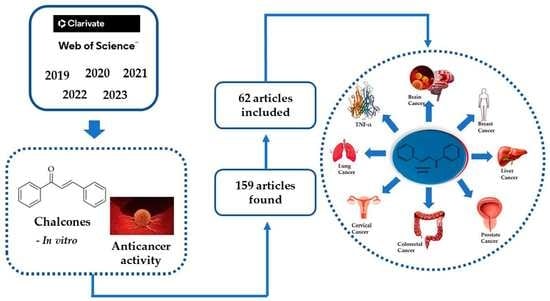
Anticancer Activity of Chalcones and Its Derivatives: Review and In Silico Studies
Abstract
:1. Introduction
2. Review Approaches
- (1)
- The first type refers to the description of the complete action mechanism of chalcones with emphasis on the biological targets and signaling pathways involved. Ouyang et al. (2021) [19] carried out a summary of recent advances in compounds belonging to the class of chalcones as potential anticancer agents and reported on the action mechanisms of these compounds. Furthermore, the authors presented future applications and scope of the chalcone family for cancer treatment and prevention. It is important to mention that in this work the authors emphasized the need for a complete description of the toxicity of chalcones. Also, it was possible to observe that the authors mentioned chalcones of natural origin, but emphasized the importance of the anticancer activity of chalcone hybrid compounds, mentioning the biological activity of artemisinin-chalcone hybrids, chalcone-azole hybrids, chalcone-coumarin hybrids, and chalcone-indole hybrids. In addition, the biological activity was addressed by describing the mechanism of action emphasizing the pathways responsible for anti-inflammatory activity, inhibition of MDR (multidrug resistance) channels, and anti-angiogenic, apoptotic, and tubulin polymerization. The authors emphasized the excellent activity of synthetic derivatives such as sofalcone (antiulcer agent) and methoxychalcone (choleretic drug), which both represent a promising strategy for developing chalcones as new anticancer agents. Another point mentioned referred to the lack of studies on chalcones in natural marine products. Thus, the authors concluded that chalcones are easy to synthesize, as well as easy to chemically modify, being able to generate compounds with a wide variety and structural diversity.
- (2)
- The second approach observed mainly emphasized synthetic compounds with the analysis of the chemical group related to activity, characterizing an approach of structure and activity approach. Among these articles, the main objective of the work developed by Shukla et al. (2021) [20] was to analyze the antitumor activity of chalcones 1,3-diaryl-2-propen-1-one through different mechanisms. The chemical groups related to activity and mentioned as being of greater importance referred to chalcone analogues with electron donating groups, indolyl, quinolone, pyrazolol, hydroxyaminobenzamide, hydroxamic acid, and pyridyl-indole groups. Among the activities evaluated were mechanisms related to apoptosis (emphasizing the importance of mitochondrial pathways), microtubule binding and cell cycle regulation, and inhibition of drug-metabolizing enzymes and new signaling pathways such as Notch (cell-surface Notch receiver). Thus, the authors concluded that the presence of electron donating groups such as OCH3, OH, halogens in the ring A or B of chalcones, induce apoptosis through the intrinsic or extrinsic pathway as they stabilize the enzyme-inhibitor complex through electrostatic interactions. Several chalcones with indolyl, quinolone, and pyrazole act as potential anti-thymicrotubule agents and interrupt the cell cycle, mainly in the G2/M phase. Apoptosis by enzyme inhibition is achieved by hydroxyaminobenzamide, hydroxamic acid groups, and pyridylindole.
- (3)
- The third approach found refers to synthetic chalcones and the reactions involved in their production. An article developed by Mastachi-Loza et al. (2021) [21] also emphasizes synthetic chalcones, but the authors mention cycloaddition mechanisms [4+2], which correspond to cycloadditions between a diene and a dienophile. The authors concluded that chalcones have not only been used as precursors of natural and synthetic molecules, but also in the development of new protocols and catalysts for asymmetric Diels–Alder nullification, reflecting their versatility. Furthermore, it is also possible that chalcones behave like dienes in Diels–Alder cycloadditions with inverse electron demand kinetics, as well as formal [4+2] cycloadditions, which are Diels–Alder cancellations. Therefore, due to their dual role in Diels–Alder cycloadditions, chalcones have wide possibilities in organic synthesis. Rani et al. (2019) [22] also addressed synthetic and conjugated chalcones, but presented the addition of a relationship and activity study as a differential, with an emphasis on their mechanism of action and anchorage studies along with their future therapeutic applications.
- (4)
- The fourth approach deals with the elucidation of the mechanism and performance of in vivo studies. This form of work was observed in the review conducted by Souza et al. (2021) [23], in which the authors aimed to describe the anticancer potential of chalcones related to some of the characteristics of cancer with emphasis on sustaining proliferative signaling, tumor-promoting inflammation, activation of invasion and metastasis, induction of angiogenesis and resistance to cell death; however, in this work no emphasis was shown on the chemical structure–the discussion was made in relation to the mechanism under study, and the chalcones which stood out were natural chalcones such as flavokawain B. Thus, the authors concluded that the chalcones and their derivatives had an anticancer effect by acting on the tumor microenvironment.
3. Chalcones with Anticancer Activity
3.1. TNF-α
3.2. Colon Cancer
3.3. Lung Cancer
3.4. Breast Cancer
3.5. Oral Cancer
3.6. Leukemia
3.7. Hepatocarcinoma
3.8. Cervical Cancer
3.9. Glioblastoma
3.10. Melanoma
4. Other Studies
5. Chemometric Analyses
- -
- The W1 descriptor belongs to the hydrophilic volume descriptor block, which describes the accessible molecular envelope that interacts attractively with water molecules.
- -
- The FLEX descriptor is related to flexibility parameters and represents the maximum flexibility of a molecule.
- -
- Another descriptor is the POL, which represents an estimate of the average molecular polarizability and is based on the structure of the compounds.
- -
- Molecular globularity (G) is related to molecular flexibility and is defined as S/Sequiv with Sequiv = surface area of a sphere of volume V, where S and V are the molecular surface and volume described above, respectively.
6. Discussion
7. Material and Methods
7.1. Literature Review
7.2. Data Set
7.3. Chemometric Studies
7.4. Partial Least Squares (PLS)
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Neto, R.A.d.M.; Santos, C.B.R.; Henriques, S.V.C.; Machado, L.d.O.; Cruz, J.N.; da Silva, C.H.T.d.P.; Federico, L.B.; de Oliveira, E.H.C.; de Souza, M.P.C.; da Silva, P.N.B. Novel Chalcones Derivatives with Potential Antineoplastic Activity Investigated by Docking and Molecular Dynamics Simulations. J. Biomol. Struct. Dyn. 2022, 40, 2204–2216. [Google Scholar] [CrossRef] [PubMed]
- Helmy, M.T.; Sroor, F.M.; Mahrous, K.F.; Mahmoud, K.; Hassaneen, H.M.; Saleh, F.M.; Abdelhamid, I.A.; Mohamed Teleb, M.A. Anticancer Activity of Novel 3-(Furan-2-yl) Pyrazolyl and 3-(Thiophen-2-yl) Pyrazolyl Hybrid Chalcones: Synthesis and In Vitro Studies. Arch. Pharm. 2022, 355, 2100381. [Google Scholar] [CrossRef] [PubMed]
- Rioux, B.; Pouget, C.; Ndong-Ntoutoume, G.M.A.; Granet, R.; Gamond, A.; Laurent, A.; Pinon, A.; Champavier, Y.; Liagre, B.; Fagnère, C. Enhancement of Hydrosolubility and In Vitro Antiproliferative Properties of Chalcones Following Encapsulation into β-Cyclodextrin/Cellulose-Nanocrystal Complexes. Bioorganic Med. Chem. Lett. 2019, 29, 1895–1898. [Google Scholar] [CrossRef]
- Escribano-Ferrer, E.; Queralt Regue, J.; Garcia-Sala, X.; Boix Montanes, A.; Lamuela-Raventos, R.M. In Vivo Anti-Inflammatory and Antiallergic Activity of Pure Naringenin, Naringenin Chalcone, and Quercetin in Mice. J. Nat. Prod. 2019, 82, 177–182. [Google Scholar] [CrossRef]
- Alaaeldin, R.; Mustafa, M.; Abuo-Rahma, G.E.A.; Fathy, M. In Vitro Inhibition and Molecular Docking of a New Ciprofloxacin-chalcone against SARS-CoV-2 Main Protease. Fundam. Clin. Pharmacol. 2022, 36, 160–170. [Google Scholar] [CrossRef]
- Durairaju, P.; Umarani, C.; Periyasami, G.; Vivekanand, P.A.; Rahaman, M. Synthesis and In Vitro Antimicrobial Evaluation of Photoactive Multi—Block Chalcone Conjugate Phthalimide and 1, 8-Naphthalimide Novolacs. Polymers 2021, 13, 1859. [Google Scholar] [CrossRef] [PubMed]
- Noser, A.A.; Shehadi, I.A.; Abdelmonsef, A.H.; Salem, M.M. Newly Synthesized Pyrazolinone Chalcones as Anticancer Agents via Inhibiting the PI3K/Akt/ERK1/2 Signaling Pathway. ACS Omega 2022, 7, 25265–25277. [Google Scholar] [CrossRef] [PubMed]
- Aktar, W.; Sengupta, D.; Chowdhury, A. Impact of Pesticides Use in Agriculture: Their Benefits and Hazards. Interdiscip. Toxicol. 2009, 2, 1–12. [Google Scholar] [CrossRef]
- Silva, P.T.; Lopes, P.T.; Xavier, L.M.A.; Carvalho, M.C.S.; Moraes, M.O.; Pessoa, C.; Barros, F.W.A.; Bandeira, P.N.; Cavalvante, C.S.P.; Teixeira, A.M.R. Atividade Citotóxica e Antifúngica de Chalconas Sintetizadas a Partir de Uma Acetofenona Natural Isolada de Croton Anisodontus. Rev. Virtual Quim. 2020, 12, 712–723. [Google Scholar]
- Alman, A.A.; Daniel, K.; Killedar, S.G. Chalcone-Promising Entity for Anticancer Activity: An Overview. Int. J. Pharm. Sci. Res 2020, 11, 2027–2041. [Google Scholar]
- Constantinescu, T.; Lungu, C.N. Anticancer Activity of Natural and Synthetic Chalcones. Int. J. Mol. Sci. 2021, 22, 11306. [Google Scholar] [CrossRef]
- Dandawate, P.; Ahmed, K.; Padhye, S.; Ahmad, A.; Biersack, B. Anticancer Active Heterocyclic Chalcones: Recent Developments. Anti-Cancer Agents Med. Chem. 2021, 21, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Phang, C.-W.; Abd Malek, S.N.; Karsani, S.A. Flavokawain C Exhibits Anti-Tumor Effects on In Vivo HCT 116 Xenograft and Identification of Its Apoptosis-Linked Serum Biomarkers via Proteomic Analysis. Biomed. Pharmacother. 2021, 137, 110846. [Google Scholar] [CrossRef]
- World Health Organization (WHO). CANCER. Available online: https://www.who.int/health-topics/cancer#tab=tab_1 (accessed on 23 February 2023).
