Applicability and Limitations of a Capillary-LC Column-Switching System Using Hybrid Graphene-Based Stationary Phases
Abstract
:1. Introduction
2. Results and Discussion
2.1. Selection of the Analytes
2.2. Loading Method
2.3. Linearity of the Calibration Curve
2.4. Intra-Day and Inter-Day Accuracy and Precision
2.5. Matrix Effects
2.6. System Life Use
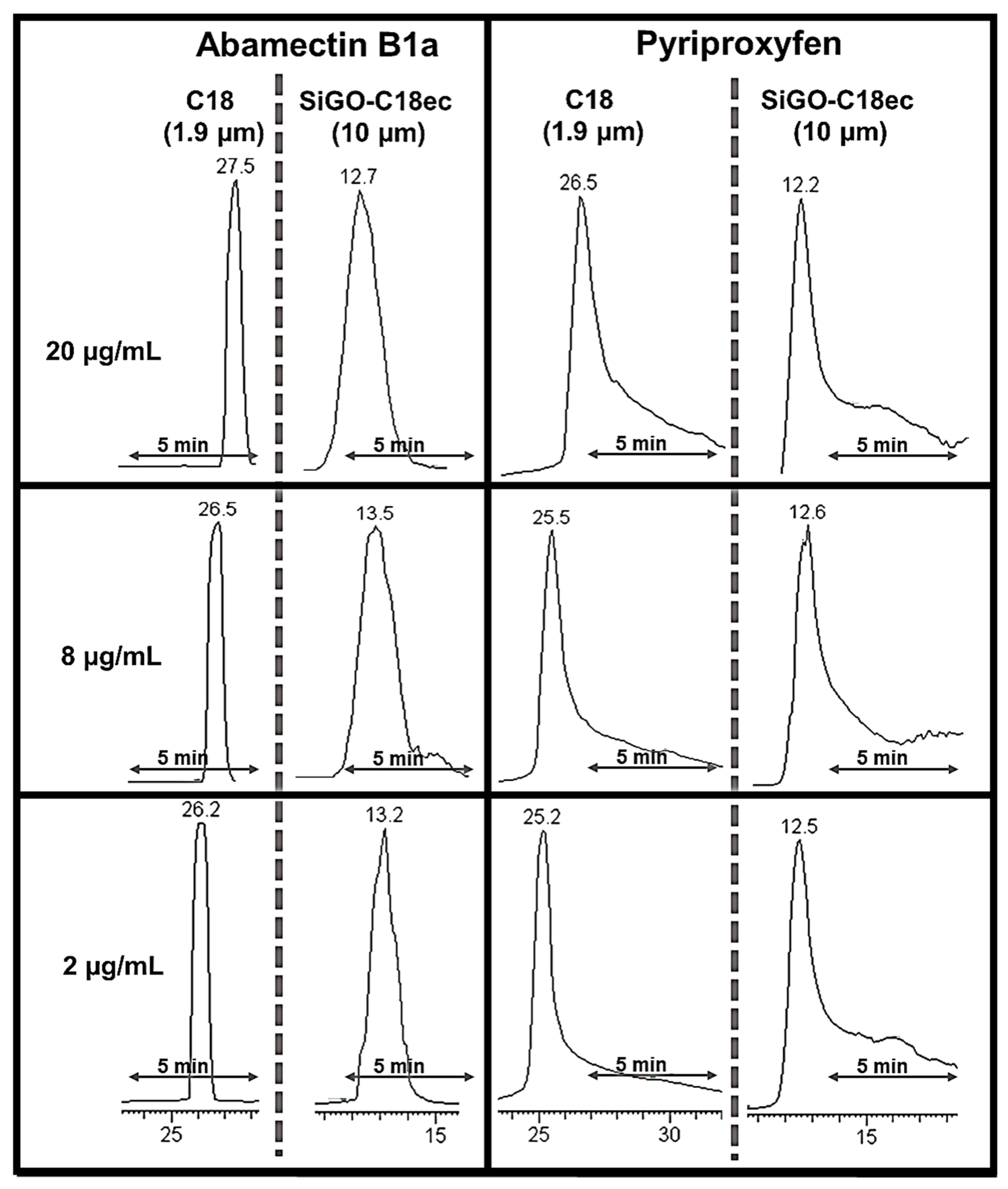
2.7. Peak Shape Comparison with C18 Analytical Column
3. Material and Methods
3.1. Reagents and Standards
3.2. Sample Preparation
3.3. Analytical Instrumentation
3.4. Analytical Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Joshi, D.J.; Koduru, J.R.; Malek, N.I.; Hussain, C.M.; Kailasa, S.K. Surface Modifications and Analytical Applications of Graphene Oxide: A Review. TrAC Trends Anal. Chem. 2021, 144, 116448. [Google Scholar] [CrossRef]
- Justino, C.I.L.; Gomes, A.R.; Freitas, A.C.; Duarte, A.C.; Rocha-Santos, T.A.P. Graphene Based Sensors and Biosensors. TrAC Trends Anal. Chem. 2017, 91, 53–66. [Google Scholar] [CrossRef]
- Huang, T.; Tang, X.; Luo, K.; Wu, Y.; Hou, X.; Tang, S. An Overview of Graphene-Based Nanoadsorbent Materials for Environmental Contaminants Detection. TrAC Trends Anal. Chem. 2021, 139, 116255. [Google Scholar] [CrossRef]
- Li, X.; Li, S.; Bai, J.; Peng, Y.; Ning, B.; Shi, H.; Kang, W.; Zhou, H.; Gao, Z. Determination of Bisphenol A by High-Performance Liquid Chromatography Based on Graphene Magnetic Dispersion Solid Phase Extraction. J. Chromatogr. Sci. 2020, 58, 280–286. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Y.; Tang, Y.; Li, X.; Zhang, X.; Li, C.; Xu, S. Magnetic Solid-Phase Extraction Based on Fe3O4/Graphene Oxide Nanoparticles for the Determination of Malachite Green and Crystal Violet in Environmental Water Samples by HPLC. Int. J. Env. Anal. Chem. 2018, 98, 215–228. [Google Scholar] [CrossRef]
