Polyphenols and Neurodegenerative Diseases: Potential Effects and Mechanisms of Neuroprotection
Abstract
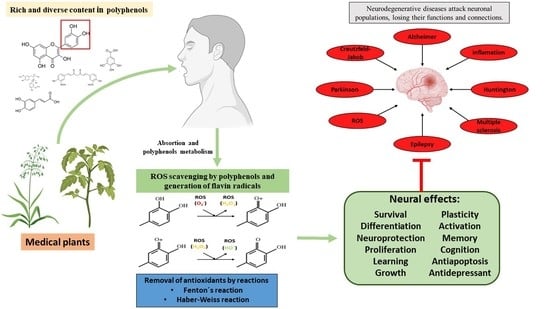
:1. Introduction
1.1. Neurodegenerative Diseases (NDs)
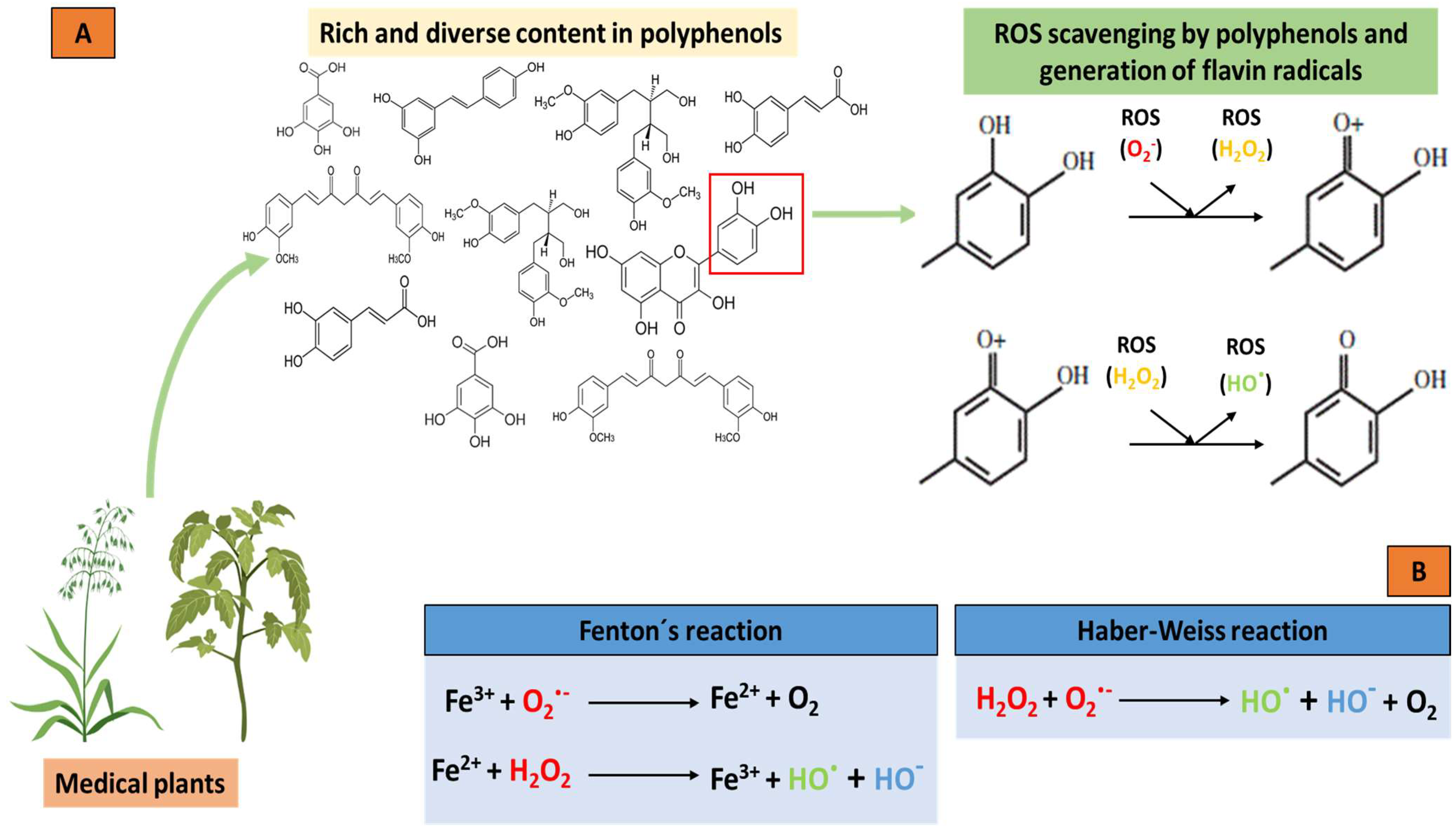
1.2. Medicinal Plants and Secondary Metabolites
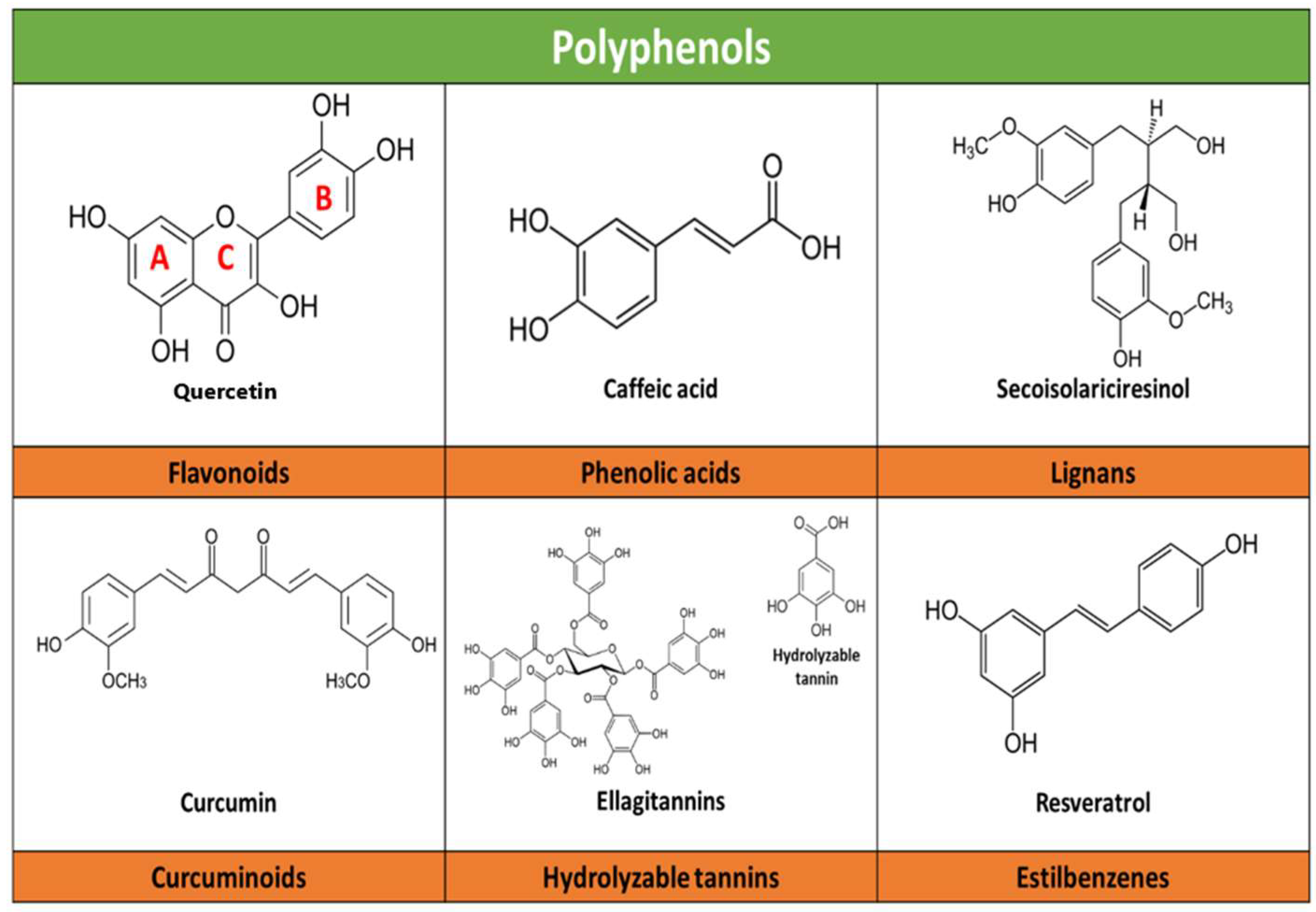
2. Polyphenol Synthesis and Classification
2.1. Digestion, Absorption and Distribution
2.2. Safety of Polyphenol Consumption
2.3. Polyphenols as Antioxidants
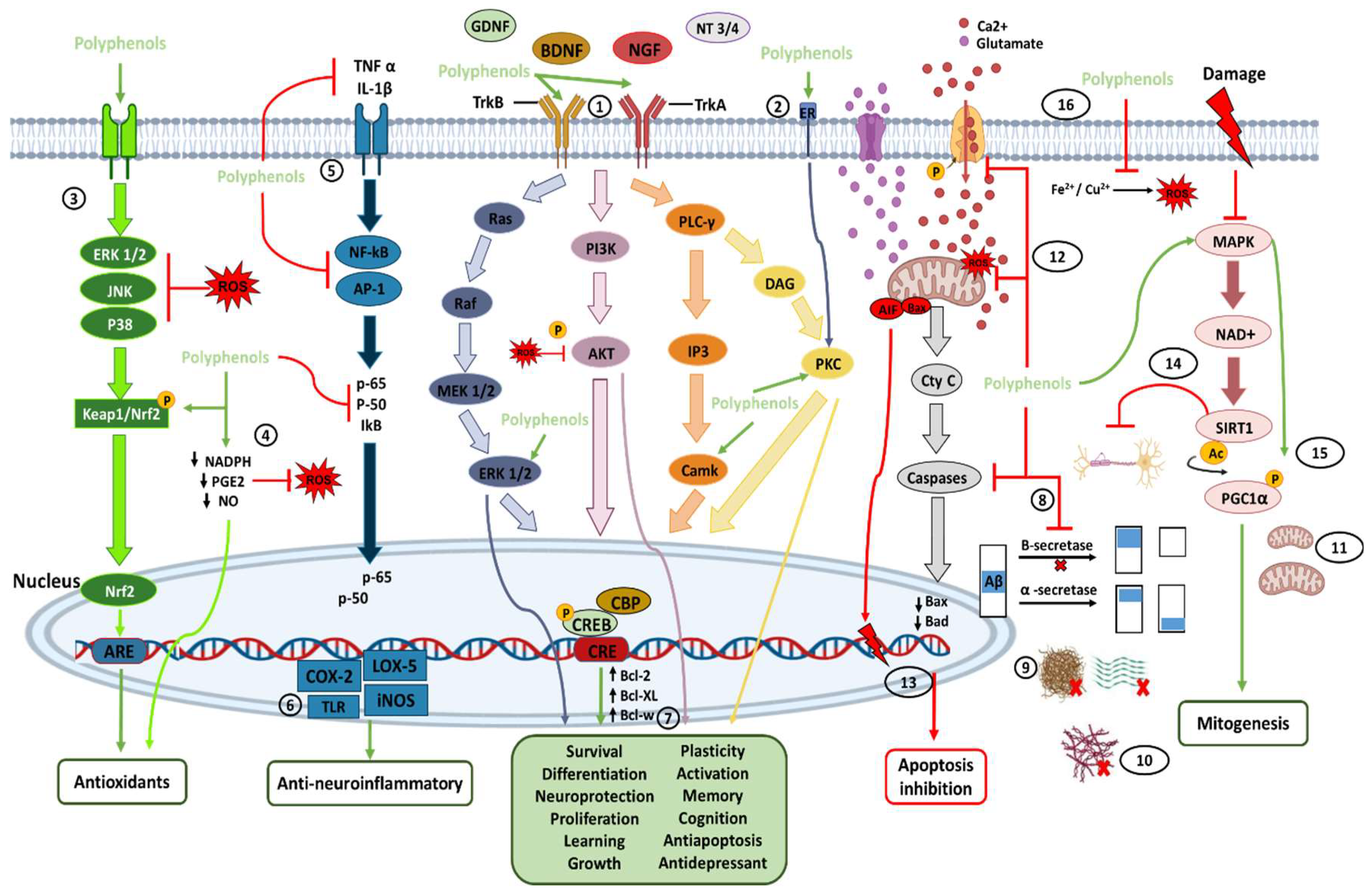
3. Polyphenols in the Nervous System and Their Neuroprotective Effects
4. Other Neuroprotection Approaches (Indirect Mechanisms)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Heemels, M.T. Neurodegenerative diseases. Nature 2016, 539, 179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banjari, I.; Marček, T.; Tomić, S.; Waisundara, V.Y. Forestalling the Epidemics of Parkinson’s Disease Through Plant-Based Remedies. Front. Nutr. 2018, 30, 95. [Google Scholar] [CrossRef] [PubMed]
- Reith, W. Neurodegenerative diseases. Radiologe 2018, 58, 241–258. [Google Scholar] [CrossRef] [PubMed]
- Rojas-García, A.; Fernández-Ochoa, Á.; Cádiz-Gurrea, M.L.; Arráez-Román, D.; Segura-Carretero, A. Neuroprotective Effects of Agri-Food By-Products Rich in Phenolic Compounds. Nutrients 2023, 15, 449. [Google Scholar] [CrossRef] [PubMed]
- Scapagnini, G.; Vasto, S.; Abraham, N.G.; Caruso, C.; Zella, D.; Fabio, G. Modulation of Nrf2/ARE pathway by food polyphenols: A nutritional neuroprotective strategy for cognitive and neurodegenerative disorders. Mol. Neurobiol. 2011, 44, 192–201. [Google Scholar] [CrossRef] [Green Version]
- Macready, A.L.; Kennedy, O.B.; Ellis, J.A.; Williams, C.M.; Spencer, J.P.; Butler, L.T. Flavonoids and cognitive function: A review of human randomized controlled trial studies and recommendations for future studies. Genes Nutr. 2009, 4, 227–242. [Google Scholar] [CrossRef] [Green Version]
- Gomez-Pinilla, F.; Nguyen, T.T. Natural mood foods: The actions of polyphenols against psychiatric and cognitive disorders. Nutr. Neurosci. 2012, 15, 127–133. [Google Scholar] [CrossRef] [Green Version]
- Silva, R.F.M.; Pogačnik, L. Polyphenols from Food and Natural Products: Neuroprotection and Safety. Antioxidants 2020, 9, 61. [Google Scholar] [CrossRef] [Green Version]
- Chahar, M.K.; Sharma, N.; Dobhal, M.P.; Joshi, Y.C. Flavonoids: A versatile source of anticancer drugs. Pharmacogn. Rev. 2011, 5, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Leclerc, M.; Dudonné, S.; Calon, F. Can Natural Products Exert Neuroprotection without Crossing the Blood-Brain Barrier? Int. J. Mol. Sci. 2021, 22, 3356. [Google Scholar] [CrossRef]