- World Health Organization. Cancer Burden Rise to 18.1 Million New Cases and 9.6 Million Cancer Deaths in 2018. Int. Agency Res. Cancer 2018, 2018, 3. [Google Scholar]
- Dos Santos, M.B.; Bertholin Anselmo, D.; de Oliveira, J.G.; Jardim-Perassi, B.V.; Alves Monteiro, D.; Silva, G.; Gomes, E.; Lucia Fachin, A.; Marins, M.; de Campos Zuccari, D.A.P. Antiproliferative Activity and P53 Upregulation Effects of Chalcones on Human Breast Cancer Cells. J. Enzym. Inhib. Med. Chem. 2019, 34, 1093–1099. [Google Scholar] [CrossRef] [PubMed]
- Saquib, M.; Baig, M.H.; Khan, M.F.; Azmi, S.; Khatoon, S.; Rawat, A.K.; Dong, J.J.; Asad, M.; Arshad, M.; Hussain, M.K. Design and Synthesis of Bioinspired Benzocoumarin-Chalcones Chimeras as Potential Anti-Breast Cancer Agents. ChemistrySelect 2021, 6, 8754–8765. [Google Scholar] [CrossRef]
- Dallavalle, S.; Dobričić, V.; Lazzarato, L.; Gazzano, E.; Machuqueiro, M.; Pajeva, I.; Tsakovska, I.; Zidar, N.; Fruttero, R. Improvement of Conventional Anti-Cancer Drugs as New Tools against Multidrug Resistant Tumors. Drug Resist. Updat. 2020, 50, 100682. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Y.; Li, J.; Chen, X.; Fu, X.; Sun, S.; Wu, Q. Chalcone Derivatives: Role in Anticancer Therapy. Biomolecules 2021, 11, 894. [Google Scholar] [CrossRef]
- Shukla, S.; Sood, A.K.; Goyal, K.; Singh, A.; Sharma, V.; Guliya, N.; Gulati, S.; Kumar, S. Chalcone Scaffolds as Anticancer Drugs: A Review on Molecular Insight in Action of Mechanisms and Anticancer Properties. Anti-Cancer Agents Med. Chem. 2021, 21, 1650–1670. [Google Scholar] [CrossRef]
- Mastachi-Loza, S.; Ramírez-Candelero, T.I.; Benítez-Puebla, L.J.; Fuentes-Benítes, A.; González-Romero, C.; Vázquez, M.A. Chalcones, a Privileged Scaffold: Highly Versatile Molecules in [4+2] Cycloadditions. Chem. Asian J. 2022, 17, e202200706. [Google Scholar] [CrossRef]
- Rani, A.; Anand, A.; Kumar, K.; Kumar, V. Recent Developments in Biological Aspects of Chalcones: The Odyssey Continues. Expert Opin. Drug Discov. 2019, 14, 249–288. [Google Scholar] [CrossRef]
- de Souza, P.S.; Bibá, G.C.C.; Melo, E.D.d.N.; Muzitano, M.F. Chalcones against the Hallmarks of Cancer: A Mini-Review. Nat. Prod. Res. 2021, 36, 4803–4820. [Google Scholar] [CrossRef] [PubMed]
- Elkanzi, N.A.A.; Hrichi, H.; Alolayan, R.A.; Derafa, W.; Zahou, F.M.; Bakr, R.B. Synthesis of Chalcones Derivatives and Their Biological Activities: A Review. ACS Omega 2022, 7, 27769–27786. [Google Scholar] [CrossRef] [PubMed]
- Leonte, D.; Coman, F.-M.; Fotso, G.W.; Ngadjui, B.T.; Zaharia, V.; Ngameni, B. Heterocycles 49. Synthesis, Chemical Behaviour and Biological Properties of Heterocyclic Chalcones. Review from Our Research. Farmacia 2021, 69, 821–836. [Google Scholar] [CrossRef]
- Yadav, G.D.; Wagh, D.P. Claisen-Schmidt Condensation Using Green Catalytic Processes: A Critical Review. ChemistrySelect 2020, 5, 9059–9085. [Google Scholar] [CrossRef]
- Liu, B.; Zhao, D.; Xu, D.; Xu, Z. Facile Aldol Reaction between Unmodified Aldehydes and Ketones in Bronsted Acid Ionic Liquids. Chem. Res. Chinese Univ. 2007, 23, 549–553. [Google Scholar] [CrossRef]
- Josephs, S.F.; Ichim, T.E.; Prince, S.M.; Kesari, S.; Marincola, F.M.; Escobedo, A.R.; Jafri, A. Unleashing Endogenous TNF-Alpha as a Cancer Immunotherapeutic. J. Transl. Med. 2018, 16, 242. [Google Scholar] [CrossRef]
- Cruceriu, D.; Baldasici, O.; Balacescu, O.; Berindan-Neagoe, I. The Dual Role of Tumor Necrosis Factor-Alpha (TNF-α) in Breast Cancer: Molecular Insights and Therapeutic Approaches. Cell. Oncol. 2020, 43, 1–18. [Google Scholar] [CrossRef]
- Koh, J.-J.; Lin, S.; Aung, T.T.; Lim, F.; Zou, H.; Bai, Y.; Li, J.; Lin, H.; Pang, L.M.; Koh, W.L.; et al. Amino Acid Modified Xanthone Derivatives: Novel, Highly Promising Membrane-Active Antimicrobials for Multidrug-Resistant Gram-Positive Bacterial Infections. J. Med. Chem. 2015, 58, 739–752. [Google Scholar] [CrossRef]
- Verza, F.A.; Das, U.; Fachin, A.L.; Dimmock, J.R.; Marins, M. Roles of Histone Deacetylases and Inhibitors in Anticancer Therapy. Cancers 2020, 12, 1664. [Google Scholar] [CrossRef]
- Rashid, H.U.; Xu, Y.; Ahmad, N.; Muhammad, Y.; Wang, L. Promising Anti-Inflammatory Effects of Chalcones via Inhibition of Cyclooxygenase, Prostaglandin E2, Inducible NO Synthase and Nuclear Factor Κb Activities. Bioorganic Chem. 2019, 87, 335–365. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, H.H.H.; Abd El-Hafeez, A.A.; Ebeid, K.; Mekkawy, A.I.; Abourehab, M.A.S.; Wafa, E.I.; Alhaj-Suliman, S.O.; Salem, A.K.; Ghosh, P.; Abuo-Rahma, G.E.-D.A. New 1, 2, 3-Triazole Linked Ciprofloxacin-Chalcones Induce DNA Damage by Inhibiting Human Topoisomerase I& II and Tubulin Polymerization. J. Enzym. Inhib. Med. Chem. 2022, 37, 1346–1363. [Google Scholar]
- Păușescu, I.; Kántor, I.; Babos, G.; May, Z.; Fodor-Kardos, A.; Miskolczy, Z.; Biczók, L.; Péter, F.; Medeleanu, M.; Feczkó, T. Halochromic Behavior and Anticancer Effect of New Synthetic Anthocyanidins Complexed with β-Cyclodextrin Derivatives. Int. J. Mol. Sci. 2022, 23, 8103. [Google Scholar] [CrossRef] [PubMed]
- Palko-Łabuz, A.; Kostrzewa-Susłow, E.; Janeczko, T.; Środa-Pomianek, K.; Poła, A.; Uryga, A.; Michalak, K. Cyclization of Flavokawain B Reduces Its Activity against Human Colon Cancer Cells. Hum. Exp. Toxicol. 2020, 39, 262–275. [Google Scholar] [CrossRef] [PubMed]
