Advances in Natural Product Extraction Techniques, Electrospun Fiber Fabrication, and the Integration of Experimental Design: A Comprehensive Review
Abstract
:1. Introduction
2. Extraction
2.1. Conventional Extraction Methods
2.1.1. Soxhlet Extraction
2.1.2. Maceration
2.1.3. Percolation
2.1.4. Decoction
2.2. Non-Conventional Extraction Methods
2.2.1. Microwave-Assisted Extraction (MAE)
2.2.2. Supercritical Fluid Extraction (SFE)
2.2.3. Ultrasound-Assisted Extraction (UAE)
2.3. Parameters Affecting the Quality of Extracts Obtained from UAE
2.3.1. Frequency
2.3.2. Power and Amplitude
2.3.3. Solvent
2.3.4. Processing Time
2.3.5. Extraction Temperature
2.3.6. Solvent-to-Solid Ratio
3. Techniques for Separation and Chemical Characterization of Extracts
3.1. High-Performance Liquid Chromatography-Diode Array Detection (HPLC-DAD)
3.2. High-Performance Liquid Chromatography-Mass Spectrometry (HPLC-MS)
3.3. High-Performance Liquid Chromatography-Diode Array Detection-Mass Spectrometry (HPLC-DAD-MS)
3.4. High-Performance Liquid Chromatography-Nuclear Magnetic Resonance (HPLC-NMR)
3.5. High-Performance Liquid Chromatography-Diode Array Detection-Solid Phase Extraction-Nuclear Magnetic Resonance (HPLC-DAD-SPE-NMR)
3.6. High-Performance Liquid Chromatography-Photodiode Array Detection-Mass Spectrometry-Solid-Phase Extraction-Nuclear Magnetic Resonance (HPLC-PDA-MS-SPE-NMR)
3.7. Gas Chromatography-Flame Ionization Detection (GC-FID)
3.8. Gas Chromatography-Mass Spectrometry (GC-MS)
4. Quality Control, Standardization and Biological Activity of Extracts
5. Examples of the Extraction and Chemical Analysis of Natural Products
5.1. Extraction and Chemical Analysis of Plant Extracts
5.2. Extraction and Chemical Analysis of Extracts from Animal Tissues
6. Electrospinning
6.1. Principles of the Electrospinning
- Appropriate solvents for dissolving the polymer should be available. The viscosity and surface tension of the solvent should not be too high to prevent the formation of a jet, nor should they be too low to allow the polymer solution to flow freely from the nozzle.
- The applied voltage must be sufficient to overcome the viscosity and surface tension of the polymer solution in order to create and maintain a jet from the syringe tip.
- The length between the spinneret and the grounded surface should be sufficient for solvent evaporation in time for fiber formation, but not so narrow as to produce sparks between the electrodes.
- Electrospun nanofibers with their high porosity and similarity to the natural extracellular matrix (ECM) can promote cell adhesion, proliferation, migration, and differentiation.
- A high specific surface area is advantageous for wound exudate, the dispersion of bioactive compounds, and enhancing the solubility of substances that are poorly water-soluble.
- Fiber morphology is advantageously utilized as a multifunctional wound dressing material.
- Enhancement of bioactive compound encapsulation effectiveness.
- Variable surface architecture.
- Decreased burst release and the potential for efficient surface functionalization through the appropriate selection of a bioactive compound-polymer-solvent system.
6.2. Electrospinnable Polymers
6.2.1. Natural Polymers
6.2.2. Synthetic Polymers
6.2.3. Blend Polymers
6.3. Development of Shellac-Based Electrospun Fibers
6.4. Parameters Affecting the Quality of Electrospun Fibers
6.4.1. Polymer Solution Parameters
- Viscosity
- Surface tension
- Electrical conductivity
6.4.2. Processing Parameters
- Applied electrical voltage.
- Solution feed rate
- Distance between the needle tip and collector
6.4.3. Environmental Parameters
- Temperature and humidity
7. Design of Experiment (DOE)
7.1. Experimental Design and Its Terminology
7.2. Process of the Experimental Design
7.2.1. Screening Design
- Full factorial design
- Fractional factorial design
- Plackett-Burman design
7.2.2. Optimization Design
- Box-Behnken design
- Central composite design
7.2.3. Verification of an Optimization Model
7.2.4. Statistical Analysis for Experimental Design
8. Application of Experimental Design
8.1. Experimental Design Applications in the UAE Procedure
8.2. Experimental Design Applications in Electrospun Fiber Production
9. Discussion
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Hussein, R.A.; El-Anssary, A.A. The Key Drivers of the Pharmacological Actions of Medicinal Plants. In Herbal Medicine, 1st ed.; Philip, F.B., Ed.; Intech: Rijeka, Croatia, 2018; Volume 1, pp. 11–30. [Google Scholar]
- Fomo, G.; Madzimbamuto, T.N.; Ojumu, T.V. Applications of nonconventional green extraction technologies in process industries: Challenges, limitations and perspectives. Sustainability 2020, 12, 5244. [Google Scholar] [CrossRef]
- Kumar, K.; Srivastav, S.; Sharanagat, V.S. Ultrasound assisted extraction (UAE) of bioactive compounds from fruit and vegetable processing by-products: A review. Ultrason. Sonochem. 2021, 70, 105325. [Google Scholar] [CrossRef] [PubMed]
- Kassing, M.; Jenelten, U.; Schenk, J.; Strube, J. A new approach for process development of plant-based extraction processes. Chem. Eng. Technol. 2010, 33, 377–387. [Google Scholar] [CrossRef]
- Kettaneh-Wold, N. Use of experimental design in the pharmaceutical industry. J. Pharm. Biomed. Anal. 1991, 9, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Lozada-Ramírez, J.D.; Ortega-Regules, A.E.; Hernández, L.R.; de Parrodi, C.A. Spectroscopic and spectrometric applications for the identification of bioactive compounds from vegetal Extracts. Appl. Sci. 2021, 11, 3039. [Google Scholar] [CrossRef]
- Dar, A.A.; Raina, A.; Kumar, A. Development, method validation and simultaneous quantification of eleven bioactive natural products from high-altitude medicinal plant by high-performance liquid chromatography. Biomed. Chromatogr. 2022, 36, e5408. [Google Scholar] [CrossRef]
- Islama, M.M.; Varshini, H.R.; Bhavani, P.D.; Goudanavar, P.S.; Naveen, N.R.; Ramesh, B.; Fattepur, S.; Shiroorkar, P.N.; Habeebuddin, M.; Meravanige, G.; et al. Optimization of process parameters for fabrication of electrospun nanofibers containing neomycin sulfate and Malva sylvestris extract for a better diabetic wound healing. Drug Deliv. 2022, 29, 3370–3383. [Google Scholar] [CrossRef]
- Tahir, M.; Vicini, S.; Sionkowska, A. Electrospun materials based on polymer and biopolymer blends-A review. Polymers 2023, 15, 1654. [Google Scholar] [CrossRef]
- Abubakar, A.R.; Haque, M. Preparation of medicinal plants: Basic extraction and fractionation procedures for experimental purposes. J. Pharm. Bioallied Sci. 2020, 12, 1–10. [Google Scholar] [CrossRef]
- Handa, S.S. An Overview of Extraction Techniques for Medicinal and Aromatic Plants. In Extraction Technologies for Medicinal and Aromatic Plants, 1st ed.; Handa, S.S., Khanuja, S.P.S., Longo, G., Rakesh, D.D., Eds.; International Centre for Science and High Technology: Trieste, Italy, 2008; Volume 1, pp. 21–25. [Google Scholar]
- Zhang, Q.-W.; Lin, L.-G.; Ye, W.-C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13, 20. [Google Scholar] [CrossRef] [Green Version]
- Picot-Allain, C.; Mahomoodally, M.F.; Ak, G.; Zengin, G. Conventional versus green extraction techniques—A comparative perspective. Curr. Opin. Food Sci. 2021, 40, 144–156. [Google Scholar] [CrossRef]
- Rasul, M.G. Extraction, isolation and characterization of natural products from medicinal plants. Int. J. Basic Sci. Appl. Comput. 2018, 2, 1–6. [Google Scholar]
- Rasul, M.G. Conventional extraction methods use in medicinal plants, their advantages and disadvantages. Int. J. Basic Sci. Appl. Comput. 2018, 2, 10–14. [Google Scholar]
- Mandal, V.; Mohan, Y.; Hemalatha, S. Microwave assisted extraction—An innovative and promising extraction tool for medicinal plant research. Pharmacogn. Rev. 2007, 1, 7–18. [Google Scholar]
- Bachtler, S.; Bart, H.-J. Increase the yield of bioactive compounds from elder bark and annatto seeds using ultrasound and microwave assisted extraction technologies. Food Bioprod. Process. 2021, 125, 1–13. [Google Scholar] [CrossRef]
- Ahmad, T.; Masoodi, F.A.; Rather, S.A.; Wani, S.M.; Gull, A. Supercritical fluid extraction: A review. J. Biol. Chem. Chron. 2019, 5, 114–122. [Google Scholar] [CrossRef]
- Mehta, N.; Jeyapriya, S.; Kumar, P.; Verma, A.K.; Umaraw, P.; Khatkar, S.K.; Khatkar, A.B.; Pathak, D.; Kaka, U.; Sazili, A.Q. Ultrasound-assisted extraction and the encapsulation of bioactive components for food applications. Foods 2022, 11, 2973. [Google Scholar] [CrossRef]
- Fu, X.; Belwal, T.; Cravotto, G.; Luo, Z. Sono-physical and sono-chemical effects of ultrasound: Primary applications in extraction and freezing operations and influence on food components. Ultrason. Sonochem. 2020, 60, 104726. [Google Scholar] [CrossRef]
- Ranjha, M.M.A.N.; Irfan, S.; Lorenzo, J.M.; Shafique, B.; Kanwal, R.; Pateiro, M.; Arshad, R.N.; Wang, L.; Nayik, G.A.; Roobab, U.; et al. Sonication, a potential technique for extraction of phytoconstituents: A systematic review. Processes 2021, 9, 1406. [Google Scholar] [CrossRef]
- Han, H.; Wang, S.; Rakita, M.; Wang, Y.; Han, Q.; Xu, Q. Effect of ultrasound-assisted extraction of phenolic compounds on the characteristics of walnut shells. Food Nutr. Sci. 2018, 9, 1034–1045. [Google Scholar] [CrossRef] [Green Version]
- Doughari, J.H. Phytochemicals: Extraction Methods, Basic Structures and Mode of Action as Potential Chemotherapeutic Agents. In Phytochemicals, 1st ed.; Rao, V., Ed.; Intech: Rijeka, Croatia, 2012; Volume 1, pp. 1–29. [Google Scholar] [CrossRef] [Green Version]
- Moradi-kheibari, N.; Ahmadzadeh, H.; Talebi, A.F.; Hosseini, M.; Murry, M.A. Recent Advances in Lipid Extraction for Biodiesel Production. In Advances in Feedstock Conversion Technologies for Alternative Fuels and Bioproducts, 1st ed.; Hosseini, M., Ed.; Woodhead Publishing: Cambridge, UK, 2019; Volume 1, pp. 179–198. [Google Scholar] [CrossRef]
- Carreira-Casais, A.; Otero, P.; Garcia-Perez, P.; Garcia-Oliveira, P.; Pereira, A.G.; Carpena, M.; Soria-Lopez, A.; Simal-Gandara, J.; Prieto, M.A. Benefits and drawbacks of ultrasound-assisted extraction for the recovery of bioactive compounds from marine algae. Int. J. Environ. Res. Public Health 2021, 18, 9153. [Google Scholar] [CrossRef] [PubMed]
- Usman, I.; Hussain, M.; Imran, A.; Afzaal, M.; Saeed, F.; Javed, M.; Afzal, A.; Ashfaq, I.; Jbawi, E.A.; Saewan, S.A. Traditional and innovative approaches for the extraction of bioactive compounds. Int. J. Food Prop. 2022, 25, 1215–1233. [Google Scholar] [CrossRef]
- Yu, H.; Liu, Y.; Li, L.; Guo, Y.; Xie, Y.; Cheng, Y.; Yao, W. Ultrasound-involved emerging strategies for controlling foodborne microbial biofilms. Trends Food Sci. Technol. 2020, 96, 91–101. [Google Scholar] [CrossRef]
- Tušek, A.J.; Šamec, D.; Šalić, A. Modern techniques for flavonoid extraction—To optimize or not to optimize? Appl. Sci. 2022, 12, 11865. [Google Scholar] [CrossRef]
