, 783–789. [Google Scholar] [CrossRef]
- Nasser, A.L.M.; Lopes, L.M.X.; Eberlin, M.N.; Monteiro, M. Identification of oligomers in polyethyleneterephthalate bottles for mineral water and fruit juice: Development and validation of a high-performance liquid chromatographic method for the determination of first series cyclic trimer. J. Chromatogr. A 2005, 1097, 130–137. [Google Scholar] [CrossRef]
- Begley, T.H.; Hollifield, H.C. Liquid chromatographic determination of residual reactants and reaction by-products in polyethylene terephthalate. J. Assoc. Off. Anal. Chem. Int. 1989, 72, 468–470. [Google Scholar] [CrossRef]
- Ohkado, Y.; Kawamura, Y.; Mutsuga, M.; Tamura, H.O.; Tanamoto, K. Analysis of formaldehyde, acetaldehyde and oligomers in recycled polyethylene terephthalate. J. Food Hyg. Soc. Jpn. 2005, 46, 218–223. [Google Scholar] [CrossRef] [Green Version]
- Diamantidou, D.; Mastrogianni, O.; Tsochatzis, E.; Theodoridis, G.; Raikos, N.; Gika, H.; Kalogiannis, S. Liquid chromatography-mass spectrometry method for the determination of polyethylene terephthalate and polybutylene terephthalate cyclic oligomers in blood samples. Anal. Bioanal. Chem. 2022, 414, 1503–1512. [Google Scholar] [CrossRef] [PubMed]
- Pinter, E.; Welle, F.; Mayrhofer, E.; Pechhacker, A.; Motloch, L.; Lahme, V.; Grant, A.; Tacker, M. Circularity study on PET bottle-to-bottle recycling. Sustainability 2021, 13, 7370. [Google Scholar] [CrossRef]
- Roduit, B.; Borgeat, C.H.; Cavin, S.; Fragnière, C.; Dudler, V. Application of finite element analysis (FEA) for the simulation of release of additives from multilayer polymeric packaging structures. Food Addit. Contam. 2005, 22, 945–955. [Google Scholar] [CrossRef] [PubMed]
- Beldi, G.; Senaldi, C.; Robouch, N.; Hoekstra, E. Testing Conditions for Kitchenware Articles in Contact with Foodstuffs: Plastics, Metals, Silicone and Rubber, The EURL-FCM Harmonised Approach Series. 2021. Available online: https://joint-research-centre.ec.europa.eu/document/download/cd8e6c6f-c982-4fe4-946c-40596a0d2c1e_en?filename=kitchenware-v3-final-20210702.pdf (accessed on 29 October 2022).

| PET Oligomer (Acronym) | Common Oligomer Name | Molecular Weight MW [g/mol] | Predicted Molecular Volume MV [Å3] |
|---|---|---|---|
| C[TPA+EG] | First series cyclic monomer | 192.17 | 162.74 |
| C[TPA+EG]2 | First series cyclic dimer | 384.34 | 323.69 |
| C[TPA+EG]3 | First series cyclic trimer | 576.51 | 484.64 |
| C[TPA+EG]4 | First series cyclic tetramer | 768.68 | 645.59 |
| C[TPA+EG]5 | First series cyclic pentamer | 960.85 | 806.54 |
| C[TPA+EG]6 | First series cyclic hexamer | 1153.02 | 967.49 |
| C[TPA+EG]7 | First series cyclic heptamer | 1345.19 | 1128.43 |
| C[TPA+EG]8 | First series cyclic octamer | 1537.36 | 1289.38 |
| C[TPA+DEG] | Second series cyclic monomer | 236.22 | 205.33 |
| C[TPA+EG]+[TPA+DEG] | Second series cyclic dimer | 428.39 | 366.28 |
| C[TPA+EG]2+[TPA+DEG] | Second series cyclic trimer | 620.56 | 527.23 |
| C[TPA+EG]3+[TPA+DEG] | Second series cyclic tetramer | 812.73 | 688.18 |
| C[TPA+EG]4+[TPA+DEG] | Second series cyclic pentamer | 1004.9 | 849.12 |
| C[TPA+EG]5+[TPA+DEG] | Second series cyclic hexamer | 1197.07 | 1010.07 |