- Luković, J.; Mitrović, M.; Popović, S.; Milosavljević, Z.; Stanojević-Pirković, M.; Anđelković, M.; Zelen, I.; Šorak, M.; Muškinja, J.; Ratković, Z. Antitumor Effects of Vanillin Based Chalcone Analogues In Vitro. Acta Pol. Pharm. Res. 2020, 77, 57–67. [Google Scholar] [CrossRef]
- Al-Hamashi, A.A.; Koranne, R.; Dlamini, S.; Alqahtani, A.; Karaj, E.; Rashid, M.S.; Knoff, J.R.; Dunworth, M.; Pflum, M.K.H.; Casero Jr, R.A. A New Class of Cytotoxic Agents Targets Tubulin and Disrupts Microtubule Dynamics. Bioorganic Chem. 2021, 116, 105297. [Google Scholar] [CrossRef]
- Mphahlele, M.J.; Gildenhuys, S.; Agbo, E.N. In Vitro Evaluation and Docking Studies of 5-Oxo-5H-Furo [3, 2-g] Chromene-6-Carbaldehyde Derivatives as Potential Anti-Alzheimer’s Agents. Int. J. Mol. Sci. 2019, 20, 5451. [Google Scholar] [CrossRef]
- Sherikar, A.S.; Bhatia, M.S.; Dhavale, R.P. Identification and Investigation of Chalcone Derivatives as Calcium Channel Blockers: Pharmacophore Modeling, Docking Studies, In Vitro Screening, and 3D-QSAR Analysis. Curr. Comput. Aided. Drug Des. 2021, 17, 676–686. [Google Scholar] [CrossRef]
- El-Wakil, M.H.; Khattab, S.N.; El-Yazbi, A.F.; El-Nikhely, N.; Soffar, A.; Khalil, H.H. New Chalcone-Tethered 1, 3, 5-Triazines Potentiate the Anticancer Effect of Cisplatin against Human Lung Adenocarcinoma A549 Cells by Enhancing DNA Damage and Cell Apoptosis. Bioorganic Chem. 2020, 105, 104393. [Google Scholar] [CrossRef]
- Kumar, K.S.; Kotra, V.; Kola, P.K.; Devi, C.H.B.P.; Anusha, N.; Babu, B.H.; Adil, S.F.; Shaik, M.R.; Khan, M.; Al-Warthan, A. ZnCl2 Catalyzed New Coumarinyl-Chalcones as Cytotoxic Agents. Saudi J. Biol. Sci. 2021, 28, 386–394. [Google Scholar] [CrossRef]
- Hseu, Y.; Huang, Y.; Thiyagarajan, V.; Mathew, D.C.; Lin, K.; Chen, S.; Liu, J.; Hsu, L.; Li, M.; Yang, H. Anticancer Activities of Chalcone Flavokawain B from Alpinia Pricei Hayata in Human Lung Adenocarcinoma (A549) Cells via Induction of Reactive Oxygen Species-mediated Apoptotic and Autophagic Cell Death. J. Cell. Physiol. 2019, 234, 17514–17526. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Liu, Z.; Huang, X.; Chen, Y.; Wang, Y.; Kong, J.; Yang, Y.; Yu, C.; Li, J.; Wang, X. Dual-Target Platinum (IV) Complexes Exhibit Antiproliferative Activity through DNA Damage and Induce ER-Stress-Mediated Apoptosis in A549 Cells. Bioorganic Chem. 2021, 110, 104741. [Google Scholar] [CrossRef] [PubMed]
- Fathi, E.M.; Sroor, F.M.; Mahrous, K.F.; Mohamed, M.F.; Mahmoud, K.; Emara, M.; Elwahy, A.H.M.; Abdelhamid, I.A. Design, Synthesis, In Silico and In Vitro Anticancer Activity of Novel Bis-Furanyl-Chalcone Derivatives Linked through Alkyl Spacers. ChemistrySelect 2021, 6, 6202–6211. [Google Scholar] [CrossRef]
- Bukhari, S.N.A. Synthesis and Evaluation of New Chalcones and Oximes as Anticancer Agents. RSC Adv. 2022, 12, 10307–10320. [Google Scholar] [CrossRef]
- Gaur, R.; Yadav, D.K.; Kumar, S.; Darokar, M.P.; Khan, F.; Singh Bhakuni, R. Molecular Modeling Based Synthesis and Evaluation of in Vitro Anticancer Activity of Indolyl Chalcones. Curr. Top. Med. Chem. 2015, 15, 1003–1012. [Google Scholar] [CrossRef]
- Parmar, V.S.; Bracke, M.E.; Vanhoecke, B.W.A.; Derycke, L.; Bolca, S.; Possemiers, S.; Heyerick, A.; Stevens, C.V.; Keukeleire, D.D.; Depypere, H.T. Plant Polyphenolics as Anti-Invasive Cancer Agents. Anti-Cancer Agents Med. Chem. 2008, 8, 171–185. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Liu, W.; Gong, Z.; Huang, Y.; Li, Y.; Peng, Z. Design, Synthesis, Biological Evaluation and Molecular Docking Studies of New Chalcone Derivatives Containing Diaryl Ether Moiety as Potential Anticancer Agents and Tubulin Polymerization Inhibitors. Bioorganic Chem. 2020, 95, 103565. [Google Scholar] [CrossRef]
- Guruswamy, D.K.M.; Jayarama, S. Proapoptotic and Anti-Angiogenic Activity of (2E)-3-(2-Bromo-6-Hydroxy-4-Methoxyphenyl)-1-(Naphthalene-2-Yl) Prop-2-En-1-One in MCF7 Cell Line. Chem. Pap. 2020, 74, 2229–2237. [Google Scholar] [CrossRef]
- Homerin, G.; Nica, A.S.; Farce, A.; Dubois, J.; Ghinet, A. Ultrasounds-Mediated 10-Seconds Synthesis of Chalcones as Potential Farnesyltransferase Inhibitors. Bioorganic Med. Chem. Lett. 2020, 30, 127149. [Google Scholar] [CrossRef]
- Harshitha, K.R.; Sarojini, B.K.; Narayana, B.; Lobo, A.G.; Kalal, B.S. Molecular Docking of 4-Ethoxychalcones on Oxidoreductase/Pirin Inhibitors and Cytotoxic Evaluation on Breast/Skin Cancer Cell Lines. Lett. Drug Des. Discov. 2020, 17, 1245–1260. [Google Scholar] [CrossRef]
- Al-Kaabi, M.M.; Rady Al-Hazam, H.A.; Arwa, H.M. Microwave Assisted Synthesis, Characterization and Biochemical Study of New Chalcones. Egypt. J. Chem. 2021, 64, 4027–4035. [Google Scholar] [CrossRef]
- Mangoud, M.M.; Hussein, M.Z.; El-Bordany, E.A. Design and Synthesis of Novel Pyrazoles, Pyrazolines, and Pyridines from Chalcone Derivatives with Evaluation of Their In Vitro Anticancer Activity Against T-47D and UACC-257 Cell Lines. Egypt. J. Chem. 2020, 63, 5203–5218. [Google Scholar]
- Ahn, S.; Truong, V.N.-P.; Kim, B.; Yoo, M.; Lim, Y.; Cho, S.K.; Koh, D. Design, Synthesis, and Biological Evaluation of Chalcones for Anticancer Properties Targeting Glycogen Synthase Kinase 3 Beta. Appl. Biol. Chem. 2022, 65, 17. [Google Scholar] [CrossRef]