- Bu, X.; Alheshibri, M. The effect of ultrasound on bulk and surface nanobubbles: A review of the current status. Ultrason. Sonochem. 2021, 76, 105629. [Google Scholar] [CrossRef]
- Wen, C.; Zhang, J.; Zhang, H.; Dzah, C.S.; Zandile, M.; Duan, Y.; Ma, H.; Luo, X. Advances in ultrasound assisted extraction of bioactive compounds from cash crops—A review. Ultrason. Sonochem. 2018, 48, 538–549. [Google Scholar] [CrossRef]
- Suhaimi, S.H.; Hasham, R.; Idris, M.K.H.; Ismail, H.F.; Ariffin, N.H.M.; Majid, F.A.A. Optimization of ultrasound-assisted extraction conditions followed by solid phase extraction fractionation from Orthosiphon stamineus Benth (Lamiace) leaves for antiproliferative effect on prostate cancer cells. Molecules 2019, 24, 4183. [Google Scholar] [CrossRef] [Green Version]
- Sun, A.; Chi, X.; Yang, X.; Feng, J.; Li, Y.; Zhou, J. Applications and prospects of ultrasound-assisted extraction in Chinese herbal medicine. J. Biomed. Sci. 2019, 1, 5–15. [Google Scholar] [CrossRef] [Green Version]
- Linares, G.; Rojas, M.L. Ultrasound-assisted extraction of natural pigments from food processing by-products: A review. Front. Nutr. 2022, 9, 891462. [Google Scholar] [CrossRef]
- Vernès, L.; Vian, M.; Chemat, F. Ultrasound and Microwave as Green Tools for Solid-liquid Extraction. In Liquid-phase Extraction, 1st ed.; Poole, C., Ed.; Elsevier: Amsterdam, Netherlands, 2020; Volume 1, pp. 355–374. [Google Scholar] [CrossRef]
- Kobus, Z.; Krzywicka, M.; Starek-Wójcicka, A.; Sagan, A. Effect of the duty cycle of the ultrasonic processor on the efficiency of extraction of phenolic compounds from Sorbus intermedia. Sci. Rep. 2022, 12, 8311. [Google Scholar] [CrossRef]
- Gullón, B.; Eibes, G.; Moreira, M.T.; Herrera, R.; Labidi, J.; Gullón, P. Yerba mate waste: A sustainable resource of antioxidant compounds. Ind. Crops Prod. 2018, 113, 398–405. [Google Scholar] [CrossRef]
- Heinrich, M.; Jalil, B.; Abdel-Tawab, M.; Echeverria, J.; Kulić, Ž.; McGaw, L.J.; Pezzuto, J.M.; Potterat, O.; Wang, J.-B. Best Practice in the chemical characterisation of extracts used in pharmacological and toxicological research—The ConPhyMP—Guidelines. Front. Pharmacol. 2022, 13, 953205. [Google Scholar] [CrossRef]
- Wilson, I.D.; Poole, C.F. Planar chromatography—Current practice and future prospects. J. Chromatogr. B 2023, 1214, 123553. [Google Scholar] [CrossRef]
- Latif, Z.; Sarker, S.D. Isolation of natural products by preparative high performance liquid chromatography (prep-HPLC). Methods Mol. Biol. 2012, 864, 255–274. [Google Scholar] [CrossRef]
- Özek, T.; Demirci, F. Isolation of natural products by preparative gas chromatography. Methods Mol. Biol. 2012, 864, 275–300. [Google Scholar] [CrossRef]
- Mao, I.L.; Martin-Pernier, J.; Bautista, C.; Lacampagne, S.; Richard, T.; Costa, G.D. 1H-NMR metabolomics as a tool for winemaking monitoring. Molecules 2021, 26, 6771. [Google Scholar] [CrossRef]
- Bross-Walch, N.; Kühn, T.; Moskau, D.; Zerbe, O. Strategies and tools for structure determination of natural products using modern methods of NMR spectroscopy. Chem. Biodivers. 2005, 2, 147–177. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, M.; Saroja, S.G.; Khan, I.A. NMR technique and methodology in botanical health product analysis and quality control. J. Pharm. Biomed. Anal. 2022, 207, 114376. [Google Scholar] [CrossRef]
- Přichystal, J.; Schug, K.A.; Lemr, K.; Novák, J.; Havlíček, V. Structural analysis of natural products. Anal. Chem. 2016, 88, 10338–10346. [Google Scholar] [CrossRef] [Green Version]
- Tshepelevitsh, S.; Hernits, K.; Jenčo, J.; Hawkins, J.M.; Muteki, K.; Solich, P.; Leito, I. Systematic optimization of liquid-liquid extraction for isolation of unidentified components. ACS Omega 2017, 2, 7772–7776. [Google Scholar] [CrossRef]
- Teipel, J.C.; Hausler, T.; Sommerfeld, K.; Scharinger, A.; Walch, S.G.; Lachenmeier, D.W.; Kuballa, T. Application of 1H nuclear magnetic resonance spectroscopy as spirit drinks screener for quality and authenticity control. Foods 2020, 9, 1355. [Google Scholar] [CrossRef] [PubMed]
- Elamin, M.M. Solvent extraction and spectroscopy identification of bioactive compounds from medicinal shrub Tamarix gallica. Funct. Foods Health Dis. 2020, 10, 456–464. [Google Scholar] [CrossRef]
- Zschocke, S.; Klaiber, I.; Bauer, R.; Vogler, B. HPLC-coupled spectroscopic techniques (UV, MS, NMR) for the structure elucidation of phthalides in Ligusticum chuanxiong. Mol. Divers. 2005, 9, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Li, D.-X.; Gan, L.; Bronja, A.; Schmitz, O.J. Gas chromatography coupled to atmospheric pressure ionization mass spectrometry (GC-API-MS): Review. Anal. Chim. Acta 2015, 891, 43–61. [Google Scholar] [CrossRef]
- Wolfender, J.-L. HPLC in natural product analysis: The detection issue. Planta Med. 2009, 75, 719–734. [Google Scholar] [CrossRef]
- Lindon, J.C.; Nicholson, J.K.; Wilson, I.D. The development and application of coupled HPLC-NMR spectroscopy. Adv. Chromatogr. 1996, 36, 315–382. [Google Scholar] [CrossRef]
- Keskes, H.; Belhadj, S.; Jlail, L.; Feki, A.E.; Damak, M.; Sayadi, S.; Allouche, N. LC-MS–MS and GC-MS analyses of biologically active extracts and fractions from Tunisian Juniperus phoenice leaves. Pharm. Biol. 2017, 55, 88–95. [Google Scholar] [CrossRef] [Green Version]
- Rather, M.A.; Ganai, B.A.; Kamili, A.N.; Qayoom, M.; Akbar, S.; Masood, A.; Rasool, R.; Wani, S.H.; Qurishi, M.A. Comparative GC–FID and GC–MS analysis of the mono and sesquiterpene secondary metabolites produced by the field grown and micropropagated plants of Artemisia amygdalina Decne. Acta Physiol. Plant. 2012, 34, 885–890. [Google Scholar] [CrossRef]
- Sarker, S.D.; Nahar, L. Hyphenated Techniques and Their Applications in Natural Products Analysis. In Natural Products Isolation (Methods in Molecular Biology), 3rd ed.; Sarker, S.D., Nahar, L., Eds.; Humana Press: Totowa, NJ, USA, 2012; Volume 864, pp. 301–340. [Google Scholar] [CrossRef]
- Es’haghi, Z. Photodiode Array Detection in Clinical Applications; Quantitative Analyte Assay Advantages, Limitations and Disadvantages. In Photodiodes—Communications, Bio-Sensings, Measurements and High-Energy Physics, 1st ed.; Jin-Wei, S., Ed.; Intech: Rijeka, Croatia, 2011; Volume 1, pp. 161–182. [Google Scholar] [CrossRef] [Green Version]
- Tasfiyati, A.N.; Antika, L.D.; Dewi, R.T.; Septama, A.W.; Sabarudin, A.; Ernawati, T. An experimental design approach for the optimization of scopoletin extraction from Morinda citrifolia L. using accelerated solvent extraction. Talanta 2022, 238, 123010. [Google Scholar] [CrossRef]
- Gomes, S.V.F.; Portugal, L.; dos Anjos, J.P.; de Jesus, O.N.; de Oliveira, E.J.; David, J.P.; David, J.M. Accelerated solvent extraction of phenolic compounds exploiting a Box-Behnken design and quantification of five flavonoids by HPLC-DAD in Passiflora species. Microchem. J. 2017, 132, 28–35. [Google Scholar] [CrossRef]
- Krongrawa, W.; Limmatvapirat, S.; Saibua, S.; Limmatvapirat, C. Optimization of ultrasound-assisted extraction of yields and total methoxyflavone contents from Kaempferia parviflora rhizomes. Molecules 2022, 27, 4162. [Google Scholar] [CrossRef]
- Sashidhara, K.V.; Rosaiah, J.N. Various dereplication strategies using LC-MS for rapid natural product lead identification and drug discovery. Nat. Prod. Commun. 2007, 2, 193–202. [Google Scholar] [CrossRef]
- Siuzdak, G. An introduction to mass spectrometry ionization: An excerpt from the expanding role of mass spectrometry in biotechnology. J. Assoc. Lab. Autom. 2005, 9, 50–63. [Google Scholar] [CrossRef] [Green Version]
- Dabetic, N.; Todorovic, V.; Malenovic, A.; Sobajic, S.; Markovic, B. Optimization of extraction and HPLC–MS/MS profiling of phenolic compounds from red grape seed extracts using conventional and deep eutectic solvents. Antioxidants 2022, 11, 1595. [Google Scholar] [CrossRef]
- Herderich, M.; Richling, E.; Roscher, R.; Schneider, C.; Schwab, W.; Humpf, H.-U.; Schreier, P. Application of atmospheric pressure ionization HPLC–MS–MS for the analysis of natural products. Chromatographia 1997, 45, 127–132. [Google Scholar] [CrossRef]
- Taamalli, A.; Arráez-Román, D.; Ibañez, E.; Zarrouk, M.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Optimization of microwave-assisted extraction for the characterization of olive leaf phenolic compounds by using HPLC-ESI-TOF-MS/IT-MS2. J. Agri. Food Chem. 2012, 60, 791–798. [Google Scholar] [CrossRef]
- Zhong, J.-S.; Wan, J.-Z.; Ding, W.-J.; Wu, X.-F.; Xie, Z.-Y. Multi-responses extraction optimization combined with high-performance liquid chromatography-diode array detection–electrospray ionization-tandem mass spectrometry and chemometrics techniques for the fingerprint analysis of Aloe barbadensis Miller. J. Pharm. Biomed. Anal. 2015, 107, 131–140. [Google Scholar] [CrossRef]
- Zielinski, A.A.F.; Haminiuk, C.W.I.; Beta, T. Multi-response optimization of phenolic antioxidants from white tea (Camellia sinensis L. Kuntze) and their identification by LC–DAD–Q-TOF–MS/MS. LWT Food Sci. Technol. 2016, 65, 897–907. [Google Scholar] [CrossRef] [Green Version]
- Jaroszewski, J.W. Hyphenated NMR Methods in Natural Products Research, Part 1: Direct Hyphenation. Planta Med. 2005, 71, 691–700. [Google Scholar] [CrossRef]
- Exarchou, V.; Krucker, M.; van Beek, T.A.; Vervoort, J.; Gerothanassis, I.P.; Albert, K. LC–NMR coupling tenchnology: Recent advancements and applications in natural products analysis. Magn. Reson. Chem. 2005, 43, 681–687. [Google Scholar] [CrossRef]
- Fukushi, E. Advanced NMR approaches for a detailed structure analysis of natural products. Biosci. Biotechnol. Biochem. 2006, 70, 1803–1812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwasa, K.; Takahashi, T.; Nishiyama, Y.; Moriyasu, M.; Sugiura, M.; Takeuchi, A.; Tode, C.; Tokuda, H.; Takeda, K. Online structural elucidation of alkaloids and other constituents in crude extracts and cultured cells of Nandina domestica by combination of LC–MS/MS, LC–NMR, and LC–CD analyses. J. Nat. Prod. 2008, 71, 1376–1385. [Google Scholar] [CrossRef] [PubMed]
- Wolfender, J.L.; Ndjoko, K.; Hostettmann, K. The Potential of LC-NMR in Phytochemical Analysis. Phytochem. Anal. 2001, 12, 2–22. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Powers, R.; Tymiak, A.; Espina, R.; Roongta, V. Introduction to NMR and Its Application in Metabolite Structure Determination. In Drug Metabolism in Drug Design and Development: Basic Concepts and Practice, 1st ed.; Zhang, D., Zhu, M., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2008; Volume 1, pp. 369–405. [Google Scholar] [CrossRef]
- Gebretsadik, T.; Linert, W.; Thomas, M.; Berhanu, T.; Frew, R. LC–NMR for natural product analysis: A journey from an academic curiosity to a robust analytical tool. Science 2021, 3, 6. [Google Scholar] [CrossRef]
- Mudaser, B.; Mumtaz, M.W.; Akhtar, M.T.; Mukhtar, H.; Raza, S.A.; Shami, A.A.; Touqeer, T. Response surface methodology based extraction optimization to improve pharmacological properties and 1H NMR based metabolite profiling of Azadirachta indica. Phytomed. Plus 2021, 1, 100015. [Google Scholar] [CrossRef]