| C[TPA+DEG]2 | Third series cyclic dimer | 472.45 | 408.87 |
| C[TPA+EG]+[TPA+DEG]2 | Third series cyclic trimer | 664.62 | 569.82 |
| C[TPA+EG]2+[TPA+DEG]2 | Third series cyclic tetramer | 856.79 | 730.76 |
| C[TPA+EG]3+[TPA+DEG]2 | Third series cyclic pentamer | 1048.96 | 891.71 |
| C[TPA+EG]4+[TPA+DEG]2 | Third series cyclic hexamer | 1241.13 | 1052.66 |
| C[TPA+EG]+[TPA+DEG]3 | Fourth series cyclic tetramer | 900.84 | 773.35 |
| L[TPA+EG] | First series linear monomer | 210.19 | 180.63 |
| L[TPA+EG]2 | First series linear dimer | 402.36 | 341.58 |
| L[TPA+EG]3 | First series linear trimer | 594.52 | 502.53 |
| L[TPA+EG]4 | First series linear tetramer | 786.70 | 663.48 |
| L[TPA+EG]5 | First series linear pentamer | 978.87 | 824.43 |
| L[TPA+EG]6 | First series linear hexamer | 1171.04 | 985.38 |
| L[TPA+EG]7 | First series linear heptamer | 1363.20 | 1146.33 |
| L[TPA+EG]8 | First series linear octamer | 1555.38 | 1307.28 |
| L[TPA+DEG] | Second series linear monomer | 254.24 | 223.22 |
| L[TPA+DEG]+EG | Second series linear monomer + EG | 298.29 | 265.81 |
| L[TPA+EG]+[TPA+DEG] | Second series linear dimer | 446.41 | 384.17 |
| L[TPA+EG]2+[TPA+DEG] | Second series linear trimer | 638.58 | 545.12 |
| L[TPA+EG]3+[TPA+DEG] | Second series linear tetramer | 830.75 | 706.07 |
| L[TPA+EG]4+[TPA+DEG] | Second series linear pentamer | 1022.92 | 867.02 |
| L[TPA+EG]5+[TPA+DEG] | Second series linear hexamer | 1215.09 | 1027.97 |
| L[TPA+DEG]2 | Third series linear dimer | 490.46 | 426.76 |
| L[TPA+EG]+[TPA+DEG]2 | Third series linear trimer | 682.63 | 587.71 |
| L[TPA+EG]2+[TPA+DEG]2 | Third series linear tetramer | 874.80 | 748.66 |
| L[TPA+EG]3+[TPA+DEG]2 | Third series linear pentamer | 1066.97 | 909.61 |
| L[TPA+EG]4+[TPA+DEG]2 | Third series linear hexamer | 1259.14 | 1070.55 |
| L[TPA+EG]+EG | First series linear monomer + EG | 254.24 | 223.22 |
| L[TPA+EG]2+EG | First series linear dimer + EG | 446.41 | 384.17 |
| L[TPA+EG]3+EG | First series linear trimer + EG | 638.58 | 545.12 |
| L[TPA+EG]4+EG | First series linear tetramer + EG | 830.75 | 706.07 |
| L[TPA+EG]5+EG | First series linear pentamer + EG | 1022.92 | 867.02 |
| L[TPA+EG]6+EG | First series linear hexamer + EG | 1215.09 | 1027.97 |
| L[TPA+EG]+TPA | First series linear monomer + TPA | 358.30 | 299.00 |
| L[TPA+EG]2+TPA | First series linear dimer + TPA | 550.47 | 459.94 |
| L[TPA+EG]3+TPA | First series linear trimer + TPA | 742.64 | 620.89 |
| L[TPA+EG]4+TPA | First series linear tetramer + TPA | 934.81 | 781.84 |
| L[TPA+EG]5+TPA | First series linear pentamer + TPA | 1126.98 | 942.79 |
| L[TPA+EG]6+TPA | First series linear hexamer + TPA | 1319.15 | 1103.74 |
| Temperature [°C] | DP Measured [cm2/s] | DP Predicted [cm2/s] | ||
|---|---|---|---|---|
| EA Based Model | AP Model (Realistic Case) | AP Model (Upper Limit) | ||
| 176 | 2.9 × 10−9 | 1.4 × 10−9 | 2.5 × 10−10 | 6.9 × 10−9 |
| 149 | 6.6 × 10−10 | 3.8 × 10−11 | 4.6 × 10−11 | 1.2 × 10−9 |
| 115 | 1.2 × 10−12 | 2.0 × 10−13 | 3.8 × 10−12 | 1.0 × 10−10 |
| PET Oligomer (Acronym) | MW [g/mol] | MV [Å3] | Impact of the Partition Coefficient (KP/F = 1 and KP/F = 1000) on Migration in % Condition/Model * | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1/EA | 2/EA | 3/EA | 4/EA | 5/EA | 1/AP | 2/AP | 3/AP | 4/AP | 5/AP | |||