- Rajeswari, K.; Manturthi, S.; Sirisha, K.; Velidandi, A.N. Anchoring and Hydrophobic Nature of Coumarin in Newer Coumarin Based Chalcones: Synthesis, In Silico, and In Vitro Cell Viability Studies. Russ. J. Bioorganic Chem. 2022, 48, 636–642. [Google Scholar] [CrossRef]
- Kode, J.; Kovvuri, J.; Nagaraju, B.; Jadhav, S.; Barkume, M.; Sen, S.; Kasinathan, N.K.; Chaudhari, P.; Mohanty, B.S.; Gour, J. Synthesis, Biological Evaluation, and Molecular Docking Analysis of Phenstatin Based Indole Linked Chalcones as Anticancer Agents and Tubulin Polymerization Inhibitors. Bioorganic Chem. 2020, 105, 104447. [Google Scholar] [CrossRef]
- Gul, H.I.; Yamali, C.; Gunesacar, G.; Sakagami, H.; Okudaira, N.; Uesawa, Y.; Kagaya, H. Cytotoxicity, Apoptosis, and QSAR Studies of Phenothiazine Derived Methoxylated Chalcones as Anticancer Drug Candidates. Med. Chem. Res. 2018, 27, 2366–2378. [Google Scholar] [CrossRef]
- Mphahlele, M.J.; Maluleka, M.M.; Choong, Y.S.; Monchusi, B.A.; Mbazima, V.G. An In Vitro Study of the 5-Methyl-and 5-Bromo/Chloro Substituted 2-Hydroxy-3-Nitrochalcones as α-Glucosidase and/or α-Amylase Inhibitors with Potential Anti-Inflammatory Activity. Med. Chem. Res. 2022, 31, 2243–2259. [Google Scholar] [CrossRef]
- Kudličková, Z.; Takáč, P.; Sabolová, D.; Vilková, M.; Baláž, M.; Béres, T.; Mojžiš, J. Novel 1-Methoxyindole-and 2-Alkoxyindole-Based Chalcones: Design, Synthesis, Characterization, Antiproliferative Activity and DNA, BSA Binding Interactions. Med. Chem. Res. 2021, 30, 897–912. [Google Scholar] [CrossRef]
- Del Rosario, H.; Saavedra, E.; Brouard, I.; González-Santana, D.; García, C.; Spínola-Lasso, E.; Tabraue, C.; Quintana, J.; Estévez, F. Structure-Activity Relationships Reveal a 2-Furoyloxychalcone as a Potent Cytotoxic and Apoptosis Inducer for Human U-937 and HL-60 Leukaemia Cells. Bioorganic Chem. 2022, 127, 105926. [Google Scholar] [CrossRef]
- Li, Y.; Sun, Y.; Zhou, Y.; Li, X.; Zhang, H.; Zhang, G. Discovery of Orally Active Chalcones as Histone Lysine Specific Demethylase 1 Inhibitors for the Treatment of Leukaemia. J. Enzym. Inhib. Med. Chem. 2021, 36, 207–217. [Google Scholar] [CrossRef]
- Petrov, O.I.; Ivanova, Y.B.; Gerova, M.S.; Momekov, G.T. Synthesis and Cytotoxicity of New Mannich Bases of 6-[3-(3,4,5-Trimetoxyphenyl)-2-Propenoyl]-2 (3H)-Benzoxazolone. Lett. Drug Des. Discov. 2020, 17, 512–517. [Google Scholar] [CrossRef]
- Insuasty, D.; García, S.; Abonia, R.; Insuasty, B.; Quiroga, J.; Nogueras, M.; Cobo, J.; Borosky, G.L.; Laali, K.K. Design, Synthesis, and Molecular Docking Study of Novel Quinoline-based Bis-chalcones as Potential Antitumor Agents. Arch. Pharm. 2021, 354, 2100094. [Google Scholar] [CrossRef] [PubMed]
- Vrontaki, E.; Melagraki, G.; Voskou, S.; Phylactides, M.S.; Mavromoustakos, T.; Kleanthous, M.; Afantitis, A. Development of a Predictive Pharmacophore Model and a 3D-QSAR Study for an In Silico Screening of New Potent Bcr-Abl Kinase Inhibitors. Mini Rev. Med. Chem. 2017, 17, 188–204. [Google Scholar] [CrossRef] [PubMed]
- Mercader, A.G.; Pomilio, A.B. (Iso)Flav(an)Ones, Chalcones, Catechins, and Theaflavins as Anticarcinogens: Mechanisms, Anti-Multidrug Resistance and QSAR Studies. Curr. Med. Chem. 2012, 19, 4324–4347. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Ma, T.; Cheng, X.; Liu, D. Design, Synthesis, and Apoptosis-Promoting Effect Evaluation of Chalcone Derivatives Containing Aminoguanidine Units. Anti-Cancer Agents Med. Chem. 2022, 22, 2116–2124. [Google Scholar]
- Huang, X.; Liu, Z.; Wang, M.; Yin, X.; Wang, Y.; Dai, L.; Wang, H. Platinum (IV) Complexes Conjugated with Chalcone Analogs as Dual Targeting Anticancer Agents: In Vitro and In Vivo Studies. Bioorganic Chem. 2020, 105, 104430. [Google Scholar] [CrossRef]
- Mourad, A.A.E.; Mourad, M.A.E.; Jones, P.G. Novel HDAC/Tubulin Dual Inhibitor: Design, Synthesis and Docking Studies of α-Phthalimido-Chalcone Hybrids as Potential Anticancer Agents with Apoptosis-Inducing Activity. Drug Des. Devel. Ther. 2020, 14, 3111–3130. [Google Scholar] [CrossRef]
- Al Zahrani, N.A.; El-Shishtawy, R.M.; Elaasser, M.M.; Asiri, A.M. Synthesis of Novel Chalcone-Based Phenothiazine Derivatives as Antioxidant and Anticancer Agents. Molecules 2020, 25, 4566. [Google Scholar] [CrossRef]
- Sangpheak, K.; Mueller, M.; Darai, N.; Wolschann, P.; Suwattanasophon, C.; Ruga, R.; Chavasiri, W.; Seetaha, S.; Choowongkomon, K.; Kungwan, N. Computational Screening of Chalcones Acting against Topoisomerase IIα and Their Cytotoxicity towards Cancer Cell Lines. J. Enzym. Inhib. Med. Chem. 2019, 34, 134–143. [Google Scholar] [CrossRef]
- Vongdeth, K.; Han, P.; Li, W.; Wang, Q.-A. Synthesis and Antiproliferative Activity of Natural and Non-Natural Polymethoxychalcones and Polymethoxyflavones. Chem. Nat. Compd. 2019, 55, 11–17. [Google Scholar] [CrossRef]
- Mendanha, D.; Vieira de Castro, J.; Moreira, J.; Costa, B.M.; Cidade, H.; Pinto, M.; Ferreira, H.; Neves, N.M. A New Chalcone Derivative with Promising Antiproliferative and Anti-Invasion Activities in Glioblastoma Cells. Molecules 2021, 26, 3383. [Google Scholar] [CrossRef] [PubMed]
- Custodio, J.M.F.; Moura, A.F.; de Moraes, M.O.; Perez, C.N.; Napolitano, H.B. On the In Silico and In Vitro Anticancer Activity of Sulfonamide Chalcones: Potential JNKK3 Inhibitors. New J. Chem. 2020, 44, 3294–3309. [Google Scholar] [CrossRef]
