Design, Synthesis and Anticancer Evaluation of Nitroimidazole Radiosensitisers
Abstract
:1. Introduction
2. Results
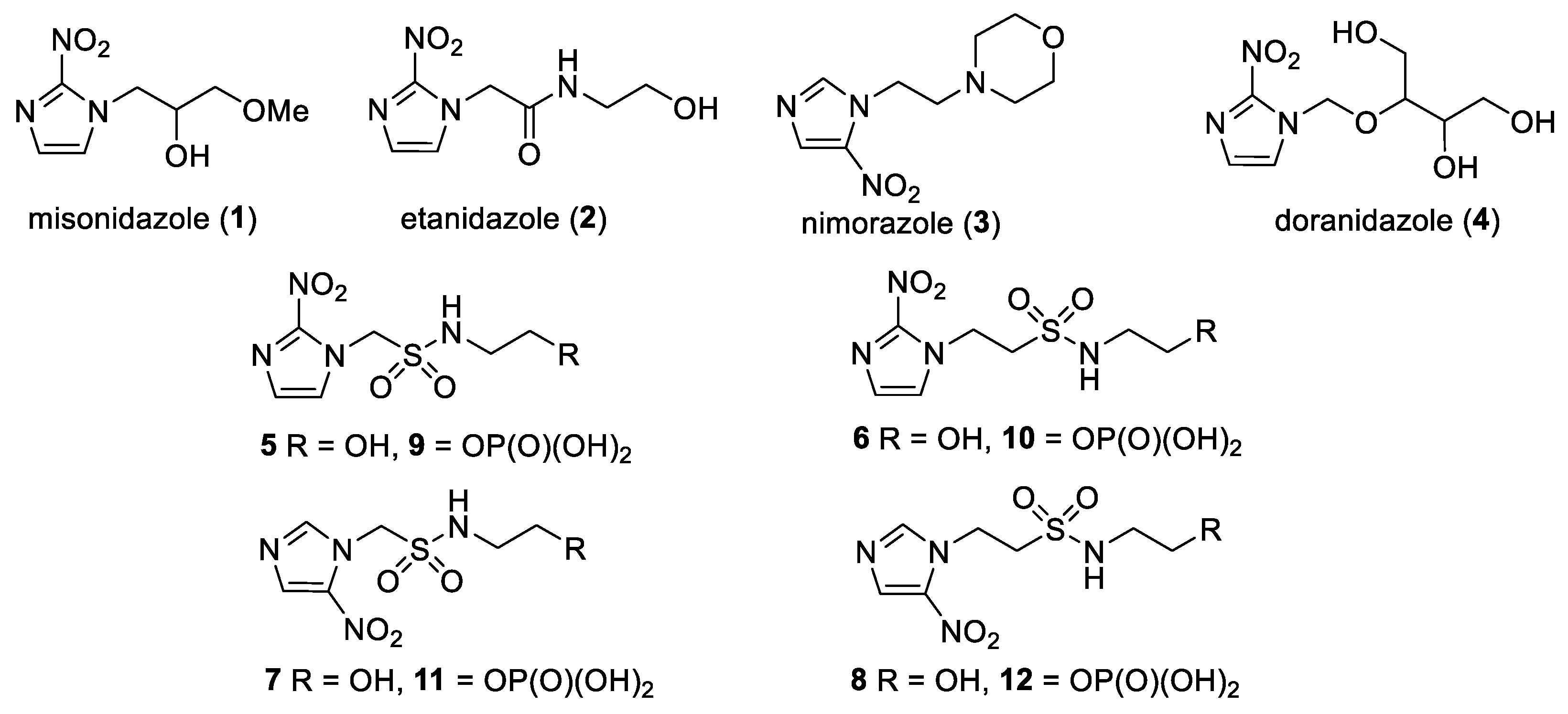
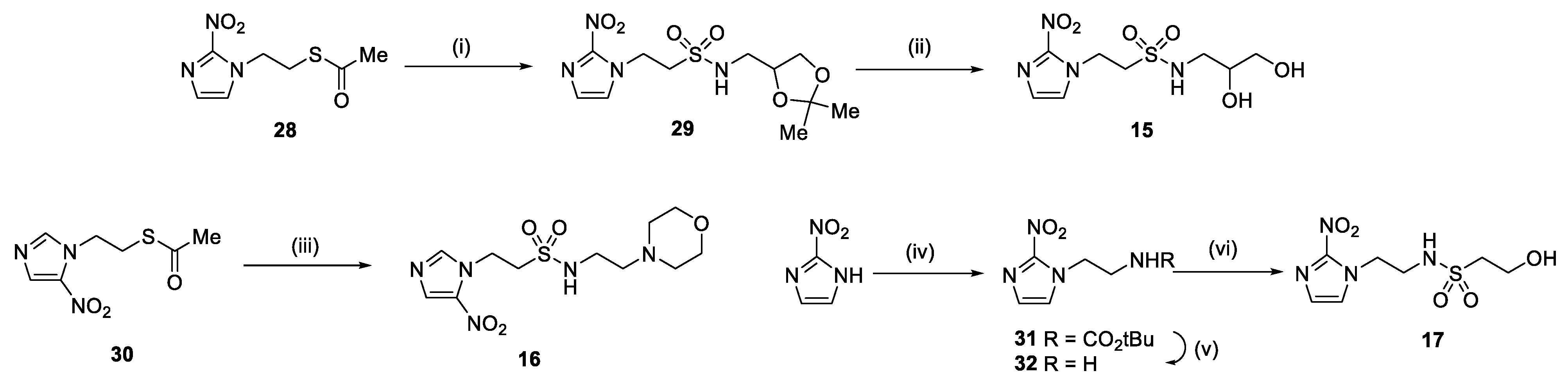
2.1. Synthesis
2.2. Physicochemical Properties
2.3. In Vitro Cytotoxicity
2.4. In Vitro Radiosensitisation
2.5. In Vivo Pharmacokinetics
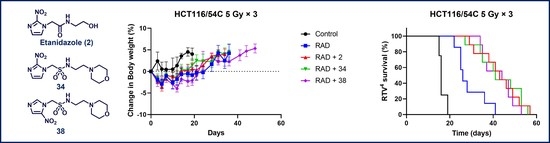
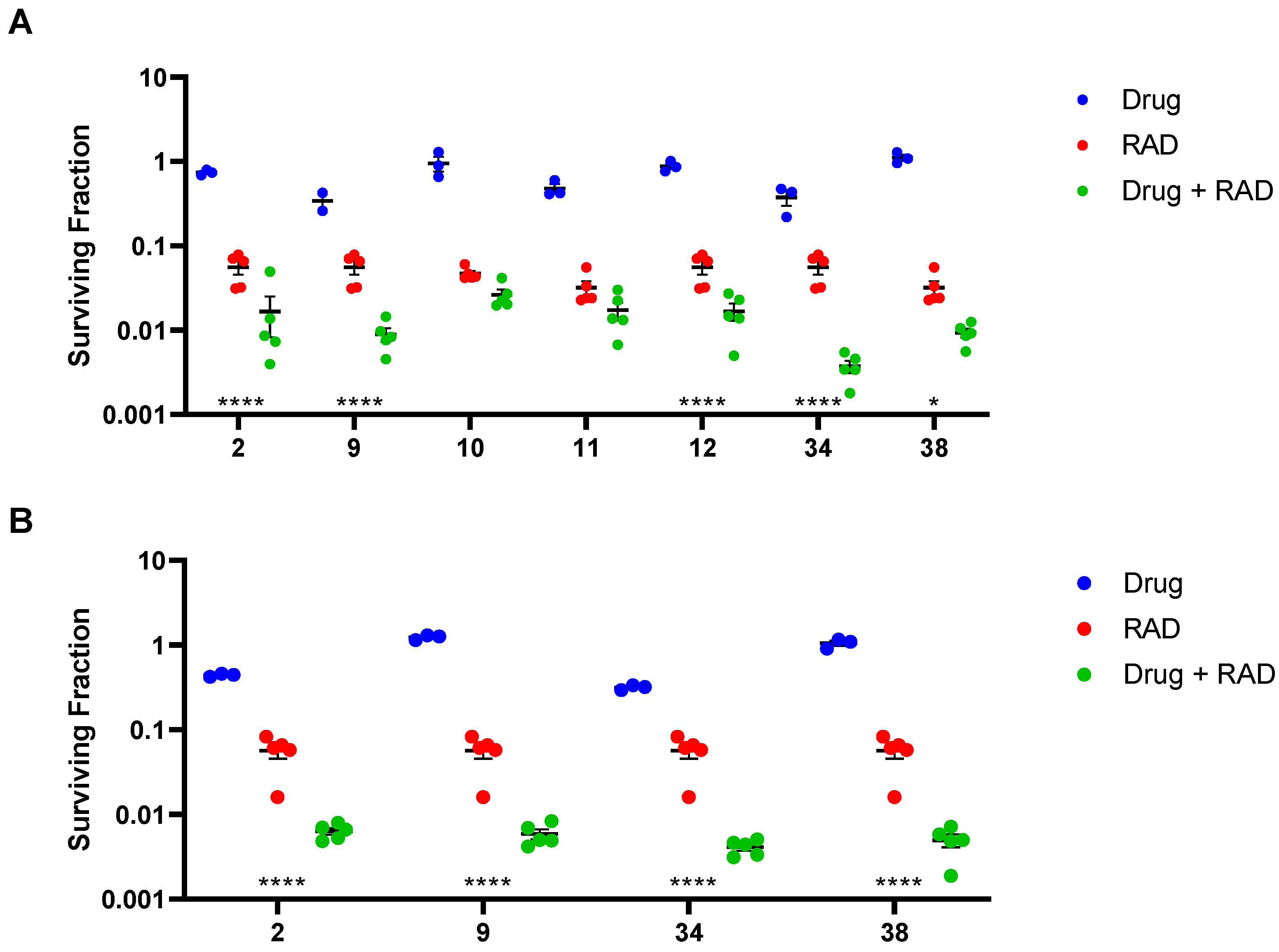
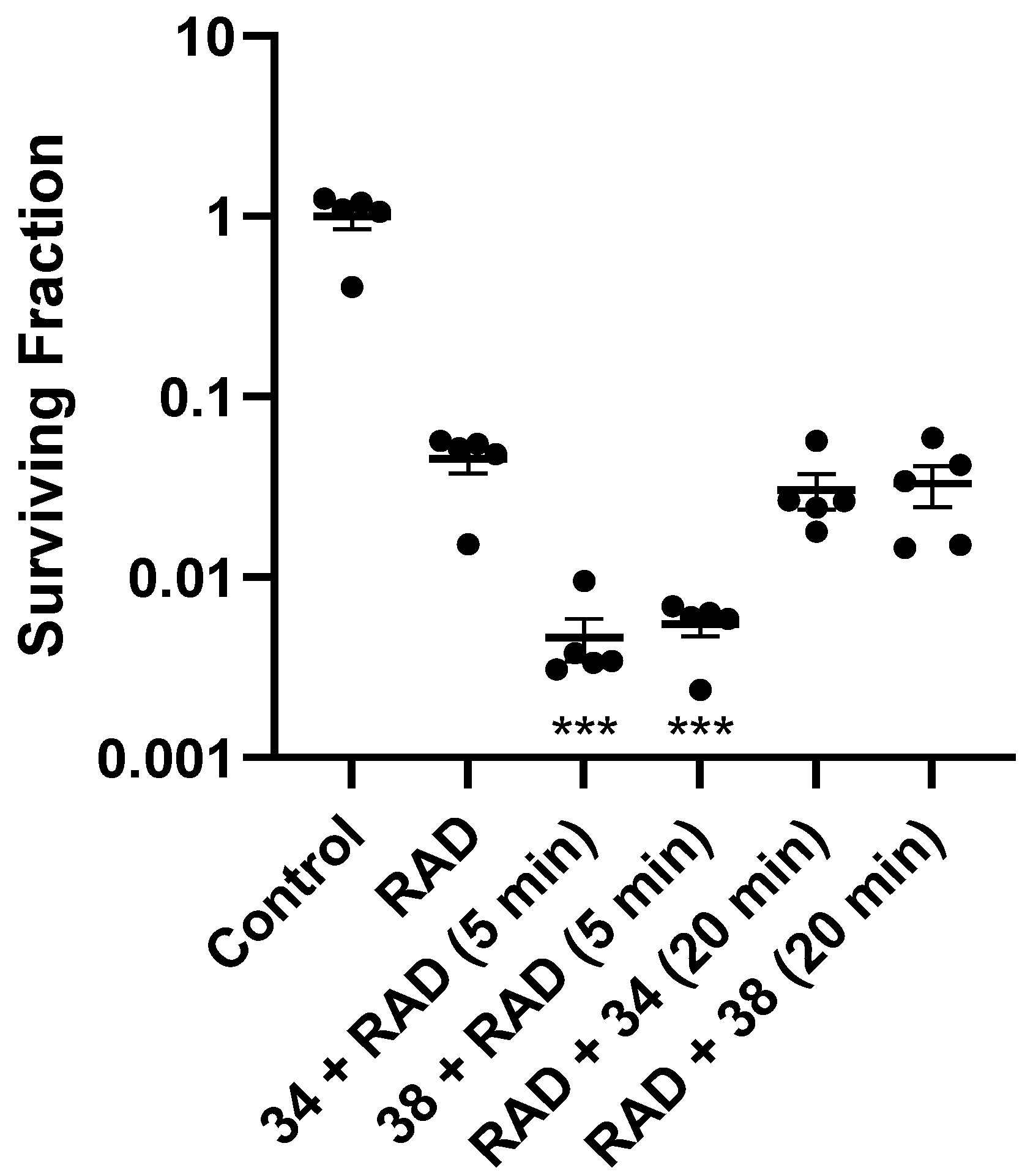
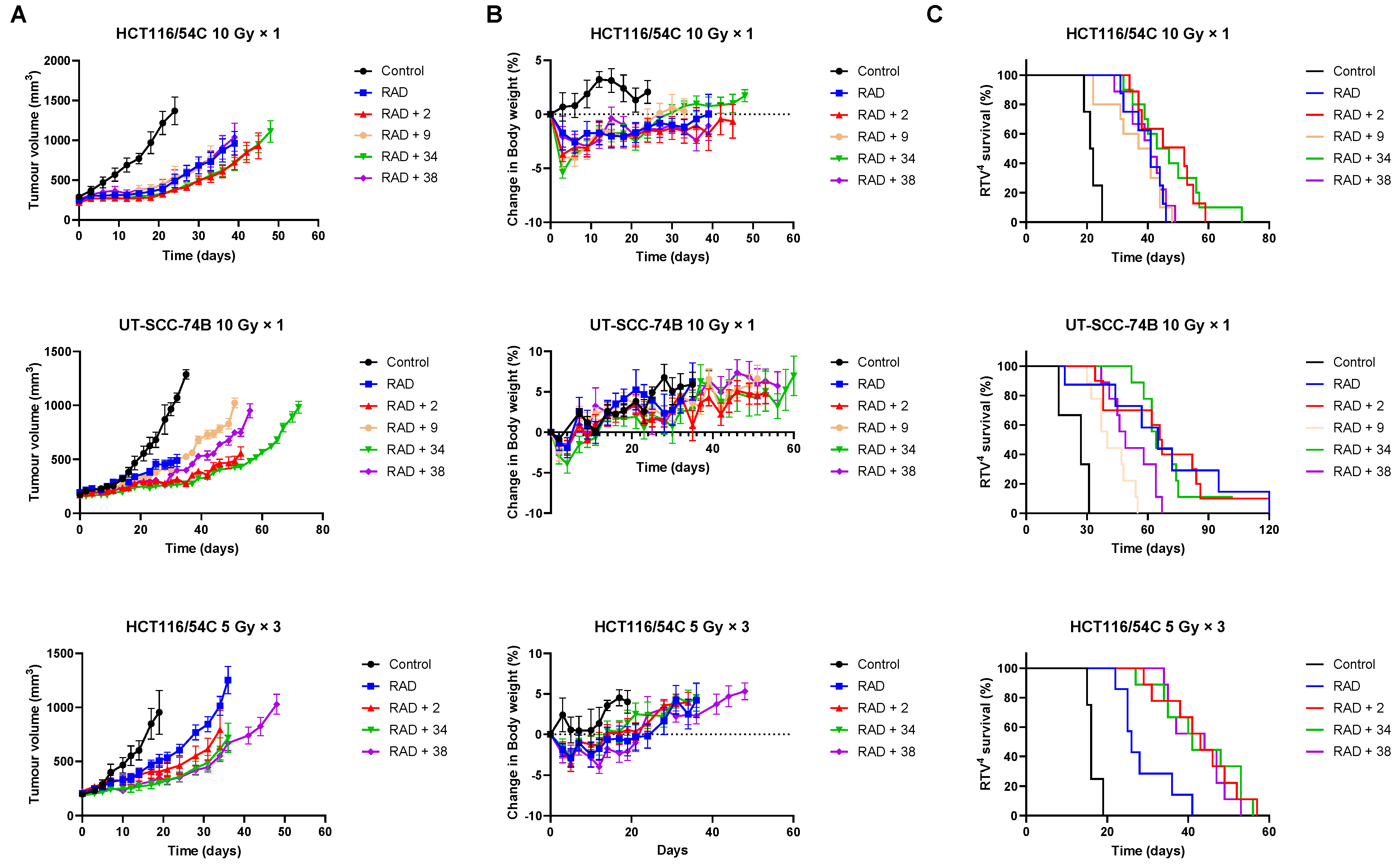
2.6. In Vivo Radiosensitisation
2.7. Inhibition of Tumour Growth
3. Discussion
4. Materials and Methods
4.1. Compounds
4.1.1. General Information
4.1.2. Compound Synthesis
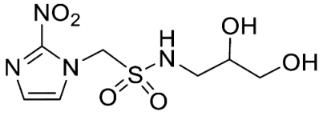