- Yang, L.; Wen, K.S.; Ruan, X.; Zhao, Y.X.; Wei, F.; Wang, Q. Response of Plant Secondary Metabolites to Environmental Factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef] [Green Version]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [Green Version]
- Di Meo, F.; Valentino, A.; Petillo, O.; Peluso, G.; Filosa, S.; Crispi, S. Bioactive Polyphenols and Neuromodulation: Molecular Mechanisms in Neurodegeneration. Int. J. Mol. Sci. 2020, 21, 2564. [Google Scholar] [CrossRef] [Green Version]
- Campos-Esparza, M.R.; Torres-Ramos, M. Neuroprotection by Natural Polyphenols: Molecular Mechanisms. Cent. Nerv. Syst. Agents Med. Chem. 2010, 10, 269–277. [Google Scholar] [CrossRef]
- Seward, M.E.; Swanson, E.; Norambuena, A.; Reimann, A.; Cochran, J.N.; Li, R.; Roberson, E.D.; Bloom, G.S. Amyloid-β signals through tau to drive ectopic neuronal cell cycle re-entry in Alzheimer’s disease. J. Cell Sci. 2013, 126, 1278–1286. [Google Scholar] [CrossRef] [Green Version]
- Chi, H.; Chang, H.Y.; Sang, T.K. Neuronal Cell Death Mechanisms in Major Neurodegenerative Diseases. Int. J. Mol. Sci. 2018, 19, 3082. [Google Scholar] [CrossRef] [Green Version]
- Uddin, M.S.; Al Mamun, A.; Kabir, M.T.; Ahmad, J.; Jeandet, P.; Sarwar, M.S.; Ashraf, G.M.; Aleya, L. Neuroprotective role of polyphenols against oxidative stress-mediated neurodegeneration. Eur. J. Pharmacol. 2020, 886, 173412. [Google Scholar] [CrossRef]
- Dugger, B.N.; Hentz, J.G.; Adler, C.H.; Sabbagh, M.N.; Shill, H.A.; Jacobson, S.; Caviness, J.N.; Belden, C.; Driver-Dunckley, E.; Davis, K.J.; et al. Clinicopathological outcomes of prospectively followed normal elderly brain bank volunteers. J. Neuropathol. Exp. Neurol. 2014, 73, 244–252. [Google Scholar] [CrossRef] [Green Version]
- Dugger, B.N.; Dickson, D.W. Pathology of Neurodegenerative Diseases. Cold Spring Harb. Perspect. Biol. 2017, 9, a028035. [Google Scholar] [CrossRef] [Green Version]
- Kovacs, G.G. Concepts and classification of neurodegenerative diseases. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2017; Volume 145, pp. 301–307. [Google Scholar] [CrossRef]
- Figueira, I.; Menezes, R.; Macedo, D.; Costa, I.; Dos Santos, C.N. Polyphenols Beyond Barriers: A Glimpse into the Brain. Curr. Neuropharmacol. 2017, 15, 562–594. [Google Scholar] [CrossRef] [Green Version]
- Assunção, M.; Santos-Marques, M.J.; Carvalho, F.; Lukoyanov, N.V.; Andrade, J.P. Chronic green tea consumption prevents age-related changes in rat hippocampal formation. Neurobiol. Aging 2011, 32, 707–717. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, Y.; Li, J.; Fu, C.; Zhang, X. The Neuroprotective Effect of Tea Polyphenols on the Regulation of Intestinal Flora. Molecules 2021, 26, 3692. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Fang, P.; An, J.; Lin, H.; Liang, Y.; Shen, W.; Leng, X.; Zhang, C.; Zheng, Y.; Qiu, S. Micro-structural white matter abnormalities in type 2 diabetic patients: A DTI study using TBSS analysis. Neuroradiology 2016, 58, 1209–1216. [Google Scholar] [CrossRef] [PubMed]