- Hernández-Bolio, G.I.; Kutzner, E.; Eisenreich, W.; Torres-Acostac, J.F.J.; Peña-Rodríguez, L.M. The use of 1H-NMR metabolomics to optimize the extraction and preliminary identification of anthelmintic products from the leaves of Lysiloma latisiliquum. Phytochem. Anal. 2018, 29, 413–420. [Google Scholar] [CrossRef]
- Lambert, M.; Stærk, D.; Hansen, S.H.; Jaroszewski, J.W. HPLC–SPE–NMR hyphenation in natural products research: Optimization of analysis of Croton membranaceus extract. Magn. Reson. Chem. 2005, 43, 771–775. [Google Scholar] [CrossRef] [Green Version]
- Miliauskas, G.; van Beek, T.A.; de Waard, P.; Venskutonis, R.P.; Sudholter, E.J.R. Identification of radical scavenging compounds in Rhaponticum carthamoides by means of LC-DAD-SPE-NMR¨. J. Nat. Prod. 2005, 68, 168–172. [Google Scholar] [CrossRef]
- Clarkson, C.; Stærk, D.; Hansen, S.H.; Jaroszewski, J.W. Hyphenation of solid-phase extraction with liquid chromatography and nuclear magnetic resonance: Application of HPLC-DAD-SPE-NMR to identification of constituents of Kanahia laniflora. Anal. Chem. 2005, 77, 3547–3553. [Google Scholar] [CrossRef]
- Sprogøe, K.; Stærk, D.; Ziegler, H.L.; Jensen, T.H.; Holm-Møller, S.B.; Jaroszewski, J.W. Combining HPLC-PDA-MS-SPE-NMR with circular dichroism for complete natural product characterization in crude extracts: Levorotatory gossypol in Thespesia danis. J. Nat. Prod. 2008, 71, 516–519. [Google Scholar] [CrossRef]
- Wang, Z.; Paré, J.R.J. Chapter 3 gas chromatography (GC): Principles and applications. In Instrumental Methods in Food Analysis, 1st ed.; Paré, J.R.J., Bélanger, J.M.R., Eds.; Elsevier Science B.V.: Amsterdam, The Netherlands, 1997; Volume 18, pp. 61–91. [Google Scholar] [CrossRef]
- Miao, Q.; Kong, W.; Zhao, X.; Yang, S.; Yang, M. GC-FID coupled with chemometrics for quantitative and chemical fingerprinting analysis of Alpinia oxyphylla oil. J. Pharm. Biomed. Anal. 2015, 102, 436–442. [Google Scholar] [CrossRef]
- Buenoa, P.C.P.; Juniorb, M.G.; Bastos, J.K. A GC-FID validated method for the quality control of Eucalyptus globulus raw material and its pharmaceutical products, and GC-MS fingerprinting of 12 Eucalyptus species. Nat. Prod. Commun. 2014, 9, 1787–1790. [Google Scholar] [CrossRef] [Green Version]
- Alvarez-Rivera, G.; Ballesteros-Vivas, D.; Parada-Alfonso, F.; Ibañez, E.; Cifuentes, A. Recent applications of high resolution mass spectrometry for the characterization of plant natural products. TrAC Trends Anal. Chem. 2019, 112, 87–101. [Google Scholar] [CrossRef]
- Moldoveanu, S.C.; David, V. Derivatization Methods in GC and GC/MS, In Gas Chromatography—Derivatization, Sample Preparation, Application, 1st ed.; Kusch, P., Ed.; Intech: Rijeka, Croatia, 2018; Volume 1, pp. 1–33. [Google Scholar] [CrossRef] [Green Version]
- Madhumita, M.; Guha, P.; Nag, A. Extraction of betel leaves (Piper betle L.) essential oil and its bio-actives identification: Process optimization, GC-MS analysis and anti-microbial activity. Ind. Crops Prod. 2019, 138, 111578. [Google Scholar] [CrossRef]
- Demirpolat, A.; Akman, F.; Kazachenko, A.S. An experimental and theoretical study on essential oil of Aethionema sancakense: Characterization, molecular properties and RDG analysis. Molecules 2022, 27, 6129. [Google Scholar] [CrossRef]
- Castro-Vázquez, L.; Leon-Ruiz, V.; Alañon, M.E.; Pérez-Coello, M.S.; González-Porto, A.V. Floral origin markers for authenticating Lavandin honey (Lavandula angustifolia x latifolia). Discrimination from Lavender honey (Lavandula latifolia). Food Control 2014, 37, 362–370. [Google Scholar] [CrossRef]
- Robotti, E.; Campo, F.; Riviello, M.; Bobba, M.; Manfredi, M.; Mazzucco, E.; Gosetti, G.; Calabrese, F.; Sangiorgi, E.; Marengo, E. Optimization of the extraction of the volatile fraction from honey samples by SPME-GC-MS, experimental design, and multivariate target functions. J. Chem. 2017, 2017, 6437857. [Google Scholar] [CrossRef] [Green Version]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; The International Natural Product Sciences Taskforce; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Lahlou, M. The success of natural products in drug discovery. Pharmacol. Pharm. 2013, 4, 33502. [Google Scholar] [CrossRef] [Green Version]
- Mihaylova, D.; Popova, A. Phytochemicals of natural products: Analysis and biological activities. Horticulturae 2023, 9, 167. [Google Scholar] [CrossRef]
- Panda, L.; Duarte-Sierra, A. Recent advancements in enhancing antimicrobial activity of plant-derived polyphenols by biochemical means. Horticulturae 2022, 8, 401. [Google Scholar] [CrossRef]
- Madrigal-Santillán, E.; Portillo-Reyes, J.; Madrigal-Bujaidar, E.; Sánchez-Gutiérrez, M.; Mercado-Gonzalez, P.E.; Izquierdo-Vega, J.A.; Vargas-Mendoza, N.; Álvarez-González, I.; Fregoso-Aguilar, T.; Delgado-Olivares, L.; et al. Opuntia genus in human health: A comprehensive summary on its pharmacological, therapeutic and preventive properties. part 1. Horticulturae 2022, 8, 88. [Google Scholar] [CrossRef]
- Ekiert, H.M.; Szopa, A. Biological activities of natural products. Molecules 2020, 25, 5769. [Google Scholar] [CrossRef] [PubMed]
- Barba-Ostria, C.; Carrera-Pacheco, S.E.; Gonzalez-Pastor, R.; Heredia-Moya, J.; Mayorga-Ramos, A.; Rodríguez-Pólit, C.; Zúñiga-Miranda, J.; Arias-Almeida, B.; Guamán, L.P. Evaluation of biological activity of natural compounds: Current trends and methods. Molecules 2022, 27, 4490. [Google Scholar] [CrossRef] [PubMed]
- Moklis, M.H.; Cheng, S.; Cross, J.S. Current and future trends for crude glycerol upgrading to high value-added products. Sustainability 2023, 15, 2979. [Google Scholar] [CrossRef]
- Piersen, C.E.; Booth, N.L.; Sun, Y.; Liang, W.; Burdette, J.E.; Breemen, R.B.v.; Geller, S.E.; Gu, C.; Banuvar, S.; Shulman, L.P.; et al. Chemical and biological characterization and clinical evaluation of botanical dietary supplements: A phase I red clover extract as a model. Curr. Med. Chem. 2004, 11, 1361–1374. [Google Scholar] [CrossRef]
- Van Breemen, R.B.; Fong, H.H.S.; Farnsworth, N.R. The role of quality assurance and standardization in the safety of botanical dietary supplements. Chem. Res. Toxicol. 2007, 20, 577–582. [Google Scholar] [CrossRef] [Green Version]
- Kellogg, J.J.; Paine, M.F.; McCune, J.S.; Oberlies, N.H.; Cech, N.B. Selection and characterization of botanical natural products for research studies: A NaPDI center recommended approach. Nat. Prod. Rep. 2019, 36, 1196–1221. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Mesa, M.; Moreno-González, D. Current role of mass spectrometry in the determination of pesticide residues in food. Separations 2022, 9, 148. [Google Scholar] [CrossRef]
- Limmatvapirat, C.; Limmatvapirat, S.; Charoenteeraboon, J.; Wessapan, C.; Kumsum, A.; Jenwithayaamornwech, S.; Luangthuwapranit, P. Comparison of eleven heavy metals in Moringa oleifera Lam. products. Indian J. Pharm. Sci. 2015, 77, 485–490. [Google Scholar] [CrossRef] [Green Version]
- Limmatvapirat, C.; Nateesathittarn, C.; Dechasathian, K.; Moohummad, T.; Chinajitphan, P.; Limmatvapirat, S. Phytochemical analysis of baby corn silk extracts. J. Ayurveda Integr. Med. 2020, 11, 344–351. [Google Scholar] [CrossRef]
- Krongrawa, W.; Limmatvapirat, S.; Pongnimitprasert, N.; Meetam, P.; Limmatvapirat, C. Formulation and evaluation of gels containing coconut kernel extract for topical application. Asian J. Pharm. Sci. 2018, 13, 415–424. [Google Scholar] [CrossRef]
- Nyeem, M.A.B.; Mannan, M.A.; Nuruzzaman, M.; Kamrujjaman, K.M.; Das, S.K. Indigenous king of bitter (Andrographis paniculata): A review. J. Med. Plants Stud. 2017, 5, 318–324. [Google Scholar]
- Ahmad, W.; Jantan, I.; Bukhari, S.N.A. Tinospora crispa (L.) Hook. f. & Thomson: A review of its ethnobotanical, phytochemical, and pharmacological aspects. Front. Pharmacol. 2016, 7, 59. [Google Scholar] [CrossRef] [Green Version]
- Limmatvapirat, C.; Limmatvapirat, S.; Chansatidkosol, S.; Krongrawa, W.; Liampipat, N.; Leechaiwat, S.; Lamaisri, P.; Siangjong, L.; Meetam, P.; Tiankittumrong, K. Preparation and properties of anti-nail-biting lacquers containing shellac and bitter herbal extract. Int. J. Polym. Sci. 2021, 2021, 8537544. [Google Scholar] [CrossRef]
- Aung, W.W.; Krongrawa, W.; Ponphaiboon, J.; Kulpicheswanich, P.; Limmatvapirat, C. Yields, phytochemicals, and biological activities of different solvent extracts of Senna alata leaves. Key Eng. Mater. 2022, 914, 135–140. [Google Scholar] [CrossRef]
- Aung, W.W.; Panich, K.; Watthanophas, S.; Naridsirikul, S.; Ponphaiboon, J.; Krongrawa, W.; Kulpicheswanich, P.; Limmatvapirat, S.; Limmatvapirat, C. Preparation of bioactive de-chlorophyll rhein-rich Senna alata extract. Antibiotics 2023, 12, 181. [Google Scholar] [CrossRef]
- Limmatvapirat, C.; Rodhetbhai, P.; Somsakraksanti, K.; Danpongprasert, P.; Poonsub, S.; Krongrawa, W. Limmatvapirat, S.; Meepan, M. Chemical constituents, antioxidant activities, and element concentrations of rusa deer velvet antler extracts. J. Chem. 2020, 2020, 3287347. [Google Scholar] [CrossRef]
- Limmatvapirat, C.; Limmatvapirat, S.; Krongrawa, W.; Ponphaiboon, J.; Witchuchai, T.; Jiranuruxwong, P.; Theppitakpong, P.; Pathomcharoensukchai, P. Beef tallow: Extraction, physicochemical property, fatty acid composition, antioxidant activity, and formulation of lotion bars. J. Appl. Pharm. Sci. 2021, 11, 018–028. [Google Scholar] [CrossRef]
- Pavlić, B.; Kaplan, M.; Zeković, Z.; Canli, O.; Jovičić, N.; Kovačević, D.B.; Markovinović, A.B.; Putnik, P.; Bera, O. Kinetics of microwave-assisted extraction process applied on recovery of peppermint polyphenols: Experiments and modeling. Plants 2023, 12, 1391. [Google Scholar] [CrossRef]
- Chan, C.-H.; Yusoff, R.; Ngoh, G.-C.; Kung, F.W.-L. Microwave-assisted extractions of active ingredients from plants. J. Chromatogr. A 2011, 1218, 6213–6225. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.P.; Fathordoobady, F.; Guo, Y.; Singh, A.; Kitts, D.D. Antioxidants help favorably regulate the kinetics of lipid peroxidation, polyunsaturated fatty acids degradation and acidic cannabinoids decarboxylation in hempseed oil. Sci. Rep. 2020, 10, 10567. [Google Scholar] [CrossRef] [PubMed]
- Ponphaiboon, J.; Limmatvapirat, S.; Chaidedgumjorn, A.; Limmatvapirat, C. Physicochemical property, fatty acid composition, and antioxidant activity of ostrich oils using different rendering methods. LWT Food Sci. Technol. 2018, 93, 45–50. [Google Scholar] [CrossRef]
- Reddy, V.S.; Tian, Y.; Zhang, C.; Ye, Z.; Roy, K.; Chinnappan, A.; Ramakrishna, S.; Liu, W.; Ghosh, R. A review on electrospun nanofibers based advanced applications: From health care to energy devices. Polymers 2021, 13, 3746. [Google Scholar] [CrossRef]
- Nangare, S.; Jadhav, N.; Ghagare, P.; Muthane, T. Pharmaceutical applications of electrospinning. Ann. Pharm. Fr. 2020, 78, 1–11. [Google Scholar] [CrossRef]