- Synthesis of N-(2,3-Dihydroxypropyl)-1-(2-nitro-1H-imidazol-1-yl)methanesulfonamide (13) (Scheme 1).
- 1-Chloro-N-((2,2-dimethyl-1,3-dioxolan-4-yl)methyl)methanesulfonamide (18).
- N-((2,2-Dimethyl-1,3-dioxolan-4-yl)methyl)-1-(2-nitro-1H-imidazol-1-yl)methanesulfonamide (19).
- N-(2,3-Dihydroxypropyl)-1-(2-nitro-1H-imidazol-1-yl)methanesulfonamide (13).
- Synthesis of 1-(2-Nitro-1H-imidazol-1-yl)-N-(1,3,4-trihydroxybutan-2-yl)methanesulfonamide (14) (Scheme 1).

- cis-2-Butene-1,4-di(tert-butyldimethylsilyl) ether (20) [37].
- 2,3-Bis(((tert-butyldimethylsilyl)oxy)methyl)oxirane (21) [37].
- 2-Azido 1,3,4-tri-((tert-butyldimethylsilyl)oxy)butane (22).
- 1,3,4-Tri-((tert-butyldimethylsilyl)oxy)butan-2-amine (25).
- 1-Bromo-N-(1,3,4-tri-((tert-butyldimethylsilyl)oxy)butan-2-yl)methanesulfonamide (26).
