Simultaneous Estimation of Quercetin and trans-Resveratrol in Cissus quadrangularis Extract in Rat Serum Using Validated LC-MS/MS Method: Application to Pharmacokinetic and Stability Studies
Abstract
:1. Introduction
2. Results
2.1. Mass Spectrometry Conditions
2.2. Liquid Chromatography Conditions
2.3. Serum Samples Processing
2.4. Method Validation
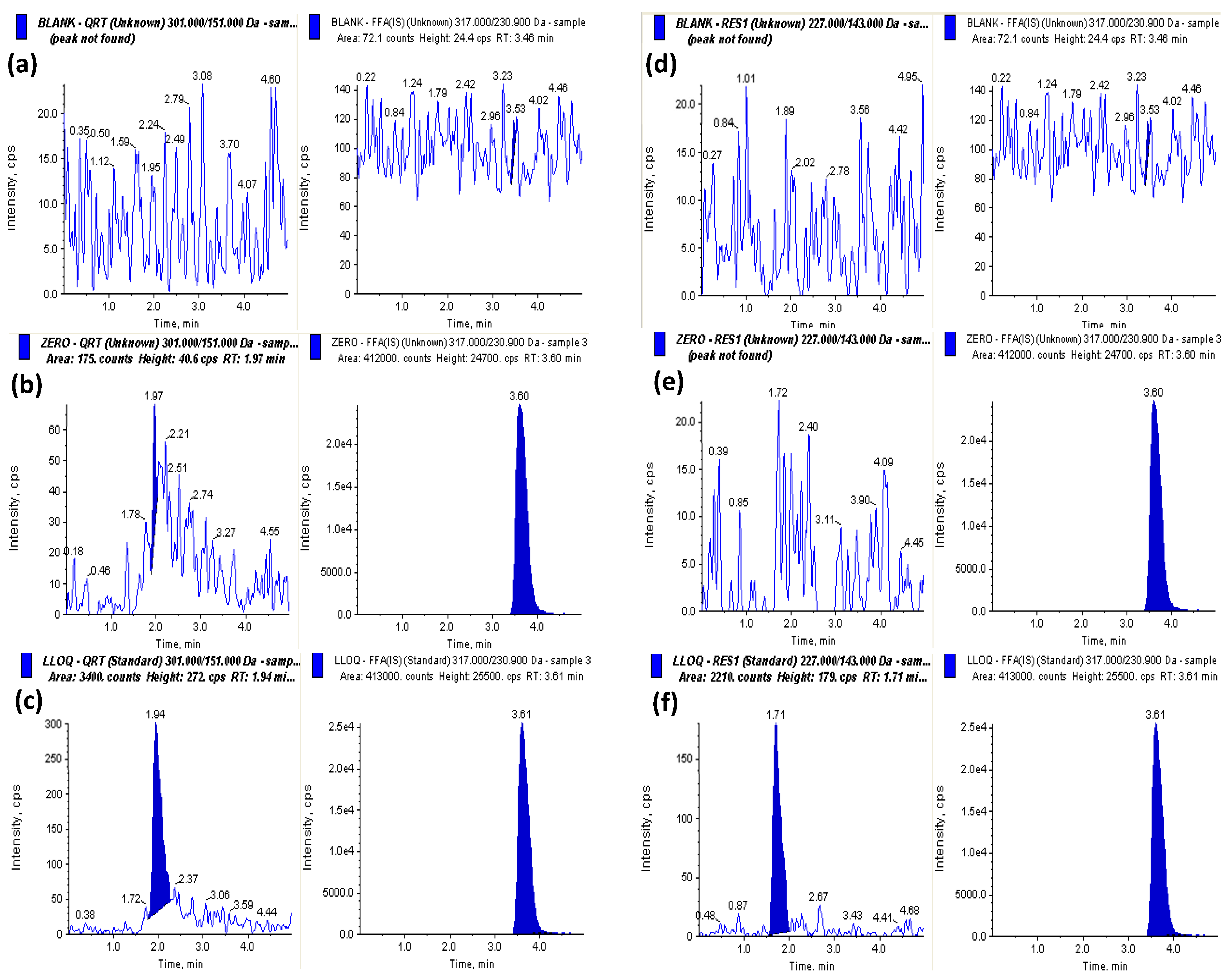
2.4.1. Selectivity and Specificity
2.4.2. Linearity and Sensitivity
2.4.3. Accuracy, Precision, and Carryover Effect
2.4.4. Dilution Integrity, Recovery and Matrix Effect
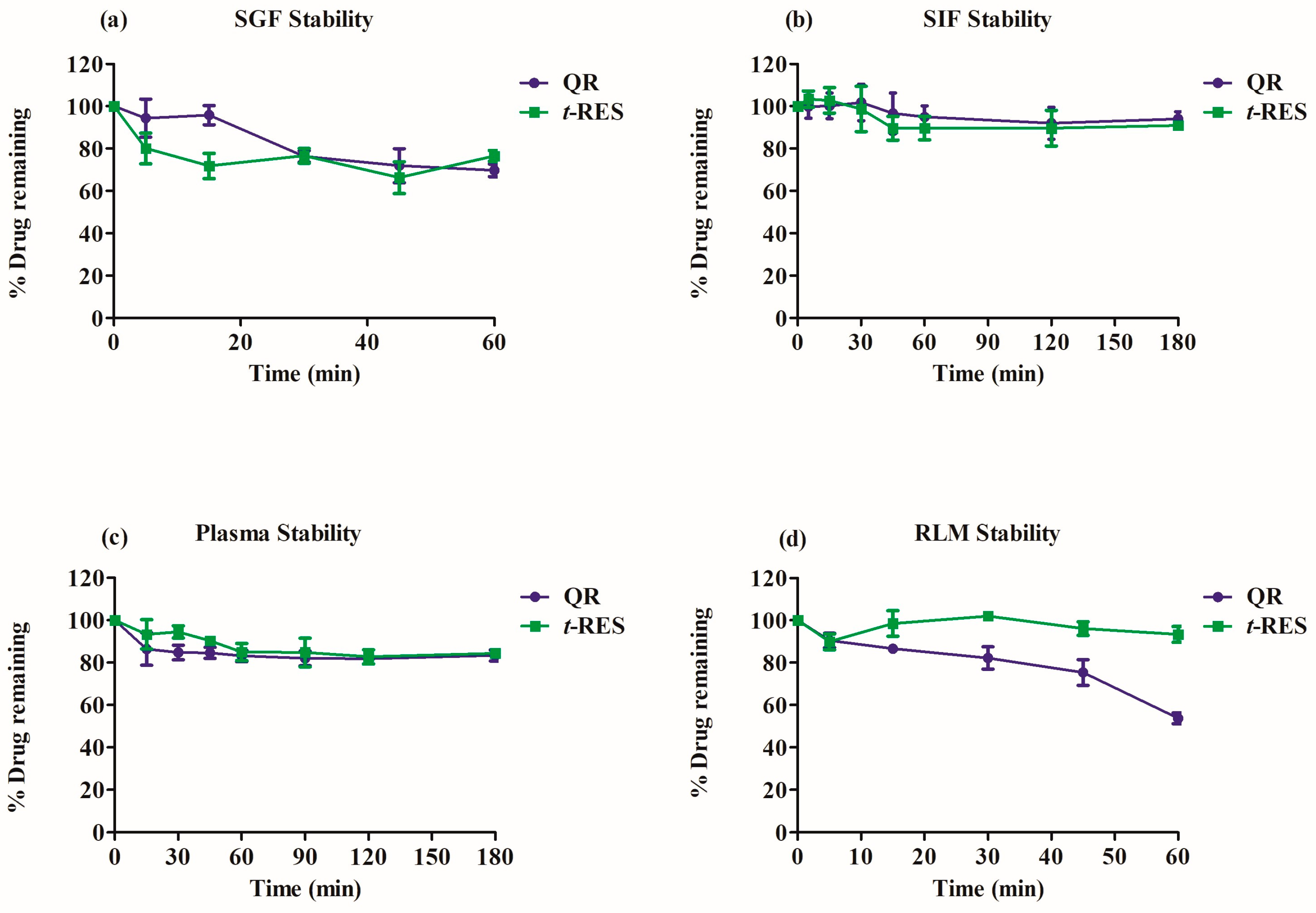
2.4.5. Stability
2.4.6. Stability of Quercetin and t-res in Simulated Gastrointestinal Fluids
2.4.7. Stability Studies in Plasma
2.4.8. Stability Study in Rat Liver Microsome
2.4.9. In-Vivo Pharmacokinetic Study in Rats
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Animals
4.3. LC-MS/MS Analysis
4.4. Characterization of Cissus Quadrangularis Extract
4.5. Standard Solutions, Calibration Curve Quality Control (QC) Samples
4.6. Serum Sample Extraction Procedure
4.7. Validation Parameters
4.7.1. Selectivity and Specificity
4.7.2. Calibration Curve
4.7.3. Accuracy and Precision
4.7.4. Recovery
4.7.5. Matrix Effect
4.7.6. Carry over and Dilution Integrity
4.7.7. Stability Studies
4.7.8. Stability in Simulated Gastric and Intestinal Fluids
4.7.9. Plasma Stability
4.7.10. Metabolic Stability in Rat Liver Microsomes (RLM)
4.7.11. In-Vivo Pharmacokinetic Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Syed, A.A.; Reza, M.I.; Kalleti, N.; Husain, A.; Singh, P.; Rath, S.K.; Gayen, J.R. Evaluation of mutagenic, cytotoxic, mitochondrial dysfunction, apoptotic activity, and acute toxicity of ethanolic extract of Cissus quadrangularis. Toxicology 2023, 483, 153373. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, S.C. Quercetin: Potentials in the prevention and therapy of disease. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Attele, A.S.; Zhou, Y.-P.; Xie, J.-T.; Wu, J.A.; Zhang, L.; Dey, L.; Pugh, W.; Rue, P.A.; Polonsky, K.S.; Yuan, C.-S. Antidiabetic effects of Panax ginseng berry extract and the identification of an effective component. Diabetes 2002, 51, 1851–1858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fennell, C.; Lindsey, K.; McGaw, L.; Sparg, S.; Stafford, G.; Elgorashi, E.; Grace, O.; Van Staden, J. Assessing African medicinal plants for efficacy and safety: Pharmacological screening and toxicology. J. Ethnopharmacol. 2004, 94, 205–217. [Google Scholar] [CrossRef]