- Ferris, J.K.; Inglis, J.T.; Madden, K.M.; Boyd, L.A. Brain and Body: A Review of Central Nervous System Contributions to Movement Impairments in Diabetes. Diabetes 2020, 69, 3–11. [Google Scholar] [CrossRef]
- Erus, G.; Battapady, H.; Zhang, T.; Lovato, J.; Miller, M.E.; Williamson, J.D.; Launer, L.J.; Bryan, R.N.; Davatzikos, C. Spatial patterns of structural brain changes in type 2 diabetic patients and their longitudinal progression with intensive control of blood glucose. Diabetes Care 2015, 38, 97–104. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Xue, R.; Hu, R. The neuroprotective effect and action mechanism of polyphenols in diabetes mellitus-related cognitive dysfunction. Eur. J. Nutr. 2020, 59, 1295–1311. [Google Scholar] [CrossRef]
- Biessels, G.J.; Strachan, M.W.; Visseren, F.L.; Kappelle, L.J.; Whitmer, R.A. Dementia and cognitive decline in type 2 diabetes and prediabetic stages: Towards targeted interventions. Lancet Diabetes Endocrinol. 2014, 2, 246–255. [Google Scholar] [CrossRef]
- Bergantin, L.B. Hypertension, Diabetes and Neurodegenerative Diseases: Is there a Clinical Link through the Ca2+/cAMP Signalling Interaction? Curr. Hypertens. Rev. 2019, 15, 32–39. [Google Scholar] [CrossRef]
- Ungvari, Z.; Toth, P.; Tarantini, S.; Prodan, C.I.; Sorond, F.; Merkely, B.; Csiszar, A. Hypertension-induced cognitive impairment: From pathophysiology to public health. Nat. Rev. Nephrol. 2021, 17, 639–654. [Google Scholar] [CrossRef]
- Chukwuma, C.I.; Matsabisa, M.G.; Ibrahim, M.A.; Erukainure, O.L.; Chabalala, M.H.; Islam, M.S. Medicinal plants with concomitant anti-diabetic and anti-hypertensive effects as potential sources of dual acting therapies against diabetes and hypertension: A review. J. Ethnopharmacol. 2019, 235, 329–360. [Google Scholar] [CrossRef]
- Hachkova, H.; Nagalievska, M.; Soliljak, Z.; Kanyuka, O.; Kucharska, A.Z.; Sokół-Łętowska, A.; Belonovskaya, E.; Buko, V.; Sybirna, N. Medicinal Plants Galega officinalis L. and Yacon Leaves as Potential Sources of Antidiabetic Drugs. Antioxidants 2021, 10, 1362. [Google Scholar] [CrossRef]
- Williams, D.H.; Stone, M.J.; Hauck, P.R.; Rahman, S.K. Why are secondary metabolites (natural products) biosynthesized? J. Nat. Prod. 1989, 52, 1189–1208. [Google Scholar] [CrossRef]
- Madariaga-Mazón, A.; Hernández-Alvarado, R.B.; Noriega-Colima, K.O.; Osnaya-Hernández, A.; Martinez-Mayorga, K. Toxicity of secondary metabolites. Phys. Sci. Rev. 2019, 4, 20180116. [Google Scholar] [CrossRef]
- Erb, M.; Kliebenstein, D.J. Plant Secondary Metabolites as Defenses, Regulators, and Primary Metabolites: The Blurred Functional Trichotomy. Plant Physiol. 2020, 184, 39–52. [Google Scholar] [CrossRef]