- 1-(2-Nitro-1H-imidazol-1-yl)-N-(1,3,4-tri-((tert-butyldimethylsilyl)oxy)butan-2-yl)methanesulfonamide (27).
- 1-(2-Nitro-1H-imidazol-1-yl)-N-(1,3,4-trihydroxybutan-2-yl)methanesulfonamide (14).
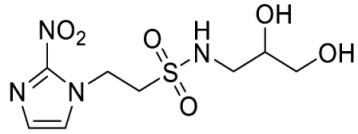
- Synthesis of N-(2,3-Dihydroxypropyl)-2-(2-nitro-1H-imidazol-1-yl)ethane-1-sulfonamide (15) (Scheme 2).
- N-((2,2-Dimethyl-1,3-dioxolan-4-yl)methyl)-2-(2-nitro-1H-imidazol-1-yl)ethane-1-sulfonamide (29).
- N-(2,3-Dihydroxypropyl)-2-(2-nitro-1H-imidazol-1-yl)ethane-1-sulfonamide (15).
- Synthesis of N-(2-Morpholinoethyl)-2-(5-nitro-1H-imidazol-1-yl)ethane-1-sulfonamide (16) (Scheme 2).

- tert-Butyl (2-(2-nitro-1H-imidazol-1-yl)ethyl)carbamate (31).
- 2-(2-Nitro-1H-imidazol-1-yl)ethan-1-amine (32).