- Syed, A.A.; Reza, M.I.; Shafiq, M.; Kumariya, S.; Singh, P.; Husain, A.; Hanif, K.; Gayen, J.R. Naringin ameliorates type 2 diabetes mellitus-induced steatohepatitis by inhibiting RAGE/NF-κB mediated mitochondrial apoptosis. Life Sci. 2020, 257, 118118. [Google Scholar] [CrossRef]
- Syed, A.A.; Lahiri, S.; Mohan, D.; Valicherla, G.R.; Gupta, A.P.; Riyazuddin, M.; Kumar, S.; Maurya, R.; Hanif, K.; Gayen, J.R. Evaluation of anti-hypertensive activity of Ulmus wallichiana extract and fraction in SHR, DOCA-salt-and L-NAME-induced hypertensive rats. J. Ethnopharmacol. 2016, 193, 555–565. [Google Scholar] [CrossRef]
- Arojojoye, O.A.; Alao, O.O.; Owoeye, O.A.; Adejuwon, A.O.; John-Dewole, O.O.; Jemiseye, Y.J. Heavy metal and phytochemical screening of anti-jaundice and anti-malaria concoctions and genotoxicity assessment of the concoctions using Allium cepa assay. Trop. Plant Res. 2015, 2, 127–133. [Google Scholar]
- Golla, U.; Bhimathati, S.S.R. Evaluation of antioxidant and DNA damage protection activity of the hydroalcoholic extract of Desmostachya bipinnata L. Stapf. Sci. World J. 2014, 2014, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Sirasanagandla, S.R.; Pai, K.S.R.; Potu, B.K.; Bhat, K.M. Protective effect of Cissus quadrangularis Linn. on diabetes induced delayed fetal skeletal ossification. J. Ayurveda Integr. Med. 2014, 5, 25. [Google Scholar]
- Syed, A.A.; Reza, M.I.; Garg, R.; Goand, U.K.; Gayen, J.R. Cissus quadrangularis extract attenuates diabetic nephropathy by altering SIRT1/DNMT1 axis. J. Pharm. Pharmacol. 2021, 73, 1442–1450. [Google Scholar] [CrossRef]
- Syed, A.A.; Reza, M.I.; Shafiq, M.; Kumariya, S.; Katekar, R.; Hanif, K.; Gayen, J.R. Cissus quadrangularis extract mitigates diabetic cardiomyopathy by inhibiting RAAS activation, inflammation and oxidative stress. Biomarkers 2022, 27, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Syed, A.A.; Reza, M.I.; Singh, P.; Husain, A.; Dadge, S.; Gayen, J.R. Polyphenolic-rich Cissus quadrangularis extract ameliorates insulin resistance by activating AdipoR1 in peri-/post-menopausal rats. Exp. Gerontol. 2022, 159, 111681. [Google Scholar] [CrossRef] [PubMed]
- Syed, A.A.; Shafiq, M.; Reza, M.I.; Bharati, P.; Husain, A.; Singh, P.; Hanif, K.; Gayen, J.R. Ethanolic extract of Cissus quadrangularis improves vasoreactivity by modulation of eNOS expression and oxidative stress in spontaneously hypertensive rats. Clin. Exp. Hypertens. 2022, 44, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Zaki, S.; Chaithra, M.l.; Bansal, S.; Latha, V.; Bajpai, M.; Malathi, R.; Sibi, G. In vitro anti-inflammatory, anti-diabetic and antioxidant potential of Cissus quadrangularis along with its orexigenic activity in Drosophila melanogaster. J. Appl. Nat. Sci. 2021, 13, 962–969. [Google Scholar] [CrossRef]