- Arowolo, M.A.; He, J. Use of probiotics and botanical extracts to improve ruminant production in the tropics: A review. Anim. Nutr. 2018, 4, 241–249. [Google Scholar] [CrossRef]
- Chen, D.; Mubeen, B.; Hasnain, A.; Rizwan, M.; Adrees, M.; Naqvi, S.A.H.; Iqbal, S.; Kamran, M.; El-Sabrout, A.M.; Elansary, H.O.; et al. Role of Promising Secondary Metabolites to Confer Resistance Against Environmental Stresses in Crop Plants: Current Scenario and Future Perspectives. Front. Plant Sci. 2022, 13, 881032. [Google Scholar] [CrossRef]
- Debbab, A.; Aly, A.H.; Proksch, P. Bioactive secondary metabolites from endophytes and associated marine derived fungi. Fungal Divers. 2011, 49, 1–12. [Google Scholar] [CrossRef]
- Croteau, R.; Kutchan, T.; Lewis, N.G. Natural products (secondary metabolites). In Biochemistry & Molecular Biology of Plants; Buchanan, B., Gruissem, W., Jones, R., Eds.; American Society of Plant Physiologists: Rockville, MD, USA, 2000; pp. 1250–1319. [Google Scholar]
- Orozco, M.F.; Vázquez-Hernández, A.; Fenton-Navarro, B. Active compounds of medicinal plants, mechanism for antioxidant and beneficial effects. Phyton 2019, 88, 1–10. [Google Scholar] [CrossRef]
- Saldivar, J.C.; Hamperl, S.; Bocek, M.J.; Chung, M.; Bass, T.E.; Cisneros-Soberanis, F.; Samejima, K.; Xie, L.; Paulson, J.R.; Earnshaw, W.C.; et al. An intrinsic S/G2 checkpoint enforced by ATR. Science 2018, 361, 806–810. [Google Scholar] [CrossRef] [Green Version]
- Renaud, J.; Martinoli, M.G. Considerations for the Use of Polyphenols as Therapies in Neurodegenerative Diseases. Int. J. Mol. Sci. 2019, 20, 1883. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Zhao, W.; Wang, J.; Xu, Q.; Li, S.; Yin, C. Imaging Diagnosis of Central Nervous System Damage in Patients with T2DM. Neurosci. Lett. 2020, 33, 135092. [Google Scholar] [CrossRef] [PubMed]
- Tzin, V.; Galili, G. New insights into the shikimate and aromatic amino acids biosynthesis pathways in plants. Mol. Plant 2010, 3, 956–972. [Google Scholar] [CrossRef] [PubMed]
- Mattioli, R.; Francioso, A.; Mosca, L.; Silva, P. Anthocyanins: A Comprehensive Review of Their Chemical Properties and Health Effects on Cardiovascular and Neurodegenerative Diseases. Molecules 2020, 25, 3809. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Dudareva, N. The shikimate pathway and aromatic amino Acid biosynthesis in plants. Annu. Rev. Plant Biol. 2012, 63, 73–105. [Google Scholar] [CrossRef]
- Lamport, D.J.; Williams, C.M. Polyphenols and Cognition In Humans: An Overview of Current Evidence from Recent Systematic Reviews and Meta-Analyses. Brain Plast. 2021, 6, 139–153. [Google Scholar] [CrossRef]