- Gao, C.; Zhang, L.; Wang, J.; Jin, M.; Tang, Q.; Chen, Z.; Cheng, Y.; Yang, R.; Zhao, G. Electrospun nanofibers promote wound healing: Theories, techniques, and perspectives. J. Mater. Chem. B 2021, 9, 3106–3130. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and electrospun nanofibers: Methods, materials, and applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef]
- Ramakrishna, S.; Fujihara, K.; Teo, W.; Lim, T.; Ma, Z. Electrospinning Process. In An introduction to electrospinning and nanofibers, 1st ed.; Ramakrishna, S., Fujihara, K., Teo, W., Lim, T., Ma, Z., Eds.; World Scientific: Singapore, 2005; Volume 1, pp. 90–154. [Google Scholar] [CrossRef]
- Ye, P.; Wei, S.; Luo, C.; Wang, Q.; Li, A.; Wei, F. Long-term effect against methicillin-resistant Staphylococcus aureus of emodin released from coaxial electrospinning nanofiber membranes with a biphasic profile. Biomolecules 2020, 10, 362. [Google Scholar] [CrossRef] [Green Version]
- Lai, G.-J.; Shalumon, K.T.; Chen, S.-H.; Chen, I.-P. Composite chitosan/silk fibroin nanofibers for modulation of osteogenic differentiation and proliferation of human mesenchymal stem cells. Carbohydr. Polym. 2014, 111, 288–297. [Google Scholar] [CrossRef]
- Paipitak, K.; Pornpra, T.; Mongkontalang, P.; Techitdheer, W.; Pecharapa, W. Characterization of PVA-chitosan nanofibers prepared by electrospinning. Procedia Eng. 2011, 8, 101–105. [Google Scholar] [CrossRef] [Green Version]
- Rogina, A. Electrospinning process: Versatile preparation method for biodegradable and natural polymers and biocomposite systems applied in tissue engineering and drug delivery. Appl. Surf. Sci. 2014, 296, 221–230. [Google Scholar] [CrossRef]
- Jose, M.V.; Thomas, V.; Johnson, K.T.; Dean, D.R.; Nyairo, E. Aligned PLGA/HA nanofibrous nanocomposite scaffolds for bone tissue engineering. Acta Biomater. 2009, 5, 305–315. [Google Scholar] [CrossRef]
- Keshvardoostchokami, M.; Majidi, S.S.; Huo, P.; Ramachandran, R.; Chen, M.; Liu, B. Electrospun nanofibers of natural and synthetic polymers as artificial extracellular matrix for tissue engineering. Nanomaterials 2020, 11, 21. [Google Scholar] [CrossRef]
- Chinatangkul, N.; Limmatvapirat, C.; Limmatvapirat, S. Electrospun nanofibers from natural polymers and their application. Sci. Eng. Health Stud. 2021, 15, 21010005. [Google Scholar] [CrossRef]
- Wang, X.; Yu, D.-G.; Li, X.-Y.; Bligh, S.W.A.; Williams, G.R. Electrospun medicated shellac nanofibers for colon-targeted drug delivery. Int. J. Pharm. 2015, 490, 384–390. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Dong, Q.; Chen, J.; Li, L. Effects of coaxial electrospun eugenol loaded core-sheath PVP/shellac fibrous films on postharvest quality and shelf life of strawberries. Postharvest Biol. Technol. 2020, 159, 111028. [Google Scholar] [CrossRef]
- Chinatangkul, N.; Limmatvapirat, C.; Nunthanid, J.; Luangtana-Anan, M.; Sriamornsak, P.; Limmatvapirat, S. Design and characterization of monolaurin loaded electrospun shellac nanofibers with antimicrobial activity. Asian J. Pharm. Sci. 2018, 13, 459–471. [Google Scholar] [CrossRef]
- Chinatangkul, N.; Pengon, S.; Krongrawa, W.; Chansatidkosol, S.; Limmatvapirat, C.; Limmatvapirat, S. Designing electrospun shellac nanofibers with mupirocin using the Box-Behnken approach for topical wound care. J. Drug Deliv. Sci. Technol. 2022, 76, 103720. [Google Scholar] [CrossRef]
- Wu, Y.H.; Yu, D.G.; Huang, S.M.; Zha, D.P.; Wang, M.L.; Wang, S.J. Ultra-thin shellac fibers fabricated using two different electrospinning processes. Adv. Mat. Res. 2014, 1015, 51–55. [Google Scholar] [CrossRef]
- Chinatangkul, N.; Pengon, S.; Piriyaprasarth, S.; Limmatvapirat, C.; Limmatvapirat, S. Development of electrospun shellac and hydroxypropyl cellulose blended nanofibers for drug carrier application. Key Eng. Mater. 2020, 859, 239–243. [Google Scholar] [CrossRef]
- Chinatangkul, N.; Tubtimsri, S.; Panchapornpon, D.; Akkaramongkolporn, P.; Limmatvapirat, C.; Limmatvapirat, S. Design and characterisation of electrospun shellac-polyvinylpyrrolidone blended micro/nanofibres loaded with monolaurin for application in wound healing. Int. J. Pharm. 2019, 562, 258–270. [Google Scholar] [CrossRef] [PubMed]
- Limmatvapirat, S.; Limmatvapirat, C.; Luangtana-anan, M.; Nunthanid, J.; Oguchi, T.; Tozuka, Y.; Yamamoto, K.; Puttipipatkhachorn, S. Modification of physicochemical and mechanical properties of shellac by partial hydrolysis. Int. J. Pharm. 2004, 278, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; He, N.; Xue, Q.; Guo, Q.; Dong, L.; Haruna, M.H.; Zhang, X.; Li, B.; Li, L. Shellac: A promising natural polymer in the food industry. Trends Food Sci.Technol. 2021, 109, 139–153. [Google Scholar] [CrossRef]
- Badhani, G.; Yadav, S.; Reji, E.; Adimurthy, S. Recovery of lac resin from the aqueous effluent of shellac industry. Sustain. Chem. 2023, 4, 1–7. [Google Scholar] [CrossRef]
- Thammachat, T.; Sriamornsak, P.; Luangtana-Anan, M.; Nunthanid, J.; Limmatvapirat, C.; Limmatvapirat, S. Preparation and characterization of shellac fiber as a novel material for controlled drug release. Adv. Mat. Res. 2011, 152-153, 1232–1235. [Google Scholar] [CrossRef]
- Krongrawa, W.; Limmatvapirat, S.; Vollrath, M.K.; Kittakoop, P.; Saibua, S.; Limmatvapirat, C. Fabrication, optimization, and characterization of antibacterial electrospun shellac fibers loaded with Kaempferia parviflora extract. Pharmaceutics 2023, 15, 123. [Google Scholar] [CrossRef]
- Nasouri, K.; Haji, A.; Shoushtari, A.M.; Kaflou, A. A novel study of electrospun nanofibers morphology as a function of polymer solution properties. In Proceedings of the International Conference Nanomaterials Applications and Properties, Alushta, Ukraine, 16–21 September 2013. [Google Scholar]
- Okutan, N.; Terzi, P.; Altay, F. Affecting parameters on electrospinning process and characterization of electrospun gelatin nanofibers. Food Hydrocoll. 2014, 39, 19–26. [Google Scholar] [CrossRef]
- Haider, A.; Haider, S.; Kang, I.-K. A comprehensive review summarizing the effect of electrospinning parameters and potential applications of nanofibers in biomedical and biotechnology. Arabian J. Chem. 2018, 11, 1165–1188. [Google Scholar] [CrossRef]
- Sill, T.J.; Recum, H.A. Electrospinning: Applications in drug delivery and tissue engineering. Biomaterials 2008, 29, 1989–2006. [Google Scholar] [CrossRef]
- Williams, G.R.; Raimi-Abraham, B.T.; Luo, C.J. Electrospinning Fundamentals. In Nanofibres in Drug Delivery, 1st ed.; Williams, G.R., Raimi-Abraham, B.T., Luo, C.J., Eds.; UCL Press: London, UK, 2018; Volume 1, pp. 24–59. [Google Scholar] [CrossRef] [Green Version]
- Sun, B.; Long, Y.Z.; Zhang, H.D.; Li, M.M.; Duvail, J.L.; Jiang, X.Y.; Yin, H.L. Advances in three-dimensional nanofibrous macrostructures via electrospinning. Prog. Polym. Sci. 2014, 39, 862–890. [Google Scholar] [CrossRef]
- Hoseyni, S.Z.; Jafari, S.M.; Tabarestani, H.S.; Ghorbani, M.; Assadpour, E.; Sabaghi, M. Production and characterization of catechin-loaded electrospun nanofibers from Azivash gum- polyvinyl alcohol. Carbohydr. Polym. 2020, 235, 115979. [Google Scholar] [CrossRef]
- Al-Abduljabbar, A.; Farooq, I. Electrospun polymer nanofibers: Processing, properties, and applications. Polymers 2023, 15, 65. [Google Scholar] [CrossRef]
- Robb, B.; Lennox, B. The Electrospinning Process, Conditions and Control. In Electrospinning for Tissue Regeneration, 1st ed.; Bosworth, L.A., Downes, S., Eds.; Woodhead Publishing: Sawston, UK, 2011; Volume 1, pp. 51–66. [Google Scholar] [CrossRef]
- Mehta, P.P.; Pawar, V.S. Electrospun Nanofiber Scaffolds: Technology and Applications. In Applications of Nanocomposite Materials in Drug Delivery, 1st ed.; Inamuddin, Asiri, A.M., Mohammad, A., Eds.; Woodhead Publishing: Sawston, UK, 2018; Volume 1, pp. 509–573. [Google Scholar]
- Khajavi, R.; Abbasipour, M. Controlling nanofiber morphology by the electrospinning process. In Electrospun Nanofibers, 1st ed.; Afshari, M., Ed.; Woodhead Publishing: Sawston, UK, 2017; Volume 1, pp. 109–123. [Google Scholar] [CrossRef]
- Beachley, V.; Wen, X. Polymer nanofibrous structures: Fabrication, biofunctionalization, and cell interactions. Prog. Polym. Sci. 2010, 35, 868–892. [Google Scholar] [CrossRef] [Green Version]
- Nayak, R.; Padhye, R.; Arnold, L. Recent advances in nanofibre fabrication techniques. Text. Res. J. 2011, 82, 129–147. [Google Scholar] [CrossRef]
- Gunst, R.F.; Mason, R.L. Fractional factorial design. WIREs Comput. Stat. 2009, 1, 234–244. [Google Scholar] [CrossRef]
- Das, A.K.; Dewanjee, S. Optimization of Extraction Using Mathematical Models and Computation. In Computational Phytochemistry, 1st ed.; Sarker, S.D., Nahar, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 1, pp. 75–106. [Google Scholar] [CrossRef]
- Sharif, K.M.; Rahman, M.M.; Azmir, J.; Mohamed, A.; Jahurul, M.H.A.; Sahena, F.; Zaidul, I.S.M. Experimental design of supercritical fluid extraction–a review. J. Food Eng. 2014, 124, 105–116. [Google Scholar] [CrossRef]
- Frey, D.D.; Wang, H. Adaptive one-factor-at-a-time experimentation and expected value of improvement. Technometrics 2006, 48, 418–431. [Google Scholar] [CrossRef]
- Douaik, A. Design of experiments for screening and optimizing factors influencing extraction of bioactive compounds from saffron. Acta Hortic. 2017, 1184, 151–158. [Google Scholar] [CrossRef]
- Narenderan, S.T.; Meyyanathan, S.N.; Karri, V.V.S.R. Experimental design in pesticide extraction methods: A review. Food Chem. 2019, 289, 384–395. [Google Scholar] [CrossRef]
- Politis, S.N.; Colombo, P.; Colombo, G.; Rekkas, D.M. Design of experiments (DoE) in pharmaceutical development. Drug Dev. Ind. Pharm. 2017, 43, 889–901. [Google Scholar] [CrossRef]
- Baş, D.; Boyacı, I.H. Modeling and optimization I: Usability of response surface methodology. J. Food Eng. 2007, 78, 836–845. [Google Scholar] [CrossRef]
- Ferreira, S.L.C.; Bruns, R.E.; Ferreira, H.S.; Matos, G.D.; David, J.M.; Brandão, G.C.; da Silva, E.G.P.; Portugal, L.A.; dos Reis, P.S.; Souza, A.S.; et al. Box-Behnken design: An alternative for the optimization of analytical methods. Anal. Chim. Acta 2007, 597, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S. Central Composite Design for Response Surface Methodology and Its Application in Pharmacy. In Response Surface Methodology in Engineering Science, 1st ed.; Kayaroganam, P., Ed.; Intech: Rijeka, Croatia, 2021; Volume 1, pp. 1–19. [Google Scholar] [CrossRef]
- Tiwari, R.; Pathak, K. Nanostructured lipid carrier versus solid lipid nanoparticles of simvastatin: Comparative analysis of characteristics, pharmacokinetics and tissue uptake. Int. J. Pharm. 2011, 415, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Antony, J. Case Studies. In Design of Experiments for Engineers and Scientists, 2nd ed.; Antony, J., Ed.; Elsevier: Oxford, UK, 2014; Volume 1, pp. 125–188. Available online: https://www.researchgate.net/publication/362080058 (accessed on 17 April 2023).