- Nie, T.W.; Shukkoor, M.S.A.; Nair, R.S.; Amiruddin, F.K.B.; Ramasamy, S. Involvement of opioidergic and serotonergic systems in the analgesic activity of Cissus quadrangularis L. stem extract in mice. J. Basic Clin. Physiol. Pharmacol. 2015, 26, 35–41. [Google Scholar] [CrossRef]
- Stohs, S.J.; Ray, S.D. A review and evaluation of the efficacy and safety of Cissus quadrangularis extracts. Phytother. Res. 2013, 27, 1107–1114. [Google Scholar] [CrossRef]
- Rajeshkumar, S.; Menon, S.; Ponnanikajamideen, M.; Ali, D.; Arunachalam, K. Anti-inflammatory and antimicrobial potential of Cissus quadrangularis-assisted copper oxide nanoparticles. J. Nanomater. 2021, 2021, 5742981. [Google Scholar] [CrossRef]
- Dhanasekaran, S. Phytochemical characteristics of aerial part of Cissus quadrangularis (L) and its in-vitro inhibitory activity against lukemic cells and antioxidant properties. Saudi J. Biol. Sci. 2020, 27, 1302–1309. [Google Scholar] [CrossRef]
- Adesanya, S.A.; Nia, R.; Martin, M.-T.; Boukamcha, N.; Montagnac, A.; Païs, M.J. Stilbene derivatives from Cissus quadrangularis. J. Nat. Prod. 1999, 62, 1694–1695. [Google Scholar] [CrossRef]
- Rao, G.; Annamalai, T.; Mukhopadhyay, T.; Madhavi, M.S.L. Chemical constituents and melanin promotion activity of Cissus quadrangularis Linn. Res. J. Chem. Sci. 2011, 1, 25–29. [Google Scholar]
- Sawangjit, R.; Puttarak, P.; Saokaew, S.; Chaiyakunapruk, N. Efficacy and safety of Cissus quadrangularis L. in clinical use: A systematic review and meta-analysis of randomized controlled trials. Phytother. Res. 2017, 31, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Bafna, P.S.; Patil, P.H.; Maru, S.K.; Mutha, R.E. Cissus quadrangularis L: A comprehensive multidisciplinary review. J. Ethnopharmacol. 2021, 279, 114355. [Google Scholar] [CrossRef] [PubMed]
- Biasutto, L.; Zoratti, M. Prodrugs of Quercetin and Resveratrol: A Strategy Under development. Curr. Drug Metabolism. 2014, 19, 75–95. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [Green Version]