- Silveira, A.C.; Dias, J.P.; Santos, V.M.; Oliveira, P.F.; Alves, M.G.; Rato, L.; Silva, B.M. The Action of Polyphenols in Diabetes Mellitus and Alzheimer’s Disease: A Common Agent for Overlapping Pathologies. Curr. Neuropharmacol. 2019, 17, 590–613. [Google Scholar] [CrossRef] [Green Version]
- Koudoufio, M.; Desjardins, Y.; Feldman, F.; Spahis, S.; Delvin, E.; Levy, E. Insight into Polyphenol and Gut Microbiota Crosstalk: Are Their Metabolites the Key to Understand Protective Effects against Metabolic Disorders? Antioxidants 2020, 9, 982. [Google Scholar] [CrossRef]
- Caruso, G.; Godos, J.; Privitera, A.; Lanza, G.; Castellano, S.; Chillemi, A.; Bruni, O.; Ferri, R.; Caraci, F.; Grosso, G. Phenolic Acids and Prevention of Cognitive Decline: Polyphenols with a Neuroprotective Role in Cognitive Disorders and Alzheimer’s Disease. Nutrients 2022, 14, 819. [Google Scholar] [CrossRef]
- Kozłowska, A.; Szostak-Wegierek, D. Flavonoids-food sources and health benefits. Rocz. Panstw. Zakl. Hig. 2014, 65, 79–85. [Google Scholar]
- Dueñas, M.; Cueva, C.; Muñoz-González, I.; Jiménez-Girón, A.; Sánchez-Patán, F.; Santos-Buelga, C.; Moreno-Arribas, M.V.; Bartolomé, B. Studies on Modulation of Gut Microbiota by Wine Polyphenols: From Isolated Cultures to Omic Approaches. Antioxidants 2015, 4, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Teng, H.; Chen, L. Polyphenols and bioavailability: An update. Crit. Rev. Food Sci. Nutr. 2019, 59, 2040–2051. [Google Scholar] [CrossRef]
- Kasprzak-Drozd, K.; Oniszczuk, T.; Stasiak, M.; Oniszczuk, A. Beneficial Effects of Phenolic Compounds on Gut Microbiota and Metabolic Syndrome. Int. J. Mol. Sci. 2021, 22, 3715. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, W.; Zhang, L.; Wang, M.; Chang, W. The Interaction of Polyphenols and the Gut Microbiota in Neurodegenerative Diseases. Nutrients 2022, 14, 5373. [Google Scholar] [CrossRef]
- Cottart, C.H.; Nivet-Antoine, V.; Laguillier-Morizot, C.; Beaudeux, J.L. Resveratrol bioavailability and toxicity in humans. Mol. Nutr. Food Res. 2010, 54, 7–16. [Google Scholar] [CrossRef]
- Czerwiecki, L. Współczesne poglady na role przeciwutleniaczy roślinnych w profilaktyce chorób cywilizacyjnych [Contemporary view of plant antioxidants role in prevention of civilization diseases]. Rocz. Panstw. Zakl. Hig. 2009, 60, 201–206. [Google Scholar]
- Uno, T.; Yasui-Furukori, N. Effect of grapefruit juice in relation to human pharmacokinetic study. Curr. Clin. Pharmacol. 2006, 1, 157–161. [Google Scholar] [CrossRef]
