Electroanalysis of Ibuprofen and Its Interaction with Bovine Serum Albumin
Abstract
:1. Introduction
2. Results and Discussion
2.1. Material Characterization
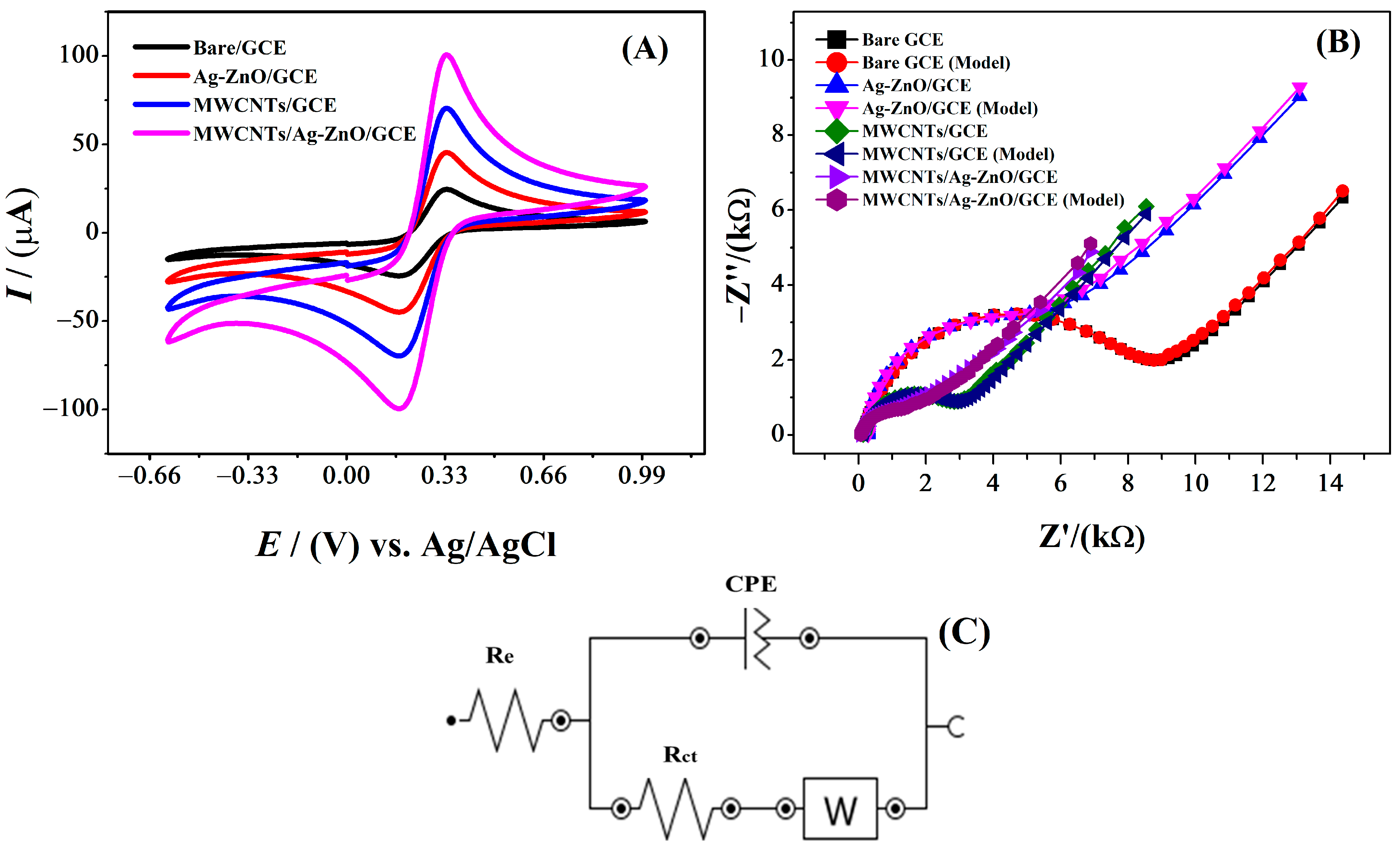
2.2. Electrochemical Characterization of IBP Using the Designed Sensor
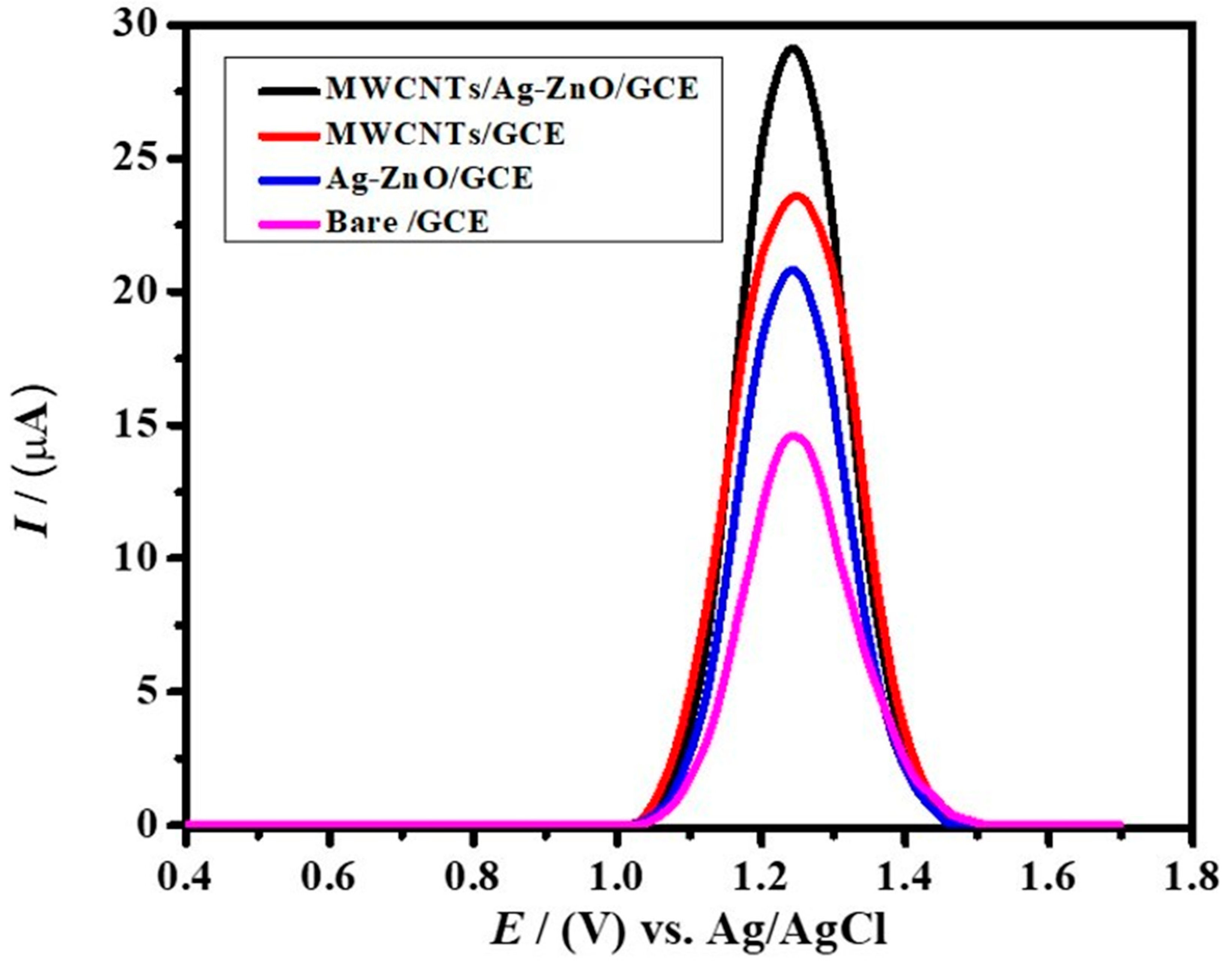
2.3. Voltammetric Analysis of the Targeted Analyte
2.4. Effect of Scan Rate
2.5. Optimization of Experimental Parameters
2.5.1. Supporting Electrolyte Optimization
2.5.2. Effect of Accumulation Potential
2.5.3. Influence of Accumulation Time
2.6. Limit of Detection of IBP and Calibration Plot
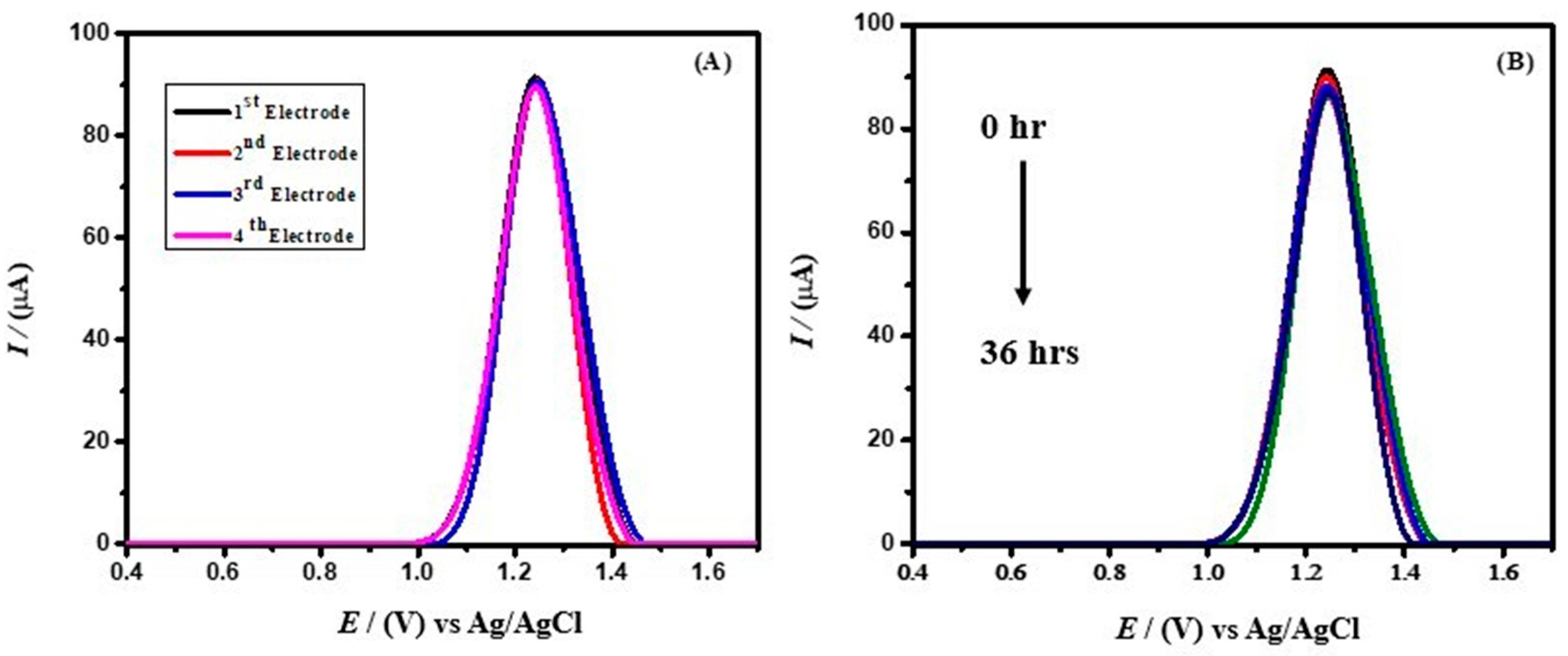
2.6.1. Estimation of the Stability of the Designed Sensor
2.6.2. Effects of Interferents for Validation of the Designed Sensor
2.7. Interaction Studies of IBP with BSA
3. Conclusions
4. Experimental Section
4.1. Materials and Methods
4.2. Synthesis of Ag-ZnO
4.3. Electrode Modification and Detection Procedure
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Pereira, J.C. Environmental issues and international relations, a new global (dis) order-the role of international relations in promoting a concerted international system. Rev. Bras. Politica Int. 2015, 58, 191–209. [Google Scholar] [CrossRef] [Green Version]
- Velusamy, S.; Roy, A.; Sundaram, S.; Mallick, T.K. A review on heavy metal ions and containing dyes removal through graphene oxide-based adsorption strategies for textile wastewater treatment. J.Chem. Rec. 2021, 21, 1570–1610. [Google Scholar] [CrossRef] [PubMed]
- Anju, A.; Ravi, S.P.; Bechan, S. Water pollution with special reference to pesticide contamination in India. J. Water Resour. Prot. 2010, 2, 17. [Google Scholar]
- Grant, R.; Combs, A.; Acosta, D. Experimental models for the investigation of toxicological mechanisms. Elsevier Sci. 2010, 73, 203–224. [Google Scholar]