- Kaidane, S.W. Application of Response Surface Methodology in Food Process Modeling and Optimization. In Response Surface Methodology in Engineering Science, 1st ed.; Kayaroganam, P., Ed.; Intech: Rijeka, Croatia, 2021; Volume 1, pp. 1–20. [Google Scholar] [CrossRef]
- Bialete, E.R.; Manuel, M.C.E.; Alcance, R.M.E.; Canlas, J.P.A.; Chico, T.-J.B.; Sanqui, J.P.; Cruz, J.C.D.; Verdadero, M.S. Characterization of the tensile strength of FDM-printed parts made from polylactic acid filament using 33 full-factorial design of experiment. In Proceedings of the 2020 IEEE 12th International Conference on Humanoid, Nanotechnology, Information Technology, Communication and Control, Environment, and Management (HNICEM), Manila, Philippines, 3–7 December 2020. [Google Scholar] [CrossRef]
- Chicco, D.; Warrens, M.J.; Jurman, G. The coefficient of determination R-squared is more informative than SMAPE, MAE, MAPE, MSE and RMSE in regression analysis evaluation. PeerJ. Comput. Sci. 2021, 7, e623. [Google Scholar] [CrossRef] [PubMed]
- Myers, R.H.; Montgomery, D.C.; Anderson-Cook, C.M. Response Surface Methodology: Process and Product Optimization Using Designed Experiments., 4th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2016; pp. 1–12. [Google Scholar]
- Fukuda, I.M.; Pinto, C.F.F.; Moreira, C.D.S.; Saviano, A.M.; Lourenço, F.R. Design of experiments (DoE) applied to pharmaceutical and analytical quality by design (QbD). Braz. J. Pharm. Sci. 2018, 54, e01006. [Google Scholar] [CrossRef]
- Carciochi, R.A.; Sologubik, C.A.; Fernández, M.B.; Manrique, G.D.; D’Alessandro, L.G. Extraction of antioxidant phenolic compounds from brewer’s spent grain: Optimization and kinetics modeling. Antioxidants 2018, 7, 45. [Google Scholar] [CrossRef] [Green Version]
- Hasnain, M.S.; Javed, M.N.; Alam, M.S.; Rishishwar, P.; Rishishwar, S.; Ali, S.; Nayak, A.K.; Beg, S. Purple heart plant leaves extract-mediated silver nanoparticle synthesis: Optimization by Box-Behnken design. Mater. Sci. Eng. C 2019, 99, 1105–1114. [Google Scholar] [CrossRef]
- Lin, X.; Wu, L.; Wang, X.; Yao, L.; Wang, L. Ultrasonic-assisted extraction for flavonoid compounds content and antioxidant activities of India Moringa oleifera L. leaves: Simultaneous optimization, HPLC characterization and comparison with other methods. J. Appl. Res. Med. Aromat. Plants 2021, 20, 100284. [Google Scholar] [CrossRef]
- Vural, N.; Cavuldak, O.A.; Kenar, A.; Akay, M.A. Green alcoholic solvent and UAE extraction of oleuropein from the Olea europaea L. leaves: Experimental design, optimization, and comparison with Pharmacopoeia method. Sep. Sci. Technol. 2020, 55, 1813–1828. [Google Scholar] [CrossRef]
- Detti, C.; Nascimento, L.B.d.S.; Brunetti, C.; Ferrini, F.; Gori, A. Optimization of a green ultrasound-assisted extraction of different polyphenols from Pistacia lentiscus L. leaves using a response surface methodology. Plants 2020, 9, 1482. [Google Scholar] [CrossRef]
- Keerthiraj, M.; Bhowmik, A.; Saha, S.; Dutta, A.; Chawla, G.; Kundu, A. Optimisation of patchoulol in the lipid-soluble concentrates of Pogostemon cablin using response surface methodology (RSM) coupled with genetic algorithms (GA). Ind. Crops Prod. 2022, 182, 114826. [Google Scholar] [CrossRef]
- Chakraborty, S.; Uppaluri, R.; Das, C. Optimization of ultrasound-assisted extraction (UAE) process for the recovery of bioactive compounds from bitter gourd using response surface methodology (RSM). Food Bioprod. Process. 2020, 120, 114–122. [Google Scholar] [CrossRef]
- Amiri, S.; Shakeri, A.; Sohrabi, M.R.; Khalajzadeh, S.; Ghasemi, E. Optimization of ultrasonic assisted extraction of fatty acids from Aesculus hippocastanum fruit by response surface methodology. Food Chem. 2019, 271, 762–766. [Google Scholar] [CrossRef]
- Fernandez-Barbero, G.; Pinedo, C.; Espada-Bellido, E.; Ferreiro-Gonzalez, M.; Carrera, C.; Palma, M.; Garcia-Barroso, C. Optimization of ultrasound-assisted extraction of bioactive compounds from jabuticaba (Myrciaria cauliflora) fruit through a Box-Behnken experimental design. Food Sci. Technol. 2019, 39, 1018–1029. [Google Scholar] [CrossRef] [Green Version]
- Vázquez-Espinosa, M.; González de Peredo, A.V.; Ferreiro-González, M.; Barroso, C.G.; Palma, M.; Barbero, G.F.; Espada-Bellido, E. Optimizing and comparing ultrasound- and microwave-assisted extraction methods applied to the extraction of antioxidant capsinoids in peppers. Agronomy 2019, 9, 633. [Google Scholar] [CrossRef] [Green Version]
- Mohammadpour, H.; Sadrameli, S.M.; Eslami, F.; Asoodeh, A. Optimization of ultrasound-assisted extraction of Moringa peregrina oil with response surface methodology and comparison with Soxhlet method. Ind. Crops Prod. 2019, 131, 106–116. [Google Scholar] [CrossRef]
- Madrera, R.R.; Valles, B.S. Development and validation of ultrasound assisted extraction (UAE) and HPLC-DAD method for determination of polyphenols in dry beans (Phaseolus vulgaris). J. Food Compos. Anal. 2020, 85, 103334. [Google Scholar] [CrossRef]
- Zhang, H.; Li, H.; Zhang, Z.; Hou, T. Optimization of ultrasound-assisted extraction of polysaccharides from perilla seed meal by response surface methodology: Characterization and in vitro antioxidant activities. J. Food Sci. 2021, 86, 306–318. [Google Scholar] [CrossRef]
- Wang, X.; Lui, X.; Shi, N.; Zhang, Z.; Chen, Y.; Yan, M.; Li, Y. Response surface methodology optimization and HPLC-ESI-QTOF-MS/MS analysis on ultrasonic-assisted extraction of phenolic compounds from okra (Abelmoschus esculentus) and their antioxidant activity. Food Chem. 2023, 405, 134966. [Google Scholar] [CrossRef]
- Panwar, D.; Panesar, P.S.; Chopra, H.K. Ultrasound-assisted extraction of pectin from Citrus limetta peels: Optimization, characterization, and its comparison with commercial pectin. Food Biosci. 2023, 51, 102231. [Google Scholar] [CrossRef]
- Nipornram, S.; Tochampa, W.; Rattanatraiwong, P.; Singanusong, R. Optimization of low power ultrasound-assisted extraction of phenolic compounds from mandarin (Citrus reticulata Blanco cv. Sainampueng) peel. Food Chem. 2018, 241, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Tungmunnithum, D.; Drouet, S.; Hano, C. Enrichment in antioxidant flavonoids of stamen extracts from Nymphaea lotus L. using ultrasonic-assisted extraction and macroporous resin adsorption. Antioxidants 2020, 9, 576. [Google Scholar] [CrossRef] [PubMed]
- Almusallam, I.A.; Ahmed, I.S.A.; Babiker, E.E.; Juhaimi, F.Y.A.; Fadimu, G.J.; Osman, M.A.; Maiman, S.A.A.; Ghafoor, K.; Alqah, H.A.S. Optimization of ultrasound-assisted extraction of bioactive properties from date palm (Phoenix dactylifera L.) spikelets using response surface methodology. LWT 2021, 140, 110816. [Google Scholar] [CrossRef]
- Thamapan, K.; Laohakunjit, N.; Kerdchoechuen, O.; Vongsawasdi, P.; Mingvanish, W. Ultrasound-assisted extraction for simultaneous quantitation of potential sweetening compounds from Derris reticulata aqueous extracts: A response surface methodology approach. J. Food Meas. Charact. 2021, 15, 2251–2263. [Google Scholar] [CrossRef]
- Thong-on, W.; Pathomwichaiwat, T.; Boonsith, S.; Koo-amornpattana, W.; Prathanturarug, S. Green extraction optimization of triterpenoid glycoside-enriched extract from Centella asiatica (L.) Urban (RSM). Sci. Rep. 2021, 11, 22026. [Google Scholar] [CrossRef]
- Koay, Y.S.; Hasham, R.; Bohari, S.P.M.; Hamid, M.A. Probe ultrasonic-assisted extraction of andrographolide rich extract from Andrographis paniculata: Processing parameters optimization using response surface methodology. Trends Sci. 2022, 19, 385. [Google Scholar] [CrossRef]
- Yang, B.; Zhang, M.; Weng, H.; Xu, Y.; Zeng, L. Optimization of ultrasound assisted extraction (UAE) of kinsenoside compound from Anoectochilus roxburghii (Wall.) Lindl by response surface methodology (RSM). Molecules 2020, 25, 193. [Google Scholar] [CrossRef] [Green Version]
- González-de-Peredo, A.V.; Vázquez-Espinosa, M.; Espada-Bellido, E.; Carrera, C.; Ferreiro-González, M.; Barbero, G.F.; Palma, M. Flavonol composition and antioxidant activity of onions (Allium cepa L.) based on the development of new analytical ultrasound-assisted extraction methods. Antioxidants 2021, 10, 273. [Google Scholar] [CrossRef]
- Ciric, A.; Krajnc, B.; Heath, D.; Ogrinc, N. Response surface methodology and artificial neural network approach for the optimization of ultrasound-assisted extraction of polyphenols from garlic. Food Chem. Toxicol. 2020, 135, 110976. [Google Scholar] [CrossRef]
- Mrkonjić, Ž.; Rakić, D.; Olgun, E.O.; Canli, O.; Kaplan, M.; Teslić, N.; Zeković, Z.; Pavlić, B. Optimization of antioxidants recovery from wild thyme (Thymus serpyllum L.) by ultrasound-assisted extraction: Multi-response approach. J. Appl. Res. Med. Aromat. Plants 2021, 24, 100333. [Google Scholar] [CrossRef]
- Jin, Y.; Liu, Z.; Liu, D.; Shi, G.; Liu, D.; Yang, Y.; Gu, H.; Yang, L.; Zhou, Z. Natural antioxidant of rosemary extract used as an additive in the ultrasound-assisted extraction of anthocyanins from lingonberry (Vaccinium vitis-idaea L.) pomace. Ind. Crops Prod. 2019, 138, 111425. [Google Scholar] [CrossRef]
- Balogh, A.; Farkas, B.; Faragó, K.; Farkas, A.; Wagner, I.; Assche, I.V.; Verreck, G.; Nagy, Z.K.; Marosi, G. Melt-blown and electrospun drug-loaded polymer fiber mats for dissolution enhancement: A comparative study. J. Pharm. Sci. 2015, 104, 1767–1776. [Google Scholar] [CrossRef] [Green Version]
- Weng, L.; Xie, J. Smart electrospun nanofibers for controlled drug release: Recent advances and new perspectives. Curr. Pharm. Des. 2015, 21, 1944–1959. [Google Scholar] [CrossRef] [Green Version]
- Azimi, B.; Maleki, H.; Zavagna, L.; Ossa, J.G.D.; Linari, S.; Lazzeri, A.; Danti, S. Bio-based electrospun fibers for wound healing. J. Funct. Biomater. 2020, 11, 67. [Google Scholar] [CrossRef]
- Yao, C.-H.; Yeh, J.-Y.; Chen, Y.-S.; Li, M.-H.; Huang, C.-H. Wound-healing effect of electrospun gelatin nanofibres containing Centella asiatica extract in a rat model. J. Tissue Eng. Regen. Med. 2017, 11, 905–915. [Google Scholar] [CrossRef]
- Sadri, M.; Arab-Sorkhi, S.; Valtani, H.; Bagheri-Pebdeni, A. New wound dressing polymeric nanofiber containing green tea extract prepared by electrospinning method. Fibers Polym. 2015, 16, 1742–1750. [Google Scholar] [CrossRef]