- Castaño, L.F.; Quiroga, J.; Abonia, R.; Insuasty, D.; Vidal, O.M.; Seña, R.; Rubio, V.; Puerto, G.; Nogueras, M.; Cobo, J. Synthesis, Anticancer and Antitubercular Properties of New Chalcones and Their Nitrogen-Containing Five-Membered Heterocyclic Hybrids Bearing Sulfonamide Moiety. Int. J. Mol. Sci. 2022, 23, 12589. [Google Scholar] [CrossRef] [PubMed]
- Bhandare, R.R.; Shaik, A.B. Assessment of the Antimicrobial and Antiproliferative Activities of Chloropyrazine-Tethered Pyrimidine Derivatives: In Vitro, Molecular Docking, and In-Silico Drug-Likeness Studies. Appl. Sci. 2021, 11, 10734. [Google Scholar] [CrossRef]
- Safwat, G.M.; Hassanin, K.; Mohammed, E.T.; Ahmed, E.K.; Abdel Rheim, M.R.; Ameen, M.A.; Abdel-Aziz, M.; Gouda, A.M.; Peluso, I.; Almeer, R. Synthesis, Anticancer Assessment, and Molecular Docking of Novel Chalcone-Thienopyrimidine Derivatives in HepG2 and MCF-7 Cell Lines. Oxid. Med. Cell. Longev. 2021, 2021, 4759821. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; He, M.; Li, Y.; Peng, Z.; Wang, G. A Review on Synthetic Chalcone Derivatives as Tubulin Polymerisation Inhibitors. J. Enzym. Inhib. Med. Chem. 2022, 37, 9–38. [Google Scholar] [CrossRef] [PubMed]
- Egbujor, M.C.; Saha, S.; Buttari, B.; Profumo, E.; Saso, L. Activation of Nrf2 Signaling Pathway by Natural and Synthetic Chalcones: A Therapeutic Road Map for Oxidative Stress. Expert Rev. Clin. Pharmacol. 2021, 14, 465–480. [Google Scholar] [CrossRef]
- Elkhalifa, D.; Alali, F.; Al Moustafa, A.-E.; Khalil, A. Targeting Triple Negative Breast Cancer Heterogeneity with Chalcones: A Molecular Insight. J. Drug Target. 2019, 27, 830–838. [Google Scholar] [CrossRef]
- WalyEldeen, A.A.; El-Shorbagy, H.M.; Hassaneen, H.M.; Abdelhamid, I.A.; Sabet, S.; Ibrahim, S.A. [1,2,4] Triazolo [3,4-a] Isoquinoline Chalcone Derivative Exhibits Anticancer Activity via Induction of Oxidative Stress, DNA Damage, and Apoptosis in Ehrlich Solid Carcinoma-Bearing Mice. Naunyn-Schmiedebergs Arch. Pharmacol. 2022, 395, 1225–1238. [Google Scholar] [CrossRef]
- Islam, M.S.; Al-Majid, A.M.; Azam, M.; Verma, V.P.; Barakat, A.; Haukka, M.; Elgazar, A.A.; Mira, A.; Badria, F.A. Construction of Spirooxindole Analogues Engrafted with Indole and Pyrazole Scaffolds as Acetylcholinesterase Inhibitors. ACS Omega 2021, 6, 31539–31556. [Google Scholar] [CrossRef]
- Cuartas, V.; Aragón-Muriel, A.; Liscano, Y.; Polo-Cerón, D.; del Pilar Crespo-Ortiz, M.; Quiroga, J.; Abonia, R.; Insuasty, B. Anticancer Activity of Pyrimidodiazepines Based on 2-Chloro-4-Anilinoquinazoline: Synthesis, DNA Binding and Molecular Docking. RSC Adv. 2021, 11, 23310–23329. [Google Scholar] [CrossRef]
- Shaik, A.B.; Prasad, Y.R.; Nissankararao, S.; Shahanaaz, S. Synthesis, Biological and Computational Evaluation of Novel 2, 3-Dihydro-2-Aryl-4-(4-Isobutylphenyl)-1, 5-Benzothiazepine Derivatives as Anticancer and Anti-EGFR Tyrosine Kinase Agents. Anti-Cancer Agents Med. Chem. 2020, 20, 1115–1128. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, G.; Bhanu, D.; Aruchamy, B.; Ramani, P.; Pandurangan, N.; Bobba, K.N.; Oh, E.J.; Chung, H.Y.; Gangadaran, P.; Ahn, B.-C. Chalcone: A Promising Bioactive Scaffold in Medicinal Chemistry. Pharmaceuticals 2022, 15, 1250. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Gao, M.; Diao, Q.; Gao, F. Chalcone Derivatives and Their Activities against Drug-Resistant Cancers: An Overview. Curr. Top. Med. Chem. 2021, 21, 348–362. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, C.-F.; Rao, G.-W. Progress in the Synthesis, Angiogenesis Activity and Mechanism of Chalcone Derivatives. Mini. Rev. Org. Chem. 2020, 17, 814–827. [Google Scholar] [CrossRef]
- Han, Y.; Dong, W.; Guo, Q.; Li, X.; Huang, L. The Importance of Indole and Azaindole Scaffold in the Development of Antitumor Agents. Eur. J. Med. Chem. 2020, 203, 112506. [Google Scholar] [CrossRef]
- Dhaliwal, J.S.; Moshawih, S.; Goh, K.W.; Loy, M.J.; Hossain, M.S.; Hermansyah, A.; Kotra, V.; Kifli, N.; Goh, H.P.; Dhaliwal, S.K.S. Pharmacotherapeutics Applications and Chemistry of Chalcone Derivatives. Molecules 2022, 27, 7062. [Google Scholar] [CrossRef]
- Abd-Alla, H.I.; Souguir, D.; Radwan, M.O. Genus Sophora: A Comprehensive Review on Secondary Chemical Metabolites and Their Biological Aspects from Past Achievements to Future Perspectives. Arch. Pharm. Res. 2021, 44, 903–998. [Google Scholar] [CrossRef]
- Rout, S.; Mahapatra, R.K. In Silico Screening of Novel Inhibitors of M17 Leucine Amino Peptidase (LAP) of Plasmodium Vivax as Therapeutic Candidate. Biomed. Pharmacother. 2016, 82, 192–201. [Google Scholar] [CrossRef]
- Constantinescu, T.; Mihis, A.G. Two Important Anticancer Mechanisms of Natural and Synthetic Chalcones. Int. J. Mol. Sci. 2022, 23, 11595. [Google Scholar] [CrossRef]
- WalyEldeen, A.A.; Sabet, S.; El-Shorbagy, H.M.; Abdelhamid, I.A.; Ibrahim, S.A. Chalcones: Promising Therapeutic Agents Targeting Key Players and Signaling Pathways Regulating the Hallmarks of Cancer. Chem. Biol. Interact. 2022, 369, 110297. [Google Scholar] [CrossRef] [PubMed]
- Othman, E.M.; Fayed, E.A.; Husseiny, E.M.; Abulkhair, H.S. Apoptosis Induction, PARP-1 Inhibition, and Cell Cycle Analysis of Leukemia Cancer Cells Treated with Novel Synthetic 1,2,3-Triazole-Chalcone Conjugates. Bioorganic Chem. 2022, 123, 105762. [Google Scholar] [CrossRef]