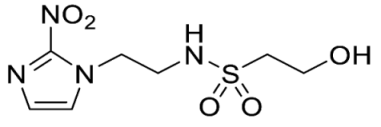
- 2-Hydroxy-N-(2-(2-nitro-1H-imidazol-1-yl)ethyl)ethane-1-sulfonamide (17).
4.2. Assays
4.2.1. Solubility and Stability
4.2.2. In Vitro Cytotoxicity Testing
4.2.3. In Vitro Radiosensitisation Testing
4.3. Animal Experiments
4.3.1. Pharmacokinetics
4.3.2. Tumour Radiosensitisation: Ex Vivo Clonogenic Assay
4.3.3. Tumour Radiosensitisation: Tumour Growth Inhibition
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
References
- Delaney, G.; Jacob, S.; Featherstone, C.; Barton, M. The role of radiotherapy in cancer treatment: Estimating optimal utilization from a review of evidence-based clinical guidelines. Cancer 2005, 104, 1129–1137. [Google Scholar] [CrossRef]
- Moding, E.J.; Kastan, M.B.; Kirsch, D.G. Strategies for optimizing the response of cancer and normal tissues to radiation. Nat. Rev. Drug Discov. 2013, 12, 526–542. [Google Scholar] [CrossRef] [PubMed]
- Pignon, J.P.; Bourhis, J.; Domenge, C.; Designe, L. Chemotherapy added to locoregional treatment for head and neck squamous-cell carcinoma: Three meta-analyses of updated individual data. MACH-NC Collaborative Group. Meta-Analysis of Chemotherapy on Head and Neck Cancer. Lancet 2000, 355, 949–955. [Google Scholar] [PubMed]
- Bentzen, S.M.; Trotti, A. Evaluation of early and late toxicities in chemoradiation trials. J. Clin. Oncol. 2007, 25, 4096–4103. [Google Scholar] [PubMed]
- Harrington, K.J.; Billingham, L.J.; Brunner, T.B.; Chan, C.S.; Hoskin, P.; Mackay, R.I.; Maughan, T.S.; J Macdougall, J.; McKenna, W.G.; Nutting, C.M.; et al. Guidelines for preclinical and early phase clinical assessment of novel radiosensitisers. Br. J. Cancer 2011, 105, 628–639. [Google Scholar] [CrossRef]
- Lawrence, Y.R.; Vikram, B.; Dignam, J.J.; Chakravarti, A.; Machtay, M.; Freidlin, B.; Takebe, N.; Curran, W.J., Jr.; Bentzen, S.M.; Okunieff, P.; et al. NCI-RTOG translational program strategic guidelines for the early-stage development of radiosensitizers. J. Natl. Cancer Inst. 2013, 105, 11–24. [Google Scholar] [CrossRef]
- Sharma, R.A.; Plummer, R.; Stock, J.K.; Greenhalgh, T.A.; Ataman, O.; Kelly, S.; Clay, R.; Adams, R.A.; Baird, R.D.; Billingham, L.; et al. Clinical development of new drug-radiotherapy combinations. Nat. Rev. Clin. Oncol. 2016, 13, 627–642. [Google Scholar]
- Vaupel, P.; Mayer, A. Hypoxia in cancer: Significance and impact on clinical outcome. Cancer Metastasis Rev. 2007, 26, 225–239. [Google Scholar]
- Dhani, N.; Fyles, A.; Hedley, D.; Milosevic, M. The clinical significance of hypoxia in human cancers. Semin. Nucl. Med. 2015, 45, 110–121. [Google Scholar] [CrossRef]
- Dewhirst, M.W.; Cao, Y.; Moeller, B. Cycling hypoxia and free radicals regulate angiogenesis and radiotherapy response. Nat. Rev. Cancer 2008, 8, 425–437. [Google Scholar] [CrossRef]
- Wardman, P. Chemical radiosensitizers for use in radiotherapy. Clin. Oncol. 2007, 19, 397–417. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, V.; Hoey, C.; Liu, L.Y.; Lalonde, E.; Ray, J.; Livingstone, J.; Lesurf, R.; Shiah, Y.J.; Vujcic, T.; Huang, X.; et al. Molecular landmarks of tumor hypoxia across cancer types. Nat. Genet. 2019, 51, 308–318. [Google Scholar] [CrossRef]
- Nordsmark, M.; Overgaard, M.; Overgaard, J. Pretreatment oxygenation predicts radiation response in advanced squamous cell carcinoma of the head and neck. Radiother. Oncol. 1996, 41, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Brizel, D.M.; Sibley, G.S.; Prosnitz, L.R.; Scher, R.L.; Dewhirst, M.W. Tumor hypoxia adversely affects the prognosis of carcinoma of the head and neck. Int. J. Radiat. Oncol. Biol. Phys. 1997, 38, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Brizel, D.M.; Dodge, R.K.; Clough, R.W.; Dewhirst, M.W. Oxygenation of head and neck cancer: Changes during radiotherapy and impact on treatment outcome. Radiother. Oncol. 1999, 53, 113–117. [Google Scholar] [CrossRef]
- Stadler, P.; Becker, A.; Feldmann, H.J.; Hansgen, G.; Dunst, J.; Wurschmidt, F.; Molls, M. Influence of the hypoxic subvolume on the survival of patients with head and neck cancer. Int. J. Radiat. Oncol. Biol. Phys. 1999, 44, 749–754. [Google Scholar] [CrossRef]
- Adams, G.E.; Dische, S.; Fowler, J.F.; Thomlinson, R.H. Hypoxic cell sensitisers in radiotherapy. Lancet 1976, 1, 186–188. [Google Scholar] [CrossRef]
- Overgaard, J. Clinical evaluation of nitroimidazoles as modifiers of hypoxia in solid tumors. Oncol. Res. 1994, 6, 509–518. [Google Scholar]
- Saunders, M.; Dische, S. Clinical results of hypoxic cell radiosensitisation from hyperbaric oxygen to accelerated radiotherapy, carbogen and nicotinamide. Br. J. Cancer 1996, 27, S271–S278. [Google Scholar]