- Murakami, A.; Ashida, H.; Terao, J. Multitargeted cancer prevention by quercetin. Cancer Lett. 2008, 269, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Biasutto, L.; Marotta, E.; Bradaschia, A.; Fallica, M.; Mattarei, A.; Garbisa, S.; Zoratti, M.; Paradisi, C. Soluble polyphenols: Synthesis and bioavailability of 3, 4′, 5-tri (α-d-glucose-3-O-succinyl) resveratrol. Bioorganic Med. Chem. Lett. 2009, 19, 6721–6724. [Google Scholar] [CrossRef] [PubMed]
- Biasutto, L.; Marotta, E.; De Marchi, U.; Zoratti, M.; Paradisi, C. Ester-based precursors to increase the bioavailability of quercetin. J. Med. Chem. 2007, 50, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Pervaiz, F.; Ahmad, M.; Minhas, M.U.; Sohail, M. Development and validation of reverse phase high performance chromatography method for determination of olanzapine in microsample rat plasma: Application to preclinical pharmacokinetic study. Trop. J. Pharm. Res. 2015, 14, 141–147. [Google Scholar] [CrossRef] [Green Version]
- Das, S.; Lin, H.-S.; Ho, P.C.; Ng, K.-Y. The impact of aqueous solubility and dose on the pharmacokinetic profiles of resveratrol. Pharm. Res. 2008, 25, 2593–2600. [Google Scholar] [CrossRef]
- Soleas, G.; Yan, J.; Goldberg, D.M. Ultrasensitive assay for three polyphenols (catechin, quercetin and resveratrol) and their conjugates in biological fluids utilizing gas chromatography with mass selective detection. J. Chromatogr. B Biomed. Sci. Appl. 2001, 757, 161–172. [Google Scholar] [CrossRef]
- Li, H.; Zhao, X.; Ma, Y.; Zhai, G.; Li, L.; Lou, H. Enhancement of gastrointestinal absorption of quercetin by solid lipid nanoparticles. J. Control. Release 2009, 133, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Lai, X.; Zhao, Y.; Liang, H.; Bai, Y.; Wang, B.; Guo, D. SPE-HPLC method for the determination of four flavonols in rat plasma and urine after oral administration of Abelmoschus manihot extract. J. Chromatogr. B 2007, 852, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Juan, M.E.; Lamuela-Raventós, R.M.; de la Torre-Boronat, M.C.; Planas, J.M. Determination of trans-resveratrol in plasma by HPLC. Anal. Chem. 1999, 71, 747–750. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Chen, X.; Wang, G.; Wang, J.; Davey, A.K. High-performance liquid chromatography spectrometric analysis of trans-resveratrol in rat plasma. J. Chromatogr. B 2006, 832, 177–180. [Google Scholar] [CrossRef] [PubMed]
- Boocock, D.J.; Faust, G.E.; Patel, K.R.; Schinas, A.M.; Brown, V.A.; Ducharme, M.P.; Booth, T.D.; Crowell, J.A.; Perloff, M.; Gescher, A.J.; et al. Phase I dose escalation pharmacokinetic study in healthy volunteers of resveratrol, a potential cancer chemopreventive agent. Cancer Epidemiol. Biomark. Prev. 2007, 16, 1246–1252. [Google Scholar] [CrossRef] [Green Version]