- Seba, V.; de Lima, G.G.; Pereira, B.L.; Silva, G.; Reinhardt, L.S.; Arantes, P.R.; Chee, B.S.; Dos Santos, M.B.; França, S.C.; Regasini, L.O.; et al. Development, Characterization and Cell Viability Inhibition of Pva Spheres Loaded with Doxorubicin and 4′-Amino-1-Naphthyl-Chalcone (D14) for Osteosarcoma. Polymers 2021, 13, 2611. [Google Scholar] [CrossRef] [PubMed]
- Gunasekaran, V.; Yuvakkumar, R.; Ganesan, R.; Palapetta, S.C.; Gurusamy, H. Biological Evaluation of Polycyclic Chalcone Based Acrylamides in Human MCF-7 and HeLa Cancer Cell Lines. Environ. Res. 2023, 222, 115395. [Google Scholar] [CrossRef]
- Khan, I.; Ganapathi, T.; Shareef, M.A.; Shaik, A.B.; Akbar, S.; Rajanna, A.; Kamal, A.; Kumar, C.G. One-Pot Synthesis and Biological Evaluation of Arylpropenone Aminochalcone Conjugates as Potential Apoptotic Inducers. ChemistrySelect 2019, 4, 4672–4678. [Google Scholar] [CrossRef]
- Jasim, H.A.; Nahar, L.; Jasim, M.A.; Moore, S.A.; Ritchie, K.J.; Sarker, S.D. Chalcones: Synthetic Chemistry Follows Where Nature Leads. Biomolecules 2021, 11, 1203. [Google Scholar] [CrossRef] [PubMed]
- Michalkova, R.; Kello, M.; Cizmarikova, M.; Bardelcikova, A.; Mirossay, L.; Mojzis, J. Chalcones and Gastrointestinal Cancers: Experimental Evidence. Int. J. Mol. Sci. 2023, 24, 5964. [Google Scholar] [CrossRef] [PubMed]
- Moreira, J.; Almeida, J.; Saraiva, L.; Cidade, H.; Pinto, M. Chalcones as Promising Antitumor Agents by Targeting the P53 Pathway: An Overview and New Insights in Drug-Likeness. Molecules 2021, 26, 3737. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Huang, G.; Xiao, J. Chalcone Hybrids as Potential Anticancer Agents: Current Development, Mechanism of Action, and Structure-activity Relationship. Med. Res. Rev. 2020, 40, 2049–2084. [Google Scholar] [CrossRef]
- Montes-González, I.; Alsina-Sánchez, A.M.; Aponte-Santini, J.C.; Delgado-Rivera, S.M.; Durán-Camacho, G.L. Perspectives of Ferrocenyl Chalcones: Synthetic Scaffolds toward Biomedical and Materials Science Applications. Pure Appl. Chem. 2019, 91, 653–669. [Google Scholar] [CrossRef]
- Gangopadhyay, A. Natural Flavans and (Iso) Flavanones with Anticancer Activity: A Review. Curr. Org. Chem. 2021, 25, 1028–1046. [Google Scholar] [CrossRef]
- Mahapatra, D.K.; Bharti, S.K.; Asati, V.; Singh, S.K. Perspectives of Medicinally Privileged Chalcone Based Metal Coordination Compounds for Biomedical Applications. Eur. J. Med. Chem. 2019, 174, 142–158. [Google Scholar] [CrossRef] [PubMed]
- Raj, V.; Aboumanei, M.H.; Rai, A.; Verma, S.P.; Singh, A.K.; Keshari, A.K.; Saha, S. Pharmacophore and 3d-Qsar Modeling of New 1, 3, 4-Thiadiazole Derivatives: Specificity to Colorectal Cancer. Pharm. Chem. J. 2020, 54, 12–25. [Google Scholar] [CrossRef]
- Lv, Y.; Liu, Z.; Jia, H.; Xiu, Y.; Liu, Z.; Deng, L. Properties of Flavonoids in the Treatment of Bladder Cancer. Exp. Ther. Med. 2022, 24, 1–19. [Google Scholar] [CrossRef]
- Jayashree, B.S.; Nikhil, P.S.; Paul, S. Bioisosterism in Drug Discovery and Development-An Overview. Med. Chem. 2022, 18, 915–925. [Google Scholar] [CrossRef] [PubMed]
- Pasricha, S.; Gahlot, P. Synthetic Strategies and Biological Potential of Coumarin-Chalcone Hybrids: A New Dimension to Drug Design. Curr. Org. Chem. 2020, 24, 402–438. [Google Scholar] [CrossRef]
- Nanjala, C.; Odago, W.O.; Rono, P.C.; Waswa, E.N.; Mutinda, E.S.; Oulo, M.A.; Muema, F.W.; Wanga, V.O.; Mkala, E.M.; Kuja, J. A Review on Ethnobotany, Phytochemistry, and Pharmacology of the Genus Didymocarpus Wall. (Gesneriaceae). J. Ethnopharmacol. 2022, 295, 115404. [Google Scholar] [CrossRef]
- Celentano, A.; Tran, A.; Testa, C.; Thayanantha, K.; Tan-Orders, W.; Tan, S.; Syamal, M.; McCullough, M.J.; Yap, T. The Protective Effects of Kava (Piper Methysticum) Constituents in Cancers: A Systematic Review. J. Oral Pathol. Med. 2019, 48, 510–529. [Google Scholar] [CrossRef]
- Hosseinzadeh, E.; Hassanzadeh, A.; Marofi, F.; Alivand, M.R.; Solali, S. Flavonoid-Based Cancer Therapy: An Updated Review. Anti-Cancer Agents Med. Chem. 2020, 20, 1398–1414. [Google Scholar] [CrossRef]
- Hassan, S.T.S.; Šudomová, M. Molecular Mechanisms of Flavonoids against Tumor Gamma-Herpesviruses and Their Correlated Cancers—A Focus on EBV and KSHV Life Cycles and Carcinogenesis. Int. J. Mol. Sci. 2022, 24, 247. [Google Scholar] [CrossRef]
- Chan, E.W.C.; Wong, S.K.; Tangah, J.; Inoue, T.; Chan, H.T. Phenolic Constituents and Anticancer Properties of Morus Alba (White Mulberry) Leaves. J. Integr. Med. 2020, 18, 189–195. [Google Scholar] [CrossRef]
- Maria Pia, G.D.; Sara, F.; Mario, F.; Lorenza, S. Biological Effects of Licochalcones. Mini Rev. Med. Chem. 2019, 19, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Dhawan, S.; Girase, P.S.; Awolade, P.; Shinde, S.R.; Karpoormath, R.; Singh, P. Recent Advances in Chalcone-Based Anticancer Heterocycles: A Structural and Molecular Target Perspective. Curr. Med. Chem. 2021, 28, 6805–6845. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Liang, J.; Zhang, J.; Wang, Y.; Chai, X. Natural Chalcones in Chinese Materia Medica: Licorice. Evid.-Based Complement. Altern. Med. 2020, 2020, 3821248. [Google Scholar] [CrossRef] [PubMed]
- Michalkova, R.; Mirossay, L.; Gazdova, M.; Kello, M.; Mojzis, J. Molecular Mechanisms of Antiproliferative Effects of Natural Chalcones. Cancers 2021, 13, 2730. [Google Scholar] [CrossRef] [PubMed]
- Hacker, M. Adverse Drug Reactions. In Pharmacology; Elsevier: Amsterdam, The Netherlands, 2009; pp. 327–352. [Google Scholar]