- Grigsby, P.W.; Winter, K.; Wasserman, T.H.; Marcial, V.; Rotman, M.; Cooper, J.; Keys, H.; Asbell, S.O.; Phillips, T.L. Irradiation with or without misonidazole for patients with stages IIIB and IVA carcinoma of the cervix: Final results of RTOG 80-05. Radiation Therapy Oncology Group. Int. J. Radiat. Oncol. Biol. Phys. 1999, 44, 513–517. [Google Scholar] [CrossRef]
- Brown, J.M.; Yu, N.Y.; Brown, D.M.; Lee, W.W. SR-2508: A 2-nitroimidazole amide which should be superior to misonidazole as a radiosensitizer for clinical use. Int. J. Radiat. Oncol. Biol. Phys. 1981, 7, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.J.; Cosmatos, D.; Marcial, V.A.; Fu, K.K.; Rotman, M.; Cooper, J.S.; Ortiz, H.G.; Beitler, J.J.; Abrams, R.A.; Curran, W.J. Results of an RTOG phase III trial (RTOG 85-27) comparing radiotherapy plus etanidazole with radiotherapy alone for locally advanced head and neck carcinomas. Int. J. Radiat. Oncol. Biol. Phys. 1995, 32, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Eschwege, F.; Sancho-Garnier, H.; Chassagne, D.; Brisgand, D.; Guerra, M.; Malaise, E.P.; Bey, P.; Busutti, L.; Cionini, L.; N’Guyen, T.; et al. Results of a European randomized trial of etanidazole combined with radiotherapy in head and neck carcinomas. Int. J. Radiat. Oncol. Biol. Phys. 1997, 39, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Overgaard, J.; Overgaard, M.; Nielsen, O.S.; Pedersen, A.K.; Timothy, A.R. A comparative investigation of nimorazole and misonidazole as hypoxic radiosensitizers in a C3H mammary carcinoma in vivo. Br. J. Cancer 1982, 46, 904–911. [Google Scholar] [CrossRef] [PubMed]
- Overgaard, J.; Hansen, H.S.; Overgaard, M.; Bastholt, L.; Berthelsen, A.; Specht, L.; Lindelov, B.; Jorgensen, K.A. A randomized double-blind phase III study of nimorazole as a hypoxic radiosensitizer of primary radiotherapy in supraglottic larynx and pharynx carcinoma. Results of the Danish Head and Neck Cancer Study (DAHANCA) Protocol 5-85. Radiother. Oncol. 1998, 46, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Hassan Metwally, M.A.; Jansen, J.A.; Overgaard, J. Study of the population pharmacokinetic characteristics of nimorazole in head and neck cancer patients treated in the DAHANCA-5 trial. Clin. Oncol. (R. Coll. Radiol.) 2015, 27, 168–175. [Google Scholar] [CrossRef]
- Adams, G.E.; Flockhart, I.R.; Smithen, C.E.; Stratford, I.J.; Wardman, P.; Watts, M.E. Electron-affinic sensitization. VII. A correlation between structures, one-electron reduction potentials, and efficiencies of nitroimidazoles as hypoxic cell radiosensitizers. Radiat. Res. 1976, 67, 9–20. [Google Scholar] [CrossRef]
- Adams, G.E.; Clarke, E.D.; Flockhart, I.R.; Jacobs, R.S.; Sehmi, D.S.; Stratford, I.J.; Wardman, P.; Watts, M.E.; Parrick, J.; Wallace, R.G.; et al. Structure-activity relationships in the development of hypoxic cell radiosensitizers. I. Sensitization efficiency. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1979, 35, 133–150. [Google Scholar] [CrossRef]
- Adams, G.E.; Clarke, E.D.; Gray, P.; Jacobs, R.S.; Stratford, I.J.; Wardman, P.; Watts, M.E.; Parrick, J.; Wallace, R.G.; Smithen, C.E. Structure-activity relationships in the development of hypoxic cell radiosensitizers. II. Cytotoxicity and therapeutic ratio. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1979, 35, 151–160. [Google Scholar] [CrossRef]
- Nishimura, Y.; Nakagawa, K.; Takeda, K.; Tanaka, M.; Segawa, Y.; Tsujino, K.; Negoro, S.; Fuwa, N.; Hida, T.; Kawahara, M.; et al. Phase I/II trial of sequential chemoradiotherapy using a novel hypoxic cell radiosensitizer, doranidazole (PR-350), in patients with locally advanced non-small-cell lung Cancer (WJTOG-0002). Int. J. Radiat. Oncol. Biol. Phys. 2007, 6, 786–792. [Google Scholar] [CrossRef]
- Sunamura, M.; Karasawa, K.; Okamoto, A.; Ogata, Y.; Nemoto, K.; Hosotani, R.; Nishimura, Y.; Matsui, K.; Matsuno, S. Phase III trial of radiosensitizer PR-350 combined with intraoperative radiotherapy for the treatment of locally advanced pancreatic cancer. Pancreas 2004, 28, 330–334. [Google Scholar] [CrossRef] [PubMed]
- Karasawa, K.; Sunamura, M.; Okamoto, A.; Nemoto, K.; Matsuno, S.; Nishimura, Y.; Shibamoto, Y. Efficacy of novel hypoxic cell sensitiser doranidazole in the treatment of locally advanced pancreatic cancer: Long-term results of a placebo-controlled randomised study. Radiother. Oncol. 2008, 87, 326–330. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, M.; Hong, C.R.; Gu, Y.; Anderson, R.F.; Wilson, W.R.; Pruijn, F.B.; Wang, J.; Hicks, K.O.; Hay, M.P. Novel nitroimidazole alkylsulfonamides as hypoxic cell radiosensitisers. Bioorg. Med. Chem. 2014, 22, 2123–2132. [Google Scholar] [CrossRef]