- Hou, X.; Ding, R.; Yan, S.; Zhao, H.; Yang, Q.; Wu, W. ZrO2 Nanoparticles and Poly(Diallyldimethylammonium Chloride)-Doped Graphene Oxide Aerogel-Coated Stainless-Steel Mesh for the Effective Adsorption of Organophosphorus Pesticides. Foods 2021, 10, 1616. [Google Scholar] [CrossRef]
- Sun, H.; Feng, J.; Feng, J.; Sun, M.; Feng, Y.; Sun, M. Carbon Aerogels Derived from Waste Paper for Pipette-Tip Solid-Phase Extraction of Triazole Fungicides in Tomato, Apple and Pear. Food Chem. 2022, 395, 133633. [Google Scholar] [CrossRef]
- Gao, Y.; Gao, M.; Chen, G.; Tian, M.; Zhai, R.; Huang, X.; Xu, X.; Liu, G.; Xu, D. Facile Synthesis of Covalent Organic Frameworks Functionalized with Graphene Hydrogel for Effectively Extracting Organophosphorus Pesticides from Vegetables. Food Chem. 2021, 352, 129187. [Google Scholar] [CrossRef]
- Ligorio, C.; Vijayaraghavan, A.; Hoyland, J.A.; Saiani, A. Acidic and Basic Self-Assembling Peptide and Peptide-Graphene Oxide Hydrogels: Characterisation and Effect on Encapsulated Nucleus Pulposus Cells. Acta Biomater. 2022, 143, 145–158. [Google Scholar] [CrossRef]
- Wang, N.; Lu, Y.; Cui, B. Preparation and Application of β-Cyclodextrin Functionalised Graphene Oxide-Grafted Silica Sorbents for Solid-Phase Extraction (SPE) of Polycyclic Aromatic Hydrocarbons from Fried Food Using a Box-Behnken Design. Food Anal. Methods 2021, 14, 1577–1589. [Google Scholar] [CrossRef]
- Song, X.-L.; Lv, H.; Wang, D.-D.; Liao, K.-C.; Wu, Y.-Y.; Li, G.-M.; Chen, Y. Graphene Oxide Composite Microspheres as a Novel Dispersive Solid-Phase Extraction Adsorbent of Bisphenols Prior to Their Quantitation by HPLC–Mass Spectrometry. Microchem. J. 2022, 172, 106920. [Google Scholar] [CrossRef]