- Charernsriwilaiwat, N.; Rojanarata, T.; Ngawhirunpat, T.; Sukma, M.; Opanasopit, P. Electrospun chitosan-based nanofiber mats loaded with Garcinia mangostana extracts. Int. J. Pharm. 2013, 452, 333–343. [Google Scholar] [CrossRef]
- Chiu, C.-M.; Nootem, J.; Santiwat, T.; Srisuwannaket, C.; Pratumyot, K.; Lin, W.-C.; Mingvanish, W.; Niamnont, N. Enhanced stability and bioactivity of Curcuma comosa Roxb. extract in electrospun gelatin nanofibers. Fibers 2019, 7, 76. [Google Scholar] [CrossRef] [Green Version]
- Leyva-Jiménez, F.-J.; Fernández-Ochoa, A.; Cádiz-Gurrea, M.L.; Lozano-Sánchez, J.; Simancas, R.O.; Alañón, M.E.; Castangia, I.; Segura-Carretero, A.; Arráez-Román, D. Application of response surface methodologies to optimize high-added value products developments: Cosmetic formulations as an example. Antioxidants 2022, 11, 1552. [Google Scholar] [CrossRef]
- Virly; Chiu, C.; Tsai, T.; Yeh, Y.; Wang, R. Encapsulation of β-glucosidase within PVA Fibers by CCD-RSM-guided coelectrospinning: A novel approach for specific mogroside sweetener production. J. Agri. Food Chem. 2020, 68, 11790–11801. [Google Scholar] [CrossRef]
- Yao, F.; Gao, Y.; Chen, F.; Xia, Y. Effects of electrospinning parameters on peanut protein isolate nanofibers diameter. CYTA-J. Food 2021, 19, 729–738. [Google Scholar] [CrossRef]
- Baghersad, S.; Bahrami, S.H.; Mohammadi, M.R.; Mojtahedi, M.R.M.; Milan, P.B. Development of biodegradable electrospun gelatin/aloe-vera/poly (ε-caprolactone) hybrid nanofibrous scaffold for application as skin substitutes. Mater. Sci. Eng. C 2018, 93, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Salimbeigi, G.; Oliveira, R.N.; McGuinness, G.B. Electrospun poly(e-caprolactone)/propolis fiber morphology: A process optimisation study. J. Appl. Polym. Sci. 2022, 139, 52131. [Google Scholar] [CrossRef]
- Varsei, M.; Tanha, N.R.; Gorji, M.; Mazinani, S. Fabrication and optimization of PCL/PVP nanofibers with Lawsonia inermis for antibacterial wound dressings. Polym. Polym. Compos. 2021, 29, S1403–S1413. [Google Scholar] [CrossRef]




| Topic | Bath Sonicator | Probe Sonicator |
|---|---|---|
| Characteristics of the tool |  |  |
| Direct/indirect contact method | A water bath is equipped to deliver energy to the samples. Thus, it is an indirect contact method. | A probe is inserted into the mixture of sample and solvent. Thus, it is a direct contact method. |
| The delivery of energy | A water bath equipped with transducers at the machine’s base transfers energy to the samples. | A probe is responsible for transferring energy to the samples. |
| Cross-contamination possibility | No | Yes |
| Number of samples per time | A large number of vessels simultaneously | One sample at a time |
| Extraction Methods | Advantages | Disadvantage |
|---|---|---|
| Conventional methods | ||
| Soxhlet Extraction [11,14,15] | A very simple technique Large quantities of plant materials can be extracted simultaneously No need to filter the solvent after extraction Can be used on both small and large scales | Probability of thermal decomposition of heat-labile substances Extensive extraction time Work intensive Permit constrained manipulation of variables Need a large amount of solvent |
| Maceration [15] | A simple method using non-complicated utensils and equipment Operator skill is not required Energy-saving process Because of the prolonged contact time, it is appropriate for substances that are very poorly soluble in the solvent A method suitable for less potent and cheaper extracts | In some instances, the extraction process can last for weeks Not entirely extracting the substance Very sluggish and time-consuming procedure More solvents are required |
| Percolation [11,15] | Less labor-intensive than maceration The extraction of thermolabile components is possible A method suitable for potent and expensive crude drugs Rapid and more thorough extraction | Longer duration than Soxhlet extraction More solvent uses A skilled individual is required Throughout the process, the particle size of the material should be given special consideration |
| Decoction [12,15] | Compatible with heat-resistant substances Expensive additional equipment is not required. Simple to execute No need for a trained operator | Not appropriate for the extraction of heat-sensitive components |
| Non-conventional methods | ||
| Microwave-assisted extraction (MAE) [12,13,23,24] | Improve extract quality by enhancing bioactive compound purity and stability Enabling the use of fewer hazardous solvents Reduced administrative costs The very rapid rate of extraction Reduce power and solvent consumption | Specific equipment is required Low selectivity Depending on the solvent type and extraction temperature The reaction is inevitable at high temperatures |
| Supercritical fluid extraction (SFE) [12,23] | Increase the extraction rate. Greater selectivity of desired substances Low solvent consumption Automatic sample processing Superior extractive efficacy Due to the complete evaporation of CO2, a commonly used supercritical fluid, there are no solvent residues left in the extract | Quite expensive technology Require high pressures Unusual operating conditions Complicated phase behavior |
| Ultrasound-assisted extraction (UAE) [13,17,20,25] | Low power and solvent utilization Enhance extraction yield Reduced extraction time and lower temperatures | The heat generated during the extraction procedure can deteriorate heat-sensitive substances The effect of the ultrasound waves depends on the position of the container containing the matrix and solvent within the bath The inefficiency of energy transfer within the vessel containing the sample and solvent is a result of insufficient bath temperature and inadequate power control |
| Electrospinning Parameters | Effects of Different Parameters on the Diameter and Structural Morphology of Nanofibers |
|---|---|
| Flow rate | The polymer solution has sufficient time for polarization when the flow rate is modest, resulting in fibers with small diameters. If it is excessively high, rapid drying and minimum stretching result in the formation of bead fibers with large diameters [148]. |
| Applied voltage | In general, a higher voltage favors the formation of fibers with smaller diameters, but it can also induce the ejection of more polymer fluid, resulting in fibers with larger diameters. When the voltage is increased beyond a critical value, the diameter initially decreases before increasing after a certain point. Increased repulsion forces account for the initial decrease in diameter [115]. |
| Distance | The working distance between the spinneret and collector determines the stage of instability at which the jet is deposited on the collector. In order for the jet to fully extend and solidify, resulting in the formation of solid fibers, a sufficiently long distance is required. In fact, the long distance can cause fibers to become thinner. Jet solidification prevents the fiber from becoming thinner as the distance increases beyond a certain threshold. At a short distance, there is insufficient time for solvent evaporation. Consequently, nanofibers with flattened structures are produced. Beaded morphology occurs when the distance is insufficient [118]. |
| Relative molecular mass | The molecular weight of a polymer has a significant effect on the rheological and electrical properties of electrospun solutions. Due to the limited chain entanglement, low molecular weight polymers tend to generate beads rather than fibers [117]. |
| Viscosity | The low viscosity of the electrospun solution made it easier to produce thinner fibers. A high number of solvent molecules led to fewer chain entanglements and a lower density of surface charges, which allowed for the formation of beaded nanofibers. However, in the event that the viscosity was extraordinarily high, it would be difficult to expel the solution from the spinneret [118]. |
| Surface tension | The reduced surface tension of the electrospun solution facilitates jet initiation. In general, decreasing surface tension enabled the production of thinner fibers and the gradual disappearance of beads [115]. |
| Relative volatility | A polymer solution with a very high volatility is unsuitable for fiber spinning because the jet may solidify immediately upon exiting the spinneret. If the volatility is insufficient, the fibers will remain wet when they are deposited on the collector. The appearance of a porous microstructure is a result of increased volatility [117]. |
| Conductivity | The high charge-carrying capacity of the electrospun solution results in a higher applied voltage due to its high conductivity. The higher the voltage, the smaller the diameter, and the wider the fiber diameter distribution [115]. |
| Solubility of solvent | Critical to the formation of a homogeneous polymer solution is the solvent’s solubility, but a solvent with a high solubility does not necessarily produce an electrospinning-compatible solution. The volatility or vapor pressure of the solvent dictates its evaporation rate and, consequently, the jet’s rate of solidification [117]. |
| Temperature | At a higher temperature, the polymer solution’s surface tension and viscosity decrease, allowing for the formation of thinner fibers. Nevertheless, at a higher temperature, the solvent evaporates more quickly, limiting the jet’s extension. The temperature has two contradictory effects on fiber diameter that must be carefully optimized [117]. |
| Relative humidity | The relative humidity influences the solvent’s evaporation rate and the jet’s rate of solidification. Lower relative humidity encourages the formation of thinner, drier fibers. If the relative humidity is too low, the solvent evaporates rapidly, thereby limiting the extension of the jet. When the relative humidity reaches a certain level, however, water vapor in the air can enter the jet and cause morphological changes in the nanofibers [117]. |
| p | Fraction | Number of Experiments |
|---|---|---|
| 0 (full factorial design) | 1 | 256 |
| 1 | 2 | 128 |
| 2 | 4 | 64 |
| 3 | 8 | 32 |
| 4 | 16 | 16 |
| Design | Advantages | Disadvantages |
|---|---|---|