- Bonnet, M.; Hong, C.R.; Wong, W.W.; Liew, L.P.; Shome, A.; Wang, J.; Gu, Y.; Stevenson, R.J.; Qi, W.; Anderson, R.F.; et al. Next-generation hypoxic cell radiosensitizers: Nitroimidazole alkylsulfonamides. J. Med. Chem. 2018, 61, 1241–1254. [Google Scholar] [CrossRef]
- Murayama, C.; Suzuki, A.; Suzuki, T.; Miyata, Y.; Sakaguchi, M.; Tanabe, Y.; Tanaka, N.; Mori, T. Radiosensitization by a new nucleoside analogue: 1-[2-hydroxy-1-(hydroxymethyl)ethoxy]methyl-2-nitroimidazole (RP-170). Int. J. Radiat. Oncol. Biol. Phys. 1989, 17, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Murayama, C.; Suzuki, A.; Sato, C.; Tanabe, Y.; Shoji, T.; Miyata, Y.; Nishio, A.; Suzuki, T.; Sakaguchi, M.; Mori, T. Radiosensitization by a new potent nucleoside analog: 1-(1′,3′,4′-trihydroxy-2′-butoxy)methyl-2-nitroimidazole(RP-343). Int. J. Radiat. Oncol. Biol. Phys. 1993, 26, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Tran, V.T.; Woerpel, K.A. Nucleophilic addition to silyl-protected five-membered ring oxocarbenium ions governed by stereoelectronic effects. J. Org. Chem. 2013, 78, 6609–6621. [Google Scholar] [CrossRef] [PubMed]
- Veisi, H.; Ghorbani-Vaghei, R.; Hemmati, S.; Mahmoodi, J. Convenient one-pot synthesis of sulfonamides and sulfonyl azides from thiols using N-chlorosuccinimide. Synlett 2011, 16, 2315–2320. [Google Scholar] [CrossRef]
- Oya, N.; Shibamoto, Y.; Sasai, K.; Shibata, T.; Murata, R.; Takagi, T.; Iwai, H.; Suzuki, T.; Abe, M. Optical isomers of a new 2-nitroimidazole nucleoside analog (PR-350 series): Radiosensitization efficiency and toxicity. Int. J. Radiat. Oncol. Biol. Phys. 1995, 33, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Kallman, R.F. The phenomenon of reoxygenation and its implications for fractionated radiotherapy. Radiology 1972, 105, 135–142. [Google Scholar] [CrossRef]
- Hill, R.P. Sensitizers and radiation dose fractionation: Results and interpretations. Int. J. Radiat. Oncol. Biol. Phys. 1986, 12, 1049–1054. [Google Scholar] [CrossRef] [PubMed]
- Kallman, R.F.; Dorie, M.J. Tumor oxygenation and reoxygenation during radiation therapy: Their importance in predicting tumor response. Int. J. Radiat. Oncol. Biol. Phys. 1986, 12, 681–685. [Google Scholar] [CrossRef] [PubMed]
- Coleman, C.N.; Urtasun, R.C.; Wasserman, T.H.; Hancock, S.; Harris, J.W.; Haley, J.; Hirst, V.K. Initial report of the phase I trial of the hypoxic cell radiosensitiser SR2508. Int. J. Radiat. Oncol. Biol. Phys. 1984, 10, 1749–1753. [Google Scholar] [CrossRef]
- Coleman, C.N.; Halsey, J.; Cox, R.S.; Hirst, V.K.; Blaschke, T.; Howes, A.E.; Wasserman, T.H.; Urtasun, R.C.; Pajak, T.; Hancock, S.; et al. Relationship between the neurotoxicity of the hypoxic cell radiosensitizer SR 2508 and the pharmacokinetic profile. Cancer Res. 1987, 47, 319–322. [Google Scholar] [PubMed]
- Lo, S.S.; Fakiris, A.J.; Teh, B.S.; Cardenes, H.R.; Henderson, M.A.; Forquer, J.A.; Papiez, L.; McGarry, R.C.; Wang, J.Z.; Li, K.; et al. Stereotactic body radiation therapy for oligometastases. Expert. Rev. Anticancer Ther. 2009, 9, 621–635. [Google Scholar] [CrossRef]
- Lo, S.S.; Fakiris, A.J.; Chang, E.L.; Mayr, N.A.; Wang, J.Z.; Papiez, L.; Teh, B.S.; McGarry, R.C.; Cardenes, H.R.; Timmerman, R.D. Stereotactic body radiation therapy: A novel treatment modality. Nat. Rev. Clin. Oncol. 2010, 7, 44–54. [Google Scholar] [CrossRef]
- Dahele, M.; Senan, S. The role of stereotactic ablative radiotherapy for early-stage and oligometastatic non-small cell lung cancer: Evidence for changing paradigms. Cancer Res. Treat. 2011, 43, 75–82. [Google Scholar] [CrossRef]
- Tree, A.C.; Khoo, V.S.; Eeles, R.A.; Ahmed, M.; Dearnaley, D.P.; Hawkins, M.A.; Huddart, R.A.; Nutting, C.M.; Ostler, P.J.; Van As, N.J. Stereotactic body radiotherapy for oligometastases. Lancet Oncol. 2013, 14, e28–e37. [Google Scholar] [CrossRef]
- Lim, C.M.; Clump, D.A.; Heron, D.E.; Ferris, R.L. Stereotactic Body Radiotherapy (SBRT) for primary and recurrent head and neck tumors. Oral. Oncol. 2013, 49, 401–406. [Google Scholar] [CrossRef]
- Siddiqui, F.; Raben, D.; Lu, J.J.; Grecula, J.C.; Lo, S.S.; Huang, Z.; Mayr, N.A.; Teh, B.S.; Yao, M. Emerging applications of stereotactic body radiation therapy for head and neck cancer. Expert. Rev. Anticancer Ther. 2011, 11, 1429–1436. [Google Scholar] [CrossRef]
- Amini, A.; McDermott, J.D.; Gan, G.; Bhatia, S.; Sumner, W.; Fisher, C.M.; Jimeno, A.; Bowles, D.W.; Raben, D.; Karam, S.D. Stereotactic body radiotherapy as primary therapy for head and neck cancer in the elderly or patients with poor performance. Front. Oncol. 2014, 4, 274. [Google Scholar] [CrossRef]
- Brown, J.M.; Diehn, M.; Loo, B.W. Stereotactic ablative radiotherapy should be combined with a hypoxic cell radiosensitizer. Int. J. Radiat. Oncol. Biol. Phys. 2010, 78, 323–327. [Google Scholar] [CrossRef]