- Sun, M.; Han, S.; Maloko Loussala, H.; Feng, J.; Li, C.; Ji, X.; Feng, J.; Sun, H. Graphene Oxide-Functionalized Mesoporous Silica for Online in-Tube Solid-Phase Microextraction of Polycyclic Aromatic Hydrocarbons from Honey and Detection by High Performance Liquid Chromatography-Diode Array Detector. Microchem. J. 2021, 166, 106263. [Google Scholar] [CrossRef]
- Pei, J.; Ren, T.; Huang, Y.; Chen, R.; Jin, W.; Shang, S.; Wang, J.; Liu, Z.; Liang, Y.; Abd El-Aty, A.M. Application of Graphene and Its Derivatives in Detecting Hazardous Substances in Food: A Comprehensive Review. Front. Chem. 2022, 10, 894759. [Google Scholar] [CrossRef]
- Hu, H.; Wen, W.; Ou, J. Z Construction of Adsorbents with Graphene and Its Derivatives for Wastewater Treatment: A Review. Environ. Sci. Nano 2022, 9, 3226–3276. [Google Scholar] [CrossRef]
- Li, Y.; Lan, S.; Zhu, T. Recent Advances of Graphene-Based Sorptive Materials in Extraction: A Review. TrAC Trends Anal. Chem. 2021, 142, 116319. [Google Scholar] [CrossRef]
- Manousi, N.; Kabir, A.; Zachariadis, G.A. Recent Advances in the Extraction of Triazine Herbicides from Water Samples. J. Sep. Sci. 2022, 45, 113–133. [Google Scholar] [CrossRef]
- Musarurwa, H.; Tawanda Tavengwa, N. Recent Progress in the Application of PH-Responsive Polymers in Separation Science. Microchem. J. 2022, 179, 107503. [Google Scholar] [CrossRef]
- Jiang, X.; Ruan, G.; Huang, Y.; Chen, Z.; Yuan, H.; Du, F. Assembly and Application Advancement of Organic-functionalized Graphene-based Materials: A Review. J. Sep. Sci. 2020, 43, 1544–1557. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wang, R.; Chen, Z. Stir Bar Sorptive Extraction with a Graphene Oxide Framework-Functionalized Stainless-Steel Wire for the Determination of Sudan Dyes in Water Samples. Anal. Methods 2019, 11, 2050–2056. [Google Scholar] [CrossRef]
- Wang, X.; Han, Y.; Cao, J.; Yan, H. Headspace Solid-Phase-Microextraction Using a Graphene Aerogel for Gas Chromatography–Tandem Mass Spectrometry Quantification of Polychlorinated Naphthalenes in Shrimp. J. Chromatogr. A 2022, 1672, 463012. [Google Scholar] [CrossRef] [PubMed]