- Kress, J.P.; Gehlbach, B.; Lacy, M.; Pliskin, N.; Pohlman, A.S.; Hall, J.B. The long-term psychological effects of daily sedative interruption on critically ill patients. Am. J. Respir. Crit. Care Med. 2003, 168, 1457–1461. [Google Scholar] [CrossRef] [PubMed]
- Taschereau-Dumouchel, V.; Michel, M.; Lau, H.; Hofmann, S.G.; LeDoux, J.P. Putting the “mental” back in “mental disorders”: A perspective from research on fear and anxiety. Mol. Psychiatry 2022, 27, 1322–1330. [Google Scholar] [CrossRef]
- Takakura, Y.; Hashida, M. Macromolecular carrier systems for targeted drug delivery: Pharmacokinetic considerations on biodistribution. J. Pharm. Res. 1996, 13, 820–831. [Google Scholar]
- Marchlewicz, A.; Guzik, U.; Wojcieszynska, D. Over-the-counter monocyclic non-steroidal anti-inflammatory drugs in environment sources, risks, biodegradation. Wat. Air Soil Poll. 2015, 226, 335. [Google Scholar] [CrossRef] [Green Version]
- Fent, K.; Weston, A.A.; Caminada, D. Ecotoxicology of human pharmaceuticals. Aquat. Toxicol. Aquat. Toxicol. 2006, 76, 122–159. [Google Scholar] [CrossRef]
- Abbas, A.; Ali, M.; Yosef, A.; Abdalmageed, O.; Shaaban, O.F. Sterility, Can the response to three months ibuprofen in controlling heavy menstrual bleeding with copper intrauterine device be predicted at baseline visit. Clin. Infect. Dis. 2017, 108, 123–124. [Google Scholar]
- Bierma-Zeinstra, S.; Brew, J.; Stoner, K.; Wilson, R.; Kilbourn, A.; Conaghan, P.O. Cartilage, A new lipid formulation of low dose ibuprofen shows non-inferiority to high dose standard ibuprofen the FLARE study (flaring arthralgia relief evaluation in episodic flaring knee pain)–a randomised double-blind study. Osteoarthr. Cartil. 2017, 25, 1942–1951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ju, Z.; Li, M.; Xu, J.; Howell, D.C.; Li, Z.; Chen, F.E. Recent development on COX-2 inhibitors as promising anti-inflammatory agents: The past 10 years. Acta Pharm. Sin. B 2022, 12, 2790–2807. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Chai, T.; Yin, Z.; Zhang, X.; Zhang, W.; Qian, Y.; Qiu, J.P. Stereoselective effects of ibuprofen in adult zebrafish (Danio rerio) using UPLC-TOF/MS-based metabolomics. Environ. Pollut. 2018, 241, 730–739. [Google Scholar] [CrossRef] [PubMed]
- Bouissou-Schurtz, C.; Houeto, P.; Guerbet, M.; Bachelot, M.; Casellas, C.; Mauclaire, A.-C.; Panetier, P.; Delval, C.; Masset, D.J.R.T. Pharmacology, Ecological risk assessment of the presence of pharmaceutical residues in a French national water survey. Regul. Toxicol. Pharmacol. 2014, 69, 296–303. [Google Scholar] [CrossRef]
- Kappus, H. Irreversible protein binding of 14C-imipramine in rats in vivo. Arch. Toxicol. 1976, 37, 75–80. [Google Scholar] [CrossRef]
- Nakagawa, H.; Yamamoto, O.; Oikawa, S.; Higuchi, H.; Watanabe, A.; Katoh, N.S. Detection of serum haptoglobin by enzyme-linked immunosorbent assay in cows with fatty liver. Res. Vet. Sci. 1997, 62, 137–141. [Google Scholar] [CrossRef]
- Ravelli, D.; Isernia, P.; Acquarulo, A.; Profumo, A.; Merli, D.C. Voltammetric Determination of Binding Constant and Stoichiometry of Albumin (Human, Bovine, Ovine)–Drug Complexes. J. Anal. Chem. 2019, 91, 10110–10115. [Google Scholar] [CrossRef]
- Ghalkhani, M.; Kaya, S.I.; Bakirhan, N.K.; Ozkan, Y.; Ozkan, S.A. Application of nanomaterials in development of electrochemical sensors and drug delivery systems for anticancer drugs and cancer biomarkers. Crit. Rev. Anal. Chem. 2022, 52, 481–503. [Google Scholar] [CrossRef]
- Ahmad, K.; Shah, A.H.; Adhikari, B.; Rana, U.A.; Vijayaratnam, C.; Muhammad, N.; Shujah, S.; Rauf, A.; Hussain, H.; Badshah, A. pH-dependent redox mechanism and evaluation of kinetic and thermodynamic parameters of a novel anthraquinone. RSC Adv. 2014, 4, 31657–31665. [Google Scholar] [CrossRef]