- Koga, N.; Ohta, C.; Kato, Y.; Haraguchi, K.; Endo, T.; Ogawa, K.; Ohta, H.; Yano, M. In vitro metabolism of nobiletin, a polymethoxy-flavonoid, by human liver microsomes and cytochrome P450. Xenobiotica 2011, 41, 927–933. [Google Scholar] [CrossRef]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, R.; Khurana, N.; Singh, S.K.; Khurana, S.; Verma, S.; Sharma, N.; Vyas, M.; Dua, K.; Khursheed, R.; et al. Improved neuroprotective activity of Fisetin through SNEDDS in ameliorating the behavioral alterations produced in rotenone-induced Parkinson’s model. Environ. Sci. Pollut. Res. Int. 2022, 29, 50488–50499. [Google Scholar] [CrossRef]
- Ward, R.J.; Dexter, D.T.; Crichton, R.R. Ageing, neuroinflammation and neurodegeneration. Front. Biosci. 2015, 7, 189–204. [Google Scholar] [CrossRef]
- Santos, V.H.; Minatel, I.O.; Lima, G.P.; Silva, R.M.; Chen, C.Y.O. Antioxidant capacity and phytochemical characterization of Spathodea campanulata growing in different climatic zones in Brazil. Biocatal. Agric. Biotechnol. 2020, 24, 101536–101544. [Google Scholar] [CrossRef]
- Mandel, S.; Youdim, M.H. Catechin polyphenols: Neurodegeneration and neuroprotection in neurodegenerative diseases. Free Radic. Biol. Med. 2004, 37, 304–317. [Google Scholar] [CrossRef] [PubMed]
- Quiñones, M.; Miguel, M.; Aleixandre, A. Los polifenoles, compuestos de origen natural con efectos saludables sobre el sistema cardiovascular [The polyphenols, naturally occurring compounds with beneficial effects on cardiovascular disease]. Nutr. Hosp. 2012, 27, 76–89. [Google Scholar] [CrossRef] [PubMed]
- Bhullar, K.S.; Rupasinghe, H.P. Polyphenols: Multipotent therapeutic agents in neurodegenerative diseases. Oxid. Med. Cell. Longev. 2013, 2013, 891748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Youdim, K.A.; Qaiser, M.Z.; Begley, D.J.; Rice-Evans, C.A.; Abbott, N.J. Flavonoid permeability across an in situ model of the blood-brain barrier. Free Radic. Biol. Med. 2004, 36, 592–604. [Google Scholar] [CrossRef]
- Frade, J.G.; Ferreira, N.R.; Barbosa, R.M.; Laranjinha, J. Mechanisms of neuroprotection by polyphenols. Cent. Nerv. Syst. Agents Med. Chem. 2005, 5, 307–318. [Google Scholar] [CrossRef]
- Rendeiro, C.; Rhodes, J.S.; Spencer, J.P. The mechanisms of action of flavonoids in the brain: Direct versus indirect effects. Neurochem. Int. 2015, 89, 126–139. [Google Scholar] [CrossRef]
- Vauzour, D.; Vafeiadou, K.; Rodriguez-Mateos, A.; Rendeiro, C.; Spencer, J.P. The neuroprotective potential of flavonoids: A multiplicity of effects. Genes Nutr. 2008, 3, 115–126. [Google Scholar] [CrossRef] [Green Version]
- Bari, A.; Shah, S.M.M.; Al-Joufi, F.A.; Shah, S.W.A.; Shoaib, M.; Shah, I.; Zahoor, M.; Ahmed, M.N.; Ghias, M.; Shah, S.M.H.; et al. Effects of Artemisia macrocephala Jacquem on Memory Deficits and Brain Oxidative Stress in Streptozotocin-Induced Diabetic Mice. Molecules 2022, 27, 2399. [Google Scholar] [CrossRef]
- Bastianetto, S.; Zheng, W.H.; Quirion, R. The Ginkgo biloba extract (EGb 761) protects and rescues hippocampal cells against nitric oxide-induced toxicity: Involvement of its flavonoid constituents and protein kinase C. J. Neurochem. 2000, 74, 2268–2277. [Google Scholar] [CrossRef]