- Jones, D.; Lim, C.; Ferry, D.; Gescher, A. Determination of quercetin in human plasma by HPLC with spectrophotometric or electrochemical detection. Biomed. Chromatogr. 1998, 12, 232–235. [Google Scholar] [CrossRef]
- Ishii, K.; Furuta, T.; Kasuya, Y. High-performance liquid chromatographic determination of quercetin in human plasma and urine utilizing solid-phase extraction and ultraviolet detection. J. Chromatogr. B 2003, 794, 49–56. [Google Scholar] [CrossRef]
- Vitaglione, P.; Sforza, S.; Galaverna, G.; Ghidini, C.; Caporaso, N.; Vescovi, P.P.; Fogliano, V.; Marchelli, R. Bioavailability of trans-resveratrol from red wine in humans. Mol. Nutr. Food Res. 2005, 49, 495–504. [Google Scholar] [CrossRef]
- Moon, Y.J.; Wang, L.; DiCenzo, R.; Morris, M.E. Quercetin pharmacokinetics in humans. Biopharm. Drug Dispos. 2008, 29, 205–217. [Google Scholar] [CrossRef]
- Sheweita, S.A. Drug-metabolizing enzymes mechanisms and functions. Curr. Drug Metab. 2000, 1, 107–132. [Google Scholar] [CrossRef] [Green Version]
- Coe, K.J.; Koudriakova, T.R. Metabolic stability assessed by liver microsomes and hepatocytes. Optim. Drug Discov. Vitr. Methods 2014, 87–99. [Google Scholar] [CrossRef]
- Chaudhuri, S.; Banerjee, A.; Basu, K.; Sengupta, B.; Sengupta, P.K. Interaction of flavonoids with red blood cell membrane lipids and proteins: Antioxidant and antihemolytic effects. Food Res. Int. 2007, 41, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Blache, D.; Rustan, I.; Durand, P.; Lesgards, G.; Loreau, N. Gas chromatographic analysis of resveratrol in plasma, lipoproteins and cells after in vitro incubations. J. Chromatogr. B Biomed. Sci. Appl. 1997, 702, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Ou-yang, Z.; Cao, X.; Wei, Y.; Zhao, M.; Duan, J.-A. Pharmacokinetic study of rutin and quercetin in rats after oral administration of total flavones of mulberry leaf extract. Rev. Bras. Farm. 2013, 23, 776–782. [Google Scholar] [CrossRef]
- Chavari, M.; Góes, P.R.N.D.; Lachi-Silva, L.; Barth, A.B.; Silva, A.O.F.D.; Longhini, R.; Mello, J.C.P.D.; Kimura, E.; Diniz, A. Simultaneous liquid chromatography-tandem mass spectrometry method to quantify epicatechin and procyanidin B2 in rat plasma after oral administration of Trichilia catigua (catuaba) extract and its application to a pharmacokinetic study. Rev. Bras. Farmacogn. 2019, 29, 457–463. [Google Scholar] [CrossRef]