- Shah, A.; Ullah, A.; Rauf, A.; Rehman, Z.U.; Shujah, S.; Shah, S.M.; Waseem, A. Detailed electrochemical probing of a biologically active isoquinoline. J. Electrochem. Soc. 2013, 160, 597. [Google Scholar] [CrossRef]
- Hung, V.W.; Veloso, A.J.; Chow, A.M.; Ganesh, H.V.; Seo, K.; Kenduezler, E.; Brown, I.R.; Kerman, K. Electrochemical impedance spectroscopy for monitoring caspase-3 activity. Electrochem. Acta 2015, 162, 79–85. [Google Scholar] [CrossRef]
- Mamuru, S.A.; Saki, N.; Bello, D.M.; Dalen, M.B. Square Wave Voltammetric Detection of Nitrite on Platinum Electrode Modified with Moringa oleifera Mediated Biosynthesized Nickel Nanoparticles. J. Adv. Electrochem. 2018, 4, 168–171. [Google Scholar] [CrossRef]
- Amin, M.A.; Abd El-Rehim, S.S.; El-Sherbini, E.; Bayoumi, R. The inhibition of low carbon steel corrosion in hydrochloric acid solutions by succinic acid: Part I. Weight loss, polarization, EIS, PZC, EDX and SEM studies. Electrochem. Acta 2007, 52, 3588–3600. [Google Scholar] [CrossRef]
- Randviir, E.P.; Banks, C.E. Electrochemical impedance spectroscopy: An overview of bioanalytical applications. Anal. Methods 2013, 5, 1098–1115. [Google Scholar] [CrossRef]
- Kokab, T.; Shah, A.; Iftikhar, F.J.; Nisar, J.; Akhter, M.S.; Khan, S.B. Amino acid-fabricated glassy carbon electrode for efficient simultaneous sensing of zinc (II), cadmium (II), copper (II), and mercury (II) ions. ACS Omega 2019, 4, 22057–22068. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Yang, B.; Wang, H.; Yang, P.; Du, Y. Highly sensitive electrochemical determination of Sunset Yellow based on gold nanoparticles/graphene electrode. Anal. Chim. Acta 2015, 893, 41–48. [Google Scholar] [CrossRef]
- Akbari, M.; Mohammadnia, M.S.; Ghalkhani, M.; Aghaei, M.; Sohouli, E.; Rahimi-Nasrabadi, M.; Arbabi, M.; Banafshe, H.R.; Sobhani-Nasab, A. Development of an electrochemical fentanyl nanosensor based on MWCNT-HA/Cu-H3BTC nanocomposite. J. Ind. Eng. Chem. 2022, 114, 418–426. [Google Scholar] [CrossRef]