- Letenneur, L.; Proust-Lima, C.; Le Gouge, A.; Dartigues, J.F.; Barberger-Gateau, P. Flavonoid intake and cognitive decline over a 10-year period. Am. J. Epidemiol. 2007, 165, 1364–1371. [Google Scholar] [CrossRef]
- Murillo Ortíz, B.; Ramírez Emiliano, J.; Ramos-Rodríguez, E.; Martínez-Garza, S.; Macías-Cervantes, H.; Solorio-Meza, S.; Pereyra-Nobara, T.A. Brain-derived neurotrophic factor plasma levels and premature cognitive impairment/dementia in type 2 diabetes. World J. Diabetes 2016, 7, 615–620. [Google Scholar] [CrossRef]
- Bathina, S.; Das, U.N. Brain-derived neurotrophic factor and its clinical implications. Arch. Med. Sci. 2015, 11, 1164–1178. [Google Scholar] [CrossRef]
- Zeng, P.; Fang, M.; Zhao, H.; Guo, J. A network pharmacology approach to uncover the key ingredients in Ginkgo Folium and their anti-Alzheimer’s disease mechanisms. Aging 2021, 13, 18993–19012. [Google Scholar] [CrossRef]
- Baek, S.C.; Park, M.H.; Ryu, H.W.; Lee, J.P.; Kang, M.G.; Park, D.; Park, C.M.; Oh, S.R.; Kim, H. Rhamnocitrin isolated from Prunus padus var. seoulensis: A potent and selective reversible inhibitor of human monoamine oxidase A. Bioorg. Chem. 2019, 83, 317–325. [Google Scholar] [CrossRef]
- Binder, D.K.; Scharfman, H.E. Brain-derived neurotrophic factor. Growth Factors 2004, 22, 123–131. [Google Scholar] [CrossRef] [Green Version]
- Rozanska, O.; Uruska, A.; Zozulinska-Ziolkiewicz, D. Brain-Derived Neurotrophic Factor and Diabetes. Int. J. Mol. Sci. 2020, 21, 841. [Google Scholar] [CrossRef] [Green Version]
- Zhen, Y.F.; Zhang, J.; Liu, X.Y.; Fang, H.; Tian, L.B.; Zhou, D.H.; Kosten, T.R.; Zhang, X.Y. Low BDNF is associated with cognitive deficits in patients with type 2 diabetes. Psychopharmacology 2013, 227, 93–100. [Google Scholar] [CrossRef]
- Moosavi, F.; Hosseini, R.; Saso, L.; Firuzi, O. Modulation of neurotrophic signaling pathways by polyphenols. Drug Des. Devel. Ther. 2015, 10, 23–42. [Google Scholar] [CrossRef] [Green Version]
- Mohammadi, A.; Amooeian, V.G.; Rashidi, E. Dysfunction in Brain-Derived Neurotrophic Factor Signaling Pathway and Susceptibility to Schizophrenia, Parkinson’s and Alzheimer’s Diseases. Curr. Gene Ther. 2018, 18, 45–63. [Google Scholar] [CrossRef]
- Srivastava, P.; Dhuriya, Y.K.; Kumar, V.; Srivastava, A.; Gupta, R.; Shukla, R.K.; Yadav, R.S.; Dwivedi, H.N.; Pant, A.B.; Khanna, V.K. PI3K/Akt/GSK3β induced CREB activation ameliorates arsenic mediated alterations in NMDA receptors and associated signaling in rat hippocampus: Neuroprotective role of curcumin. Neurotoxicology 2018, 67, 190–205. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Xie, D.J.; Zhou, P.; Gao, H.W.; Zhang, M.T.; Chen, D.B.; Qin, Y.P.; Lei, X.; Li, X.Q.; Liu, J.; et al. Huang-Pu-Tong-Qiao Formula Ameliorates the Hippocampus Apoptosis in Diabetic Cognitive Dysfunction Mice by Activating CREB/BDNF/TrkB Signaling Pathway. Evid. Based Complement. Altern. Med. 2021, 2021, 5514175. [Google Scholar] [CrossRef] [PubMed]