- Carlson, D.J.; Keall, P.J.; Loo, B.W., Jr.; Chen, Z.J.; Brown, J.M. Hypofractionation results in reduced tumor cell kill compared to conventional fractionation for tumors with regions of hypoxia. Int. J. Radiat. Oncol. Biol. Phys. 2011, 79, 1188–1195. [Google Scholar] [CrossRef] [PubMed]
- Wittenborn, T.R.; Horsman, M.R. Targeting tumour hypoxia to improve outcome of stereotactic radiotherapy. Acta Oncol. 2015, 54, 1385–1392. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.M.; Carlson, D.J.; Brenner, D.J. The tumor radiobiology of SRS and SBRT: Are more than the 5 Rs involved? Int. J. Radiat. Oncol. Biol. Phys. 2014, 88, 254–262. [Google Scholar] [CrossRef]
- Adams, G.E. Hypoxia-mediated drugs for radiation and chemotherapy. Cancer 1981, 48, 696–707. [Google Scholar] [CrossRef]
- Fowler, J.F. Eighth annual Juan del Regato lecture. Chemical modifiers of radiosensitivity--theory and reality: A review. Int. J. Radiat. Oncol. Biol. Phys. 1985, 11, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.M. Selective radiosensitization of the hypoxic cells of mouse tumors with the nitroimidazoles metronidazole and Ro 7-0582. Radiat. Res. 1975, 64, 633–647. [Google Scholar] [CrossRef]
- Sheldon, P.W.; Foster, J.L.; Fowler, J.F. Radiosensitization of C3H mouse mammary tumours by a 2-nitroimidazole drug. Br. J. Cancer 1974, 30, 560–565. [Google Scholar] [CrossRef]
- Jamieson, S.M.F.; Tsai, P.; Kondratyev, M.K.; Budhani, P.; Liu, A.; Senzer, N.N.; Chiorean, E.G.; Jalal, S.I.; Nemunaitis, J.J.; Kee, D.; et al. Evofosfamide for the treatment of human papillomavirus-negative head and neck squamous cell carcinoma. JCI Insight 2023, 8, e169136. [Google Scholar] [CrossRef]
- Hunter, F.W.; Wang, J.; Patel, R.; Hsu, H.L.; Hickey, A.J.; Hay, M.P.; Wilson, W.R. Homologous recombination repair-dependent cytotoxicity of the benzotriazine di-N-oxide CEN-209: Comparison with other hypoxia-activated prodrugs. Biochem. Pharmacol. 2012, 83, 574–585. [Google Scholar] [CrossRef]
- Cross, P.; Marshall, E.S.; Baguley, B.C.; Finlay, G.J.; Matthews, J.H.L.; Wilson, W.R. Proliferative assays for the assessment of radiosensitivity of tumor cell lines using 96-well microcultures. Radiat. Oncol. Investig. 1994, 1, 261–269. [Google Scholar] [CrossRef]
- Hong, C.R.; Wang, J.; Hicks, K.O.; Hay, M.P. Efficient protocol for the identification of hypoxic cell radiosensitisers. Adv. Exp. Med. Biol. 2016, 899, 269–290. [Google Scholar] [PubMed]
- Patel, K.; Lewiston, D.; Gu, Y.; Hicks, K.O.; Wilson, W.R. Analysis of the hypoxia-activated dinitrobenzamide mustard phosphate prodrug PR-104 and its alcohol metabolite PR-104A in plasma and tissues by liquid chromatography-mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2007, 856, 302–311. [Google Scholar] [CrossRef]
- Gu, Y.; Wilson, W.R. Rapid and sensitive ultra-high-pressure liquid chromatography-tandem mass spectrometry analysis of the novel anticancer agent PR-104 and its major metabolites in human plasma: Application to a pharmacokinetic study. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2009, 877, 3181–3186. [Google Scholar] [CrossRef] [PubMed]
- Hicks, K.O.; Pruijn, F.B.; Secomb, T.W.; Hay, M.P.; Hsu, R.; Brown, J.M.; Denny, W.A.; Dewhirst, M.W.; Wilson, W.R. Use of three-dimensional tissue cultures to model extravascular transport and predict in vivo activity of hypoxia-targeted anticancer drugs. J. Natl. Cancer Inst. 2006, 98, 1118–1128. [Google Scholar] [CrossRef]

| No. | Structure | Purity % | Solubility 1 (mM) | % Stability in αMEM |
|---|---|---|---|---|
| 13 | 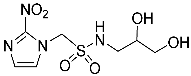 | 99.6 | 110 | 99.3 |
| 14 | 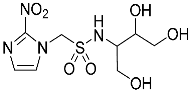 | 98.9 | 95.8 | 98.5 |
| 15 | 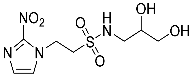 | 99.3 | 84.3 | 99.9 |
| 16 | 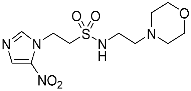 | 99.8 | 83.05 | 99.9 |
| 17 | 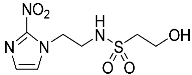 | 98.4 | >101.2 | 100.0 |
| No. | Structure | HCT116/54C | FaDu | UT-SCC-74B | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| IC50 Oxic (mM) | IC50 Anoxic (mM) | HCR | IC50 Oxic (mM) | IC50 Anoxic (mM) | HCR | IC50 Oxic (mM) | IC50 Anoxic (mM) | HCR | ||
| 1 | 1 | >25 | 1.5 ± 0.3 | >16.8 | >20 | 1.3 ± 0.2 | >15.4 | >25 | 2.1 ± 0.6 | >11.9 |
| 2 | 2 | >25 | 1.7 ± 0.2 | >14.8 | >20 | 1.5 ± 0.3 | >13.3 | - | - | - |
| 3 | 3 | >25 | >20 | - | >20 | 13.2 ± 3.4 | >1.5 | - | - | - |
| 5 |  | >6.2 | 0.29 ± 0.02 | >21 | 2.3 ± 0.2 | 0.23 ± 0.01 | 10.0 | 4.3 ± 0.8 | 0.24 ± 0.04 | 17.7 |
| 6 | 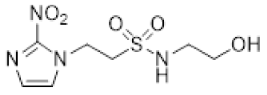 | >16 | 2.2 ± 0.04 | >7.3 | 17.8 ± 2.2 | 1.2 ± 0.2 | 14.9 | >20 | 3.0 ± 0.6 | >6.6 |