- ChemAxon Ltd. ChemAxon Marvin Sketch 22.13. 2022. Available online: https://chemaxon.com/ (accessed on 10 January 2023).
- USFC Modeller 9.20—Program for Comparative Protein Structure Modelling by Satisfaction of Spatial Restraints; University of California San Francisco: San Francisco, CA, USA, 2020.
- Cruciani, G.; Crivori, P.; Carrupt, P.A.; Testa, B. Molecular Fields in Quantitative Structure-Permeation Relationships: The VolSurf Approach. J. Mol. Struct. Theochem 2000, 503, 17–30. [Google Scholar] [CrossRef]
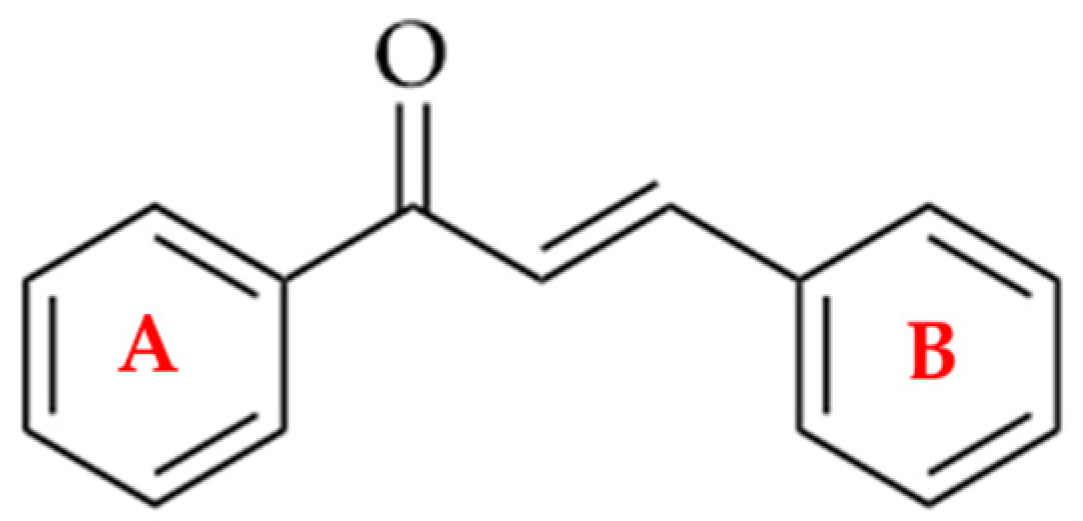
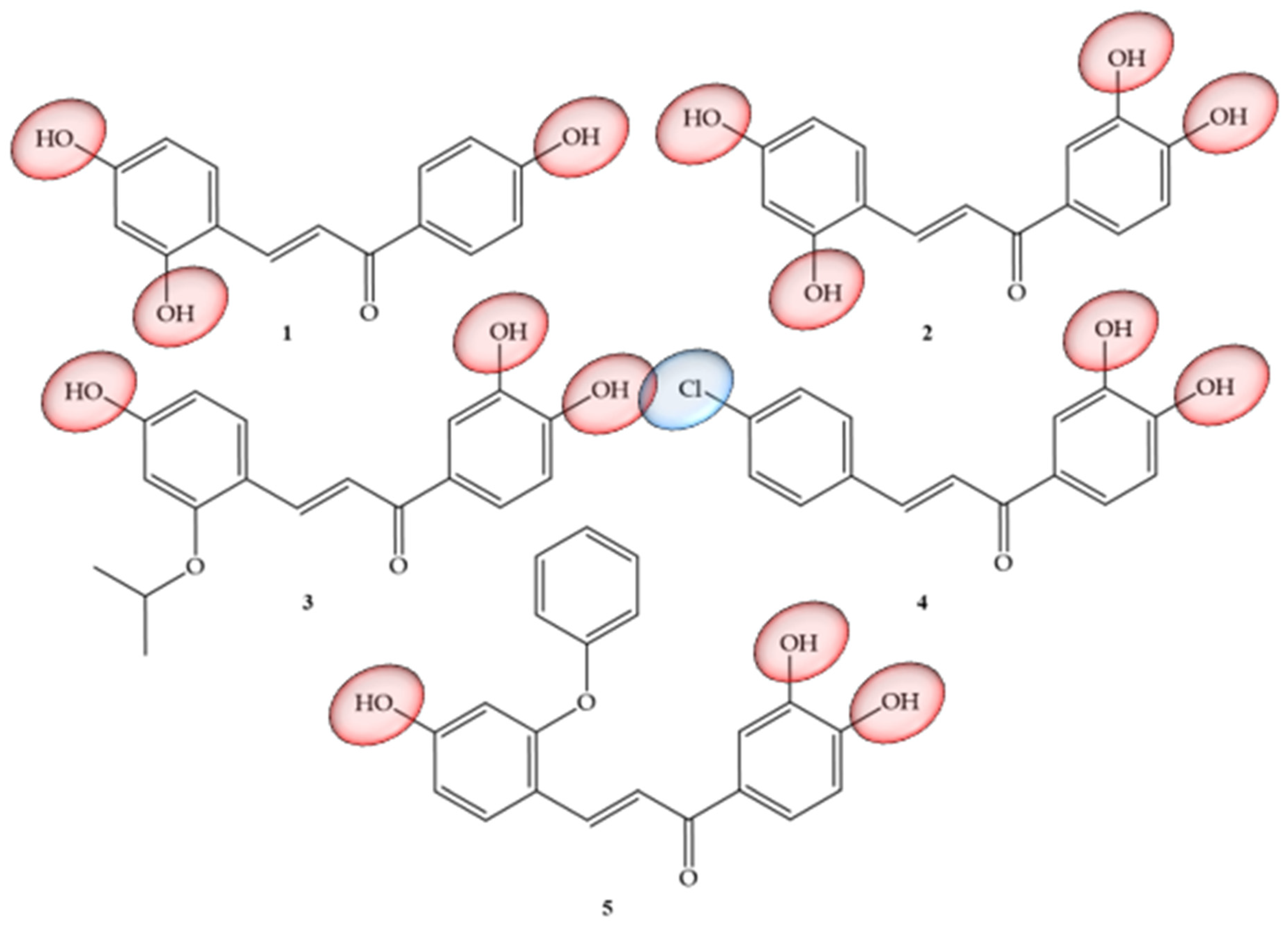
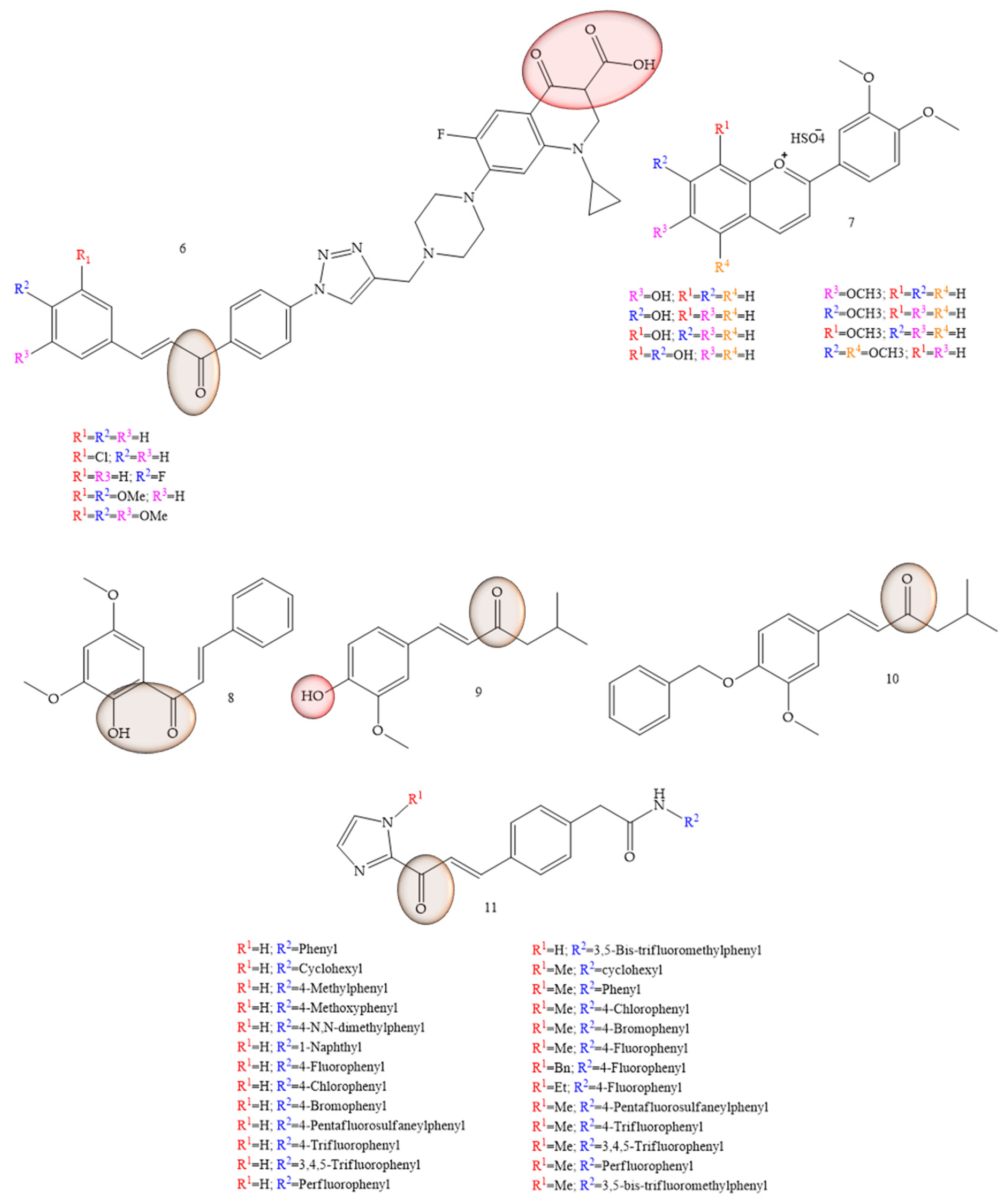


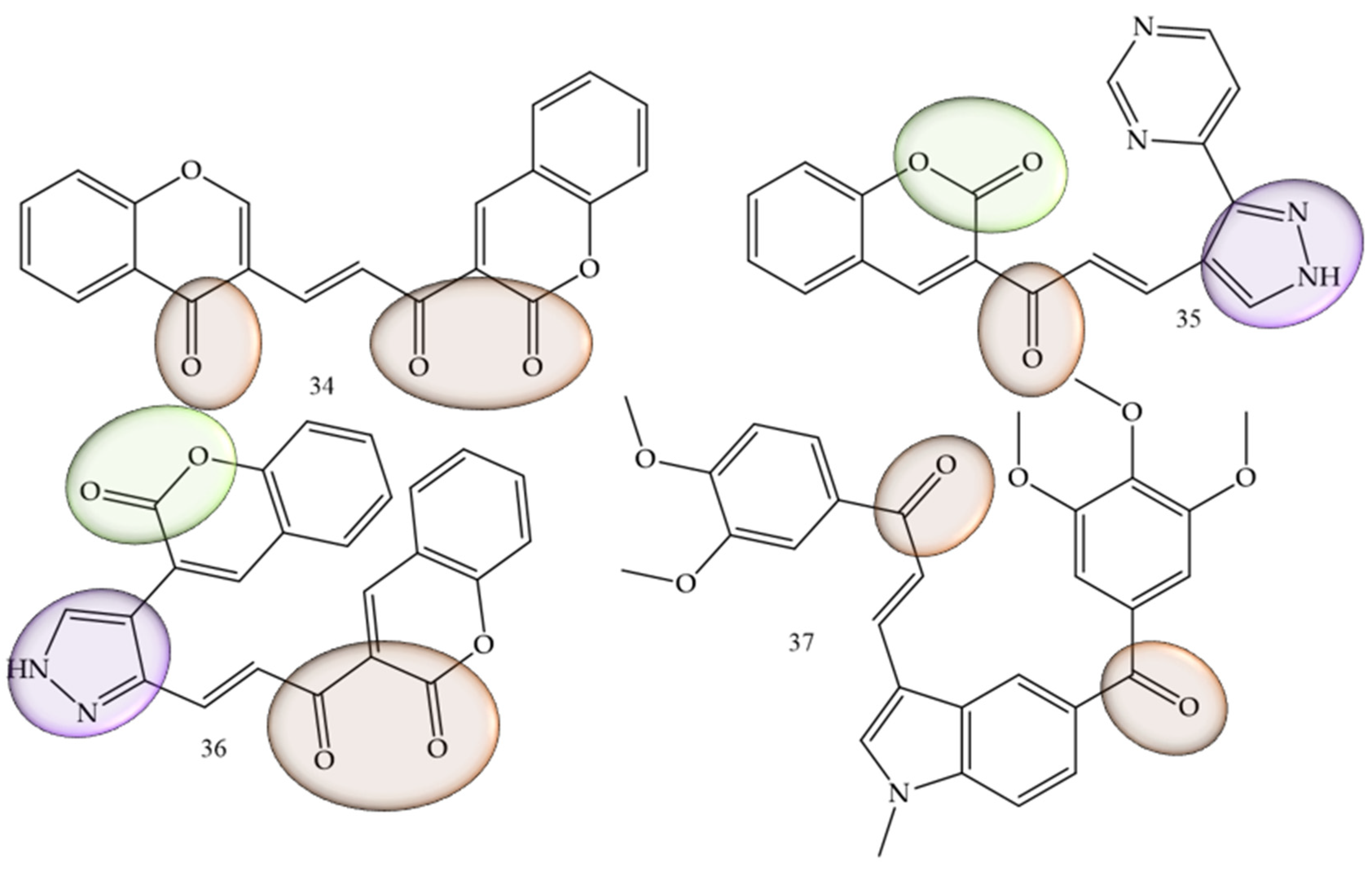
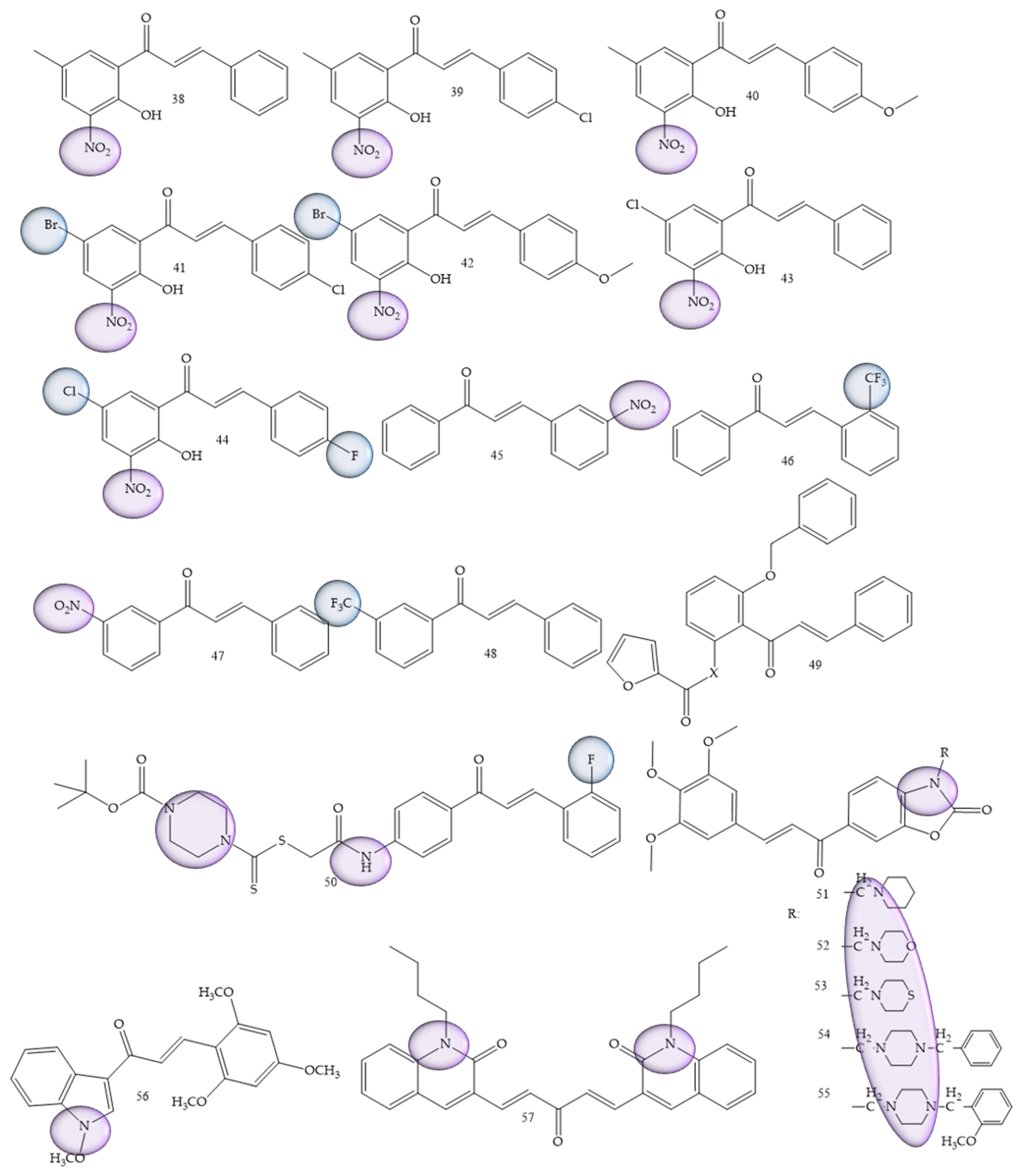
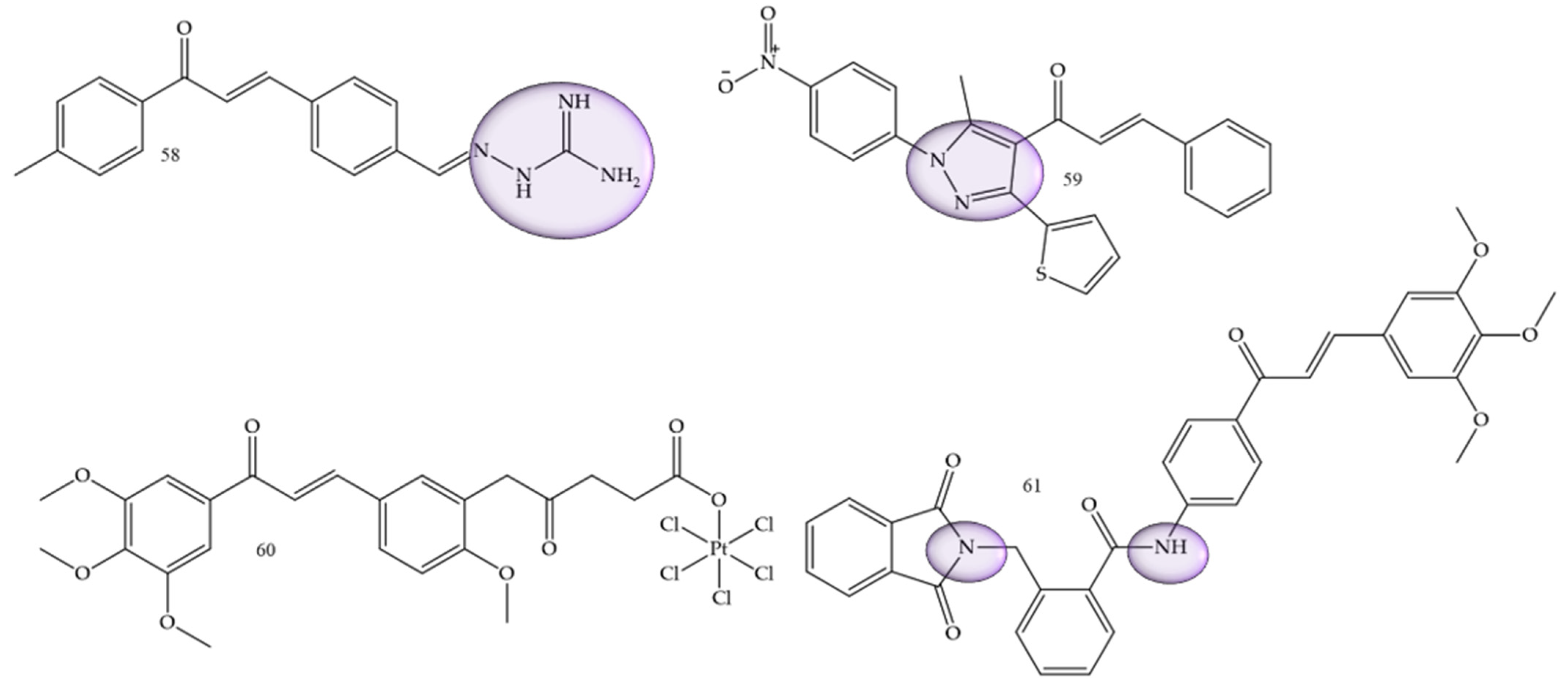
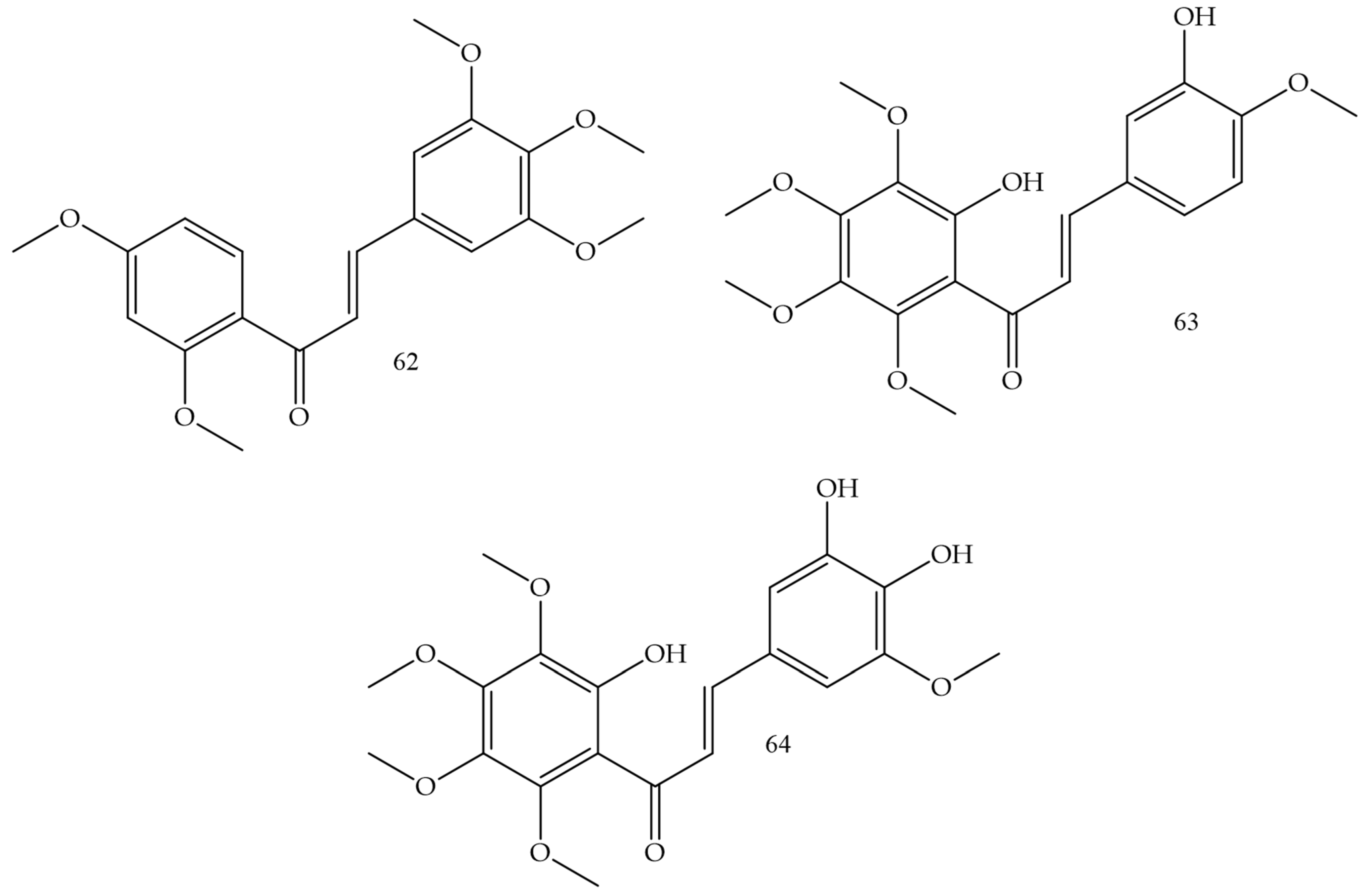
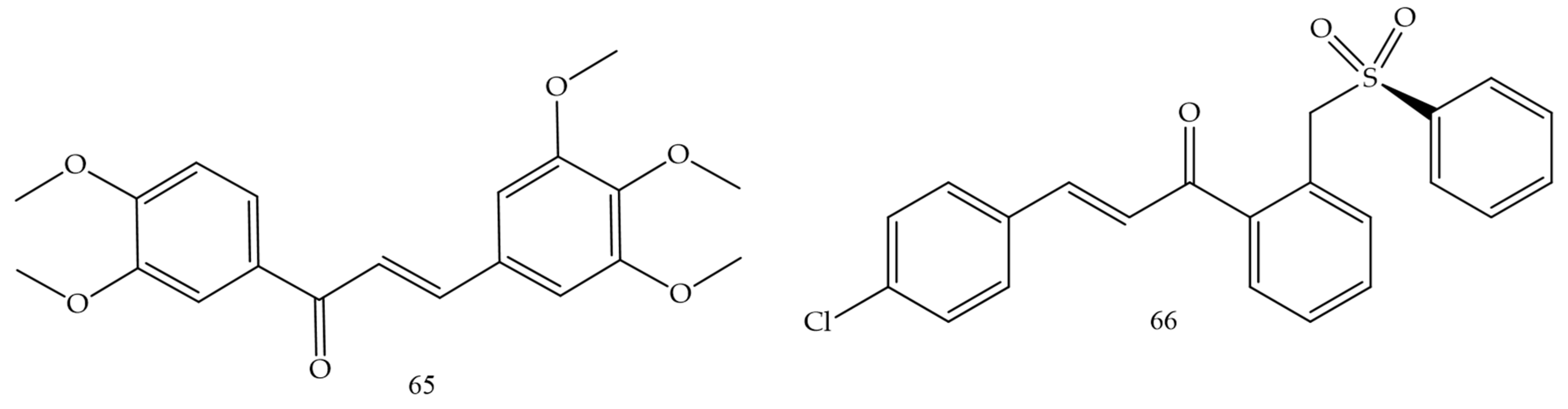

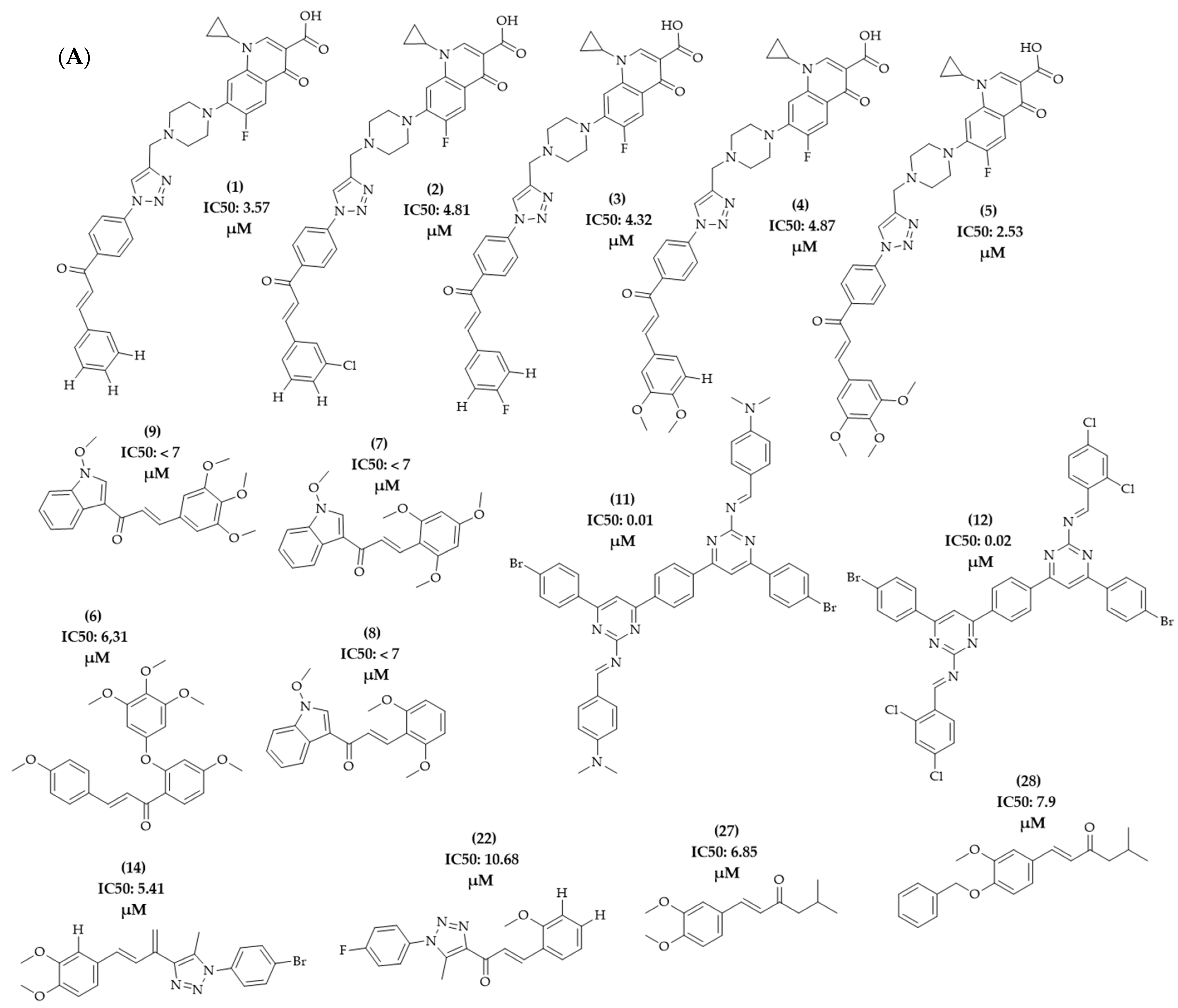


| Compounds | Number of Compounds under Study | Obtaining Method | In Vitro Activity | Cell Lineage | References |
|---|---|---|---|---|---|
| Pyrimidine derivatives linked to chloropyrazine | 18 | Synthesis | 5 ± 1 µg/mL (IC50) | DU-145 (prostate cancer) | [75] |
| chalcone-thienopyrimidine | 06 | Synthesis | 5.3 ± 2.1 µg/mL (IC50) | Hep-G2 (Hepatocarcinoma) | [76] |
| Natural chalcones | Various compounds | Literature review | - | Antimitotic activity (cell cycle inhibition in the G2/M phase) | [77] |
| 1,3-diaryl-2-propen-1-one synthetic chalcones | Various compounds | Literature review | - | Inhibition of erythroid nuclear factor-related factor 2 (Nrf2) | [78] |
| Natural, synthetic, and semi-synthetic chalcones | Various compounds | Literature review | - | Biological activity of chalcones in negative undulating breast cancers (TNBCs) | [79] |
| Isoquinoline chalcone | 01 | Synthesis | Qualitative evaluation of marker expression | Evaluation of antioxidant and antiproliferative activity, as well as the action on p-53 and BAX markers | [80] |
| Spirooxindole hybrids | 37 | Synthesis | 7 ± 0.27 µM (IC50) 5.5 ± 0.2 µM (IC50) | HCT-116 and HepG2 (colon cancer) | [81] |
| Pyrimidodiazepine derivatives containing the 2-chloro-4-anilinoquinazoline fragment | 14 | Synthesis | 0.622 μM (GI50) | K-562 (Leukemia) | [82] |
| Benzothiazepine derivatives | 20 | Synthesis | 16 ± 18 μg/mL (IC50) 12 ± 13 μg/mL (IC50) 15 ± 18 μg/mL (IC50) | HT-29 (colon cancer) MCF-7 (breast cancer) DU-145 (prostate cancer) | [83] |
| Homocyclic and heterocyclic chalcones | Various compounds | Literature review | - | Biological activity of chalcones in breast cancer. | [84] |
| Natural and synthetic chalcone derivatives | Various compounds | Literature review | 50 ± 6 nM (GI50) 1–53.4 nM (GI50) | MDR A549/T (lung cancer) HCT-116/L (colon cancer) HL60/DOX (leukemia) | [85] |
| Natural and synthetic chalcone derivatives | Various compounds | Literature review | 6.20 ± 2.82 μg/mL (IC50) | A-549 (lung cancer) HepG2 (colon cancer) MCF-7 (breast cancer) MDA-MB-231 (breast cancer) ALL-SIL (leukemia) SW1990 (pancreatic cancer) Vascular endothelial growth factor (VEGF) (anti-angiogenesis) | [86] |
| Derivatives of indole chalcones | Various compounds | Literature review | 4 μM (IC50) | PaCa2 (pancreatic carcinoma) RT112 (bladder carcinoma) | [87] |
| Substituted natural chalcones | Various compounds | Literature review | - | Evaluation of the induction of apoptosis by the caspase-3 pathway | [88] |
| Natural chalcones present in the Sophora kingdom | Various compounds | Literature review | - | It does not mention a specific cell or mechanism; it only informs that the compounds have anticancer activity | [89] |
| Glycosidic derivatives of chalcones | Various compounds | Literature review | 2.97 μM (IC50) | HL-60 (leukemia) | [90] |
| Natural and synthetic chalcone derivatives | Various compounds | Literature review | 0.17–0.19 µM (IC50) | ABCG2 transport protein inhibition | [91] |
| Natural and synthetic chalcone derivatives | Various compounds | Literature review | - | It does not mention a specific cell or mechanism; it only informs that the compounds have anticancer activity. | [92] |
| Triazole-chalcone-conjugates | 7 | Synthesis | 0.94–1.92 µM (IC50) | MCF-7 (breast cancer) Leukemia SR | [93] |
| Amino-naftil-chalcona | 1 | Synthesis | 8 µg/mL (IC50) | U2OS (humanosteosarcoma cell line) | [94] |
| Polycyclic chalcone based acrylamides | 4 | Synthesis | 38.46–48.25 µg/mL (IC50) 38.02–36.35 µg/mL (IC50) | MCF-7 (breast cancer) HeLa (cervical cancer) | [95] |
| Arylpropenone aminochalcone conjugates | 17 | Synthesis | 6.7–9.8 µM (IC50) | MCF-7 (breast cancer) | [96] |
| Synthetic chalcones | Various compounds | Review article | - | NRF2, apoptosis, and BCL2 | [97] |
| Natural and synthetic chalcone derivatives | Various compounds | Review article | - | Evaluation of the activity of chalcones in multiple mechanisms | [20] |
| Natural and synthetic chalcone derivatives | Various compounds | Review article | Survey of several studies and explanation of the mechanisms | Mechanisms related to gastric cancer | [98] |
| Synthetic chalcones | Various compounds | Review article about synthesis | Survey of several studies and explanation of the mechanisms | Various mechanisms | [19] |
| Natural and synthetic chalcone derivatives | Various compounds | Review articles on mechanisms of action of chalcones | Survey of several studies and explanation of the mechanisms | Various mechanisms | [22] |