- Bhakkiyalakshmi, E.; Dineshkumar, K.; Karthik, S.; Sireesh, D.; Hopper, W.; Paulmurugan, R.; Ramkumar, K.M. Pterostilbene-mediated Nrf2 activation: Mechanistic insights on Keap1:Nrf2 interface. Bioorg. Med. Chem. 2016, 24, 3378–3386. [Google Scholar] [CrossRef] [PubMed]
- Si, T.L.; Liu, Q.; Ren, Y.F.; Li, H.; Xu, X.Y.; Li, E.H.; Pan, S.Y.; Zhang, J.L.; Wang, K.X. Enhanced anti-inflammatory effects of DHA and quercetin in lipopolysaccharide-induced RAW264.7 macrophages by inhibiting NF-κB and MAPK activation. Mol. Med. Rep. 2016, 14, 499–508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fonseca-Kelly, Z.; Nassrallah, M.; Uribe, J.; Khan, R.S.; Dine, K.; Dutt, M.; Shindler, K.S. Resveratrol neuroprotection in a chronic mouse model of multiple sclerosis. Front. Neurol. 2012, 3, 84. [Google Scholar] [CrossRef] [Green Version]
- Ho, D.J.; Calingasan, N.Y.; Wille, E.; Dumont, M.; Beal, M.F. Resveratrol protects against peripheral deficits in a mouse model of Huntington’s disease. Exp. Neurol. 2010, 225, 74–84. [Google Scholar] [CrossRef]
- Schneider, S.A.; Hardy, J.; Bhatia, K.P. Syndromes of neurodegeneration with brain iron accumulation (NBIA): An update on clinical presentations, histological and genetic underpinnings, and treatment considerations. Mov. Disord. 2012, 27, 42–53. [Google Scholar] [CrossRef]
- Filosa, S.; Di Meo, F.; Crispi, S. Polyphenols-gut microbiota interplay and brain neuromodulation. Neural Regen. Res. 2018, 13, 2055–2059. [Google Scholar] [CrossRef]
- Banc, R.; Rusu, M.E.; Filip, L.; Popa, D.S. The Impact of Ellagitannins and Their Metabolites through Gut Microbiome on the Gut Health and Brain Wellness within the Gut-Brain Axis. Foods 2023, 12, 270. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arias-Sánchez, R.A.; Torner, L.; Fenton Navarro, B. Polyphenols and Neurodegenerative Diseases: Potential Effects and Mechanisms of Neuroprotection. Molecules 2023, 28, 5415. https://doi.org/10.3390/molecules28145415
Arias-Sánchez RA, Torner L, Fenton Navarro B. Polyphenols and Neurodegenerative Diseases: Potential Effects and Mechanisms of Neuroprotection. Molecules. 2023; 28(14):5415. https://doi.org/10.3390/molecules28145415
Chicago/Turabian StyleArias-Sánchez, Raziel Alejandro, Luz Torner, and Bertha Fenton Navarro. 2023. "Polyphenols and Neurodegenerative Diseases: Potential Effects and Mechanisms of Neuroprotection" Molecules 28, no. 14: 5415. https://doi.org/10.3390/molecules28145415