| 2-Level full factorial design | -Every possible combination of experimental runs is carried out. -The primary effects and their interactions are assessed. | -As the number of factors increases, the number of experimental runs increases exponentially. |
| Fractional factorial design | -A fractional factorial design requires fewer experimental iterations than a full factorial design. | -Certain interactions, particularly higher-order interactions that are insignificant compared to the main effects, can be disregarded by this design. |
| Plackett-Burman design | -With minimal runs, a large number of factors can be determined. | -This design is only useful for examining the main effects; interaction effects are not taken into account. |
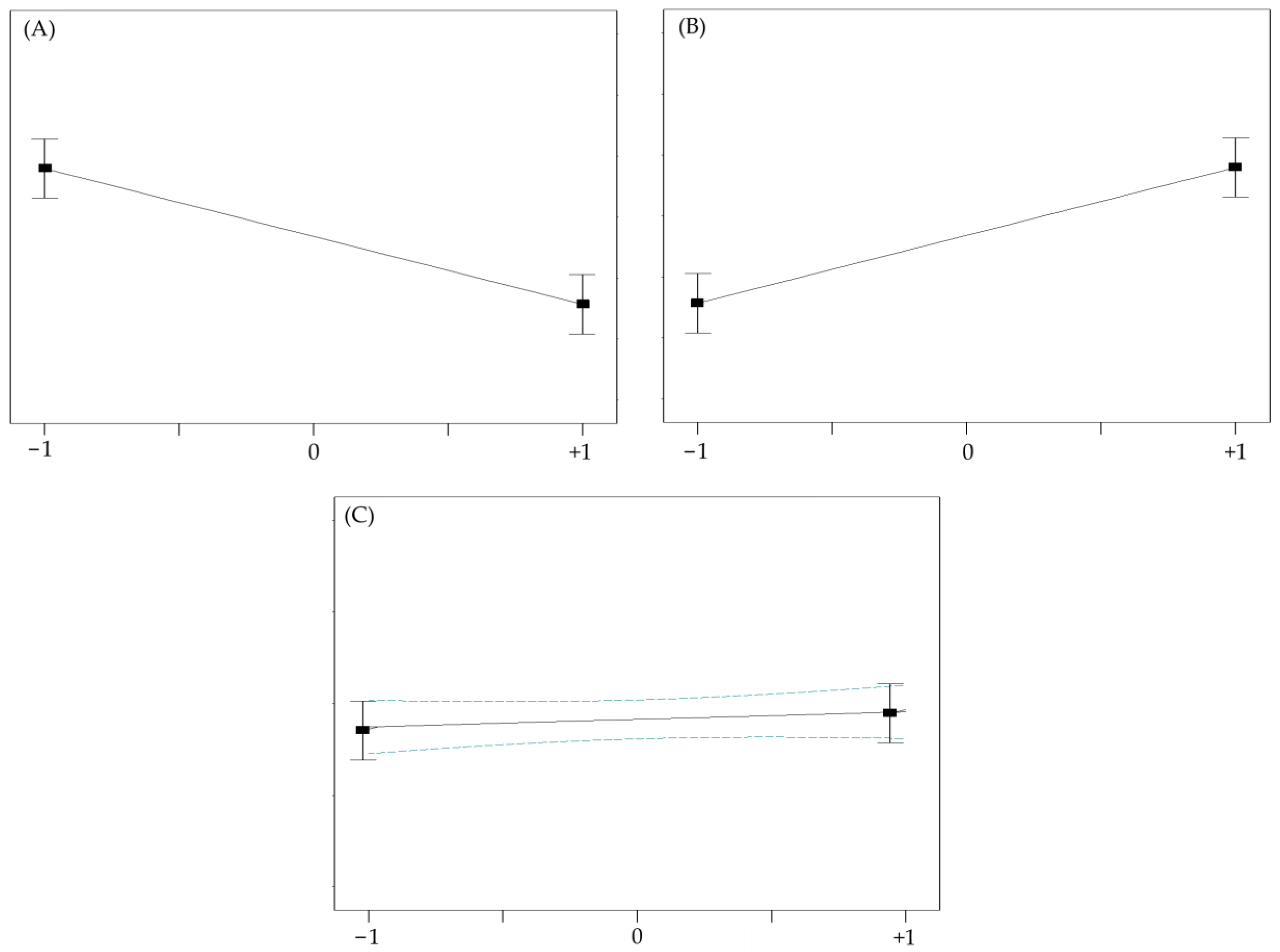
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | Conclusions | Remarks |
|---|---|---|---|---|---|---|---|
| Model | 11037.31 | 9 | 1126.37 | 91.23 | <0.0001 | Significant | R2 = 0.9915 |
| A | 9524.77 | 1 | 9524.77 | 708.85 | <0.0001 | Significant | Adjusted- = 0.9807 |
| B | 1127.80 | 1 | 1127.80 | 83.89 | <0.0001 | Significant | Predicted-R2 = 0.8928 Adeq. precision = 28.34 |
| C | 168.39 | 1 | 168.39 | 12.53 | 0.0095 | Significant | |
| D | 70.66 | 1 | 70.66 | 5.26 | 0.0456 | Significant | |
| E | 29.05 | 1 | 29.05 | 2.16 | 0.1850 | Not significant | |
| F | 12.90 | 1 | 12.90 | 0.96 | 0.3600 | Not significant | |
| G | 8.07 × 10−3 | 1 | 8.07 × 10−3 | 6.01 × 10−4 | 0.9811 | Not significant | |
| Residual | 94.10 | 7 | 13.44 | ||||
| Lack of fit | 72.44 | 3 | 24.15 | 4.46 | 0.0914 | Not significant | |
| Pure error | 21.66 | 4 | 5.42 |
| Design * | Plant Materials | Ultrasound Source | Parameters | Levels | Optimal UAE Conditions | Responses/Results | Ref. |
|---|---|---|---|---|---|---|---|
| OFAT and CCD | Moringa oleifera L. leaves | Ultrasonic probe | -Frequency -Power -Solvent/solid -Temperature -Time -Extraction solvent | ND 80–240 W 25–40 mL/g 30–60 °C 5–25 min 52% v/v ethanol | ND 188 W 40 mL/g 52 °C 20 min 52% v/v ethanol | -The extract contained eight flavonoids with the highest concentrations of D-(+)-catechin, hyperoside, and kaempferol-3-O-rutinoside and had a higher total flavonoid content (TFC) and antioxidant activities (DPPH, ABTS, and FRAP) than those extracted by stirring-assisted extraction, Soxhlet-assisted extraction, and microwave-assisted extraction (MAE). | [170] |
| CCD | Olea europaea L. leaves | Ultrasonic bath | -Frequency -Power -Solvent/solid -Temperature -Time -Extraction solvent | 28 kHz 250 and 500 W 0.5–18.5 mL/g 15–75 °C 0–100 min 5–85 % v/v methanol | 28 kHz 250 W 12.8 mL/g 58.3 °C 71.25 min 61.75% v/v methanol | -The extract was superior to that obtained using the European Pharmacopoeia method in terms of oleuropein content, TPC (26.80 ± 1.96 mg GAE/g leaf), and antioxidant property (DPPH). | [171] |
| FFD and BBD | Pistacia lentiscus leaves | Ultrasonic bath | -Frequency -Power -Solvent/solid -Temperature -Time -Extraction solvent | 39 kHz 100 W 100–200 mL/g 30–50 °C 15–30 min 30–50% v/v ethanol | 39 kHz 100 W 130 mL/g 50 °C 15 min 50% v/v ethanol | -The extract contained the highest TFC (10.2 ± 0.8 mg/g). | [172] |
| BBD | Pogostemon cablin leaves | Ultrasonic bath | -Frequency -Power -Solvent/solid -Temperature -Time -Extraction solvent | 30–50 kHz ND 20–40 mL/g ND 10–20 min 100% v/v hexane | 48.84 kHz ND 26.99 mL/g ND 17.78 min 100% v/v hexane | -The optimal conditions resulted in the highest yield (182.24 mg/g) of a lipid-soluble extract with the highest patchoulol content (48.84%) and excellent fungal mycelial inhibition against virulent strains of Aspergillus flavus and A. fumigatus due to the disintegration of the matrix cell wall with numerous fractures, resulting in better phytochemical solubility in the extraction solvent (hexane). -To produce lipid-soluble extracts with a higher patchoulol content, the UAE has been favored over MAE and maceration with the same extraction solvent. | [173] |
| BBD | Senna alata leaves | Ultrasonic bath | -Frequency -Power -Solvent/solid -Temperature -Time -Extraction solvent | 40 kHz 160 W 20–40 mL/g 40–60 °C 10–20 min 95% v/v ethanol | 40 kHz 160 W 25.48 mL/g 59.52 °C 18.4 min 95% v/v ethanol | -The most rhein (10.44 mg/g DW) was found in the optimized extract, which was responsible for their antioxidant, anti-inflammatory, and antibacterial activities. | [107] |
| CCD | Momordica charantia fruits | Ultrasonic bath | -Frequency -Power -Sonication mode -Solvent/solid -Temperature -Time -Extraction solvent | ND ND normal and pulsed modes 0.1–0.5 mg/L 20–80 °C 10–15 min Deionized water | ND ND pulsed mode 0.25 mL/g 68.4 °C 12 min Deionized water | -The pulsed mode sonication yielded the best results in terms of optimal bioactive compound concentrations (antioxidant activity of 77.9%, total polyphenol content of 104.5 mg GAE/g, and total soluble protein content of 42.1 mg/1000 mL), which contributed to oxidative stress reduction. | [174] |
| BBD | Aesculus hippocastanum fruits | Ultrasonic bath | -Frequency -Power -Solvent/solid -Temperature -Time -Extraction solvent | 40 kHz 200 W 15–45 mL/g 30–60 °C 10–60 min 100% v/v methanol | 40 kHz 200 W 22.5 mL/g 60 °C 56.5 min 100% v/v methanol | -The UAE extraction yield was 21.683 ± 0.452% with a high concentration of pentadecanoic acid (19.34%), which was significantly higher than the Soxhlet extraction yield (19.455 ± 0.477%). | [175] |
| BBD | Myrciaria cauliflora fruits | Ultrasonic probe | Anthocyanins -Frequency -Power -Amplitude -Solvent/solid -Temperature -Time -Extraction solvent -Cycle -pH Phenolics -Frequency -Power -Amplitude -Solvent/solid -Temperature -Time -Extraction solvent -Cycle -pH | 24 kHz 200 W 30–70% 6.67–13.33 mL/g 10–70 °C 10 min 25–75% v/v methanol 2–7 s−1 3–7 24 kHz 200 W 30–70% 6.67–13.33 mL/g 10–70 °C 10 min 25–75% v/v methanol 2–7 s−1 3–7 | 24 kHz 200 W 34% 13.33 mL/g 39.8 °C 10 min 51% v/v methanol 0.47 s−1 7 24 kHz 200 W 68.5% 13.33 mL/g 26.0 °C 10 min 72% v/v methanol 0.50 s−1 7 | -The concentration of methanol was found to be the most influential variable in the extraction of anthocyanins and phenolics. Temperature and extraction cycle were additional variables that impacted anthocyanins. The optimal extraction time of 10 min was sufficient to extract both compounds quantitatively. -Due to their extreme temperature sensitivity, anthocyanins can be severely degraded during high-temperature extraction. | [176] |
| BBD | Capsicum chinense fruits | Ultrasonic bath | -Frequency -Power -Solvent/solid -Temperature -Time -Extraction solvent -pH | ND ND 25–75 mL/g 5–55 °C 5–15 min methanol: ethyl acetate (42: 58) 2–8 | ND ND 72.5 mL/g 5.5 °C 5 min methanol: ethyl acetate (42: 58) 8 | -UAE is sufficient, rapid, and efficient for extracting heat-sensitive capsinoids from peppers. -The capsiate content was found to be 1323 µg/g greater than in the previous report without optimization. | [177] |
| OFAT and CCD | Moringa peregrina seeds | Ultrasonic bath | -Frequency -Power -Solvent/solid -Temperature -Time -Extraction solvent | 20 kHz 348 W 5–20 mL/g 30–60 °C 5–30 min 80% v/v methanol | 20 kHz 348 W 17.8 mL/g 30 °C 26.3 min 80% v/v methanol | -The maximum yield of oil extraction was 53.101%, which was greater than the Soxhlet method yield of 43% after 11 h of extraction. -The UAE approach produced superior M. peregrina oil properties, including peroxide value, antioxidant activity (DPPH), total phenolic content (TPC), and iodine value (IV), compared to the Soxhlet method. | [178] |
| BBD | Phaseolus vulgaris seeds | Ultrasonic probe | -Frequency -Power -Amplitude -Solvent/solid -Temperature -Time -Extraction solvent -Cycle | 26 kHz 200 W 60–100% 20 mL/g 30 °C 10–30 min 40–80% v/v ethanol 2–7 s−1 | 26 kHz 200 W 100% 20 mL/g 30 °C 10.3 min 46% v/v ethanol 4 s−1 | -The extract contained the highest quantitative recovery of hydroxycinnamic acids, anthocyanins, and flavonols. | [179] |
| BBD | Perilla frutescens seeds | Ultrasonic bath | -Frequency -Power -Solvent/solid -Temperature -Time -Extraction solvent | ND 165–255 W 20–30 mL/g 30–50 °C 40–60 min 100% v/v water | ND 229 W 26 mL/g 43 °C 52 min 100% v/v water | -The yield of polysaccharides with substantial antioxidant activity was 6.14 ± 0.062%. -Scanning electron microscopy analysis revealed the formation of numerous holes on the surface of perilla seed meal after UAE. | [180] |
| CCD | Abelmoschus esculentus pulps | Ultrasonic bath | -Frequency -Power -Solvent/solid -Temperature -Time -Extraction solvent | 40 kHz 96–192 W 15–25 mL/g 40–70 °C 20–50 min 70% v/v ethanol | 40 kHz 142 W 25 mL/g 46 °C 40 min 70% v/v ethanol | -The UAE extract had a higher TPC (7.02 mg GAE/g dry weight) than the MAE extract (3.89 mg GAE/g dry weight) when extracted at the same temperature, time, solvent/solid ratio, and ethanol concentration. It also possessed exceptional abilities to scavenge free radicals and mitigate oxidative damage, which can be attributed primarily to hydroxycoumarin and quercetin derivatives. | [181] |
| BBD | Citrus limetta peels | Ultrasonic probe | -Frequency -Power -Amplitude -Solvent/solid -Temperature -Time -Extraction solvent -pH | 40 kHz 500 W 10–50% 30 mL/g 30–50 °C 10–30 min Water acidified with citric acid 1–3 | 40 kHz 500 W 37% 30 mL/g 40 °C 24 min Water acidified with citric acid 1.9 | -Pectin extracted under optimal conditions exhibited superior antioxidant, water/oil retention, emulsifying, and thermal properties compared to commercial pectin. UAE-obtained, extracted pectin may be utilized as a food ingredient. | [182] |
| BBD | Citrus reticulata Blanco cv. Sainampueng peels | Ultrasonic bath | -Frequency -Power -Amplitude -Solvent/solid -Temperature -Time -Extraction solvent | 38.5 kHz 30.34–59.36 W 10–50% 20 mL/g 30–50 °C 20–40 min 80% v/v acetone | 38.5 kHz 56.71 W 37% 20 mL/g 48 °C 40 min 80% v/v acetone | -Low power UAE has a higher extraction efficiency for total phenolic content (152.63 mg GAE/g DW) and hesperidin content (64.36 mg/g DW) than MAE at the same extraction temperature and time. | [183] |