- Suresh, E.; Sundaram, K.; Kavitha, B.; Kumar, S. Square wave voltammetry sensing of ibuprofen on glassy carbon electrode. Int. J. Pharmtech Res. 2016, 9, 182–188. [Google Scholar]
- Montes, R.H.; Lima, A.P.; Cunha, R.R.; Guedes, T.J.; dos Santos, W.T.; Nossol, E.; Richter, E.M.; Munoz, R.A.A. Size effects of multi-walled carbon nanotubes on the electrochemical oxidation of propionic acid derivative drugs: Ibuprofen and naproxen. J. Electroanal. Chem. 2016, 775, 342–349. [Google Scholar] [CrossRef]
- Abbas Momtazi, A.; Sahebkar, A. Difluorinated curcumin: A promising curcumin analogue with improved anti-tumor activity and pharmacokinetic profile. Curr. Pharm. Des. 2016, 22, 4386–4397. [Google Scholar] [CrossRef]
- Mekassa, B.; Tessema, M.; Chandravanshi, B.S.; Tefera, M. Square wave voltammetric determination of ibuprofen at poly (l-aspartic acid) modified glassy carbon electrode. IEEE Sens. J. 2017, 18, 37–44. [Google Scholar] [CrossRef]
- Rivera-Hernandez, S.I.; Alvarez-Romero, G.A.; Corona-Avendano, S.; Páez-Hernndez, M.E.; Galán-Vidal, C.A.; Romero-Romo, M. Technology, Voltammetric determination of ibuprofen using a carbon paste–multiwalled carbon nanotube composite electrode. Instru. Sci. Technol. 2016, 44, 483–494. [Google Scholar] [CrossRef]
- Ţuchiu, B.-M.; Stefan-van Staden, R.-I.; van Staden, J. Recent Trends in Ibuprofen and Ketoprofen Electrochemical Quantification–A Review. Crit. Rev. Anal. Chem. 2022, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Motoc, S.; Manea, F.; Iacob, A.; Martinez-Joaristi, A.; Gascon, J.; Pop, A.; Schoonman, J.J.S. Electrochemical selective and simultaneous detection of diclofenac and ibuprofen in aqueous solution using HKUST-1 metal-organic framework-carbon nanofiber composite electrode. Sensors 2016, 16, 1719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Svorc, Ľ.; Strezova, I.; Kianickova, K.; Stankovic, D.M.; Otrisal, P.; Samphao, A. An advanced approach for electrochemical sensing of ibuprofen in pharmaceuticals and human urine samples using a bare boron-doped diamond electrode. J. Electroanal. Chem. 2018, 822, 144–152. [Google Scholar] [CrossRef]