- Almeida, L.; Vaz-da-Silva, M.; Falcão, A.; Soares, E.; Costa, R.; Loureiro, A.I.; Fernandes-Lopes, C.; Rocha, J.F.; Nunes, T.; Wright, L.; et al. Pharmacokinetic and safety profile of trans-resveratrol in a rising multiple-dose study in healthy volunteers. Mol. Nutr. Food Res. 2009, 53, S7–S15. [Google Scholar] [CrossRef]
- Goldberg, D.M.; Yan, J.; Soleas, G.J. Absorption of three wine-related polyphenols in three different matrices by healthy subjects. Clin. Biochem. 2003, 36, 79–87. [Google Scholar] [CrossRef]
- Walle, T.; Hsieh, F.; DeLegge, M.H.; Oatis, J.E.; Walle, U.K. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab. Dispos. 2004, 32, 1377–1382. [Google Scholar] [CrossRef] [Green Version]
- Syed, A.; Reza, M.; Husain, A.; Singh, P.; Gayen, J.R. Inhibition of NOX4 by Cissus quadrangularis extract protects from Type 2 diabetes induced-steatohepatitis. Phytomed. Plus 2021, 1, 100021. [Google Scholar] [CrossRef]
- FDA. Guidance for industry: Bioanalytical method validation. In Bioanalytical Method Validation Guidance for Industry; FDA: Silver Spring, ML, USA, 2001; pp. 1–27. [Google Scholar]
- Di, L.; Kerns, E.H. Solution stability-plasma, gastrointestinal, bioassay. Curr. Drug Metab. 2008, 9, 860–868. [Google Scholar] [CrossRef]
- Cheruvu, H.S.; Yadav, N.K.; Valicherla, G.R.; Arya, R.K.; Hussain, Z.; Sharma, C.; Arya, K.R.; Singh, R.K.; Datta, D.; Gayen, J.R. LC-MS/MS method for the simultaneous quantification of luteolin, wedelolactone and apigenin in mice plasma using hansen solubility parameters for liquid-liquid extraction: Application to pharmacokinetics of Eclipta alba chloroform fraction. J. Chromatogr. B 2018, 1081, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Riyazuddin, M.; Valicherla, G.R.; Husain, A.; Hussain, M.K.; Shukla, M.; Katekar, R.; Gupta, A.P.; Singh, P.; Banerjee, D.; Hajela, K.; et al. Elucidation of pharmacokinetics of novel DNA ligase I inhibitor, S012-1332 in rats: Integration of in vitro and in vivo findings. J. Pharm. Biomed. Anal. 2019, 162, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Chhonker, Y.S.; Chandasana, H.; Bala, V.; Mukkavilli, R.; Kumar, D.; Vangala, S.; Bhatta, R.S.J. In-vitro metabolism, CYP profiling and metabolite identification of E- and Z- guggulsterone, a potent hypolipidmic agent. J. Pharm. Biomed. Anal. 2018, 160, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Coughlin, J.E.; Pandey, R.K.; Padmanabhan, S.; O’Loughlin, K.G.; Marquis, J.; Green, C.E.; Mirsalis, J.C.; Iyer, R.P. Metabolism, Pharmacokinetics, Tissue Distribution, and Stability Studies of the Prodrug Analog of an Anti-Hepatitis B Virus Dinucleoside Phosphorothioate. Drug Metab. Dispos. 2012, 40, 970–981. [Google Scholar] [CrossRef] [PubMed] [Green Version]