| 7 | 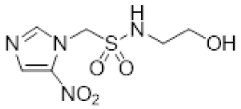 | 4.6 ± 0.3 | 1.4 ± 0.1 | 3.3 | 5.4 ± 0.7 | 1.4 ± 0.02 | 4.0 | 4.2 ± 0.9 | 1.3 ± 0.04 | 3.3 |
| 8 | 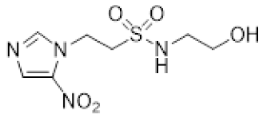 | >22 | 9.0 ± 0.9 | >2.4 | >24 | 6.6 ± 0.6 | >3.6 | >22 | 10.9 ± 1.3 | >2.0 |
| 13 | 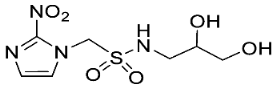 | 14.5 ± 2.9 | 4.1 ± 0.1 | 3.5 | 8.7 ± 1.1 | 4.3 ± 0.3 | 2.0 | 10.3 ± 1.7 | 2.6 ± 0.1 | 4.0 |
| 14 | 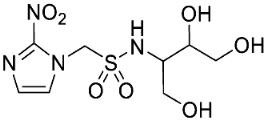 | 16.4 ± 3.0 | 12.6 ± 0.5 | 1.3 | 5.3 ± 1.1 | 7.9 ± 1.1 | 0.67 | - | - | - |
| 15 |  | >25 | 12.8 ± 4.4 | >1.9 | >25 | 7.3 ± 0.9 | >3.4 | - | - | - |
| 16 | 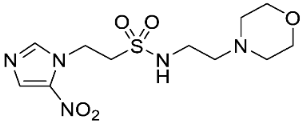 | 12.8 ± 1.5 | 12.9 ± 0.9 | 1.0 | >16 | 6.1 ± 0.8 | >2.6 | 14.1 ± 2.1 | 10.3 ± 2.6 | 1.4 |
| 17 | 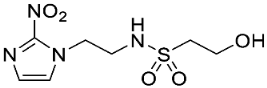 | >17 | 3.2 ± 0.2 | >5.3 | >18 | 2.8 ± 0.6 | >6.4 | >20 | 2.9 ± 0.5 | >6.8 |
| 33 | 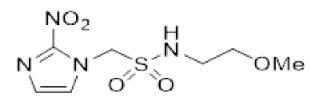 | >6.2 | 0.12 ± 0.002 | >51 | 4.0 ± 1.2 | 0.09 ± 0.01 | 46.6 | 3.7 ± 0.8 | 0.19 ± 0.02 | 19.9 |
| 34 | 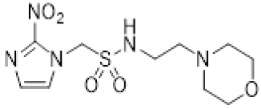 | 6.4 ± 1.0 | 0.17 ± 0.01 | 38.8 | 3.7 ± 0.1 | 0.16 ± 0.01 | 23.3 | 12.6 ± 1.8 | 0.51 ± 0.08 | 24.5 |
| 35 | 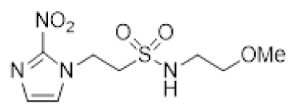 | >16 | 0.97 ± 0.16 | >16 | 16.5 ± 1.6 | 0.81 ± 0.12 | 20.4 | - | - | - |
| 36 | 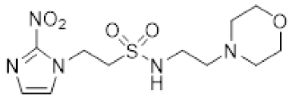 | 9.2 ± 1.1 | 0.71 ± 0.13 | 12.9 | 7.3 ± 0.6 | 0.69 ± 0.04 | 10.6 | 11.9 ± 1.5 | 1.6 ± 0.3 | 7.6 |
| 37 | 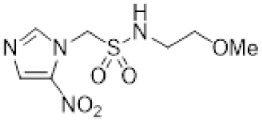 | 11.4 ± 1.0 | 4.0 ± 0.7 | 2.9 | 12.0 ± 1.9 | 2.1 ± 0.04 | 5.7 | - | - | - |
| 38 | 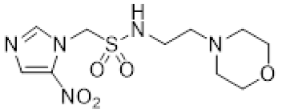 | 13.7 ± 2.0 | 1.2 ± 0.1 | 11.2 | 10.6 ± 0.6 | 1.8 ± 0.1 | 5.8 | 13.1 ± 2.8 | 1.5 ± 0.3 | 8.6 |
| 39 |  | >25 | 13.0 ± 1.3 | >1.9 | >19 | 7.2 ± 0.2 | >2.6 | - | - | - |
| 40 | 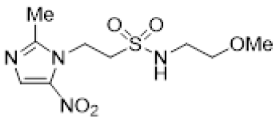 | >20 | 11.0 ± 0.4 | >1.8 | >22 | 8.0 ± 0.5 | >2.7 | - | - | - |
| 41 | 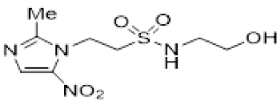 | >18 | 15.5 ± 2.6 | >1.1 | >25 | >22 | - | >21 | 13.7 ± 3.7 | >1.5 |
| 42 | 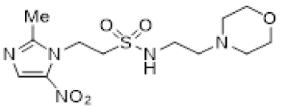 | 7.6 ± 0.4 | 4.3 ± 0.2 | 1.8 | 9.8 ± 1.2 | 7.8 ± 0.2 | 1.3 | 8.9 ± 1.6 | 6.6 ± 0.6 | 1.4 |
| 43 |  | 11.7 ± 1.3 | 9.9 ± 0.3 | 1.2 | 13.3 ± 2.5 | 8.6 ± 0.6 | 1.6 | - | - | - |
| 44 | 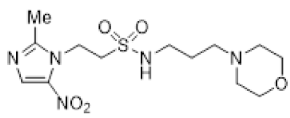 | >21 | 10.3 ± 1.5 | >2.0 | >16 | 7.8 ± 0.7 | >2.0 | 13.2 ± 2.0 | 7.1 ± 1.5 | 1.9 |
| 45 |  | 11.8 ± 1.5 | 2.4 ± 0.4 | 5.0 | 4.8 ± 0.3 | 1.7 ± 0.4 | 2.8 | - | - | - |
| 46 |  | 16.9 ± 1.4 | 2.0 ± 0.3 | 8.6 | 9.5 ± 2.0 | 1.3 ± 0.1 | 7.3 | 8.1 ± 0.9 | 1.9 ± 0.2 | 4.4 |
| Compound | SER 1 at 1 mM | |
|---|---|---|
| Misonidazole 1 | 1.46 ± 0.01 | |
| Etanidazole 2 | 1.18 ± 0.00 | |
| Nimorazole 3 | 1.20 ± 0.01 | |
| 5 | 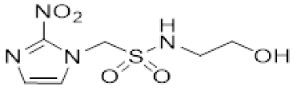 | 1.72 ± 0.06 |
| 6 | 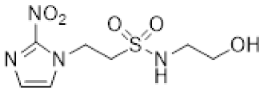 | 1.39 ± 0.01 |
| 7 | 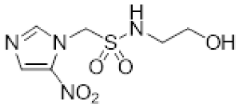 | 1.41 ± 0.02 |
| 8 | 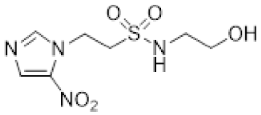 | 1.27 ± 0.00 |
| 13 | 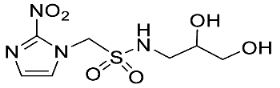 | 1.00 ± 0.00 |
| 14 |  | 1.00 ± 0.00 |
| 16 | 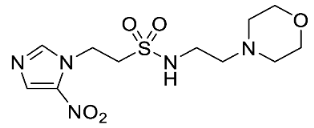 | 1.31 ± 0.01 |
| 17 | 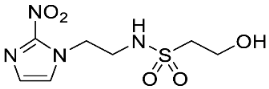 | 1.35 ± 0.02 |
| 34 | 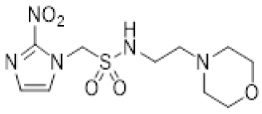 | 1.92 ± 0.01 |
| 36 | 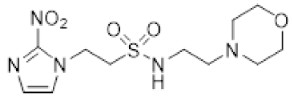 | 1.57 ± 0.00 |
| 38 | 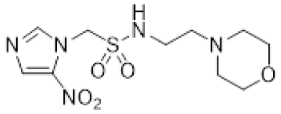 | 1.39 ± 0.01 |
| 41 | 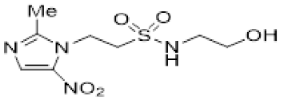 | 1.16 ± 0.01 |
| 42 | 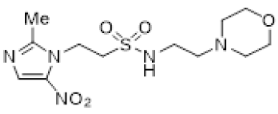 | 1.16 ± 0.01 |
| Compound | Plasma Cmax (mM) | T1/2 (h) | AUC (mM·h) |
|---|---|---|---|
| 34 | 1.53 | 1.52 | 0.96 |
| 2 1 | 3.64 | 1.05 | 1.82 |
| 9 1 | 0.92 | 2.33 | 0.55 |
| 10 1 | 1.14 | 2.21 | 0.64 |
| 11 1 | 1.10 | 1.72 | 0.71 |
| Compound 1 | Sensitisation Ratio 2 | |
|---|---|---|
| HCT116/54C | UT-SCC-74B | |
| 2 | 3.34 | 8.94 |
| 9 | 6.22 | 9.64 |
| 10 | 1.78 | - |
| 11 | 1.85 | - |
| 12 | 3.32 | - |
| 34 | 14.9 | 13.8 |
| 38 | 3.43 | 11.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liew, L.P.; Shome, A.; Wong, W.W.; Hong, C.R.; Hicks, K.O.; Jamieson, S.M.F.; Hay, M.P. Design, Synthesis and Anticancer Evaluation of Nitroimidazole Radiosensitisers. Molecules 2023, 28, 4457. https://doi.org/10.3390/molecules28114457
Liew LP, Shome A, Wong WW, Hong CR, Hicks KO, Jamieson SMF, Hay MP. Design, Synthesis and Anticancer Evaluation of Nitroimidazole Radiosensitisers. Molecules. 2023; 28(11):4457. https://doi.org/10.3390/molecules28114457
Chicago/Turabian StyleLiew, Lydia P., Avik Shome, Way W. Wong, Cho R. Hong, Kevin O. Hicks, Stephen M. F. Jamieson, and Michael P. Hay. 2023. "Design, Synthesis and Anticancer Evaluation of Nitroimidazole Radiosensitisers" Molecules 28, no. 11: 4457. https://doi.org/10.3390/molecules28114457