- Nair, A.S.; Sooraj, M. Molecular imprinted polymer-wrapped AgNPs-decorated acid-functionalized graphene oxide as a potent electrochemical sensor for ibuprofen. J. Mater. Sci. 2020, 55, 3700–3711. [Google Scholar] [CrossRef]
- Vuignier, K.; Schappler, J.; Veuthey, J.L.; Carrupt, P.-A.; Martel, S. Drug–protein binding: A critical review of analytical tools. Anal. Bioanal. Chem. 2010, 398, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Leuna, J.B.M.; Sop, S.K.; Makota, S.; Njanja, E.; Ebelle, T.C.; Azebaze, A.G.; Ngameni, E.; Nassi, A. Voltammetric behavior of Mammeisin (MA) at a glassy carbon electrode and its interaction with Bovine Serum Albumin (BSA). Bioelectrochemistry 2018, 119, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.K.; Jain, R.; Radhapyari, K.; Jadon, N.; Agarwal, S.B. Voltammetric techniques for the assay of pharmaceuticals—A review. Anal. Biochem. 2011, 408, 179. [Google Scholar] [CrossRef]
- Maruthamuthu, M.; Kishore, S. Binding of ketoprofen with bovine serum albumin. Proc. Indian Acad. Sci.-Chem. Sci. 1987, 99, 187–193. [Google Scholar] [CrossRef]
- Pacifici, G.; Viani, A.; Schulz, H.U.; Frercks, H.P. Plasma protein binding of furosemide in the elderly. Eur. J. Clin. Pharmacol. 1987, 32, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Prasanth, S.; Sudarsanakumar, C. Elucidating the interaction of L-cysteine-capped selenium nanoparticles and human serum albumin: Spectroscopic and thermodynamic analysis. New J. Chem. 2017, 41, 9521–9530. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Y.; He, L.L.; Liu, B.; Zhang, S.Y.; Ye, X.; Jing, J.J.; Zhang, J.