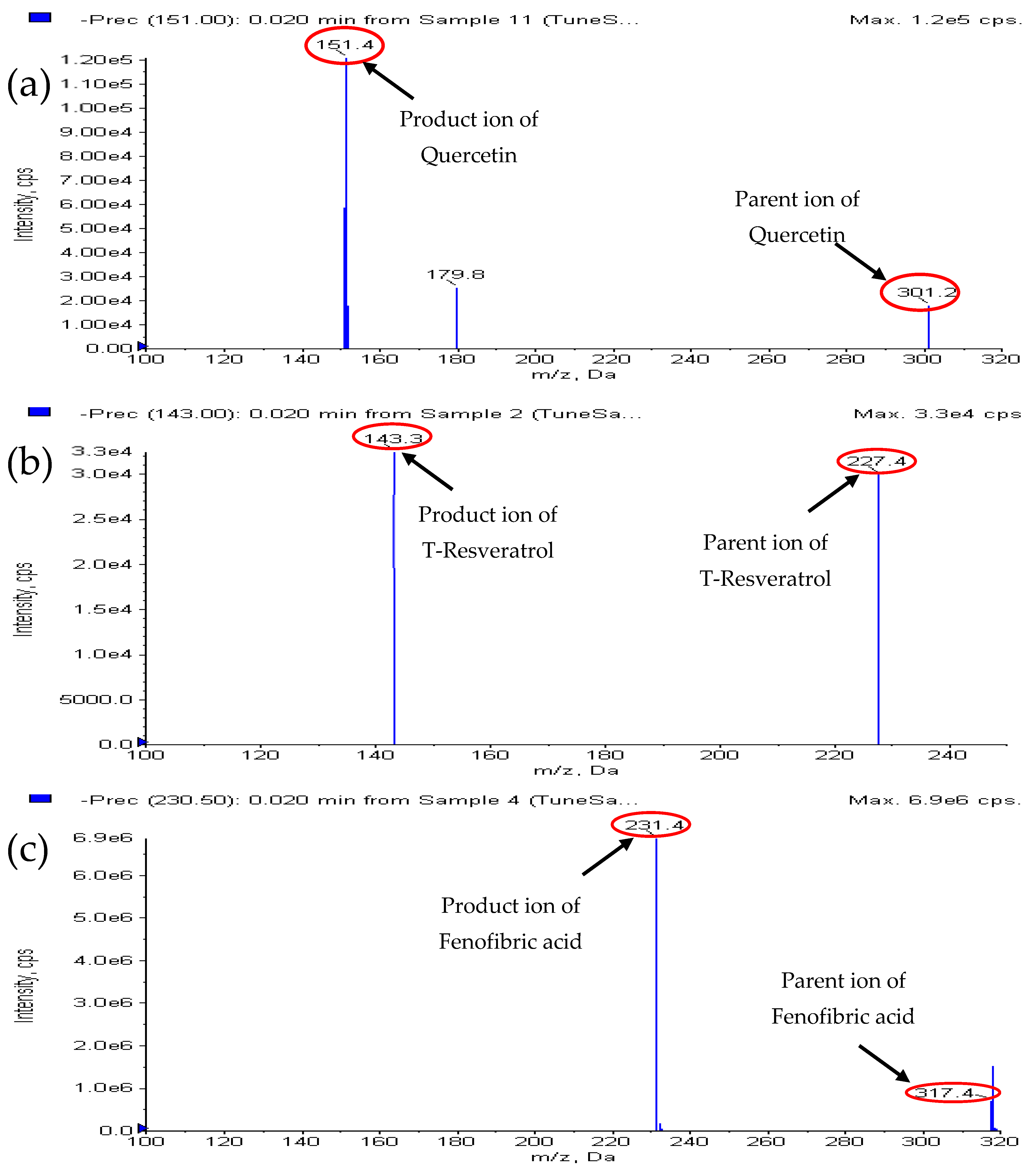

| Compound Parameters | trans-Resveratrol | Quercetin | Fenofibric Acid (I.S) |
|---|---|---|---|
| Negative Mode | [M-H]−1 | ||
| Parent ion Q1 m/z) | 227 | 301 | 317 |
| Product ion Q3 (m/z) | 143 | 151 | 230.9 |
| DT (ms) | 150 | 150 | 150 |
| DP (eV) | −98 | −105 | −53 |
| EP (eV) | −10 | −10 | −12 |
| CE (eV) | −38 | −30 | −18 |
| CXP (eV) | −6 | −6 | −10 |
| Source Parameters: CUR = 25, CAD = Medium, IS = −4500 V, Temp = 400 °C, GS1 and GS2 = 50:50 | |||
| Analyte | Linearity Ranges (ng/mL) | LLOQ (ng/mL) | Regression Equation | Correlation Coefficient (r2) |
|---|---|---|---|---|
| Quercetin | 5–500 | 5 | 0.00336x + 0.0006 | ≥0.9974 |
| trans-resveratrol | 5–500 | 5 | 0.00242x + 0.00084 | ≥0.9938 |
| Analyte | Spiked Conc. (ng/mL) | Intraday Assay | Interday Assay | ||||
|---|---|---|---|---|---|---|---|
| Measured Conc. (ng/mL) | Accuracy (% RE) | Precision (% RSD) | Measured Conc. (ng/mL) | Accuracy (% RE) | Precision (% RSD) | ||
| Quercetin | 5 | 5.04 ± 0.45 | 100.72 | 8.86 | 4.89 ± 0.37 | 97.88 | 7.52 |
| 30 | 29.99 ± 1.91 | 99.97 | 6.37 | 30.09 ± 2.62 | 100.30 | 8.71 | |
| 203 | 188.33 ± 12.07 | 92.77 | 6.41 | 193.02 ± 18.34 | 95.08 | 9.50 | |
| 375 | 372.42 ± 19.04 | 99.31 | 5.11 | 387.40 ± 30.35 | 103.31 | 7.84 | |
| trans-resveratrol | 5 | 5.20 ± 0.29 | 104.03 | 5.66 | 4.68 ± 0.20 | 93.68 | 4.35 |
| 30 | 30.61 ± 1.57 | 102.04 | 5.14 | 28.72 ± 2.76 | 95.74 | 9.61 | |
| 203 | 189.03 ± 8.70 | 93.12 | 4.60 | 186.83 ± 12.75 | 92.03 | 6.82 | |
| 375 | 392.19 ± 13.01 | 104.58 | 3.32 | 402.73 ± 24.59 | 107.39 | 6.11 | |
| Analyte | Spiked Conc. (ng/mL) | Extraction Recovery (%, Mean ± SD) | Matrix Effect (%, Mean ± SD) |
|---|---|---|---|
| Quercetin | 30 | 84.52 ± 5.04 | 101.32 ± 9.62 |
| 203 | 87.96 ± 3.01 | 91.76 ± 5.75 | |
| 375 | 87.87 ± 7.35 | 91.40 ± 6.56 | |
| trans-resveratrol | 30 | 86.32 ± 10.14 | 102.32 ± 5.87 |
| 203 | 80.52 ± 7.53 | 96.51 ± 5.55 | |
| 375 | 82.35 ± 4.08 | 106.41 ± 8.00 |
| Autosampler Stability (4 ± 2 °C, 24 h) | Benchtop Stability (24 ± 4 °C, 8 h) | Long-Term Stability (−70 ± 10 °C, 30 Days) | Freeze-Thaw Stability (−70 ± 10 °C, 3 Cycles) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Analyte | Nominal Conc. (ng/mL) | Measured Conc. (ng/mL) | RSD (%) | Accuracy (%) | Measured Conc. (ng/mL) | RSD (%) | Accuracy (%) | Measured Conc. (ng/mL) | RSD (%) | Accuracy (%) | Measured Conc. (ng/mL) | RSD (%) | Accuracy (%) |