- Pei, M.; Shi, X.; Wu, J.; Huang, X. Graphene Reinforced Multiple Monolithic Fiber Solid-Phase Microextraction of Phenoxyacetic Acid Herbicides in Complex Samples. Talanta 2019, 191, 257–264. [Google Scholar] [CrossRef]
- de Toffoli, A.L.; Fumes, B.H.; Lanças, F.M. Packed In-Tube Solid Phase Microextraction with Graphene Oxide Supported on Aminopropyl Silica: Determination of Target Triazines in Water Samples. J. Env. Sci. Health B 2018, 53, 434–440. [Google Scholar] [CrossRef]
- Sun, R.; Lu, F.; Yu, C.; Yang, Y.; Qiao, L.; Liu, A. Peanut Shells-Derived Biochars as Adsorbents for the Pipette-Tip Solid-Phase Extraction of Endocrine-Disrupting Phenols in Water, Milk and Beverage. J. Chromatogr. A 2022, 1673, 463101. [Google Scholar] [CrossRef] [PubMed]
- Grajek, H.; Jonik, J.; Witkiewicz, Z.; Wawer, T.; Purchała, M. Applications of Graphene and Its Derivatives in Chemical Analysis. Crit. Rev. Anal. Chem. 2020, 50, 445–471. [Google Scholar] [CrossRef]
- Costa Queiroz, M.E.; Donizeti de Souza, I.; Marchioni, C. Current Advances and Applications of In-Tube Solid-Phase Microextraction. TrAC Trends Anal. Chem. 2019, 111, 261–278. [Google Scholar] [CrossRef]
- Ponce-Rodríguez, H.D.; Verdú-Andrés, J.; Herráez-Hernández, R.; Campíns-Falcó, P. Innovations in Extractive Phases for In-Tube Solid-Phase Microextraction Coupled to Miniaturized Liquid Chromatography: A Critical Review. Molecules 2020, 25, 2460. [Google Scholar] [CrossRef]
- Technical Analytical Methods Committee Briefs What Causes Most Errors in Chemical Analysis? Anal. Methods 2013, 5, 2914–2915. [CrossRef] [PubMed]
- Liang, X.; Hou, X.; Chan, J.H.M.; Guo, Y.; Hilder, E.F. The Application of Graphene-Based Materials as Chromatographic Stationary Phases. TrAC Trends Anal. Chem. 2018, 98, 149–160. [Google Scholar] [CrossRef]