| Factorial design | Nymphaea lotus stamens | Ultrasonic bath | -Frequency -Power -Solvent/solid -Temperature -Time -Extraction solvent | 0–45 kHz 400 W 10 mL/g 45 °C 20–60 min 50–100% v/v ethanol | 34.65 kHz 400 W 10 mL/g 45 °C 46 min 90% v/v ethanol | -The total flavonoid content was 235.45 mg/g dry weight, which was greater than the traditional heat reflux extraction yield of 169.64 mg/g dry weight. | [184] |
| BBD | Phoenix dactylifera L. Spikelets | Ultrasonic bath | -Frequency -Power -Solvent/solid -Temperature -Time -Extraction solvent | 40 kHz 110 W 100 mL/g 25–60 °C 20–40 min 25–50% v/v ethanol | 40 kHz 110 W 100 mL/g 40.80 °C 21.60 min 50% v/v ethanol | -Rutin and (+)-catechin were the major phenolic compounds in the extract under optimized UAE conditions. The total phenolic concentration in the optimized extract was 130.20 mg GAE/g DW, and DPPH radical inhibition was 87.20%. | [185] |
| BBD | Derris reticulata stems | Ultrasonic bath | -Frequency -Power -Solvent/solid -Temperature -Time -Extraction solvent | 37 kHz 120 W 10–30 mL/g 40–80 °C 20–60 min 100% v/v water | 37 kHz 120 W 10 mL/g 80 °C 60 min 100% v/v water | -The UAE extract contained gallic acid, p-coumaric acid, quercetin, and kaempferol in addition to high levels of phenolics (0.48 ± 0.03 mg GAE/g DW), flavonoids (0.15 ± 0.03 mg CE/g DW), and sugar (4.80 ± 0.65 mg/g DW) that can be used as a sweetener or sugar substitute in foods. | [186] |
| CCD | Centella asiatica L. aerial parts | Ultrasonic bath | -Frequency -Power -Solvent/solid -Temperature -Time -Extraction solvent | 20 kHz ND 10 mL/g 40–70 °C 30–90 min 40–80% v/v ethanol | 20 kHz ND 10 mL/g 48 °C 50 min 80% v/v ethanol | -The extract contained the greatest content of total triterpenoids, with 2.262 ± 0.046% w/w madecassoside, 1.325 ± 0.062% w/w asiaticoside, 0.082 ± 0.009% w/w madecassic acid, and 0.052 ± 0.007% w/w asiatic acid. | [187] |
| OFAT and BBD | Andrographis paniculate aerial parts | Ultrasonic probe | -Frequency -Power -Amplitude -Solvent/solid -Temperature -Time -Extraction solvent -Duty cycle | 40 kHz ND 10–100% 17 mL/g 70 °C 1–5 min 75% v/v ethanol 10–100% | 40 kHz ND 66% 17 mL/g 70 °C 5 min 75% v/v ethanol 11% | -The extract contained the highest concentration of andrographolide, 3.50 ± 0.17% w/w. | [188] |
| OFAT and BBD | Anoectochilus roxburghii whole plants | Ultrasonic bath | -Frequency -Power -Solvent/solid -Temperature -Time -Extraction solvent | ND 240–540 W 5–30 mL/g 10–60 °C 10–50 min 0–100% v/v methanol | ND 420 W 10.83 mL/g 35 °C 45 min 16.33% v/v methanol | -The extract had a high kinsenoside yield of 32.24% dry weight. | [189] |
| PBD and BBD | Kaempferia parviflora rhizomes | Ultrasonic bath | -Frequency -Power -Solvent/solid -Temperature -Time -Extraction solvent | 40 kHz 160 W 10–50 mL/g 30–80 °C 5–30 min 50–95% v/v ethanol | 40 kHz 160 W 50 mL/g 50 °C 15.99 min 95% v/v ethanol | -The highest concentration of total methoxyflavones was found in the extract at 327.25 mg/g. | [58] |
| BBD | Allium cepa L. bulbs | Ultrasonic probe | -Frequency -Power -Amplitude -Solvent/solid -Temperature -Time -Extraction solvent -pH -Cycle | 20 kHz 70 W 30–90% 50–100 mL/g 10–60 °C 10 min 50–100% v/v methanol 2–7 0.4–1 s−1 | 20 kHz 70 W 85% 64 mL/g 58.8 °C 10 min 76.8% v/v methanol 2 0.94 s−1 | -The optimization of UAE yielded the extract with the highest total flavonols content (8.78 ± 0.03 mg/g extract) and antioxidant activity (11.85 ± 0.11 mg trolox/g extract). | [190] |
| CCD and ANN | Allium sativum L. bulbs | Ultrasonic bath | -Frequency -Power -Solvent/solid -Temperature -Time -Extraction solvent | ND ND 10–30 mL/g 40–80 °C 10–30 min 20–100% v/v methanol | ND ND 20 mL/g 59 °C 13.5 min 71% v/v methanol | -The maximum values of the two output parameters found in the extract were 19.498 mg GAE/g fresh weight total phenolic content and 1.422 mg RUT/g fresh weight total flavonoid content. | [191] |
| CCD | Thymus serpyllum (a by-product from filter tea production) | Ultrasonic bath | -Frequency -Power -Solvent/solid -Temperature -Time -Extraction solvent | 40 kHz 42 W 20 mL/g 50–80 °C 40–70 min 45–75% v/v ethanol | 40 kHz 42 W 20 mL/g 70.28 °C 70 min 45% v/v ethanol | -In terms of yield (28.03%), TPC (4.39 mg GAE/g), and antioxidant activities (DPPH, ABTS, and FRAP), the extract was superior to the one obtained via the conventional process. | [192] |
| BBD | Vaccinium vitis-idaea L. fruit pomace | Ultrasonic bath | -Frequency -Power -Solvent/solid -Temperature -Time -Extraction solvent | 50 kHz 120–200 W 5–25 mL/g 40–60 °C 20–40 min 0–40% v/v ethanol | 50 kHz 166.86 W 20 mL/g 55.15 °C 35.54 min 40% v/v ethanol | -Anthocyanins are known to be sensitive to oxygen; therefore, antioxidants could be added to the extraction process to reduce oxidation. The UAE method with rosemary extract as an additive has been developed for extracting anthocyanins from V. vitis-idaea fruit pomace in order to increase extraction efficiency while reducing anthocyanin loss in the extract. The total concentration of anthocyanin was 4.12 ± 0.18 mg/g DW. | [193] |
| Design * | Materials | Parameters | Levels | Optimization | Responses/Results | Ref |
|---|---|---|---|---|---|---|
| CCD | Polymer -AZG -PVA Bioactive substance -Catechin | -AZG concentration -PVA concentration -Voltage -Catechin -Feed rate -Needle-to-collector distance | 2 g/L 80 g/L 16–20 kV 500–3000 mg/L 0.5–0.9 mL/h 10–14 cm | 2 g/L 80 g/L 20 kV 3000 mg/L 0.5 mL/h 11 cm | -The average diameter of fibers containing and lacking catechin was 371 nm and 89 nm, respectively. -Catechin concentrations increased nanofiber loading capacity, changing their shape and microstructure. The polymer wall reacted with catechin hydroxyl groups as concentration increased. Hydrogen bonding and molecular chain adhesion increased nanofiber heat resistance. -Electrospun nanofibers loaded with catechin could be utilized in food packaging and pharmaceuticals. | [144] |
| CCD | Polymer -PVA Biocatalyst -β-glucosidase | -PVA content -Voltage -Feed rate -Needle-to-collector distance | 8% w/v 12.5–13.5 kV 0.004–0.008 mL/min 12.5–17.5 cm | 8% w/v 13 kV 0.006 mL/min 15 cm | -The average diameter of fibers was 343 nm. -β-Glucosidase was entrapped in PVA electrospun fibers using enzyme immobilization techniques to convert mogroside V in Siraitia grosvenorii fruit extract into siamenoside I. (sweetener). | [202] |
| BBD | Polymer -PLA Bioactive substance -Peanut protein isolate | -Solution mass fraction -Voltage -Feed rate -Needle-to-collector distance | 10–14% w/w 14–18 kV 0.3–0.9 mL/h ND | 10% w/w 16 kV 0.6 mL/h ND | -The average diameter of fibers was 164 nm. -Peanut protein isolate is comprised of eight essential amino acids. Because of their high porosity, large specific surface area, biocompatibility, and degradability, electrospun nanofiber membranes loaded with this isolate could be exploited for sustained drug release and wound dressing. | [203] |
| BBD | Polymer -Gelatin -PCL Bioactive substance -Aloe vera extract | -Gel content -Extract content -PCL content -Voltage -Feed rate -Needle-to-collector distance | 5–15% w/v 0–10% w/v 12% w/v 15 kV 0.6–1.4 mL/h 15 cm | 10% w/v 7.3% w/v 12% w/v 15 kV 1.2 mL/h 15 cm | -Fibers showed an average diameter of 125 nm with a tensile strength of 5.1 MPa. -The inclusion of A. vera extract enhanced antibacterial activity (>99.9% against Gram-positive bacteria and 85.63% against Gram-negative bacteria, respectively) and facilitated appropriate in vitro biodegradation. Moreover, the addition of A. vera extract enhanced cell viability without causing toxicity. | [204] |
| BBD | Polymer -PCL Bioactive substance -Propolis | -PCL content -Propolis content -Voltage -Feed rate -Needle-to-collector distance (cm) | 8% w/w 0–7% w/w 12–18 kV 0.1–0.3 mL/h 10–17 cm | 8% w/w 6.56% w/w 15.5 kV 0.238 mL/h 14.2 cm | -The average diameter of PCL/propolis fibers produced by electrospinning was 560 nm. -Electrospun fibers provided a slow release of antibacterial propolis, thereby preventing wound infection and accelerating the healing process. | [205] |
| BBD | Polymer -SHL Bioactive substance -Kaempferia parviflora rhizome extract | -SHL content -Extract content -Voltage -Feed rate -Needle-to-collector distance | 36–40% w/w 1–5% w/w 12–24 kV 0.4–1.2 mL/h 20 cm | 37.25% w/w 1.5% w/w 18 kV 0.8 mL/h 20 cm | -The fiber diameter was 574 nm with a low bead amount (0.48 beads/fiber). -Electrospun shellac fibers loaded with K. parviflora extract were able to produce a sustained-release profile within 10 h and demonstrated antibacterial activity against S. aureus. The optimized fibers can be developed into wound dressings. | [137] |
| BBD | Polymer -PCL -PVP Bioactive substance -Lawsonia inermis extract | -PCL content (% w/v) -PVP content (% w/v) -Voltage (kV) -Feed rate (mL/h) -Needle-to-collector distance (cm) | 10–15% w/v 25–35% w/v 17 kV 1–2 mL/h 15 cm | 12.5% w/v 30% w/v 17 kV 1.5 mL/h 15 cm | -The average fiber diameter was 241.17 nm with an air permeability of 1.63 × 103 mL/s. cm2/mm. -The optimized dressing was effective against both E. coli and S. aureus without exhibiting any toxicity. Due to their excellent water vapor permeability, swelling ratio, and mechanical performance, PCL/PVP/L. inermis extract nanofibers are suitable as wound dressings for the prevention of infection and acceleration of wound healing. | [206] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ponphaiboon, J.; Krongrawa, W.; Aung, W.W.; Chinatangkul, N.; Limmatvapirat, S.; Limmatvapirat, C. Advances in Natural Product Extraction Techniques, Electrospun Fiber Fabrication, and the Integration of Experimental Design: A Comprehensive Review. Molecules 2023, 28, 5163. https://doi.org/10.3390/molecules28135163
Ponphaiboon J, Krongrawa W, Aung WW, Chinatangkul N, Limmatvapirat S, Limmatvapirat C. Advances in Natural Product Extraction Techniques, Electrospun Fiber Fabrication, and the Integration of Experimental Design: A Comprehensive Review. Molecules. 2023; 28(13):5163. https://doi.org/10.3390/molecules28135163
Chicago/Turabian StylePonphaiboon, Juthaporn, Wantanwa Krongrawa, Wah Wah Aung, Nawinda Chinatangkul, Sontaya Limmatvapirat, and Chutima Limmatvapirat. 2023. "Advances in Natural Product Extraction Techniques, Electrospun Fiber Fabrication, and the Integration of Experimental Design: A Comprehensive Review" Molecules 28, no. 13: 5163. https://doi.org/10.3390/molecules28135163