| Quercetin | 30 | 27.77 ± 1.50 | 5.41 | 92.56 | 27.09 ± 1.67 | 6.17 | 90.29 | 30.07 ± 2.06 | 6.86 | 100.22 | 29.44 ± 2.73 | 9.27 | 98.12 |
| 203 | 181.31 ± 8.65 | 4.77 | 89.32 | 184.64 ± 7.16 | 3.88 | 90.96 | 199.71 ± 8.70 | 4.35 | 98.38 | 190.17 ± 8.97 | 4.72 | 93.68 | |
| 375 | 372.92 ± 2.21 | 0.59 | 99.44 | 371.12 ± 2.43 | 0.66 | 98.96 | 373.05 ± 10.65 | 2.86 | 99.48 | 369.86 ± 4.43 | 1.20 | 98.63 | |
| trans-resveratrol | 30 | 28.36 ± 2.19 | 7.71 | 94.53 | 28.52 ± 3.05 | 10.68 | 95.08 | 30.50 ± 2.53 | 8.28 | 101.68 | 27.40 ± 4.45 | 7.43 | 91.32 |
| 204 | 190.28 ± 13.20 | 6.94 | 93.73 | 189.53 ± 8.81 | 4.65 | 93.36 | 189.08 ± 14.18 | 7.50 | 93.14 | 189.43 ± 17.82 | 9.41 | 93.32 | |
| 375 | 373.15 ± 5.84 | 1.56 | 99.51 | 368.13 ± 12.29 | 3.34 | 98.17 | 366.25 ± 11.38 | 3.11 | 97.67 | 367.92 ± 11.94 | 3.25 | 98.11 | |
| Parameter (Unit) | Quercetin (in EECQ) | trans-Resveratrol (in EECQ) |
|---|---|---|
| Cmax (ng/mL) | 57.65 ± 14.28 | 56.35 ± 13.14 |
| AUC0–t (h*ng/mL) | 447.06 ± 47.05 | 144.83 ± 20.76 |
| AUC0–∞ (h*ng/mL) | 474.23 ± 51.49 | 156.57 ± 21.09 |
| Tmax (h) | 1.17 ± 0.41 | 0.58 ± 0.20 |
| T1/2 (h) | 4.91 ± 0.69 | 1.51 ± 0.40 |
| CL/F (L/h/Kg) | 851.55 ± 89.69 | 2593.43 ± 346.71 |
| Vd/F (L/Kg) | 6017.81 ± 940.82 | 5663.48 ± 1655.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dadge, S.D.; Syed, A.A.; Husain, A.; Valicherla, G.R.; Gayen, J.R. Simultaneous Estimation of Quercetin and trans-Resveratrol in Cissus quadrangularis Extract in Rat Serum Using Validated LC-MS/MS Method: Application to Pharmacokinetic and Stability Studies. Molecules 2023, 28, 4656. https://doi.org/10.3390/molecules28124656
Dadge SD, Syed AA, Husain A, Valicherla GR, Gayen JR. Simultaneous Estimation of Quercetin and trans-Resveratrol in Cissus quadrangularis Extract in Rat Serum Using Validated LC-MS/MS Method: Application to Pharmacokinetic and Stability Studies. Molecules. 2023; 28(12):4656. https://doi.org/10.3390/molecules28124656
Chicago/Turabian StyleDadge, Shailesh D., Anees A. Syed, Athar Husain, Guru R. Valicherla, and Jiaur R. Gayen. 2023. "Simultaneous Estimation of Quercetin and trans-Resveratrol in Cissus quadrangularis Extract in Rat Serum Using Validated LC-MS/MS Method: Application to Pharmacokinetic and Stability Studies" Molecules 28, no. 12: 4656. https://doi.org/10.3390/molecules28124656