- Lanças, F.M.; Medina, D.A.V.; Pereira Dos Santos, N.G.; Sinisterra, M.J. Graphene-Based Sorbents for Modern Magnetic Solid-Phase Extraction Techniques. In Analytical Applications of Functionalized Magnetic Nanoparticles; The Royal Society of Chemistry: London, UK, 2021; pp. 174–199. [Google Scholar]
- dos Santos, N.G.P.; Maciel, E.V.S.; Mejía-Carmona, K.; Lanças, F.M. Multidimensional Capillary Liquid Chromatography-Tandem Mass Spectrometry for the Determination of Multiclass Pesticides in “Sugarcane Spirits” (Cachaças). Anal. Bioanal. Chem. 2020, 412, 7789–7797. [Google Scholar] [CrossRef]
- MacIel, E.V.S.; Mejiá-Carmona, K.; Lancas, F.M. Evaluation of Two Fully Automated Setups for Mycotoxin Analysis Based on Online Extraction-Liquid Chromatography-TandemMass Spectrometry. Molecules 2020, 25, 2756. [Google Scholar] [CrossRef]
- Mejía-Carmona, K.; Lanças, F.M. Modified Graphene-Silica as a Sorbent for in-Tube Solid-Phase Microextraction Coupled to Liquid Chromatography-Tandem Mass Spectrometry. Determination of Xanthines in Coffee Beverages. J. Chromatogr. A 2020, 1621, 461089. [Google Scholar] [CrossRef]
- Maciel, E.V.S.; Vargas-Medina, D.A.; Lancas, F.M. Analyzes of β-Lactam Antibiotics by Direct Injection of Environmental Water Samples into a Functionalized Graphene Oxide-Silica Packed Capillary Extraction Column Online Coupled to Liquid Chromatography Tandem Mass Spectrometry. Talanta Open 2023, 7, 100185. [Google Scholar] [CrossRef]
- Maciel, E.V.S.; de Toffoli, A.L.; da Silva Alves, J.; Lanças, F.M. Multidimensional Liquid Chromatography Employing a Graphene Oxide Capillary Column as the First Dimension: Determination of Antidepressant and Antiepileptic Drugs in Urine. Molecules 2020, 25, 1092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, X.; Liu, S.; Song, X.; Zhu, Y.; Jiang, S. Layer-by-Layer Self-Assembled Graphene Oxide/Silica Microsphere Composites as Stationary Phase for High Performance Liquid Chromatography. Analyst 2012, 137, 5237. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, S.; Han, Q.; Ding, M. Preparation and Retention Mechanism Study of Graphene and Graphene Oxide Bonded Silica Microspheres as Stationary Phases for High Performance Liquid Chromatography. J. Chromatogr. A 2013, 1307, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Wang, S.; Liu, S.; Liu, X.; Jiang, S. A Novel Octadecylsilane Functionalized Graphene Oxide/Silica Composite Stationary Phase for High Performance Liquid Chromatography. J. Sep. Sci. 2012, 35, 2003–2009. [Google Scholar] [CrossRef] [PubMed]
- Borsatto, J.V.B.; Maciel, E.V.S.; Cifuentes, A.; Lanças, F.M. Online Extraction Followed by LC–MS/MS Analysis of Lipids in Natural Samples: A Proof-of-Concept Profiling Lecithin in Seeds. Foods 2023, 12, 281. [Google Scholar] [CrossRef]
- Liang, X.; Wang, X.; Ren, H.; Jiang, S.; Wang, L.; Liu, S. Gold Nanoparticle Decorated Graphene Oxide/Silica Composite Stationary Phase for High-Performance Liquid Chromatography. J. Sep. Sci. 2014, 37, 1371–1379. [Google Scholar] [CrossRef]
- Li, Y.; Li, Q.; Zhu, N.; Gao, Z.; Ma, Y. Cellulose Type Chiral Stationary Phase Based on Reduced Graphene Oxide@silica Gel for the Enantiomer Separation of Chiral Compounds. Chirality 2018, 30, 996–1004. [Google Scholar] [CrossRef]
- Maciel, E.V.S.; Borsatto, J.V.B.; Mejia-Carmona, K.; Lanças, F.M. Application of an In-House Packed Octadecylsilica-Functionalized Graphene Oxide Column for Capillary Liquid Chromatography Analysis of Hormones in Urine Samples. Anal. Chim. Acta 2023, 1239, 340718. [Google Scholar] [CrossRef]