| C[TPA+EG] | 192.17 | 162.74 | 1 | 2 | 2 | 16 | 16 | 26 | 28 | 14 | 40 | 40 |
| L[TPA+EG] | 210.19 | 180.63 | 1 | 1 | 2 | 13 | 13 | 24 | 25 | 13 | 37 | 37 |
| C[TPA+DEG] | 236.22 | 205.33 | 0 | 1 | 1 | 9 | 10 | 21 | 23 | 11 | 34 | 34 |
| L[TPA+DEG] | 254.24 | 223.22 | 0 | 1 | 1 | 8 | 8 | 20 | 21 | 10 | 31 | 31 |
| L[TPA+EG]+EG | 254.24 | 223.22 | 0 | 1 | 1 | 8 | 8 | 20 | 21 | 10 | 31 | 31 |
| L[TPA+DEG]+EG | 298.29 | 265.81 | 0 | 0 | 0 | 5 | 5 | 16 | 17 | 8 | 27 | 27 |
| L[TPA+EG]+TPA | 358.3 | 299.00 | 0 | 0 | 0 | 4 | 4 | 12 | 13 | 6 | 21 | 21 |
| C[TPA+EG]2 | 384.34 | 323.69 | 0 | 0 | 0 | 3 | 3 | 11 | 12 | 5 | 19 | 19 |
| L[TPA+EG]2 | 402.36 | 341.58 | 0 | 0 | 0 | 3 | 3 | 10 | 11 | 5 | 18 | 18 |
| C[TPA+EG]+[TPA+DEG] | 428.39 | 366.28 | 0 | 0 | 0 | 2 | 2 | 9 | 10 | 4 | 16 | 16 |
| L[TPA+EG]+[TPA+DEG] | 446.41 | 384.17 | 0 | 0 | 0 | 2 | 2 | 9 | 9 | 4 | 15 | 15 |
| L[TPA+EG]2+EG | 446.41 | 384.17 | 0 | 0 | 0 | 2 | 2 | 9 | 9 | 4 | 15 | 15 |
| C[TPA+DEG]2 | 472.45 | 408.87 | 0 | 0 | 0 | 2 | 2 | 8 | 8 | 4 | 14 | 14 |
| L[TPA+DEG]2 | 490.46 | 426.76 | 0 | 0 | 0 | 2 | 2 | 7 | 8 | 3 | 13 | 13 |
| L[TPA+EG]2+TPA | 550.47 | 459.94 | 0 | 0 | 0 | 1 | 1 | 6 | 6 | 3 | 10 | 10 |
| C[TPA+EG]3 | 576.51 | 484.64 | 0 | 0 | 0 | 1 | 1 | 5 | 6 | 2 | 9 | 9 |
| L[TPA+EG]3 | 594.52 | 502.53 | 0 | 0 | 0 | 1 | 1 | 5 | 5 | 2 | 9 | 9 |
| C[TPA+EG]2+[TPA+DEG] | 620.56 | 527.23 | 0 | 0 | 0 | 1 | 1 | 4 | 5 | 2 | 8 | 8 |
| L[TPA+EG]2+[TPA+DEG] | 638.58 | 545.12 | 0 | 0 | 0 | 1 | 1 | 4 | 4 | 2 | 8 | 8 |
| L[TPA+EG]3+EG | 638.58 | 545.12 | 0 | 0 | 0 | 1 | 1 | 4 | 4 | 2 | 8 | 8 |
| C[TPA+EG]+[TPA+DEG]2 | 664.62 | 569.82 | 0 | 0 | 0 | 1 | 1 | 4 | 4 | 2 | 7 | 7 |
| L[TPA+EG]+[TPA+DEG]2 | 682.63 | 587.71 | 0 | 0 | 0 | 1 | 1 | 3 | 4 | 2 | 7 | 7 |
| L[TPA+EG]3+TPA | 742.64 | 620.89 | 0 | 0 | 0 | 1 | 1 | 3 | 3 | 1 | 5 | 5 |
| C[TPA+EG]4 | 768.68 | 645.59 | 0 | 0 | 0 | 1 | 1 | 3 | 3 | 1 | 5 | 5 |
| L[TPA+EG]4 | 786.7 | 663.48 | 0 | 0 | 0 | 1 | 1 | 2 | 3 | 1 | 5 | 5 |
| C[TPA+EG]3+[TPA+DEG] | 812.73 | 688.18 | 0 | 0 | 0 | 1 | 1 | 2 | 2 | 1 | 4 | 4 |
| L[TPA+EG]3+[TPA+DEG] | 830.75 | 706.07 | 0 | 0 | 0 | 0 | 0 | 2 | 2 | 1 | 4 | 4 |
| L[TPA+EG]4+EG | 830.75 | 706.07 | 0 | 0 | 0 | 0 | 0 | 2 | 2 | 1 | 4 | 4 |
| C[TPA+EG]2+[TPA+DEG]2 | 856.79 | 730.76 | 0 | 0 | 0 | 0 | 0 | 2 | 2 | 1 | 4 | 4 |
| L[TPA+EG]2+[TPA+DEG]2 | 874.8 | 748.66 | 0 | 0 | 0 | 0 | 0 | 2 | 2 | 1 | 3 | 4 |
| C[TPA+EG]+[TPA+DEG]3 | 900.84 | 773.35 | 0 | 0 | 0 | 0 | 0 | 2 | 2 | 1 | 3 | 3 |
| L[TPA+EG]4+TPA | 934.81 | 781.84 | 0 | 0 | 0 | 0 | 0 | 2 | 2 | 1 | 3 | 3 |
| C[TPA+EG]5 | 960.85 | 806.54 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 1 | 3 | 3 |
| L[TPA+EG]5 | 978.87 | 824.43 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 3 | 3 |
| C[TPA+EG]4+[TPA+DEG] | 1004.9 | 849.12 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 2 | 2 |
| L[TPA+EG]4+[TPA+DEG] | 1022.92 | 867.02 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 2 | 2 |
| L[TPA+EG]5+EG | 1022.92 | 867.02 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 2 | 2 |