| Natural and synthetic chalcone derivatives | Various compounds | Review articles on mechanisms the enzyme p53 | Survey of several studies and explanation of the mechanisms | Action on p-53 protein | [99] |
| Chalcone hybrids | Various compounds | Review article on obtaining hybrid chalcone compounds combined with structure-activity studies | Survey of several studies and explanation of the mechanisms | The article emphasizes various synthetic compounds and various mechanisms to achieve cancer | [100] |
| Ferrocenyl chalcones | Various synthetic compounds | Review about application | Survey of several studies and explanation of the mechanisms | The article emphasizes various synthetic compounds and various mechanisms to achieve cancer | [101] |
| Natural chalcones | Various compounds | Review on compounds reported in specific mechanism | Compost survey with action on histones | Histone deacetylase | [31] |
| Natural flavans and (iso)flavanones | Various compounds | Review and application of synthetic compounds anticancer | Survey of several studies and explanation of the mechanisms | Various mechanisms | [102] |
| Chalcone based metal cordination | Various compounds | Review and application of synthetic compounds anticancer | Survey of several studies and explanation of the mechanisms | Various mechanisms for obtaining synthetic chalcones | [103] |
| Chalcone heterocycles synthesis | Various compounds | Review and application of synthetic chalcones in cancer | Various mechanisms for obtaining synthetic chalcones | Various mechanisms for obtaining synthetic chalcones | [25] |
| Quinoline chalcone hybrids | Various compounds | Review and application of synthetic chalcones in cancer | Various mechanisms for obtaining synthetic chalcones | Various mechanisms for obtaining synthetic chalcones | [104] |
| Flavonoids overview addressing natural chalcones | Various compounds | Review about application of phytochemical constituents | - | Blade cancer | [105] |
| Chalcones and other compounds | Various compounds | Review on bioisosterism and obtaining drugs | - | Various mechanisms | [106] |
| Coumarin-chalcone hybrids | Various compounds | Review and application of synthetic chalcones in cancer | Various mechanisms for obtaining synthetic chalcones | Various mechanisms | [107] |
| Phytochemical constituents of the Didymorcarpus wall (Gesneriaceae) | Various compounds | Review about application of phytochemical constituents | Survey of several studies and explanation of the mechanisms | Various mechanisms | [108] |
| Phytochemical constituents of the Kawa (piper methistycum) | Various compounds | Review about application of phytochemical constituents | Survey of several studies and explanation of the mechanisms | Various mechanisms | [109] |
| Flavonoids overview addressing natural chalcones | Various compounds | Review about application of phytochemical constituents | Survey of several studies and explanation of the mechanisms | Various mechanisms | [110] |
| Flavonoids overview addressing natural chalcones | Various compounds | Review article that addresses the antiviral activity for Herpes virus and a correlation with cancer pictures | Survey of several studies and explanation of the mechanisms | Antiviral activity and antitumor activity | [111] |
| Phenolic compounds of the Mous alba–natural chalcones | Various compounds | Review about application of phytochemical constituents | Survey of several studies and explanation of the mechanisms | Various mechanisms | [112] |
| Licochalcones | Various compounds | Review about application of phytochemical constituents | Survey of several studies and explanation of the mechanisms | Various mechanisms | [113] |
| Chalcone heterocycles synthesis | Various compounds | Review articles on mechanisms of action of chalcones | Survey of several studies and explanation of the mechanisms | Various mechanisms | [114] |
| Natural chalcones present in licorice–Chinese materia medica | Various compounds | Review articles on mechanisms of action of chalcones and other compounds | Survey of several studies and explanation of the mechanisms | Various mechanisms | [115] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leite, F.F.; de Sousa, N.F.; de Oliveira, B.H.M.; Duarte, G.D.; Ferreira, M.D.L.; Scotti, M.T.; Filho, J.M.B.; Rodrigues, L.C.; de Moura, R.O.; Mendonça-Junior, F.J.B.; et al. Anticancer Activity of Chalcones and Its Derivatives: Review and In Silico Studies. Molecules 2023, 28, 4009. https://doi.org/10.3390/molecules28104009
Leite FF, de Sousa NF, de Oliveira BHM, Duarte GD, Ferreira MDL, Scotti MT, Filho JMB, Rodrigues LC, de Moura RO, Mendonça-Junior FJB, et al. Anticancer Activity of Chalcones and Its Derivatives: Review and In Silico Studies. Molecules. 2023; 28(10):4009. https://doi.org/10.3390/molecules28104009
Chicago/Turabian StyleLeite, Fernando Ferreira, Natália Ferreira de Sousa, Bruno Hanrry Melo de Oliveira, Gabrielly Diniz Duarte, Maria Denise Leite Ferreira, Marcus Tullius Scotti, José Maria Barbosa Filho, Luís Cezar Rodrigues, Ricardo Olímpio de Moura, Francisco Jaime Bezerra Mendonça-Junior, and et al. 2023. "Anticancer Activity of Chalcones and Its Derivatives: Review and In Silico Studies" Molecules 28, no. 10: 4009. https://doi.org/10.3390/molecules28104009