- Borsatto, J.V.B.; Maciel, E.V.S.; Lanças, F.M. Investigation of the Applicability of Silica-Graphene Hybrid Materials as Stationary Phases for Capillary Liquid Chromatography. J. Chromatogr. A 2022, 1685, 463618. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, C.; Martínez-Bueno, M.J.; Lozano, A.; Fernández-Alba, A.R. Pesticide Residue Analysis of Fruit Juices by LC–MS/MS Direct Injection. One Year Pilot Survey. Talanta 2011, 83, 1552–1561. [Google Scholar] [CrossRef] [PubMed]
- by Liquid Chromatography–Electrospray Tandem Mass Spectrometry. J. Chromatogr. A 2003, 992, 133–140. [CrossRef]
- Lee, T.D. Introduction to Modern Liquid Chromatography, Third Edition. J. Am. Soc. Mass. Spectrom. 2011, 22, 196. [Google Scholar] [CrossRef] [Green Version]
- González-Ruiz, V.; Olives, A.I.; Martín, M.A. Core-Shell Particles Lead the Way to Renewing High-Performance Liquid Chromatography. TrAC Trends Anal. Chem. 2015, 64, 17–28. [Google Scholar] [CrossRef]
- Blue, L.E.; Jorgenson, J.W. 1.1μm Superficially Porous Particles for Liquid Chromatography. Part II: Column Packing and Chromatographic Performance. J. Chromatogr. A 2015, 1380, 71–80. [Google Scholar] [CrossRef]
- Zhao, C.; Yan, S.; Liu, J.; Xiong, Z.; Zhao, L. Octadecylamine and Serine-Derived Carbon Dots-Modified Silica Gel for Reversed Phase/Hydrophilic Interaction Liquid Chromatography. Microchem. J. 2022, 183, 107987. [Google Scholar] [CrossRef]
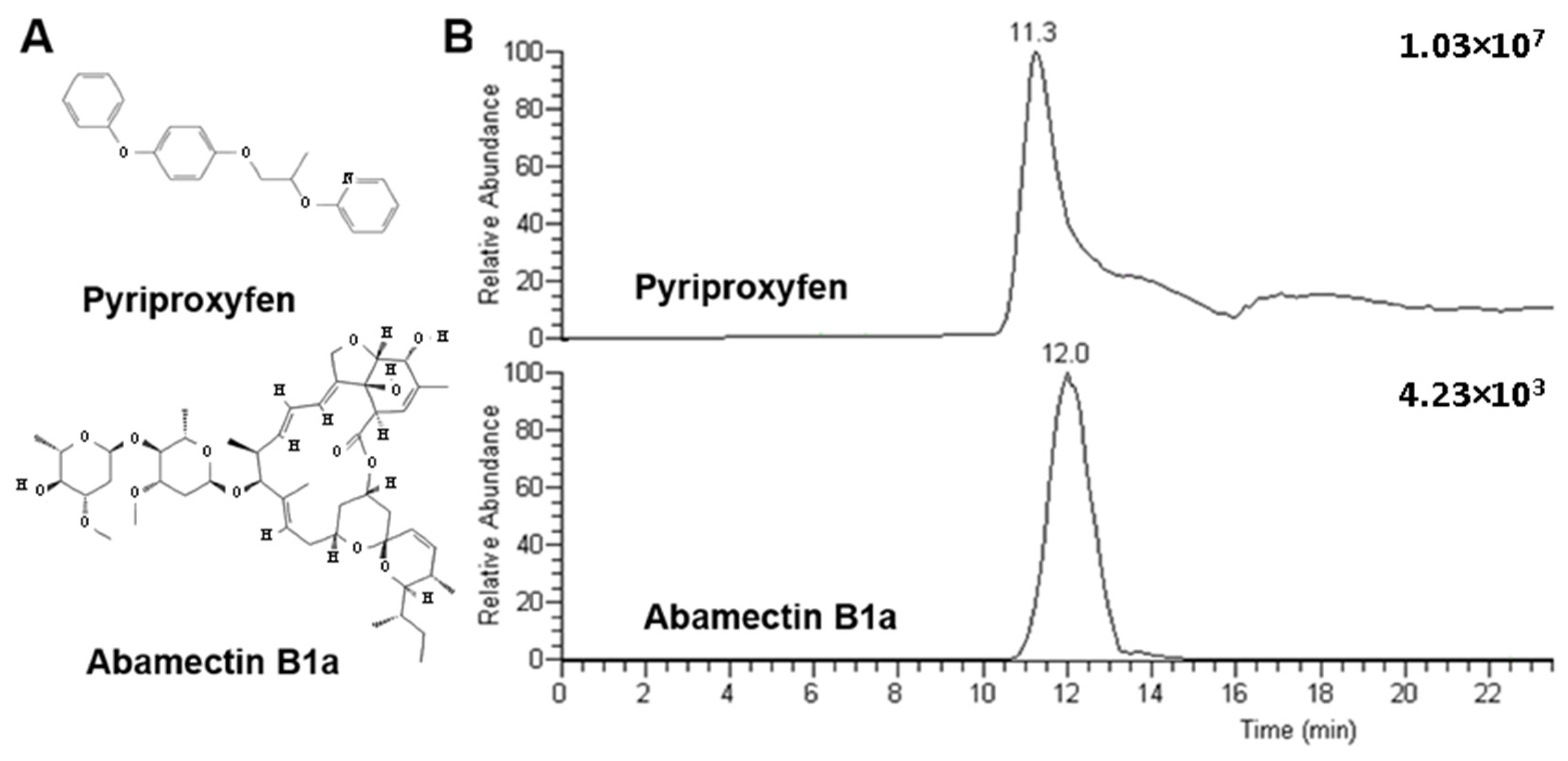
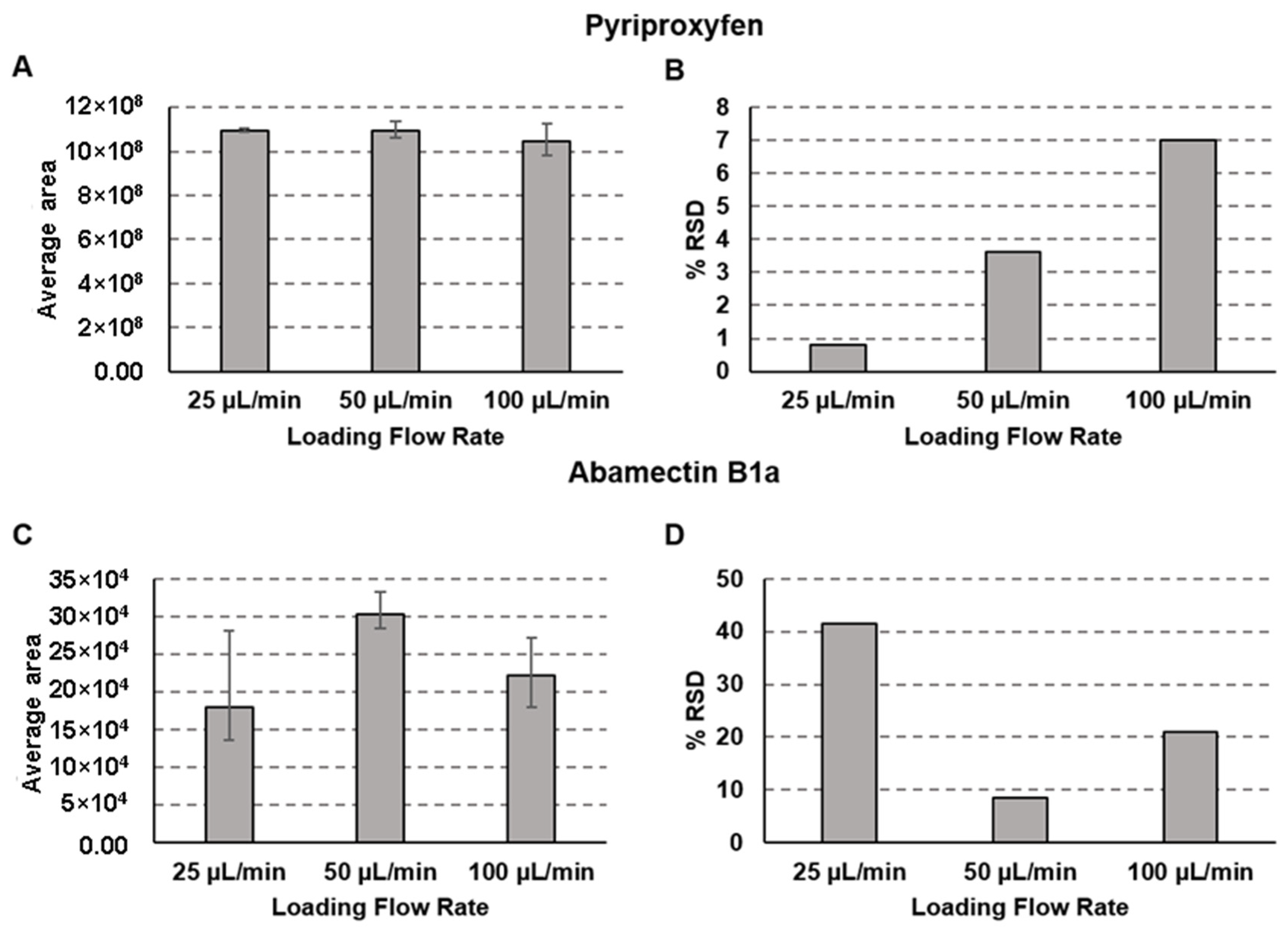
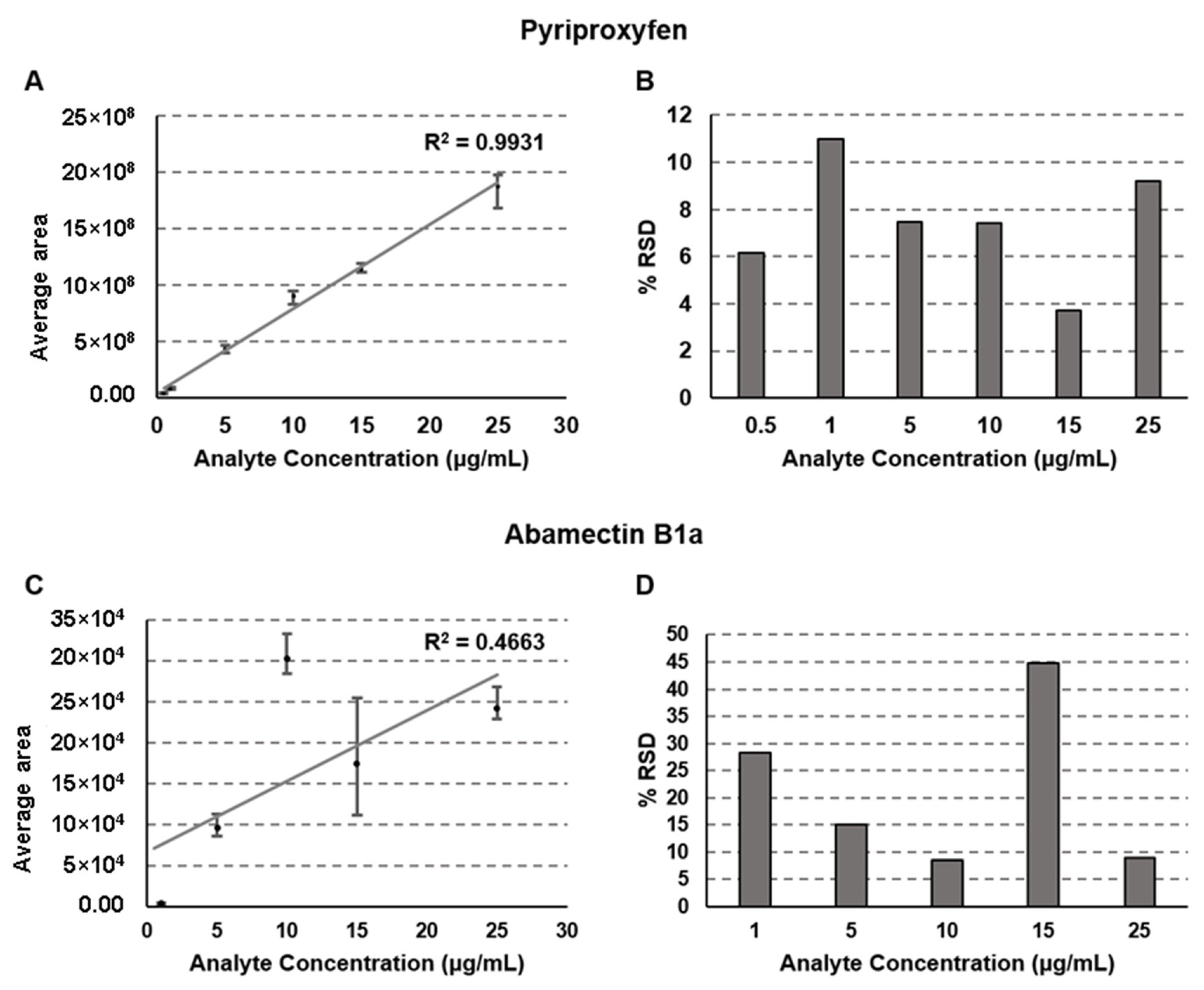

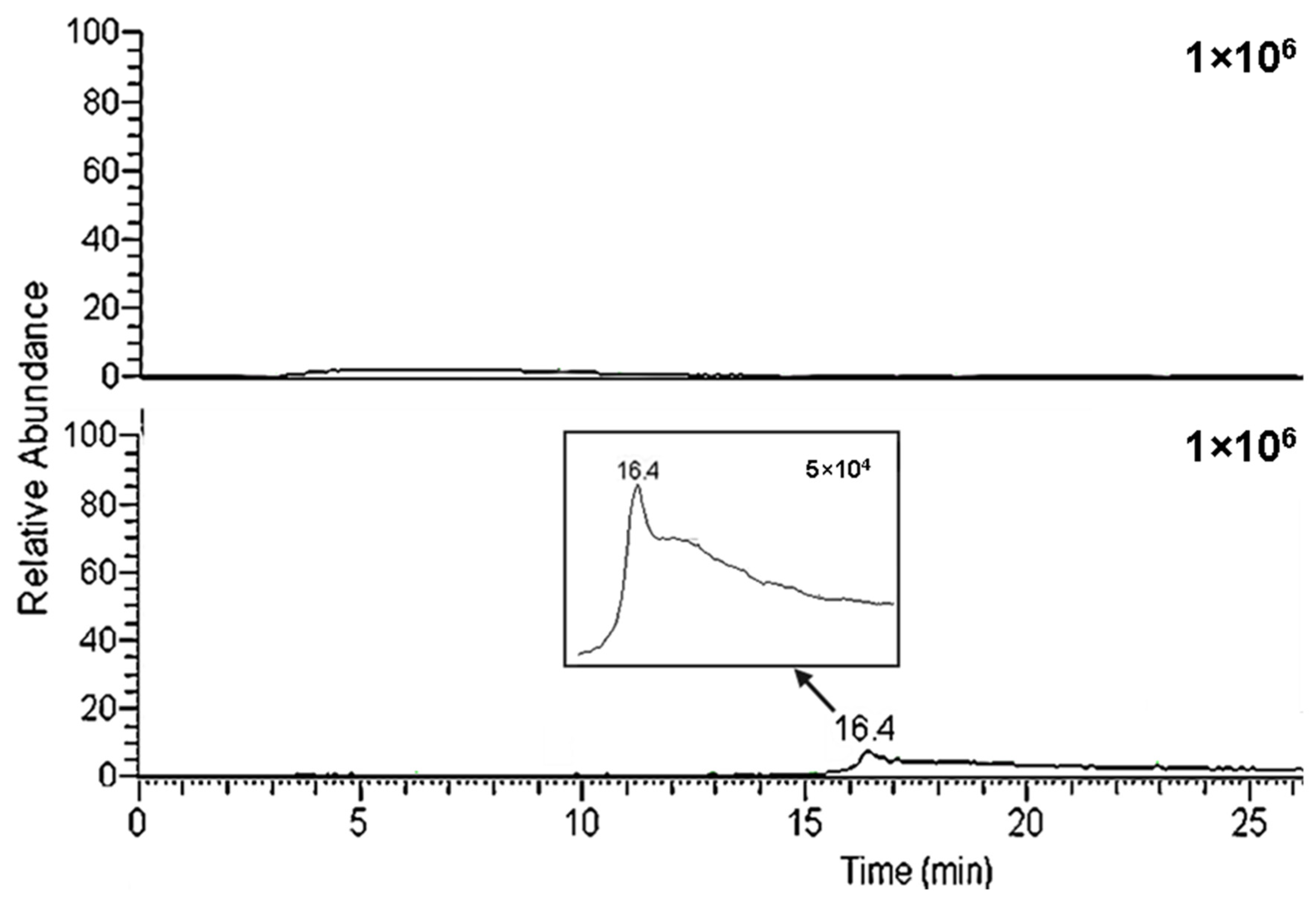

| Compound | Pyriproxyfen | Abamectin B1a | |||||
|---|---|---|---|---|---|---|---|
| Concentration (µg/mL) | 2 | 8 | 20 | 2 | 8 | 20 | |
| Expected Area * | 190,000,000 | 610,000,000 | 1,450,000,000 | 84,362 | 136,104 | 239,587 | |
| Day 1 | Area | 176,290,988 | 612,883,132 | 1,438,632,466 | 4899 | 120,972 | 274,393 |
| Intra-Day RSD % | 5.8 | 7.8 | 3.1 | 172.8 | 22.6 | 9 | |