-F.; Gao, M.J.F.; Toxicology, C. Spectroscopic investigation on the food components–drug interaction: The influence of flavonoids on the affinity of nifedipine to human serum albumin. Food Chem. Toxicol. 2015, 78, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Kariv, I.; Cao, H.; Oldenburg, K.R. Development of a high throughput equilibrium dialysis method. J. Pharm. Sci. 2001, 90, 580–587. [Google Scholar] [CrossRef]








| Sensors | Measurement Technique | Linear Range (μM) | LOD (nM) | Ref. |
|---|---|---|---|---|
| Pretreated GCE | SWSV | 1.45–3.87 | 960 | [28] |
| SD-MWCNT/GCE | FIA-AMP | 10–1000 | 1900 | [29] |
| Polyaniline nanofiber/GCE | DPSV | 0.96–1.94 | 480 | [30] |
| P(L-Asp)/GCE | SWV | 1–150 | 220 | [31] |
| MWCNT–CPE | DPV | 2.36–242 | 9100 | [32] |
| Clay-CPE | DPV | 1–1000 | 835 | [33] |
| HKUST-CNF | CV | 4.84–29.08 | 100 | [34] |
| Pd-PdO/Mt-CPE | DPV | 0.01–0.9 | 28 | [35] |
| AgNPs@Af-GO-MIP/GCE | DPV | 1–100 | 8.7 | [36] |
| MWCNTs/Ag-ZnO/GCE | DPV | 0.1–90 | 28 | This work |
| Drug Complexes | Complex Stoichiometry (m) | Binding Constant | Ref. |
|---|---|---|---|
| Ketoprofen-BSA | 3 | 2.4 × 109 | [40] |
| Ketoprofen-HAS | 1 | 1.4 × 1010 | [41] |
| Lorazepam-OVA | 3 | 2.5 × 1010 | [42] |
| Paroxetine-BSA | 4 | 5.8 × 1018 | [43] |
| Paroxetine-OVA | 3 | 2.6 × 1023 | [44] |
| Ibuprofen-BSA | 3 | 8.7 × 1013 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dilshad, M.; Shah, A.; Munir, S. Electroanalysis of Ibuprofen and Its Interaction with Bovine Serum Albumin. Molecules 2023, 28, 49. https://doi.org/10.3390/molecules28010049
Dilshad M, Shah A, Munir S. Electroanalysis of Ibuprofen and Its Interaction with Bovine Serum Albumin. Molecules. 2023; 28(1):49. https://doi.org/10.3390/molecules28010049
Chicago/Turabian StyleDilshad, Muhammad, Afzal Shah, and Shamsa Munir. 2023. "Electroanalysis of Ibuprofen and Its Interaction with Bovine Serum Albumin" Molecules 28, no. 1: 49. https://doi.org/10.3390/molecules28010049