| C[TPA+EG]3+[TPA+DEG]2 | 1048.96 | 891.71 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 2 | 2 |
| L[TPA+EG]3+[TPA+DEG]2 | 1066.97 | 909.61 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 2 | 2 |
| L[TPA+EG]5+TPA | 1126.98 | 942.79 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 2 | 2 |
| C[TPA+EG]6 | 1153.02 | 967.49 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 2 | 2 |
| L[TPA+EG]6 | 1171.04 | 985.38 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 |
| C[TPA+EG]5+[TPA+DEG] | 1197.07 | 1010.07 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 |
| L[TPA+EG]5+[TPA+DEG] | 1215.09 | 1027.97 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 |
| L[TPA+EG]6+EG | 1215.09 | 1027.97 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 |
| C[TPA+EG]4+[TPA+DEG]2 | 1241.13 | 1052.66 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 |
| L[TPA+EG]4+[TPA+DEG]2 | 1259.14 | 1070.55 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 |
| L[TPA+EG]6+TPA | 1319.15 | 1103.74 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 |
| C[TPA+EG]7 | 1345.19 | 1128.43 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 |
| L[TPA+EG]7 | 1363.2 | 1146.33 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| C[TPA+EG]8 | 1537.36 | 1289.38 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| L[TPA+EG]8 | 1555.38 | 1307.28 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| Properties and Conditions | Food Contact Articles | ||||
|---|---|---|---|---|---|
| 500 mL Bottle | 1.0 L Bottle | 1.5 L Bottle | Rectangular Tray * | Round Tray ** | |
| Contact surface [cm2] | 420 | 660 | 880 | 566 | 329 |
| Contact volume [L] | 0.5 | 1.0 | 1.5 | 0.5 | 637 |
| Surface/volume [1/cm2] | 0.84 | 0.66 | 0.59 | 1.13 | 0.52 |
| Thickness PET [µm] | 300 | 300 | 300 | 300 | 300 |
| Density PET [g/cm3] | 1.4 | 1.4 | 1.4 | 1.4 | 1.4 |
| Density food/drink [g/cm3] | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| KP/F | 1 or 1000 | 1 or 1000 | 1 or 1000 | 1 or 1000 | 1 or 1000 |
| Temperature and time(application scenarios) | Condition 1 | Condition 1 | Condition 1 | Condition 3 | Condition 3 |
| 25 °C, 365 d | 25 °C, 365 d | 25 °C, 365 d | 70 °C, 0.5 h | 70 °C, 0.5 h | |
| Condition 2 | Condition 2 | Condition 2 | Condition 4 | Condition 4 | |
| 40 °C, 60 d | 40 °C, 60 d | 40 °C, 60 d | 100 °C, 10 min | 100 °C, 10 min | |
| Condition 5 | Condition 5 | ||||
| 100 °C, 2 h | 100 °C, 2 h | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schreier, V.N.; Odermatt, A.; Welle, F. Migration Modeling as a Valuable Tool for Exposure Assessment and Risk Characterization of Polyethylene Terephthalate Oligomers. Molecules 2023, 28, 173. https://doi.org/10.3390/molecules28010173
Schreier VN, Odermatt A, Welle F. Migration Modeling as a Valuable Tool for Exposure Assessment and Risk Characterization of Polyethylene Terephthalate Oligomers. Molecules. 2023; 28(1):173. https://doi.org/10.3390/molecules28010173
Chicago/Turabian StyleSchreier, Verena N., Alex Odermatt, and Frank Welle. 2023. "Migration Modeling as a Valuable Tool for Exposure Assessment and Risk Characterization of Polyethylene Terephthalate Oligomers" Molecules 28, no. 1: 173. https://doi.org/10.3390/molecules28010173