| % Accuracy ** | 92.8 | 100.5 | 99.2 | 5.8 | 88.9 | 114.5 | |
| Day 2 | Area | 156,108,411 | 621,257,647 | 1,501,904,177 | 7836 | 99,444 | 378,336 |
| Intra-Day RSD % | 8.9 | 13.3 | 3.3 | 96.1 | 31.3 | 3 | |
| % Accuracy ** | 82.2 | 101.8 | 103.6 | 9.3 | 73.1 | 157.9 | |
| Day 3 | Area | 211,749,192 | 567,532,037 | 1,399,813,509 | 12,072 | 282,730 | 372,730 |
| Intra-Day RSD % | 3.2 | 5.3 | 5.4 | 344.6 | 18.9 | 17 | |
| % Accuracy ** | 111.4 | 93.0 | 96.5 | 14.3 | 207.7 | 155.6 | |
| Average Inter-Day Area | 181,382,863 | 600,557,605 | 1,446,783,384 | 8269 | 167,715 | 341,820 | |
| Inter-Day RSD % *** | 12.7 | 3.9 | 2.9 | 35.6 | 48.8 | 14 | |
| Inter-Day % Accuracy ** | 95.5 | 98.5 | 99.8 | 9.8 | 123.2 | 142.7 | |
| Event | Time | % B | Injection Valve Position | MS Valve Position |
|---|---|---|---|---|
| Pre-run | 0 min | 0% | Loading | Waste |
| Loading | 0 to 3 min | 0% | Inject | Waste |
| Gradient | 3 to 18 min | 50% to 100% | Inject | ESI-MS |
| Isocratic | 18 to 23 min | 100% | Inject | ESI-MS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borsatto, J.V.B.; Maciel, E.V.S.; Cifuentes, A.; Lanças, F.M. Applicability and Limitations of a Capillary-LC Column-Switching System Using Hybrid Graphene-Based Stationary Phases. Molecules 2023, 28, 4999. https://doi.org/10.3390/molecules28134999
Borsatto JVB, Maciel EVS, Cifuentes A, Lanças FM. Applicability and Limitations of a Capillary-LC Column-Switching System Using Hybrid Graphene-Based Stationary Phases. Molecules. 2023; 28(13):4999. https://doi.org/10.3390/molecules28134999
Chicago/Turabian StyleBorsatto, João Victor Basolli, Edvaldo Vasconcelos Soares Maciel, Alejandro Cifuentes, and Fernando Mauro Lanças. 2023. "Applicability and Limitations of a Capillary-LC Column-Switching System Using Hybrid Graphene-Based Stationary Phases" Molecules 28, no. 13: 4999. https://doi.org/10.3390/molecules28134999








